Abstract
Please cite this paper as: Yuan et al. (2012) Initial HRCT findings of novel influenza A (H1N1) infection. Influenza and Other Respiratory Viruses 6(601), e114–e119.
Objectives The aim of our study was to describe the presentation and illustrate the imaging features of chest high‐resolution computed tomography (HRCT) of patients with novel influenza A (H1N1) virus infection.
Methods Data were collected from 163 hospitalized patients between November 2009 and March 2011, who fulfilled the clinical criteria for H1N1 influenza infection and underwent HRCT examinations within 24 hours of admission.
Results Abnormal findings were observed in 40·5% of the patients. The patients with positive imaging findings were significantly older than patients with normal HRCT findings (P = 0·02). The most common finding was ground‐glass opacity (GGO) (n = 35). Interlobular septal thickening (n = 31) and centrilobular nodules (n = 30) were the second most frequent findings. Other common findings were consolidation, reticulation, and linear shadow. The most common imaging finding for lung involvement was GGO with a patchy pattern. Pulmonary involvement of the disease may be extensive and variable, but the total volume of affected lung was mostly <1 lobe.
Conclusion The baseline HRCT may be valuable and suggestive even for non‐severe H1N1 infections. When a severe case or a evolution is suspected, chest CT could be essential both for determining the precise extent of parenchymal damage and for monitoring its evolution.
Keywords: H1N1, high‐resolution CT, influenza, lung
Introduction
The World Health Organization (WHO) issued a public health alert in April 2009 indicating that human cases of influenza type A (H1N1) virus infection had been identified. Within few weeks, the virus spread globally. In June 2009, the WHO raised its pandemic alert level to the highest level, phase 6, indicating widespread community transmission on at least two continents. 1 The 2010/2011 influenza season arrived 8‐10 weeks later than the 2009/2010 pandemic season, but still quite early compared with historical trends. During 2010/2011, pandemic H1N1 2009 continues to predominate among circulating influenza A virus. The virus can infect the lower respiratory tract and cause rapidly progressive pneumonia, especially in children and younger adults. 2
The unique genetic and antigenic features of this virus have resulted in a high incidence of infection, which differs from those of seasonal influenza infections. However, the most common symptoms in patients with H1N1 infection, such as fever, dry cough, sore throat, headache, muscle or joint pain, chills, fatigue, diarrhea, and vomiting, are similar to seasonal influenza. Laboratory criteria according to WHO case definition encompass the diagnosis of novel influenza A, while radiological evidence is required to exclude other infectious diseases that may have a specific alternate pattern, such as tuberculosis. For the majority of patients with pneumonia, the chest radiograph provides adequate imaging information. However, an increasing number of patients undergo computed tomography (CT), especially when they demonstrate (i) high clinical suspicions for pneumonia whenever in the presence of normal or questionable radiographic findings, and (ii) subtle lung changes detected by plain radiography, which is yet insufficient for evaluation and diagnosis.
The CT findings of H1N1 have not been well described as most studies have included only a small number of patients or have focused on chest X‐rays. Moreover, precise characterization of abnormalities such as centrilobular nodules may only be described ideally with high‐resolution CT (HRCT). Hence, the aim of our study was to describe the presentation and illustrate the imaging features of chest HRCT of patients with H1N1 virus infection.
Materials and methods
The study was approved by the Institutional Review Board of our institution, and written informed consent was obtained from each subject. Data were collected for hospitalized patients admitted to the public health clinical center in Shanghai between November 2009 and March 2011, who fulfilled the clinical criteria for H1N1 influenza infection established by National Centers for Disease Control and Prevention and underwent HRCT examinations within 24 hours after admission. To avoid unnecessary radiation exposure, HRCT was taken when patients were clinically suspected for pneumonia, and plain radiography was insufficient for evaluation and diagnosis. Oropharyngeal and/or nasopharyngeal specimens of all patients were evaluated by real‐time polymerase chain reaction (PCR) and were positive for H1N1. All patients were evaluated for other respiratory viral infections, and some patients underwent additional microbiological evaluation for bacterial and fungal infections. Those with a proved additional concurrent acute illness or other preexisting medical conditions were excluded.
