Abstract
Please cite this paper as: Burmaa et al. (2012) Cumulative incidence of pandemic influenza A (H1N1) 2009 by a community‐based serological cohort study in Selenghe Province, Mongolia. Influenza and Other Respiratory Viruses 6(601), e97–e104.
Background Large community outbreaks of pandemic A (H1N1) 2009 occurred between October and December 2009 in Mongolia. A serological study was conducted among the general population by testing paired sera collected before and after the first wave of pandemic in Selenghe province, Mongolia. None of the study participants had been vaccinated for pandemic A (H1N1) 2009 before the second samples were collected.
Objective The objective of this study was to estimate cumulative incidence of pandemic A (H1N1) 2009 in different age‐groups of Selenghe province residents.
Methods After informed consent was obtained from apparently healthy volunteers, the paired sera and background information were collected. Antibody titers were measured using hemagglutinin inhibition (HI) and microneutralization (MN) assays for A/California/07/2009pdm. A fourfold rise in antibody titers was regarded as the evidence of infection.
Results The overall cumulative incidences in the study group for all ages were 28·8% (76/264) by HI, 35·2% (93/264) by MN, and 25·0% (66/264) by both HI and MN. Cumulative incidences of infection varied among age‐groups, with children aged 2–4 and 5–9 years having high cumulative incidence of infection. Overall cumulative incidences of infection in the whole population were estimated to be 23·0% (4946/21 460) by HI, 30·2% (6473/21 460) by MN, and 18·8% (4036/21 460) by both HI and MN.
Conclusions This study indicates that about one‐fourth of the total population in Selenghe province was infected with pandemic A (H1N1) 2009 virus during the first wave of the pandemic.
Keywords: Cumulative incidence, influenza, Mongolia, pandemic A (H1N1) 2009, serology
Introduction
The 2009 influenza pandemic caught many public health officials by surprise. An H1N1 virus of swine origin was largely unexpected, and the virus was detected on all continents within 9 weeks since the virus was first detected in North America. 1 When human cases of pandemic A (H1N1) 2009 were identified in Mexico and the United States, a key question was whether human population had no immunity to this virus. As seasonal H1N1 influenza had been circulating in the human population since 1977, some people could have developed immunity to the pandemic H1N1 virus if there was cross‐immunity between pandemic and seasonal H1N1 viruses. Early serological studies did find evidence of cross‐immunity particularly in older age‐groups. 2 , 3 Subsequent serological studies were conducted to estimate cumulative incidence of infection by comparing antibody prevalence before and after the outbreak. One of the first studies reported in this respect indicated the highest cumulative incidence of infection in the age‐group of 5–14 years in the UK. 4 , 5 Other studies also confirmed higher cumulative incidence of infection in school‐age children. 6 , 7 , 8 Although many serological study results have been published, 5 , 9 most were cross‐sectional studies comparing antibody prevalence in samples collected from different individuals, such as blood donors, before and after the 2009 pandemic. Only a few cohort studies comparing samples collected from the same individuals before and after the pandemic wave (i.e., paired sera) have been reported. A study conducted in Singapore tested four adult groups – the general population, military personnel, hospital staff, and staff and residents of long‐term care facilities. 10 Another study in Hong Kong also included the pediatric population and indicated high cumulative incidence of infection in the age‐group of 3–19 years. 11 Most serological studies on pandemic (H1N1) 2009 have been reported from developed countries. One exception was a serological study conducted among rural farmers in Guangxi province, China, that indicated low antibody prevalence for pandemic (H1N1) 2009 even among the elderly in samples collected before the pandemic. 12 Baseline seroprevalence before the pandemic and cumulative incidence of infection among different age‐groups might be different in developing countries.
