Abstract
Please cite this paper as: Bramley et al. Intensive care unit patients with 2009 pandemic influenza A (H1N1pdm09) virus infection – United States, 2009. Influenza and Other Respiratory Viruses 6(601), e134–e142.
Background The influenza A (H1N1pdm09) [pH1N1] virus resulted in intensive care unit (ICU) admissions, acute respiratory distress syndrome (ARDS), and death.
Objectives To describe the characteristics of ICU patients with pH1N1 virus infection in the United States during the spring and fall of 2009 and to describe the factors associated with severe complications including ARDS and death.
Patients/Methods Through two national case‐series conducted during spring and fall of 2009, medical charts were reviewed on ICU patients with laboratory‐confirmed pH1N1 infection by real‐time reverse‐transcriptase polymerase chain reaction.
Results The majority (77%) of 154 patients hospitalized in an ICU were <50 years of age, and 65% had at least one underlying medical condition. One hundred and twenty‐eight (83%) patients received influenza antiviral agents; 29% received treatment ≤2 days after illness onset. Forty‐eight (38%) patients developed ARDS and 37 (24%) died. Patients with ARDS were more likely to be morbidly obese (36% versus 19%, P = 0·04) and patients who died were less likely to have asthma (11% versus 28%, P = 0.05). Compared with patients who received treatment ≥6 days after illness onset, patients treated ≤2 days after illness onset were less likely to develop ARDS (17% versus 37%, P < 0.01) or die (7% versus 35%, P < 0·01).
Conclusions Among patients hospitalized in an ICU with pH1N1 virus infection, ARDS was a common complication, and one‐quarter of patients died. Patients with asthma had less severe outcomes. Early treatment with influenza antiviral agents was likely beneficial, especially when initiated ≤2 days after illness onset.
Keywords: Epidemiology, influenza, intensive care, pandemic
Introduction
The 2009 pandemic influenza A (H1N1pdm09) [pH1N1] virus was detected in April 2009 in the United States 1 and from April 2009 to April 2010 caused an estimated 61 million cases, 274 000 hospitalizations, and 12 500 deaths. 2 Approximately 23–34% of patients hospitalized in the United States were admitted to an intensive care unit (ICU). 3 , 4 , 5 , 6 , 7 Globally, ICU admissions were associated with chronic comorbidities, 5 , 8 , 9 , 10 pregnancy, 11 obesity, 6 , 10 pneumonia, 5 , 7 , 10 and delay in influenza antiviral treatment. 5 , 7 , 10 , 11 Acute respiratory distress syndrome (ARDS) was a common complication and a significant cause of mortality among ICU patients with pH1N1 virus infection. 5 , 7
Critically ill patients with pH1N1 infection have been previously described, 12 , 13 , 14 , 15 , 16 , 17 , 18 but a few studies have focused on ICU patients hospitalized in the United States. We describe the clinical characteristics, including severe outcomes of ARDS and death and their association with different factors such as early antiviral treatment, among children and adults hospitalized in an ICU with pH1N1 virus infection in the United States during the spring and fall pandemic waves.
Methods
The source of patient data included in this analysis was two previously described national pH1N1 virus hospitalization case‐series conducted in the United States during spring and fall 2009. 5 , 7 Patients included in these case‐series had laboratory‐confirmed pH1N1 virus by real‐time reverse‐transcriptase polymerase chain reaction; testing was clinically driven.
In the spring (May 1–June 9, 2009), the first hospitalized pH1N1 patients reported to CDC were sequentially sampled; participation from 24 states where disease occurred yielded 25% of the total cases reported during the surveillance period. 5 In the fall (September 1–October 31, 2009), patients were sampled based on probability of selection proportional to the number of hospitalized cases reported to CDC; participation from 40 states yielded <2% of the total cases reported during the surveillance period. 7 Both spring and fall case‐series were part of the emergency public health practice response to assess illness severity during the pH1N1 virus pandemic and were deemed not to be research in accordance with the federal human subjects protection regulations at 45 Code of Federal Regulations 46·101c and 46·102d and CDC’s Guidelines for Defining Public Health Research and Public Health Non‐Research; participation by the state and local health departments was voluntary.
