Abstract
Please cite this paper as: Snacken et al. (2012) Surveillance of hospitalised severe cases of Influenza A(H1N1)pdm09 and related fatalities in nine EU countries in 2010–2011. Influenza and Other Respiratory Viruses 6(601), e93–e96.
A few months into the 2009 influenza pandemic, nine European countries implemented case‐based surveillance of hospitalised severe influenza infections. In the present study, we assess the association between patient characteristics, in particular underlying conditions, and the severity level of influenza A(H1N1)pdm09 infection during the 2010‐2011 season. Patient age, the presence of underlying conditions, pneumonia, acute respiratory distress syndrome (ARDS) and the need for ventilation were significantly associated with the severity of influenza A(H1N1)pdm09 infection. Despite limitations essentially because of the heterogeneity of the data reported, this study provides insight into severe influenza cases.
Keywords: Europe, influenza A(H1N1)pdm09, risk factors, severity, surveillance
Background
The severity of influenza disease is usually estimated retrospectively through observational studies on hospitalisation rates 1 or through mortality data. 2 In addition to these, the surveillance of hospitalised severe laboratory‐confirmed influenza cases was implemented for the first time following the emergence of the influenza A(H1N1)pdm09 pandemic virus. In 2010–2011, data reported on a voluntary basis to the European Centre for Disease Prevention and Control (ECDC) were standardised across countries enabling timely pooled data analysis of severe influenza cases across a sample of EU countries, which resulted in a larger sample size of the population assessed. The objective of this study was to describe the demographic, clinical characteristics and vaccination status among hospitalised severe A(H1N1)pdm09 influenza cases.
Methods
During the 2010–2011 season (weeks 40–20), case‐based data for hospitalised influenza infections were uploaded weekly on a voluntary basis to The European Surveillance System (TESSy) by Austria, Finland, France, Ireland, Malta, Portugal, Romania, Slovakia and Spain.
The following information was collected on each case for analysis: laboratory confirmation of influenza A(H1N1)pdm09 infection, type of hospitalisation (inpatients admitted to regular care versus patients in ICU) and outcome (non‐fatal versus fatal). Patients without all of the above‐mentioned data were excluded from further analysis. In addition, demographic data (age and gender), clinical data (underlying conditions, complications and oxygen support) and vaccination status were collected. Patients were categorised into three groups according to the level of severity of disease: group 1 = non‐fatal cases admitted to regular inpatient care, group 2 = non‐fatal cases admitted to ICU and group 3 = fatal cases. Descriptive statistics, chi‐squared tests and regression models for calculating the overall statistical association across the three severity groups (P < 0·05) and correlation coefficient (R 2) were performed using Stata Statistical Software (Release 12: StataCorp 2011, College Station, TX, USA)
Results
Of the 3292 cases reported, 1021 (31%) were excluded as they did not report outcome (20·3%), type of hospitalisation (4·9%) and/or laboratory confirmation of influenza A(H1N1)pdm09 infection (10·2%). After excluding these cases, a total of 2271 hospitalised laboratory‐confirmed influenza A(H1N1)pdm09 were included in the analysis. The distribution of A(H1N1)pdm09 cases is tabulated in Table 1.
Table 1.
Distribution of A(H1N1)pdm09 cases across countries
| Countries | Number of cases | % |
|---|---|---|
| Austria | 371 | 16·3 |
| Spain | 854 | 37·6 |
| Finland | 58 | 2·6 |
| France | 441 | 19·4 |
| Ireland | 105 | 4·6 |
| Malta | 48 | 2·1 |
| Portugal | 284 | 12·5 |
| Romania | 83 | 3·7 |
| Slovakia | 27 | 1·2 |
| Total | 2271 | 100 |
The 2271 cases were divided into three groups: 1056 patients were classified as the least severe (group 1), 860 as ICU non‐fatal cases (group 2) and 355 as fatal cases (group 3). Key patient characteristics and their statistical association with severity of infection, defined by group, are displayed in Table 2.
Table 2.
Influenza A(H1N1)pdm09 patient characteristics by group and the level of association (chi‐squared tests; P‐value) across the three disease severity groups
| Patient Characteristics | Group 1 | Group 2 | Group 3 | P‐value | |||
|---|---|---|---|---|---|---|---|
| Non fatal inpatients not in ICU | Non fatal patients in ICU | Fatal cases | |||||
| n*/N** | % | n/N | % | n/N | % | ||
| Age <1 year | 66/1056 | 6·3 | 21/860 | 2·4 | 3/355 | 0·8 | *** |
| Age ≥65 year | 186/1056 | 17·6 | 104/860 | 12·1 | 80/355 | 22·5 | 0·8 |
| Male | 575/1054 | 54·6 | 484/856 | 56·5 | 200/354 | 65·5 | 0·4 |
| Vaccinated | 92/635 | 14·5 | 95/619 | 15·3 | 33/210 | 15·7 | 0·6 |
| More than 1 condition | 428/649 | 66·0 | 422/579 | 72·9 | 225/255 | 88·2 | <0·01 |
| Complications: | <0·01 | ||||||
| ARDS | 53/711 | 7·5 | 351/649 | 54·1 | 151/309 | 48·9 | <0·01 |
| Pneumonia | 448/699 | 64·1 | 291/640 | 45·5 | 164/307 | 53·4 | <0·01 |
| Need for ventilation | 10/388 | 2·6 | 451/535 | 84·3 | 171/186 | 91·9 | <0·01 |
*Observation number.