The National Influenza Center (NIC) of Mongolia was established in 1974 and was enlisted with the Global Influenza Surveillance Network (GISN) in 1978. Over time, the number of sentinel surveillance sites has been increased to 158, covering the whole country since October 2009 (National Influenza Center, Mongolia: http://flu.mn/eng/). Nasopharyngeal samples from ILI cases in sentinel sites, including those in the Selenghe province, were collected for virus detection on a weekly basis. Nasopharyngeal samples were tested for influenza viruses by real‐time polymerase chain reaction (RT‐PCR). As shown in Figure 1, the surveillance data in the Selenghe province identified increasing trend of ILI cases from week 40 of 2009, and the first confirmed cases of pandemic (H1N1) 2009 were identified in week 42 (2nd week of October). The number of ILI and confirmed cases increased rapidly and peaked in weeks 43 and 45, respectively. ILI cases dropped to baseline levels by week 48 (i.e., end of November). However, some sporadic confirmed cases occurred until February 2010, and slightly increased numbers of ILI cases were seen in January 2010.
Figure 1.
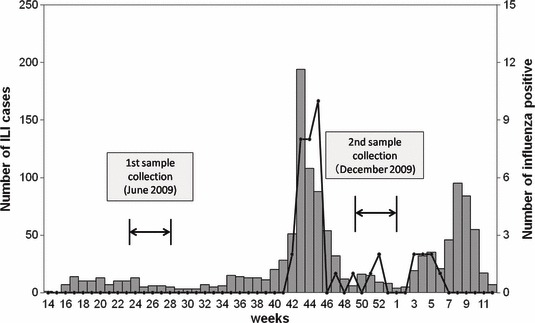
Number of influenza‐like illness and influenza virus‐positive cases from influenza surveillance data of Selenghe province (April 2009/March 2010). All influenza‐positive cases were pandemic (H1N1) 2009.
We conducted a population‐based cohort study in the Selenghe province of Mongolia by comparing antibodies in sera collected before and after the first wave of the 2009 pandemic.
Methods
We collected paired sera in June 2009 (baseline) and December 2009 (after the first wave of the pandemic) from the general population in Selenghe province, Mongolia. Selenghe province is located at the border of the Russian Federation and 300 km north of Ulaanbaatar, the capital city of Mongolia. Sukhbaatar, the capital of Selenghe province, has a population of 21 460. Randomly selected local residents in this city, who were under the care of five family group practices (FGPs), and who had not been afflicted with influenza‐like illness (ILI) in the previous 7 days, were invited to participate in the study on a voluntary basis. The proposal of this serological study was approved by the ethics committees of the National Center of Communicable Diseases (NCCD), Ministry of Health, of Mongolia and Tohoku University Graduate School of Medicine, Sendai, Japan. After informed consent was obtained from the participant or guardian, background information using a questionnaire and the blood samples were collected. The cumulative incidence of infection in each age‐group for whole population in Sukhbaatar city was extrapolated by using cumulative incidence of infection among the study participants and whole population based on 2008 population statistics. The overall population cumulative incidence of infections for Sukhbaatar city was calculated as a sum of estimated incidences of all age‐groups.
Antibody titers were measured using hemagglutinin inhibition (HI) and microneutralization (MN) assays performed at the Department of Virology, Tohoku University Graduate School of Medicine, Sendai, Japan, according to standard procedures. 13 For both HI and MN assays, antibody titers were measured against A/California/07/2009pdm. A fourfold rise in antibody titers was regarded as the evidence of infection. The standard antigens for the HI assay and the challenge virus for the MN assay were kindly provided by the Influenza Virus Research Center of National Institute of Infectious Disease (NIID), Tokyo, Japan. For HI, serum samples were pre‐treated with receptor‐destroying enzyme (RDE; Denka Seiken Co. Ltd, Tokyo Japan) and hemeadsorbed to remove non‐specific agglutinins. The serum samples were diluted twofold from 1:10 to 1:1280. After serial dilution, 25 μl of antigen (four hemagglutinin units) was added to each well and incubated for 1 hour at room temperature. After 1 hour, 0·75% of guinea pig red blood cells was added to each well and incubated for 2 hours at 4°C. After 2 hours, HI patterns were read to determine antibody titer. For the MN test, the serum was diluted twofold from 1:20 to 1:2560, and the virus was diluted to 100 TCID50/50 μl using minimum essential medium. After the serial dilution, 100 TCID50/50 μl of challenge virus was added into each well and incubated for 30 minutes at 37°C. After incubation, the serum and virus mixture was inoculated into Madin‐Darby Canine Kidney (MDCK) cells and incubated for 72 hours at 34°C. After 72 hours, the cytopathic effect was observed to determine the end point, and cells were stained using crystal violet to confirm the end point.