For this analysis, we included patients with pH1N1 infection admitted to an ICU from the overall hospitalization case‐series; this represented 34 states in the United States. Using a standard form, demographic and clinical information, including receipt of mechanical ventilation, ARDS as diagnosed by treating physicians, and death status were abstracted from medical charts by infection control practitioners, physicians, nurses, and epidemiologists at state and local health departments and reported to CDC. We collected information on all symptoms including date of onset for each symptom; the first day for any symptom was considered illness onset day 0. Day of hospital admission was considered hospital day 0; for transfer patients, date of admission related to the first hospitalization was used. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of height in meters for non‐pregnant ICU patients ≥2 years of age to determine normal range (BMI < 30·0), obesity (BMI 30·0–39·9 in adults or BMI percentile 95–100 in children), or morbid obesity (BMI ≥40 in adults only). Pneumonia status was based on admission chest radiograph reports. ARDS status was determined by review of the admission history, problem list, and discharge summary. If any of these areas of the medical chart listed ARDS, the patient was considered to have ARDS.
We conducted bivariate analyses to investigate associations with ARDS and death, using the chi‐square or Fisher’s exact test to compare categorical variables and the Wilcoxon rank‐sum test to compare continuous variables (P ≤ 0·05). All analyses were performed in sas version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Clinical characteristics
Of 527 pH1N1 hospitalized patients, 154 (30%) were admitted to an ICU, including 68/272 (26%) spring and 86/255 (34%) fall hospitalizations. The majority of patients (77%) were <50 years of age, and race/ethnicity was principally non‐Hispanic white (40%), Hispanic (22%), or black (22%) (Table 1).
Table 1.
Demographic characteristics of intensive care unit (ICU) patients with pandemic influenza A (H1N1pdm09) virus infection
| Patient demographics | Children <18 years (n = 46) | Adults ≥18 years (n = 108) | Total (n = 154) |
|---|---|---|---|
| Female, no. (%) | 23 (50) | 56 (52) | 79 (51) |
| Age, median (IQR)* | 8 (3–13) | 43 (29–52) | 30 (14–47) |
| Age group, no. (%) | |||
| 0–23 months | 9 (20) | – | 9 (6) |
| 2–4 years | 6 (13) | – | 6 (4) |
| 5–9 years | 9 (20) | – | 9 (6) |
| 10–17 years | 22 (48) | – | 22 (14) |
| 18–49 years | – | 73 (68) | 73 (47) |
| 50–64 years | – | 27 (25) | 27 (18) |
| 65+ years | – | 8 (7) | 8 (5) |
| Race and ethnicity, no. (%) | |||
| Hispanic | 12 (26) | 22 (20) | 34 (22) |
| Non‐Hispanic white | 13 (28) | 49 (45) | 62 (40) |
| Black | 11 (24) | 23 (21) | 34 (22) |
| Other** | 2 (4) | 8 (7) | 10 (7) |
| Unspecified | 8 (17) | 6 (6) | 14 (9) |
*Interquartile range.
**Includes Asian, Native Hawaiian or Pacific Islander, American Indian or Alaska Native, and Multiracial.
Forty percent of patients were admitted to the hospital ≤2 days after illness onset, including 55% of children and 34% of adults. Sixty‐four percent of patients were admitted to an ICU on the same day as hospital admission. On hospital admission, most ICU patients had a history of fever (97%), cough (89%), or shortness of breath (77%) and had documented tachypnea (54%) and tachycardia (80%) (Table 2). The median length of hospital stay was 8 days, and median ICU length of stay was 4 days.
Table 2.