All the included patients underwent HRCT examinations performed on a 16‐MSCT scanner (Siemens Medical Solutions, Erlangen, Germany) within 24 hours after admission. Protocols were as follows: 110 mAs and 120 kVp, 0·75‐mm collimations, 1‐mm slice thickness. For the young age patients (patients with <18 years old), the CT scans used the following parameters: 80–100 kVp, automatic mAs adjustment. The time intervals between onset of syndrome and HRCT scan ranged from 1 to 6 days (mean, 3 days). All CT examinations were performed without the use of contrast material. Two experienced radiologists (with at least 15 years experience in CT imaging) reviewed the HRCT examinations by consensus. All images were reviewed on a Picture Archiving and Communication System workstation. The two reviewers were blinded to the clinical data and the patients’ clinical outcomes.
The HRCT findings were classified as normal or abnormal. The distributions of lung involvement were assessed as unilateral or bilateral and as central perihilar, peripheral, or diffuse (both central perihilar and peripheral). The lobes affected by each of these findings were also recorded. The abnormal findings were air‐space consolidation (opacification obscuring the underlying vessels), ground‐glass opacity (GGO; increased attenuation without obscuring the underlying vessels), nodules (a rounded opacity measuring < 3 cm in diameter), and reticulation. Nodules were considered centrilobular when they demonstrated all of the following features: sparing of the subpleural interstitium, even spacing from one another, and association with the distal bronchovascular interstitium. Additionally, lymphadenopathy, pericardial effusion, and pleural effusion were also noted.
Results
Our study cohort included 163 patients (86 men and 77 women) with a confirmed diagnosis of H1N1 influenza virus infection who had undergone HRCT scan within 24 hours of admission. Mean age was 24·3 years (range, 4–75 years). The symptoms presented in our patients were fever, cough, sore throat, phlegm, nasal congestion, and runny nose. One hundred and one patients had a body temperature greater than 38°C. Physical signs included throat congestion (n = 154; 43 cases of which had enlarged tonsils) and coarse breath sounds of lung (n = 5). Table 1 lists the clinical characteristics of included patients.
Table 1.
Clinical characteristics of included patients with H1N1 infection
| No of patients | Percentage (%) | |
|---|---|---|
| Sign | ||
| Throat congestion | 154 | 94·5 (154/163) |
| Enlarged tonsils | 43 | 26·4 (43/163) |
| Coarse breath sounds | 5 | 3·1 (5/163) |
| Laboratory test | ||
| Elevated WBC count | 5 | 3·1 (5/163) |
| Decreased WBC count | 12 | 7·4 (12/163) |
| Higher percentage of neutrophils | 84 | 51·5 (84/163) |
| Higher percentage of Lymphocyte | 7 | 4·3 (7/163) |
| Increased CRP | 132 | 81·0 (132/163) |
| Decreased CD4 | 113 | 69·3 (113/163) |
CRP, C‐reactive protein; CD4, cluster of differentiation 4.
Ninety‐seven (59·5%) of the 163 patients had no findings that could be related to influenza. The remaining 66 (40·5%) patients had abnormal HRCT findings in lungs (n = 55), pleura (n = 32), and mediastinal lymph nodes (n = 9), which were classified as likely to be related to the influenza infection. Forty of these 66 patients were women and the other 26 were men. The patients with positive imaging findings (mean age, 26·8 years) were significantly older than patients without acute imaging findings (mean age, 22·6) (P = 0·02 < 0·05). The body temperature between the two groups showed no significant difference (P = 0·33 > 0·05).
Table 2 presents the radiologic findings and locations of lesions for the initial HRCT images. GGO was seen in 35 patients (35/66, 53·0%). Interlobular septal thickening (n = 31, 31/66, 47·0%) and centrilobular nodules (n = 30, 30/66, 45·5%) were the second most frequent findings. Other common findings were as follows: consolidation (n = 15, 15/66, 22·7%), reticulation (n = 8, 8/66, 12·1%), and linear shadow (n = 2, 2/66, 3·0%) (1, 2). The total volume of affected areas in any patient is smaller than the volume of one lobe, except for one patient with diffuse GGO lesions in both lungs (Figure 3). Reactive pleurisy were also seen (left side: five patients; right: eight patients; both sides: 19 patients), and no pleural effusion was detected. Lymphadenopathy in mediastinum and axilla was noted in seven patients. Follow‐up HRCT images were obtained from nine patients with positive initial HRCT findings who demonstrated relatively severe respiratory symptoms after admission. After 4 days of standard anti‐virus therapy, the follow‐up HRCT scans were performed. Six patients showed near‐complete clinical remission and remission of the initial abnormalities on HRCT images. One patient with linear shadow and two patients with pleurisy on the initial HRCT images showed partial remission during follow‐up, whose clinical symptoms regressed as well, and no more HRCT scans were taken thereafter. Oropharyngeal and/or nasopharyngeal specimens of the three patients were also evaluated by real‐time PCR, which were negative for pandemic novel influenza A (H1N1).