Following statistical methods were used to analyze the data; 95% confidence intervals for proportions were calculated using the adjusted Wald method, 14 and P‐values were obtained by using chi‐square test with statistical significance set at 0·05.
Results
A total of 310 individuals agreed to participate in the study and provided the first serum samples, but 46 of them failed to provide the second serum samples. Therefore, paired serum samples were obtained from 264 individuals. The first serum samples were collected in the epidemiological week 26th (first collection) and the second samples in week 49th (second collection) of 2009. Influenza surveillance data obtained from Selenghe province during the study period are shown in Figure 1. Age distribution and other background information of the study subjects are summarized in Table 1. Age of individuals ranged from 1 month to 85 years; 97 individuals (36·7%) were <5 years of age, 62 (23·5%) were 5–19 years of age, 52 (19·7%) were 20–44 years of age, 26 (9·8%) were 45–64 years of age, and 27 (10·2%) were 65 years or more. Of the 264 individuals, 125 (47·3%) were male and 139 (52·7%) were female. Only 16 had been vaccinated for seasonal influenza by vaccines containing A/Brisbane/59/2007(H1N1)‐like, A/Brisbane/10/2007(H3N2)‐like, B/Florida/4/2006‐like strains within 6 months before the first samples were collected. In Mongolia, vaccines for pandemic A (H1N1) 2009 were available after January 2010. Therefore, none of the recruited individuals had been vaccinated for pandemic A (H1N1) 2009 before the second samples were collected.
Table 1.
Background data for study population
| Number | % | |
|---|---|---|
| Age‐groups (years) | ||
| <1 | 23 | 8·7 |
| 1 | 24 | 9·1 |
| 2–4 | 50 | 18·9 |
| 5–9 | 34 | 12·9 |
| 10–19 | 28 | 10·6 |
| 20–44 | 52 | 19·7 |
| 45–64 | 26 | 9·8 |
| 65‐ | 27 | 10·2 |
| Gender | ||
| Male | 125 | 47·3 |
| Female | 139 | 52·7 |
| Vaccination history | ||
| Yes | 16 | 6·1 |
| No | 248 | 93·9 |
| Total | 264 | 100·0 |
The overall cumulative incidences of infection for all ages were 28·8% (76/264) by HI, 35·2% (93/264) by MN, and 25·0% (66/264) by both HI and MN (Figure 2, Table 2). Cumulative incidences of infection for the study population varied among age‐groups. They were higher in children aged 2–4 and 5–9 years compared with those aged 1 year and less. The rates were also lower in age‐groups of 10–19 years and 20–44 years compared with those aged 2–9 years, and they were even lower in age‐groups of 45–64 years, and 65 years or more (Figure 2 and Table 2). However, it should be noted that 10–20% of cumulative incidences of infection were observed for those aged 65 years or more. Overall cumulative incidence of infection in the whole population in Sukhbaatar city was estimated to be 23·0% (4946/21 460) by HI, 30·2% (6473/21 460) by MN, and 18·8% (4036/21 460) by both HI and MN (Table 2). More than half of the infections (2640/4946 (53·4%) by HI, 3493/6473 (54·0%) by MN, and 2453/4036 (60·8%) by both HI and MN) are estimated to have occurred in those aged <20 years. Antibody prevalence and geometric mean titer (GMT) for HI and MN in samples collected before the first wave of pandemic (H1N1) 2009 (June 2009) are shown in Figure 3. There were no significant differences in antibody prevalence and GMT, in any of age‐groups, including those aged 65 years or more. Among the 16 individuals who had been vaccinated for seasonal influenza, 14 were aged <15 years and only two were aged 70 years or more. None of the vaccinated individuals were positive by HI or MN for baseline antibody against A/California/07/2009pdm virus. Cumulative incidences of infection were compared according to baseline antibody titers to assess the protective level of baseline titers (Table 3). Cumulative incidences of infection were dependent upon baseline HI titers, and those with low baseline HI titers had higher cumulative incidences of infection. For baseline HI antibody titers, significant associations between baseline HI titer and cumulative incidences of infection (assessed by HI results and both HI and MN results) were found. However, such significant association was not observed for baseline MN titers (Table 3).