Clinical characteristics of intensive care unit (ICU) patients with pandemic influenza A (H1N1pdm09) virus infection
| Characteristics | Children <18 years (n = 46) | Adults ≥18 years (n = 108) | Total (n = 154) |
|---|---|---|---|
| Clinical signs at admission, no. (%) | |||
| Tachypnea | 32/42 (76) | 45/101 (45) | 77/143 (54) |
| Tachycardia | 39/42 (93) | 77/103 (75) | 116/145 (80) |
| Hypotension (MAP < 60) | 4/38 (11) | 10/105 (10) | 14/143 (10) |
| Illness onset to hospitalization, median (IQR),* day | 2 (1–5) (n = 44) | 3 (2–5) (n = 108) | 3 (2–5) (n = 152) |
| Admission ≤ 2 days after illness onset, no. (%) | 24/44 (55) | 37/108 (34) | 61/152 (40) |
| Length of hospital stay, median (IQR), day | 6 (3–13) (n = 45) | 9 (4–18) (n = 107) | 8 (4–16) (n = 152) |
| ICU length of stay, median (IQR), day | 3 (2–12) (n = 34) | 5 (2–11) (n = 80) | 4 (2–11) (n = 114) |
| Any underlying medical condition,** no. (%) | 28 (61) | 72 (67) | 100 (65) |
| Asthma | 12 (26) | 25 (23) | 37 (24) |
| Chronic obstructive pulmonary disease | 0 (0) | 18 (17) | 18 (12) |
| Neurological disease | 14 (30) | 12 (11) | 26 (17) |
| Diabetes | 0 (0) | 22 (20) | 22 (14) |
| Cardiac disease | 2 (4) | 21 (19) | 23 (15) |
| Immunosupression | 6 (13) | 11 (10) | 17 (11) |
| Renal disease | 1 (2) | 11 (10) | 12 (8) |
| Pregnancy | 0 (0) | 11 (10) | 11 (7) |
| Obesity,*** no. (%) | 6/20 (30) | 23/78 (29) | 29/98 (30) |
| Morbid obesity,*** no. (%) | NA | 21/78 (27) | 21/98 (21) |
| Radiographic evidence of pneumonia at admission, no. (%) | 28/41 (68) | 73/101 (72) | 101/142 (71) |
| Treatment with influenza antivirals, no. (%) | 38 (83) | 90 (83) | 128 (83) |
| ≤2 days after illness onset | 14/37 (38) | 22/88 (25) | 36/125 (29) |
| 3–5 days after illness onset | 11/37 (30) | 34/88 (39) | 45/125 (36) |
| ≥6 days after illness onset | 12/37 (32) | 32/88 (36) | 44/125 (35) |
| Antibiotic treatment, no. (%) | 41 (89) | 103 (95) | 144 (94) |
| Steroids, no. (%) | 26 (57) | 43 (40) | 69 (45) |
| Mechanical ventilation, no. (%) | 23 (50) | 66 (61) | 89 (58) |
| Sepsis syndrome, no. (%) | 9/39 (23) | 27/91 (30) | 36/130 (28) |
| Acute respiratory distress syndrome, no. (%) | 10/38 (26) | 38/90 (42) | 48/128 (38) |
| Death, no. (%) | 9 (20) | 28 (26) | 37 (24) |
*Interquartile range.
**Patients who are pregnant, immunocompromised (either because of medications or immune disorders including human immunodeficiency syndrome), or have chronic pulmonary (including asthma or chronic obstructive pulmonary disease), cardiovascular (excludes hypertension), renal, hepatic, hematological, neurological, or metabolic disease (including diabetes) are considered at high‐risk for influenza‐related complications.
***Obesity and morbid obesity are mutually exclusive.
The majority of patients (65%) had at least one underlying medical condition (Table 2). In children, neurological disease (30%), including neurocognitive dysfunction, neuromuscular disease, and seizure disorder, was most common. Asthma (23%) was most common among adult patients. Eleven (7%) patients were pregnant, eight (73%) of whom were in the third trimester. Of the 98 (73%) non‐pregnant persons aged ≥2 years for whom height and weight were available, six (30%) children and 23 (29%) adults were obese, and an additional 21 (27%) adults were morbidly obese.
Diagnostic findings
Equal proportions of patients (22%) with available admission white blood counts (n = 146) had leukopenia (white cell count < 5000 per mm3) and leukocytosis (white cell count > 11 000 per mm3). The majority of patients (71%) had radiographic findings consistent with pneumonia (Table 2). Bacterial infection was confirmed by admission blood culture, sterile respiratory site culture, or urine antigen test in 11 patients, seven of whom died, including three patients with ARDS. Organisms identified were methicillin‐resistant Staphyloccus aureus (2), methicillin‐sensitive S. aureus (2), S. aureus of unknown sensitivity (1), Streptococcus pneumoniae (3), group A streptococcus (GAS) (1), S. pneumoniae and GAS co‐infection (1), and Escherichia coli (1).
Treatment
The majority of patients received influenza antiviral agents (83%); oseltamivir (98%) was most commonly used. The median time from illness onset to antiviral treatment was 4 days among 125 ICU patients with available antiviral agent initiation dates; 29% of patients received antiviral agents ≤2 days after illness onset, including 38% of children and 25% of adults (Table 2). In addition, 36% of patients received antiviral agents between 3 and 5 days after illness onset and 35% of patients were treated ≥6 days after illness onset. Receipt of antiviral agents in relation to day of admission was the following: 5% before admission, 48% on admission, 23%≤2 days after admission, and 24% >2 days after admission.