Table 2.
The initial HRCT findings and locations of lesions of patients with H1N1 infection
| No of patients | Percentage (%) | |
|---|---|---|
| Lung | 55 | 83·3 (55/66) |
| Lung lobe | ||
| Right upper | 13 | 23·6 (13/55) |
| Right middle | 17 | 30·9 (17/55) |
| Right lower | 24 | 43·6 (24/55) |
| Left upper | 18 | 32·7 (18/55) |
| Left lower | 24 | 43·6 (24/55) |
| Lesion | ||
| GGO | 35 | 53·0 (35/55) |
| Interlobular septal thickening | 31 | 47·0 (31/55) |
| Centrilobular nodules | 30 | 45·5 (30/55) |
| Consolidation | 15 | 22·7 (15/55) |
| Reticulation | 8 | 12·1 (8/55) |
| Linear shadow | 2 | 3·0 (2/55) |
| Pleura | 32 | 48·5 (32/66) |
| Left only | 5 | 15·6 (5/32) |
| Right only | 8 | 25·0 (8/32) |
| Both sides | 19 | 59·4 (19/32) |
| Lymphadenopathy | 9 | 13·6 (9/66) |
| Mediastinum | 4 | 44·4 (4/9) |
| Axilla | 5 | 55·6 (5/9) |
HRCT, high‐resolution computed tomography; GGO, ground‐glass opacity.
Figure 1.
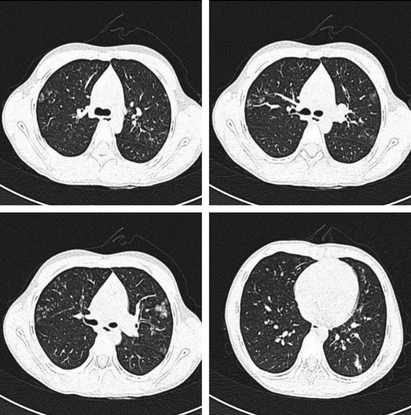
A 9‐year‐old girl, presenting with fever (38°C at admission), sore throat, throat congestion, and enlarged tonsils (I°). High‐resolution computed tomography shows tree‐in‐bud pattern, peripheral ground‐glass opacity, centrilobular nodule, and interlobular septal thickening.
Figure 2.
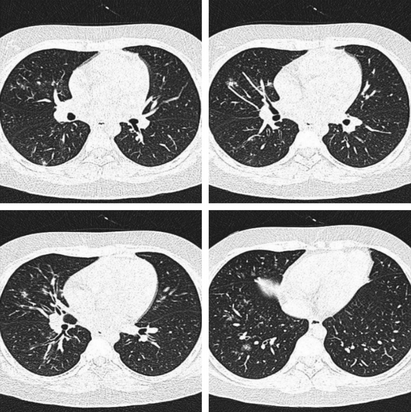
A 12‐year‐old boy, presenting with fever (37·8°C at admission) and throat congestion. High‐resolution computed tomography shows scattered ground‐glass opacity and centrilobular nodules in the right lung.
Figure 3.
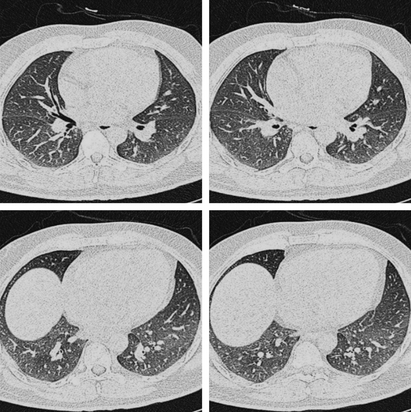
A 38‐year‐old man, presenting with fever (37·9°C at admission) and throat congestion. High‐resolution computed tomography shows diffuse area of ground‐glass opacity in both lungs.
Discussion
Although severe cases, such as those with acute respiratory distress syndrome and deaths, have been reported in 2009/2010 pandemic H1N1 infection, 3 in fact, the pandemic H1N1 influenza proved to be benign in most cases, with a mortality rate of 0·5%. The majority of H1N1 influenza cases have been mild influenza‐like illnesses and usually subclinical, 4 which is indistinguishable from seasonal influenza.