Figure 2.
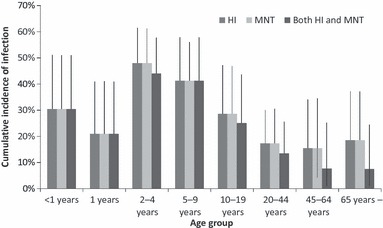
Cumulative incidences of infection (antibody titer increases by fourfold or more) by age‐groups against A/California/07/2009pdm before (June 2009) and after (December 2009) the first influenza A (H1N1) pandemic wave in Selenghe province, Mongolia. Error bars represent 95% confidence interval.
Table 2.
Estimated total incidence during the first wave of pandemic (H1N1) in Sukhbaatar city of Selenghe province, Mongolia
| Hemagglutinin inhibition (HI) test | |||||||
|---|---|---|---|---|---|---|---|
| Age‐groups (years) | Number tested | Number positive | Cumulative incidence (%) | 95% CI for cumulative incidence | Total population | Estimated incidence | 95% CI for estimated incidence |
| <1 | 23 | 7 | 30·4 | 15·4–51·1% | 356 | 108 | 55–182 |
| 1 | 24 | 5 | 20·8 | 8·8–40·9% | 384 | 80 | 34–157 |
| 2 | 50 | 24 | 48·0 | 34·8–61·5% | 1008 | 484 | 351–620 |
| 5–9 | 34 | 14 | 41·2 | 26·3–57·8% | 1919 | 790 | 506–1109 |
| 10–19 | 28 | 8 | 28·6 | 15·1–47·2% | 4124 | 1178 | 621–1948 |
| 20–44 | 52 | 9 | 17·3 | 9·2–30·0% | 9267 | 1604 | 848–2776 |
| 45–64 | 26 | 4 | 15·4 | 5·5–34·1% | 3591 | 552 | 199–1226 |
| 65‐ | 27 | 5 | 18·5 | 7·7–37·2% | 811 | 150 | 63–301 |
| Total | 264 | 76 | 28·8 | 23·7–34·5% | 21460 | 4947 | 2676–8320 |
| Microneutralization (MN) test | |||||||
| <1 | 23 | 7 | 30·4 | 15·4–51·1% | 356 | 108 | 55–182 |
| 1 | 24 | 7 | 29·2 | 14·7–49·4% | 384 | 112 | 56–190 |
| 2 | 50 | 26 | 52·0 | 38·5–65·2% | 1008 | 524 | 388–657 |
| 5–9 | 34 | 20 | 58·8 | 42·2–73·7% | 1919 | 1129 | 810–1413 |
| 10–19 | 28 | 11 | 39·3 | 23·5–57·6% | 4124 | 1620 | 970–2377 |
| 20–44 | 52 | 12 | 23·1 | 13·6–36·3% | 9267 | 2139 | 1259–3362 |
| 45–64 | 26 | 5 | 19·2 | 8·0–38·3% | 3591 | 691 | 289–1377 |
| 65‐ | 27 | 5 | 18·5 | 7·7–37·2% | 811 | 150 | 63–301 |
| Total | 264 | 93 | 35·2 | 29·7–41·2% | 21460 | 6473 | 3890–9859 |
| Both HI and MNT | |||||||
| <1 | 23 | 7 | 30·4 | 15·4–51·1% | 356 | 108 | 55–182 |
| 1 | 24 | 5 | 20·8 | 8·8–40·9% | 384 | 80 | 34–157 |
| 2 | 50 | 22 | 44·0 | 31·2–57·7% | 1008 | 444 | 314–582 |
| 5–9 | 34 | 14 | 41·2 | 26·3–57·8% | 1919 | 790 | 506–1109 |
| 10–19 | 28 | 7 | 25·0 | 12·4–43·6% | 4124 | 1031 | 512–1799 |
| 20–44 | 52 | 7 | 13·5 | 6·4–25·6% | 9267 | 1247 | 590–2371 |
| 45–64 | 26 | 2 | 7·7 | 1·0–25·3% | 3591 | 276 | 37–907 |
| 65‐ | 27 | 2 | 7·4 | 1·0–24·5% | 811 | 60 | 8–198 |
| Total | 264 | 66 | 25·0 | 20·1–30·6% | 21460 | 4037 | 2055–7305 |
Figure 3.