Outcomes
The proportion of hospitalized patients admitted to an ICU in the spring was lower than in the fall (25% versus 34%, P = 0·03). However, other indicators of severe illness were similar between the two waves including the proportion of ICU patients requiring mechanical ventilation (63% versus 53%, P = 0·2), with ARDS (43% versus 33%, P = 0·3), and who died (28% versus 21%, P = 0·3).
Eighty‐nine (58%) (23 children and 66 adults) patients were mechanically ventilated (Table 2). Patients requiring mechanical ventilation compared with patients not requiring mechanical ventilation were less likely to have asthma (18% versus 32%, P = 0·04) and more likely to have pneumonia (82% versus 54%, P < 0·01) and die (37% versus 6%, P < 0·01).
ARDS developed in 48 of 128 (38%) patients (10 children and 38 adults, Table 2). Patients with ARDS compared with patients without ARDS had a longer median time from illness onset to hospital admission (5 versus 3 days) and were more likely to be morbidly obese (36% versus 19%), have pneumonia (91% versus 56%), and die (48% versus 8%) but less likely to have a neurological disease (6% versus 20%) (Table 3). Patients with ARDS were not more likely to have asthma (19% versus 33%, P = 0·09) than patients without ARDS. Patients with ARDS and those without ARDS were equally likely to receive influenza antiviral agents, but patients with ARDS were less likely to receive treatment ≤2 days after admission (64% versus 84%, P = 0·03). Compared with patients who received treatment ≥6 days after illness onset, patients treated ≤2 days and between 3 and 5 days after illness onset were less likely to develop ARDS (Figure 1, Table 3).
Table 3.
Comparison of clinical characteristics of intensive care unit (ICU) patients with pandemic influenza A (H1N1pdm09) virus by acute respiratory distress syndrome (ARDS) and death status
| Characteristics | ARDS | Death | ||||
|---|---|---|---|---|---|---|
| Yes (n = 48) | No (n = 80) | P‐value | Yes (n = 37) | No (n = 117) | P‐value | |
| Age, median (IQR),* year | 28 (21–42) | 30 (11–49) | 0·89 | 30 (21–46) | 30 (13–49) | 0·83 |
| Age ≥ 18 years, no. (%) | 38 (79) | 52 (65) | 0·09 | 28 (76) | 80 (69) | 0·40 |
| Illness onset to admission, median (IQR), day | 5 (2–6) (n = 48) | 3 (2–4) (n = 78) | <0·01 | 5 (2–6) (n = 37) | 3 (2–5) (n = 115) | 0·03 |
| Admission ≤ 2 days after illness onset, no. (%) | 16/48 (33) | 36/78 (46) | 0·16 | 10/37 (27) | 51/115 (44) | 0·06 |
| Length of hospital stay, median (IQR), day | 16 (7–24) (n = 46) | 6 (3–10) (n = 80) | <0·01 | 7 (3–14) (n = 37) | 8 (4–16) (n = 115) | 0·33 |
| ICU length of stay, median (IQR), day | 11 (5–15) (n = 35) | 2 (1–5) (n = 59) | <0·01 | 5 (2–12) (n = 36) | 3 (2–11) (n = 78) | 0·16 |
| Any medical underlying condition,** no. (%) | 27 (56) | 53 (66) | 0·26 | 25 (68) | 75 (64) | 0·70 |
| Asthma | 9 (19) | 26 (33) | 0·09 | 4 (11) | 33 (28) | 0·05 |
| Chronic obstructive pulmonary disease | 2 (4) | 9 (11) | 0·21 | 4 (11) | 14 (12) | 1·00 |
| Neurological disease | 3 (6) | 16 (20) | 0·04 | 5 (14) | 21 (18) | 0·62 |
| Obesity,*** no. (%) | ||||||
| Obese versus not obese | 9/28 (32) | 15/54 (28) | 0·24 | 9/24 (38) | 20/74 (27) | 0·21 |
| Morbidly obese versus not obese | 10/28 (36) | 10/54 (19) | 0·04 | 6/24 (25) | 15/74 (20) | 0·36 |
| Radiographic evidence of pneumonia at admission, no. (%) | 43/47 (91) | 40/72 (56) | <0·01 | 32/35 (91) | 69/107 (64) | <0·01 |
| Treatment with influenza antivirals, no. (%) | 42 (88) | 63 (79) | 0·21 | 28 (76) | 100 (85) | 0·17 |
| ≤2 days after illness onset | 7/42 (17) | 22/60 (37) | <0·01† | 2/28 (7) | 34/97 (35) | <0·01† |
| 3–5 days after illness onset | 10/42 (24) | 26/60 (43) | <0·01† | 11/28 (39) | 34/97 (35) | 0·32† |
| ≥6 days after illness onset | 25/42 (60) | 12/60 (20) | 15/28 (54) | 29/97 (30) | ||
| Sepsis syndrome, no. (%) | 22/41 (54) | 9/80 (11) | <0·01 | 21/30 (70) | 15/100 (15) | <0·01 |
| ARDS | – | – | – | 23/29 (79) | 25/99 (25) | <0·01 |
| Death, No. (%) | 23 (48) | 6 (8) | <0·01 | – | – | – |
*Interquartile range.