The image findings of H1N1 infection often are non‐specific and may overlap with those of other infections such as bacterial, fungal, and mycobacterial disease. 5 CT could be helpful in distinguishing bacterial from atypical organisms. 6 The CT findings of viral infection in general are numerous with previously reported studies demonstrating a myriad of different findings including: consolidation, GGO, interlobular septal thickening, centrilobular nodules, airway thickening, and air trapping/mosaic perfusion. 7 , 8 It was reported that GGOs with a patchy pattern and air‐space consolidation were the mostly seen thoracic viral pneumonia findings on CT. 9 GGO is typical of alveolar damage as a reaction to the viral infection. The small airway involvement is manifested as centrilobular nodule. Reittner et al. 5 reviewed HRCT data from 114 patients with different types of pneumonia and found that viral infections are commonly associated with nodules and focal or diffuse areas of air‐space consolidation. Most of the patients in that study with viral pneumonia had centrilobular nodules. In the pathological setting, air‐space consolidation or ground‐glass attenuation with a lobular distribution is associated with hyaline membrane formation in the alveolar parenchyma around the bronchiole. 6 In this study, the most common HRCT findings of novel H1N1 included GGO, interlobular septal thickening, centrilobular nodules, and consolidation. The GGO and patchy pattern of air‐space consolidation were a prominent feature in our series, and this finding is similar to data from previous studies. Reports of influenza pneumonia radiographs have shown poorly defined patchy areas of air‐space consolidation. 9 , 10 Diffuse or patchy areas of ground‐glass attenuation mixed with consolidation were also shown on CT scans of H1N1 infection. 11
In all but one patient in our study, the affected lung area was smaller than one lobe. It could be attributed to the early stage our patients were in, as it is well known that viral infection commonly produces extensive regions of either diffuse or localized diffuse alveolar damage in late‐stage cases. Another possible explanation is that the patients in our study were all previously healthy ones with no concomitant or chronic diseases. The lung involvement was more extensive in the high‐risk group. Individuals at a higher risk for serious complications include those over 65 years of age, children younger than 5 years, in conditions such as chronic lung disease (e.g., asthma and chronic obstructive pulmonary disease), conditions associated with immunosuppression (e.g., receiving immunosuppressive medications or HIV positive), chronic cardiac disease (e.g., congenital heart disease and coronary artery disease), diabetes, obesity, pregnancy (especially during the third trimester), and in children with neurodevelopmental conditions. 12
The children and young adults (5–50 years) were reported to be more vulnerable and with the highest incidence of severe forms. 3 , 13 The proposed reasons are that a proportion of elderly people have some level of sensitized immune system following exposure to the 1918 virus or related strains that circulated until 1957. 14 Of note, however, was the fact that in this study, the patients with positive imaging findings were significantly older than patients without acute imaging findings (P = 0·02). The most possible explanation might be from the truth that most young patients with mild symptoms did not take HRCT examination to avoid radiation exposure. The chest radiography findings of H1N1 infection in children and young patients were similar to those of adults, including unilateral or bilateral GGOs and consolidations, which is consistent with previous studies. 15 , 16 , 17 , 18
Our study had some limitations. First, this study was planned prospectively, but was observational and retrospective in nature. The patients were not consecutively enrolled. Only those with available HRCT images within 24 hours of admission were included. It is possible that there may be a bias with respect to which of these individuals underwent chest CT; therefore, the results might not represent all of the CT findings of H1N1 infection, which may influence the age distribution of the patients as aforementioned. Second, no severe infection requiring mechanical ventilation was included. Therefore, we could not compare severely infected group to mild group. Third, influenza A infections are sometimes complicated by superinfection with other organisms, such as bacterial, as many of these patients were also treated empirically with anti‐bacterial agents. It was possible that some of the findings seen on initial scans were from infection other than influenza. However, bacterial sputum cultures were negative for all patients and for the HRCT findings in our study. Finally, no information on the relation between CT and histopathology could be obtained because none of the patients underwent lung biopsy or autopsy.
In conclusion, the most common patterns described are ground‐glass attenuation and air‐space consolidation with random distribution and lower lobe predominance. The patterns were non‐specificity, and the diagnosis of H1N1 infection should still rely on laboratory criteria. The baseline HRCT may be valuable and suggestive even for non‐severe H1N1 infections. However, to avoid a ‘cost’ in terms of exposure, HRCT evidence is more helpful for patients with severe clinical symptoms and for the follow‐up evaluation. When a severe case or a evolution is suspected, chest CT could be essential both for determining the precise extent of parenchymal damage and for monitoring its evolution.