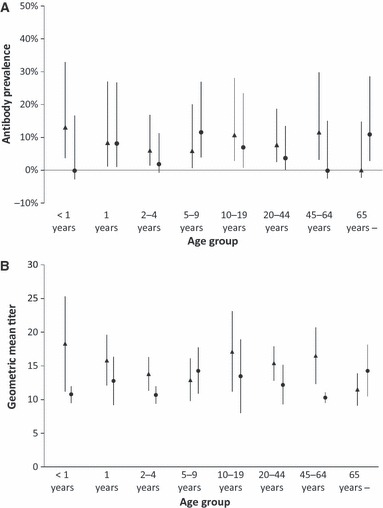
Baseline antibody profiles by age‐groups against A/California/072009pdm before (June 2009) the first influenza A(H1N1) pandemic wave in Selenghe province, Mongolia. (A) Antibody prevalence by age‐groups measured by Hemagglutinin inhibition (HI) test () and Microneutralization (MN) test (•). (B) Geometric mean titers by age‐groups measured by HI test () and MN test (•). Error bars represent 95% confidence interval.
Table 3.
Association between hemagglutinin inhibition (HI) and microneutralization titers (MNT) before the first pandemic wave (June 2009) and cumulative incidences of infection
| Yes | % | No | Total | P‐value | Yes | % | No | Total | P‐value | |
|---|---|---|---|---|---|---|---|---|---|---|
| HI titer | Cumulative incidence by HI | Cumulative incidence by both HI and MNT | ||||||||
| <10 | 15 | 32·6 | 31 | 46 | <0·005 | 14 | 30·4 | 32 | 46 | <0·05 |
| 10 | 45 | 40·2 | 67 | 112 | 37 | 33·0 | 75 | 112 | ||
| 20 | 15 | 17·4 | 71 | 86 | 14 | 16·3 | 72 | 86 | ||
| 40 | 1 | 5·6 | 17 | 18 | 1 | 5·6 | 17 | 18 | ||
| 80 | 0 | 0·0 | 2 | 2 | 0 | 0·0 | 2 | 2 | ||
| <40 | 75 | 30·7 | 169 | 244 | <0·05 | 65 | 26·6 | 179 | 244 | <0·05 |
| ≥40 | 1 | 5·0 | 19 | 20 | 1 | 5·0 | 19 | 20 | ||
| Total | 76 | 28·8 | 188 | 264 | 66 | 25·0 | 198 | 264 | ||
| MNT titer | Cumulative incidence by MNT | Cumulative incidence by both HI and MNT | ||||||||
| <20 | 86 | 36·3 | 151 | 237 | 0·52 | 60 | 25·3 | 177 | 237 | 0·44 |
| 20 | 4 | 30·8 | 9 | 13 | 4 | 30·8 | 9 | 13 | ||
| 40 | 2 | 16·7 | 10 | 12 | 1 | 8·3 | 11 | 12 | ||
| 80 | 1 | 50·0 | 1 | 2 | 1 | 50·0 | 1 | 2 | ||
| <40 | 90 | 36·0 | 160 | 250 | 0·27 | 64 | 25·6 | 186 | 250 | 0·34 |
| ≥40 | 3 | 21·4 | 11 | 14 | 2 | 14·3 | 12 | 14 | ||
| Total | 93 | 35·2 | 171 | 264 | 66 | 25·0 | 198 | 264 | ||
Discussion
We conducted a serological study of pandemic A (H1N1) 2009 in Selenghe province, Mongolia. Serological studies on pandemic A (H1N1) 2009 provide a unique opportunity to understand the transmission dynamics of influenza in the community. For seasonal influenza, high antibody cross‐reactivity with previous infections and immunity induced by vaccines make it difficult to interpret serological data. Understanding the transmission of pandemic influenza virus is important to be prepared for future pandemic. Data such as overall cumulative incidence of infection and age‐specific cumulative incidence of infection can provide useful information in being prepared for future pandemics.