**Patients who are pregnant, immunocompromised (either because of medications or immune disorders including human immunodeficiency syndrome), or have chronic pulmonary (including asthma or chronic obstructive pulmonary disease), cardiovascular (excludes hypertension), renal, hepatic, hematological, neurological, or metabolic disease (including diabetes) are considered at high‐risk for influenza‐related complications.
***Obesity categories are mutually exclusive.
†Compared with ≥6 days after illness onset.
Figure 1.
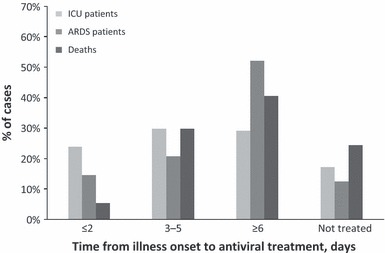
Timing of influenza antiviral treatment among intensive care unit (ICU) patients with pandemic influenza A (H1N1pdm09) virus by ICU, acute respiratory distress syndrome (ARDS), and death status (n = 151) (Antiviral timing information was not available for three patients who received antiviral treatment).
Among 37 (24%) ICU patients who died, nine were children and 28 were adults (Table 2). Patients who died compared with those who survived had a longer median time from illness onset to hospital admission (5 versus 3 days) and were more likely to have pneumonia (91% versus 64%) and ARDS (79% versus 25%) but less likely to have asthma (11% versus 28%) (Table 3). Patients who died and those who survived were equally likely to receive influenza antiviral agents, including within 2 days of admission (61% versus 78%; P = 0·08). Compared with patients who received treatment ≥6 days after illness onset, patients treated ≤2 days after illness onset were less likely to die (Figure 1, Table 3).
Discussion
We describe a large national case‐series including both children and adults hospitalized in an ICU with pH1N1 virus infection in the United States. Among 154 pH1N1 patients hospitalized in an ICU during the spring and fall pandemic waves, almost two‐thirds required mechanical ventilation, nearly 40% developed ARDS, and one‐quarter died. The majority of ICU patients in this analysis were <50 years old or had underlying medical conditions. While asthma was the most common underlying medical condition, patients with asthma were not more likely to develop ARDS or die. Early treatment with influenza antiviral agents was associated with survival and non‐progression to ARDS, especially when initiated ≤2 days after illness onset.
Critical illness has been previously described around the world; a review of key studies is highlighted in Table 4. 12 , 13 , 14 , 15 , 16 , 17 , 18 While case definitions and case ascertainment methods varied, several findings were similar across studies. Globally, critically ill pH1N1 patients were younger adults 12 , 13 , 14 , 15 , 16 with chronic lung disease 13 , 14 , 15 , 16 or obesity 12 , 13 , 14 , 15 , 16 and young children with chronic lung disease or neurological disorders. 17 , 18 The need for mechanical ventilation ranged from 65% to 93%, 12 , 13 , 15 , 16 , 17 , 18 ARDS ranged from 49% to 64%, 14 , 15 and mortality ranged from 7% to 41%. 12 , 13 , 14 , 15 , 16 , 17 , 18 Few studies were able to assess the impact of treatment with influenza antiviral agents on critical illness; however, when data have been available, antiviral treatment 12 has been associated with survival. While the critical illness literature covers a broad spectrum of populations, only a few United States ICU case‐series have been described to date.
Table 4.