Ying Yuan and Xiao‐Feng Tao contributed equally to this work.
References
- 1. Centers for Disease Control and Prevention . 2008–2009 Influenza season week 32 ending August 15, 2009, http://www.cdcgov/flu/ weekly (Accessed 29 September 2009). World Health Organization. Pandemic(H1N1) 2009‐update 58. http://www.who.int/csr/ don/2009‐07‐06/en/index.html (Accessed 29 September 2009).
- 2. Dawood FS, Jain S, Finelli L et al. Emergence of a novel swine‐origin influenza a (H1N1) virus in humans. N Engl J Med 2009; 360:2605–2615. [DOI] [PubMed] [Google Scholar]
- 3. Perez‐Padilla R, de la Rosa‐Zamboni D, Ponce de Leon S et al. Pneumonia and respiratory failure from swine‐origin influenza A (H1N1) in Mexico. N Engl J Med 2009; 361:680–689. [DOI] [PubMed] [Google Scholar]
- 4. Peiris JS, Poon LL, Guan Y. Emergence of a novel swine‐origin influenza A virus (SOIV) H1N1 virus in humans. J Clin Virol 2009; 45: 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reittner P, Ward S, Heyneman L, Johkoh T, Müller NL. Pneumonia: high‐resolution CT findings in 114 patients. Eur Radiol 2003; 13:515–521. [DOI] [PubMed] [Google Scholar]
- 6. Tanaka N, Matsumoto T, Kuramitsu T et al. High resolution CT findings in community‐acquired pneumonia. J Comput Assist Tomogr 1996; 20:600–608. [DOI] [PubMed] [Google Scholar]
- 7. Kang EY, Patz EF Jr, Muller NL. Cytomegalovirus pneumonia in transplant patients: CT findings. J Comput Assist Tomogr 1996; 20:295–299. [DOI] [PubMed] [Google Scholar]
- 8. McGuinness G, Gruden JF. Viral and pneumocystis carinii infections of the lung in the immunocompromised host. J Thorac Imaging 1999; 14:25–36. [DOI] [PubMed] [Google Scholar]
- 9. Kim EA, Lee KS, Primack SL et al. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics 2002; 22:S137–S149. [DOI] [PubMed] [Google Scholar]
- 10. Lee C, Seo J, Song J et al. Pulmonary complication of novel influenza A (H1N1) infection: imaging features in two patients. Korean J Radiol 2009; 10:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karadeli E, Koc Z, Ulusan S, Erbay G, Demiroglu YZ, Sen N. Chest radiography and CT findings in patients with the 2009 pandemic (H1N1) influenza. Diagn Interv Radiol 2011; 17:216–222. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization . Clinical management of human infection with pandemic (H1N1). revised guidance. Geneva: World Health Organization 2009; Available at http://www.who.int/csr/resources/publications/swineflu/clinical_management/en/index.html (Accessed 9 April 2010). [Google Scholar]
- 13. Ajlan AM, Quiney B, Nicolaou S, Muller NL. Swine‐origin influenza A (H1N1) viral infection: radiographic and CT findings. AJR Am J Roentgenol 2009; 193:1494–1499. [DOI] [PubMed] [Google Scholar]
- 14. Writing committee of the WHO . Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362:1708–1719. [DOI] [PubMed] [Google Scholar]
- 15. Lee EY, McAdam AJ, Chaudry G, Fishman MP, Zurakowski D, Boiselle PM. Swine‐origin influenza A (H1N1) viral infection in children: initial chest radiographic finding. Radiology 2010; 254:934–941. [DOI] [PubMed] [Google Scholar]
- 16. Agarwal PP, Cinti S, Kazerooni EA. Chest radiographic and CT findings in novel swine‐origin influenza A (H1N1) virus (S‐OIV) infection. AJR Am J Roentgenol 2009; 193:1488–1493. [DOI] [PubMed] [Google Scholar]
- 17. Andronikou S. Pathological correlation of CT‐detected mediastinal lymphadenopathy in children: the lack of size threshold criteria for abnormality. Pediatr Radiol 2002; 32:912. [DOI] [PubMed] [Google Scholar]
- 18. Mollura DJ, Asnis DS, Crupi RS et al. Imaging findings in a fatal case of pandemic swineorigin influenza A (H1N1). AJR Am J Roentgenol 2009; 193:1500–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]