In Mongolia, there was a unique epidemiological characteristic in the outbreak of pandemic A (H1N1) 2009. In many northern hemisphere countries, there were smaller outbreaks between May 2009 and August 2009, followed by larger outbreaks between September 2009 and early 2010. 15 , 16 However, in Mongolia, no confirmed case of pandemic A (H1N1) 2009 occurred until October 12, 2009, despite an extensive search for cases with pandemic A (H1N1) 2009. 17 Mongolia has a low population density, and traveling between Mongolia and other countries and within the country occurs much less than in other Asian countries. 18 Such geographical and social characteristics may have contributed to the late introduction of pandemic A (H1N1) 2009 into the country. However, once the virus was introduced, it spread rapidly, and widespread outbreaks occurred almost simultaneously in different provinces, including the Selenghe province. A very intensive outbreak occurred in mid‐November in the Selenghe province as shown in Figure 1. The present study reveals that approximately 30% of the total population in the Selenghe province was infected with pandemic A (H1N1) 2009 during the first wave of this pandemic. Our data also indicate that children aged 2–4 year and 5–9 years had higher cumulative incidences of infection than other age‐groups. The serological study in England showed that the cumulative incidences of infection in those aged 5–14 years were highest and much higher than those aged <5 years. 4 Another study in New Zealand also indicated higher cumulative incidences of infection in school‐aged children (5–9 years) compared with infants (1–4 years). 7 The fact that in the present study cumulative incidences of infection were high even in small children aged 2–4 years can be explained by different social mixing patterns in Mongolia. In countries such as the United States and the United Kingdom, schools have been implicated as a major source for community transmission of influenza. 19 , 20 In particular, with regard to pandemic A (H1N1) 2009, older children aged 10–19 years were the main target for infections in the early stages of the epidemic in 2009. 8 In Mongolia, most children aged 2 years and over go to kindergarten. Our previous analysis in Mongolia on seasonal influenza (2008–2009 season) and pandemic A (H1N1) (2009–2010 season) showed that ILI incidence was higher in children aged 1–4 years compared with those aged 5–9 years. 17 In countries like Mongolia, where in most households both parents work and the preschool system is well established, kindergarten or nursery schools may have a more important role in spreading influenza viruses; thus, there are higher incidences in smaller children. Similarly, the seroprevalence among those aged 0–5 years was high in China. 21 Social mixing patterns can be an important determinant for spread of infectious diseases including influenza. 22 , 23 However, data on social mixing patters in Mongolia are not available. Another significant finding in the present study was a high cumulative incidence of infection in the elderly: 18·5% by both HI and MN for those aged 65 years or more. In other studies, estimated cumulative incidences of infection in the elderly were significantly lower than in younger age‐groups. For example, the study in England showed that there was only a 0·9% difference between the baseline and after the first wave for those aged 65 years or more. 4 Similarly, low cumulative incidences of infection in the elderly were seen in the United States, 2 Australia, 24 , 25 and New Zealand. 7 Low cumulative incidences of infection in the elderly have been explained by preexisting cross‐immunity in the elderly population before pandemic A (H1N1) 2009. 2 , 4 In our study, there was no difference in pre‐pandemic antibody prevalence and GMT between the elderly (those more than 65 years) and younger age‐groups (Figure 2). Similar patterns were seen in rural farmers in Guangxi province, China, where the elderly over 60 years of age did not have any positive antibodies. 12 Lack of preexisting antibodies in the elderly was also confirmed in another study in China, which tested samples from larger geographic areas including urban areas. 21 There were also no preexisting antibodies in those aged between 42 and 82 years in Singapore. 26 It is still unclear why many elderly had preexisting antibodies in North America and some European countries, while the preexisting antibody prevalence in this age‐group was generally low in Asian countries. 5 However, it should be noted that all our study subjects were <80 years of age except one aged 85 years. The study in Japan indicated that only people born in 1918 had preexisting neutralizing antibodies. 27 In addition, a serological study in Finland showed that only those aged more than 80 years had significantly higher level of HI titers. 3 We might have found a higher prevalence of preexisting immunity if we had tested more samples from those over the age of 80. Further studies should be conducted to compare preexisting cross‐reactive antibody prevalence in different parts of the world.