**Total number of observations in the dataset.
***No P‐value due to low sample size.
Median patient age was significantly (P < 0·01) associated with the level of disease severity and was 41, 48 and 53 years respectively. The percentage of patients immunised against the influenza A(H1N1)pdm09 virus, with the mono‐ or trivalent vaccines available in 2010, was similar across the three groups: 14·5%, 15·3% and 15·7%, respectively. Of 1483 patients for whom information was available, 1075 (72·5%) reported at least one underlying condition and 408 (27·5%) reported no underlying condition. In addition, the prevalence of one or more underlying conditions was significantly associated with the level of severity and increased with increasing severity: 66%, 72·9% and 88·2% in the three groups, respectively. Chronic lung diseases (including asthma), diabetes mellitus, heart diseases and HIV represented 51% of patients with documented underlying conditions (n = 1483). In addition, the prevalence of obesity and pregnancy was, respectively, 14·5% and 4·9%. The vast majority of fatal cases (88·2%) occurred in patients with at least one underlying condition (n = 225) resulting in a case fatality ratio (CFR) of 20·9%, while the proportion of deaths in patients without underlying conditions (n = 30) was 11·8% (χ2 = 38·29, P < 0·001) resulting in a CFR of 7·4%. Obesity, including morbid obesity (BMI ≥ 40), was significantly associated with the disease severity. Of 215 obese persons, 46 (21·4%) had at least one additional underlying condition. Of 72 pregnant women for whom information on underlying conditions was available, one had an additional underlying condition and survived, while eleven (15·3%) had no additional underlying conditions, yet died in ICU. The prevalence of the main clinical complications, that is, secondary pneumonia and acute respiratory distress syndrome (ARDS), was significantly associated with disease severity. Oxygen ventilation was required in most of the fatal cases and in most of the non‐fatal cases admitted to the ICU, but hardly ever in the non‐fatal cases admitted to regular care. The majority (77·5%) of deaths occurred in patients aged less than 65 years. Nevertheless, the CFR increased with increasing age and was highly correlated with age (R 2 = 0·98) ranging from 3·9% in 0 to 4‐year‐olds up to 20·8% in ≥65‐year‐olds (Figure 1).
Figure 1.
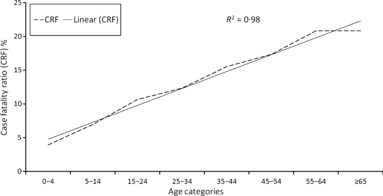
Correlation between case fatality ratio and pre‐defined age groups.
Discussion
Our study showed that age, underlying medical conditions, clinical complications and the need for ventilation were significantly associated with the severity of the influenza A(H1N1)pdm09 infection. Comparable results were reported by other studies with a similar focus on hospitalised influenza A(H1N1)pdm09 cases, even though their frameworks and settings differed from ours. Increasing age was significantly associated with the level of severity as confirmed by other studies. 3 , 4 Similar to seasonal influenza outbreaks, patients with underlying conditions, in particular chronic lung and heart disease, were at increased risk of more severe outcomes. 4 , 5 , 6 Furthermore, obesity, in particular morbid obesity, and pregnancy were also considered as particular risk factors for complications of A(H1N1)pdm09 infection. 5 , 7 , 8 , 9 As vaccination coverage was unexpectedly not significantly associated with the level of disease severity, further studies about vaccine coverage and effectiveness in severe A(H1N1)pdm09 influenza cases are needed. The low proportion of additional underlying conditions in obese patients and in pregnant women in comparison with other studies, 6 , 10 , 11 , 12 , 13 even during influenza seasons, 14 might be explained by possible under‐reporting. Additional reasons might be due to reporting differences between countries that were impossible to evaluate in this study. As observed in other studies, 4 , 10 the proportion of patients with no underlying condition is noteworthy. Not surprisingly, cases with at least one underlying condition accounted for the vast majority of deaths. The very significant linear increase of mortality with increasing age might be related to a higher proportion of underlying conditions in older patients. Severe pulmonary complications and the need for ventilation increased with the level of severity as also observed in other studies. 4 , 15
The added value of this severe influenza surveillance is its EU dimension and the first‐ever implementation of surveillance of severe influenza cases where data were uploaded to The European Surveillance System (TESSy) of ECDC in a timely fashion. Also this study provides confirmation to previously conducted observational studies based on surveillance data.