Summary of studies describing intensive care unit (ICU) or critically ill patients with pandemic influenza A (H1N1pdm09)*
| Study name (Size) | Location and time period | Case definition | Ages | Most common chronic conditions (%)** | Obesity (%)*** | Mechanical ventilation (%) | ARDS (%) | Death (%) | Antiviral agent receipt (%) |
|---|---|---|---|---|---|---|---|---|---|
| Pediatric and adult studies | |||||||||
| This study (n = 154) | United States May–June 2009; September–October 2009 | ICU admission† | Median (IQR): 30 years (14–47) | Asthma: 24 Neurologic disease: 17 | 51 | 58 | 38 | 24 | 83 |
| Dominguez‐Cherit 12 (n = 58) | Mexico March–June 2009 | Critical illness††,††† | Median (range): 44 years (10–83) | Hypertension: 26 Diabetes: 17 | 36 | 93 | – | 41 | 78 |
| Kumar 13 (n = 168) | Canada April–August 2009 | Critical illness††,‡ | Mean (SD) : 32 years (21) | Chronic lung disease: 41 Hypertension: 24 | 33 | 81 | – | 17 | 91 |
| Miller 14 (n = 47) | United States (Salt Lake County, Utah) May–June 2009 | ICU admission† | Median (range): 34 years (15–62) | Asthma: 30 Diabetes: 17 | 74 | – | 64 | 17 | 96 |
| ANZIC 15 (n = 722) | Australia and New Zealand June –August 2009 | ICU admission† | Median (IQR): 40 years (26–54) | Asthma/COPD: 33 Diabetes: 16 | 29 | 65 | 49‡‡ | 17 | – |
| Nicolay 16 (n = 77) | Ireland July 2009–May 2010 | ICU admission‡ | Median (IQR): 43 years (30–56) | Asthma/COPD: 34 Malignancy/immunosupression: 20 | 36 | 72 | – | 18 | – |
| Pediatric studies | |||||||||
| Jouvet 17 (n = 57) | Canada April–August 2009 | Critical illness††,††† | Median (range): 5 years (0·08–17) | Asthma: 25 Neurologic disease: 23 | – | 68 | – | 7 | 77 |
| Randolph 18 (n = 838) | United States April 2009–April 2010 | ICU admission‡ | Median (range): 6 years (0–20) | Asthma: 31 Neurologic disease: 31 | – | 67 | – | 9 | 97 |
*IQR, interquartile range; SD, standard deviation; COPD, chronic obstructive pulmonary disease.
**Excludes obesity and history of smoking.
***This study, Dominguez‐Cherit, Kumar, and Miller report patients with body mass index (BMI) >30; ANZIC and Nicolay report patients with BMI >35.
†Includes confirmed pH1N1cases only.
††Defined as those (1) admitted to ICU, (2) requiring mechanical ventilation, (3) having a fraction of inspired oxygen (FIO2) greater than or equal to 60%, or (4) receiving intravenous infusion of inotropic or vasopressor medication.
†††Includes confirmed, probable, and suspected pH1N1 cases.
‡Includes confirmed and probable pH1N1 cases.
‡‡Includes patients with ARDS and viral pneumonitis.
ARDS, acute respiratory distress syndrome; pH1N1, pandemic influenza A (H1N1pdm09).
In this analysis, similar to other ICU case‐series, neurological disorders and asthma were the most common underlying medical conditions in children and adults, respectively (Table 4), and are known to be high‐risk conditions for influenza‐associated complications. 19 Persons with neurological disorders infected with influenza virus are at risk for respiratory failure 20 and death 18 , 21 because of compromised respiratory function, inability to handle respiratory secretions, or malnutrition. 22 , 23 , 24 However, in this analysis, patients with neurological disorders were not more likely to develop ARDS or die; this may be due to the small number of patients with neurological disorders in our study. Interestingly, in this analysis, patients with asthma were not at greater risk for ARDS and were less likely to die. The severity and course of illness among persons with asthma with influenza infection depends on multiple factors including the immunologic response to viral infections, airway hyper‐responsiveness caused by atopy, allergens, and other environmental factors, as well as baseline control of asthma. 25 Patients included in our analysis may have been admitted to an ICU more readily if they had a history of asthma as a precaution and not because of the severity of their present illness; this would bias our analysis to demonstrating less severe outcomes in this group. In addition, morbid obesity, which emerged as a risk factor for severe influenza infection during the 2009 pandemic, 26 was more common among ARDS patients in our analysis. Adipose tissue has been hypothesized to reduce macrophage activity and cytokine production and contribute to pro‐inflammatory state predisposing obese individuals to infection. 27 Further, adipose tissue that has accumulated around the rib cage and abdomen may decrease lung compliance and increase the risk of airway closure and ventilation–perfusion mismatch. 28 While influenza vaccination is now recommended for all persons ≥6 months old, 29 in light of vaccine supply and demand and future pandemic preparedness, a better understanding of the relationship between influenza and underlying conditions that can lead to severe outcomes is necessary.