We also analyzed the effects of preexisting antibodies on cumulative incidences of infection (Table 3). Those with a higher HI antibody titer (≥1:40) had significantly lower cumulative incidences of infection than those with a lower HI antibody titer (<1:40). Our results are comparable with the cohort study conducted in adults in Singapore, which also showed a protective effect of baseline HI titers of 1:40 or more. 10 However, such significant protective effects were not observed for MN titers. Those with baseline MN titers ≥1:40 did not have significantly lower cumulative incidences of infection than those with MN titers <40. In general, MN titers are considered to be better indicators for assessing the protection level of influenza immunity. 28 , 29 It was also shown that MN results are a better indicator than HI titers in assessing cumulative incidences of infection in laboratory‐confirmed cases of pandemic (H1N1) 2009. 30 It is not clear why our MN results were not associated with cumulative incidences of infection. Although both HI and MN tests used the same reference virus (A/California/07/2009pdm), the sensitivity of our MN test might have been low, especially in detecting low levels of cross‐reacting antibodies.
There are some limitations to our study. The number of subjects in each group was relatively small, and this makes it difficult to analyze statistical differences between age‐groups. We also included samples from only one province of Mongolia, namely Selenghe province, which may not represent the pattern of whole country. Samples collected from other provinces of Mongolia are now being analyzed to compare the data between provinces. In addition, the study participants took part in the study on a voluntary basis. This might be a potential source of bias, and the data from the study participants may not represent the whole population. Despite these limitations, our study is one of a few studies that analyzed paired serum samples and assessed actual seroconversion in each individual. It was also one of a few studies that was conducted in less developed countries.
In conclusion, the community‐based serological cohort study based on paired sera revealed that approximately 30% of the population in Selenghe province of Mongolia had been infected during the first wave of pandemic A(H1N1) 2009. This overall incidence is comparable to previous studies in other countries. The cumulative incidence was higher in age‐groups of 2–4 years and 5–9 years. The baseline antibody levels were similar in all age‐groups including those >65 years.
Acknowledgments
The authors would like to thank the doctors and assistants in the territorial hospitals and FGPs in Selenghe province for collecting data and blood samples from the study participants. We are also indebted to the staff in the Department of Virology, Graduate School of Medicine, Tohoku University, Sendai, Japan; Virology Laboratory and Department of Infectious Diseases Surveillance at the National Influenza Center, National Center of Communicable Diseases and Gyals Medical Center, Mongolia, for their active collaboration and excellent technical assistance. This study was funded by the “Avian influenza and human pandemic influenza preparedness and response” World Bank project in Mongolia and partially by grants‐in‐aid for Scientific Research (Kakenhi, grant number: 21406014) of Japan Society for the Promotion of Science.
References
- 1. World Health Organization . Influenza A(H1N1)– update 51. Available at: http://www.who.int/csr/don/2009_06_19/en/index.html
- 2. Hancock K, Veguilla V, Lu X et al. Cross‐reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945–1952. [DOI] [PubMed] [Google Scholar]
- 3. Ikonen N, Strengell M, Kinnunen L et al. High frequency of cross‐reacting antibodies against 2009 pandemic influenza A(H1N1) virus among the elderly in Finland. Euro Surveill 2010; 15:pii= 19478. [PubMed] [Google Scholar]
- 4. Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross‐sectional serological study. Lancet 2010; 375:1100–1108. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Seroepidemiological studies of pandemic influenza A (H1N1) 2009 virus. Wkly Epidemiol Rec 2010; 85:229–235. [PubMed] [Google Scholar]
- 6. Wu JT, Ma ES, Lee CK et al. The infection attack rate and severity of 2009 pandemic H1N1 influenza in Hong Kong. Clin Infect Dis 2010; 51:1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bandaranayake D, Huang QS, Bissielo A et al. Risk factors and immunity in a nationally representative population following the 2009 influenza A(H1N1) pandemic. PLoS ONE 2010; 5:e13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zimmer SM, Crevar CJ, Carter DM et al. Seroprevalence following the second wave of Pandemic 2009 H1N1 influenza in Pittsburgh, PA, USA. PLoS ONE 2010; 5:e11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Broberg E, Nicoll A, Amato‐Gauci A. Seroprevalence to Influenza A(H1N1) 2009 Virus ‐ Where Are We? Clin Vaccine Immunol 2011; 18:1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen MI, Lee VJ, Lim WY et al. 2009 influenza A(H1N1) seroconversion rates and risk factors among distinct adult cohorts in Singapore. JAMA 2010; 303:1383–1391. [DOI] [PubMed] [Google Scholar]
- 11. Riley S, Kwok KO, Wu KM et al. Epidemiological characteristics of 2009 (H1N1) pandemic influenza based on paired sera from a longitudinal community cohort study. PLoS Med 2011; 8:e1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen H, Wang Y, Liu W et al. Serologic survey of pandemic (H1N1) 2009 virus, Guangxi Province, China. Emerg Infect Dis 2009; 15:1849–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . WHO Global Influenza Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza. 2011; Available at: http://whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf.