One possible limitation of the study includes, as in all facility‐based studies, the representativeness of the data. In most cases, it was impossible to determine the population denominator for the cases reported, as some countries reporting collected data from robust sentinel sites with known denominators, while other used opportunistic samples from hospitals. This limitation prevented the calculation of, for example, disease incidence rates. Also, antiviral prophylaxis, non‐pharmaceutical interventions and criteria for hospital admission differed substantially across countries and even over time. Direct comparisons of results from this study with those from other studies carried out during the pandemic season need to be interpreted with caution as the pattern and the outcome of influenza A(H1N1)pdm09 infection may be different.
In conclusion, despite the limitations, our key results provide insight into the main characteristics of hospitalised severe A(H1N1)pdm09 influenza cases and are consistent with those from other studies. These results highlight the necessity for the standardisation of the surveillance of severe influenza at EU level to support member states in developing and maintaining suitable surveillance systems.
Acknowledgements
The authors wish to thank Amparo Larrauri Cámara, Liliana Cuschieri, Concha Delgado, Lisa Domegan, Carlos Manuel de Orta Gomes, Silvia Jiménez‐Jorge, Outi Lyytikäinen, Tanya Melillo, Monika Musilová, Odette Nicolae and also members of the EISN network from Austria, Finland, France, Ireland, Malta, Portugal, Romania, Slovakia and Spain. We would like to thank the CNRL members and the ECDC’s TESSy team, in particular Silvia Sarbu, and also Anastasia Pharris for editorial assistance.
References
- 1. Thomson WW, Shay DK, Weintraub E et al. Influenza–associated hospitalizations in the United States. JAMA 2004; 292:1333–1340. [DOI] [PubMed] [Google Scholar]
- 2. Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Publ Health 1997; 87:1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Kerkhove MD, Vandemaele KAH, Shinde V et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Medicine 2011; 8:e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van‘t Klooster TM, Wielders CC, Donker T et al. Surveillance of hospitalisations for 2009 pandemic influenza A(H1N1) in the Netherlands, 5 June‐31‐December 2009. Euro Surveill 2010; 15:pii=19461. [DOI] [PubMed] [Google Scholar]
- 5. Shiley KT, Nadolski G, Mickus T, Fishman NO, Lautenbach E. Differences in the epidemiological characteristics and clinical outcomes of pandemic (H1N1) 2009 influenza, compared with seasonal influenza. Infect Control Hosp Epidemiol 2010; 31:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skarbinski J, Jain S, Bramley A et al. Hospitalized patients with 2009 Pandemic Influenza A (H1N1) virus infection in the United States—September–October 2009. Clin Infect Dis 2011; 52:S50–S59. [DOI] [PubMed] [Google Scholar]
- 7. Pebody RG, McLean E, Zhao H et al. Pandemic Influenza A (H1N1) 2009 and mortality in the United Kingdom: risk factors for death, April 2009 to March 2010. Euro Surveill 2010; 15:pii=19571. [PubMed] [Google Scholar]
- 8. Morgan OW, Bramley A, Fowlkes A et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 Pandemic Influenza A(H1N1) disease. PLoS ONE 2010; 5:e9694 Doi: 10.1371/journal.pone.0009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller RR, Markewitz BA, Rolfs RT et al. Clinical findings and demographic factors associated with ICU admission in utah due to novel 2009 influenza A(H1N1) infection. Chest 2010; 137:752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Louie JK, Acosta M, Winter K et al. Factors associated with death or hospitalization due to 2009 pandemic Influenza A(H1N1) infection in California. JAMA 2009; 302:1896–1902. [DOI] [PubMed] [Google Scholar]
- 11. Louie JK, Acosta M, Jamieson DJ, Honein MA, for the California Pandemic (H1N1) Working Group . Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med 2010; 362:27–35. [DOI] [PubMed] [Google Scholar]
- 12. Siston AM, Rasmussen SA, Honein MA et al. Pandemic 2009 influenza A(H1N1) virus illness in pregnant women in the United States. JAMA 2010; 303:1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hewagama S, Walker SP, Stuart RL et al. 2009 H1N1 influenza A and pregnancy outcomes in Victoria, Australia. Clin Infect Dis 2010; 50:686–690. [DOI] [PubMed] [Google Scholar]
- 14. Kwong JC, Campitelli MA, Rosella LC. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin Infect Dis 2011; 53:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar A, Zarychanski R, Pinto R et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302:1872–1879. [DOI] [PubMed] [Google Scholar]