ARDS affected nearly 40% of patients in our ICU case‐series. While the proportion of ARDS was not as high as in other studies of critically ill pH1N1 patients in which 49–64% of patients had ARDS, 14 , 15 this may have been due to variable study periods, study populations, ARDS definitions, and differences in assessment of ARDS (Table 4). The proportion of pH1N1 ICU patients with ARDS in this analysis was substantially higher than reported in another ICU case‐series that focused on seasonal influenza virus infection (23%). 30 Results from both animal studies and human autopsy reports indicate that pH1N1 virus infection caused severe diffuse alveolar damage that likely led to more severe lower‐tract respiratory disease and respiratory failure than has been seen with seasonal influenza infections in the past. 31 , 32 , 33 , 34
While our data is observational, it suggests that early treatment with influenza antiviral agents, especially when initiated ≤2 days after illness onset, may be beneficial in preventing ARDS or death. Patients who received antiviral treatment between 3 and 5 days after illness onset were less likely to develop ARDS but equally likely to die compared with patients treated ≥6 days after illness onset. The reasons for this discrepancy are unclear but could be related to persons dying of causes other than ARDS, misclassification of ARDS status, or because of the low number of deaths in this analysis. While the greatest benefit associated with antiviral treatment has been noted for patients who receive early antiviral therapy, some studies have shown that treatment with antiviral agents >2 days from illness onset may avert influenza‐associated complications. 35 Our results underscore the importance of early influenza antiviral treatment among hospitalized 5 , 7 , 9 , 36 and critically ill 37 patients with influenza and the need for clarity on the optimal timing in which antivirals still have benefit.
Our data are subject to limitations. The patients described were derived from two hospitalization case‐series that used different sampling methods. 5 , 7 However, data from both periods were nationally representative of hospitalizations from areas in the United States where peak disease activity was occurring at the time. Patients included in this analysis were laboratory‐confirmed for pH1N1 virus and may not be representative of all ICU patients with pH1N1 infection who were not tested. Deaths occurring after hospital discharge, which could have been related to the influenza admission, were not captured. Despite the use of a standard data collection form, not all information was collected for all patients, including influenza vaccination status (pH1N1 vaccine was not readily available during the study); this limits our ability to assess these interventions; however, the study was not designed to address these specific questions. While we attempted to collect detailed clinical information on each patient, variables that could be included in severity scores were not available for most patients. ARDS status was also not always available. In addition, variables, including ARDS and history of asthma, were based on chart review and not a standardized clinical assessment, potentially resulting in misclassification. To reduce further misclassification of ARDS, missing ARDS data were not imputed. Because not all data elements were collected for all patients, we did not have an adequate sample size to conduct a multivariable analysis.
The pH1N1 virus caused significant morbidity in the United States, 2 leading to a substantial number of ICU admissions and deaths. 3 , 4 , 5 , 6 , 7 Influenza vaccination is the primary tool for preventing infection and is recommended for all persons aged ≥6 months. 29 However, because of varying and suboptimal influenza vaccination rates, and as influenza vaccine effectiveness is not 100%, early influenza antiviral treatment is a key element needed to reduce morbidity and mortality from influenza. All persons with suspected or confirmed influenza virus infection requiring hospitalization or patients with progressive, severe, or complicated illness, including those admitted to an ICU, should be given influenza antiviral agents as early as possible, regardless of their prior vaccination status. 38 , 39
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Disclosure
No potential conflict of interest relevant to this article was reported.
Funding/Support
Influenza Division at the Centers for Disease Control and Prevention.