- 14. Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 1998; 52:119–126. [Google Scholar]
- 15. Nymadawa P, Burmaa A, Darmaa B et al. The first wave of Influenza A (H1N1) 2009 pandemics in Mongolia. Influenza Other Respi Viruses 2011; 5(Suppl 1):163–165. [Google Scholar]
- 16. McCallum L, Partridge J. Epidemiological characteristics of the influenza A(H1N1) 2009 pandemic in the Western Pacific Region. Western Pacific Surveillance and Response Journal 2010; 1:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nukiwa N, Alexanderyn B, Kamigaki T et al. Evaluating influenza disease burden during the 2008–2009 and 2009–2010 influenza seasons in Mongolia. Western Pacific Surveillance and Response Journal 2011; 2:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mendsaikhan S, Gerelt‐Od G, Erdeneuren B, et al. . Mongolian Statistical Yearbook 2010. 2011; 307–308.
- 19. Cauchemez S, Valleron AJ, Boëlle PY, Flahault A, Ferguson NM. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature 2008; 452:750–754. [DOI] [PubMed] [Google Scholar]
- 20. Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age‐specific transmission parameters for respiratory‐spread infectious agents. Am J Epidemiol 2006; 164:936–944. [DOI] [PubMed] [Google Scholar]
- 21. Xu C, Bai T, Iuliano AD et al. The seroprevalence of pandemic influenza H1N1 (2009) virus in China. PLoS ONE 2011; 6:e17919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hens N, Ayele GM, Goeyvaerts N et al. Estimating the impact of school closure on social mixing behaviour and the transmission of close contact infections in eight European countries. BMC Infect Dis 2009; 9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mossong J, Hens N, Jit M et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 2008; 5:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McVernon J, Laurie K, Nolan T et al. Seroprevalence of 2009 pandemic influenza A(H1N1) virus in Australian blood donors, October ‐ December 2009. Euro Surveill 2010; 15:pii 19678. [DOI] [PubMed] [Google Scholar]
- 25. Gilbert GL, Cretikos MA, Hueston L, Doukas G, O’Toole B, Dwyer DE. Influenza A (H1N1) 2009 antibodies in residents of New South Wales, Australia, after the first pandemic wave in the 2009 southern hemisphere winter. PLoS ONE 2010; 5:e12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang JW, Tambyah PA, Wilder‐Smith A et al. Cross‐reactive antibodies to pandemic (H1N1) 2009 virus, Singapore. Emerg Infect Dis 2010; 16:874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Itoh Y, Shinya K, Kiso M et al. In vitro and in vivo characterization of new swine‐origin H1N1 influenza viruses. Nature 2009; 460:1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull 1979; 35:69–75. [DOI] [PubMed] [Google Scholar]
- 29. de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination‐inhibiting antibody to influenza virus. Dev Biol (Basel) 2003; 115:63–73. [PubMed] [Google Scholar]
- 30. Chen MI, Barr IG, Koh GC et al. Serological response in RT‐PCR confirmed H1N1‐2009 influenza a by hemagglutination inhibition and virus neutralization assays: an observational study. PLoS ONE 2010; 5:e12474. [DOI] [PMC free article] [PubMed] [Google Scholar]