Acknowledgements
We thank the members of the 2009 Pandemic Influenza A (H1N1) Hospitalizations Investigation Team for data collection: Centers for Disease Control and Prevention: John McKenna, Victoria White; Alabama Department of Public Health: S. Davidson; Alaska Department of Health and Social Services: Donna Fearey; Arizona Department of Public Health: Sanny Chen*; Arkansas Department of Health: Linda Gladden; California Department of Public Health: Janice Louie, Allison Stone; Chicago Department of Public Health (IL): Kathleen A. Ritger; Colorado Department of Public Health and Environment: Ken Gershman; Cook County Department of Public Health: Supriya Jasuja; Delaware Division of Public Health: Paula Eggers; DuPage County Health Department (IL): Rashmi Chugh; Florida Department of Health/Bureau of Epidemiology: Patti Ragan; Georgia Division of Public Health: Kathryn E. Arnold; Hawaii Department of Health: Meera Sreenivasan*; Idaho Department of Health and Welfare: James Colborn*; Illinois Department of Public Health: Kenneth Soyemi; Indiana State Department of Health: Shawn M. Richards; Iowa Department of Public Health: Christopher Tate; Kansas Department of Public Health: Daniel Neises; Kentucky Department for Public Health: Doug Thoroughman; Louisiana Office of Public Health: Julie Hand; Maryland Department of Health and Mental Hygiene: Maya Monroe; Massachusetts Department of Health: Susan Lett, Noelle Cocoros, Molly Crockett; Michigan Department of Community Health: Eden V Wells, Jennie Finks; Minnesota Department of Public Health: Ruth Lynfield; Mississippi State Department of Health: Jannifer G Anderson; Missouri Department of Health and Senior Services: Sarah L. Patrick; Nebraska Department of Health and Human Services: Robin M. Williams; Nevada Department of Public Health: Ihsan Azzam, Carmen P. Cruz; New Hampshire Department of Health and Human Services: Elizabeth R. Daly; New Jersey Department of Health and Senior Services: Samantha Pitts; New Mexico Department of Health: Catherine Avery; New York City Department of Health and Mental Hygiene: Swine Flu Investigation Team; New York State Department of Health: Nancy L. Spina; North Carolina Department of Health and Human Services: Zack Moore; Ohio Department of Health: Shannon L. Page; Oklahoma State Department of Health: Kristy K. Bradley; Oregon Department of Health: Meredith Vandermeer; Pennsylvania Department of Health: Tina Berezansky, Bruno Petruccelli; Philadelphia Department of Public Health (PA): Colleen Burke; Rhode Island Department of Health: Tara Cooper; San Diego County Health and Human Services (CA): David E. Sugerman*; St. Luke’s South Hospital (KS): Kathleen S. Hall‐Meyer; South Carolina Department of Health and Environmental Control: Chasisity Brown Springs; South Dakota Department of Health: Vickie Horan; Public Health ‐ Seattle & King County, Seattle (WA): Jeffrey S. Duchin; Tennessee Department of Health: Tim F. Jones, David Kirschke; Texas Department of State Health Services: John D. Walker, Lesley Brannan; University of North Carolina at Chapel Hill (NC): Tiffany Wedlake; Utah Department of Health: Robert T. Rolfs, Valoree Vernon; Vermont Department of Health: Lynn Z. Blevins; Washington State Department of Health: Kathryn H. Lofy; West Virginia Bureau for Public Health: Danae Bixler, Maria DelRosario; Wisconsin Division of Public Health: Jean K. Druckenmiller, Carrie Nielsen*; Wyoming Department of Health: Aimee Geissler*.
*Epidemic Intelligence Service, Office of Workforce and Career Development, Centers for Disease Control and Prevention, Atlanta, GA.
For the 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team members are in Acknowledgements.
References
- 1. Dawood FS, Jain S, Finelli L et al. Emergence of a novel swine‐origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360:2605–2615. [DOI] [PubMed] [Google Scholar]
- 2. Shrestha SS, Swerdlow DL, Borse RH et al. Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–April 2010). Clin Infect Dis 2011; 52(Suppl 1):S75–S82. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. 2009 pandemic influenza A (H1N1) virus infections – Chicago, Illinois, April–July 2009. MMWR Morb Mortal Wkly Rep 2009; 58:913–918. [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Patients hospitalized with 2009 pandemic influenza A (H1N1) – New York City, May 2009. MMWR Morb Mortal Wkly Rep 2010; 58:1436–1440. [PubMed] [Google Scholar]
- 5. Jain S, Kamimoto L, Bramley AM et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med 2009; 361:1935–1944. [DOI] [PubMed] [Google Scholar]
- 6. Louie JK, Acosta M, Winter K et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 2009; 302:1896–1902. [DOI] [PubMed] [Google Scholar]
- 7. Skarbinski J, Jain S, Bramley A et al. Hospitalized patients with 2009 pandemic influenza A (H1N1) virus infection in the United States – September–October 2009. Clin Infect Dis 2011; 52(Suppl 1):S50–S59. [DOI] [PubMed] [Google Scholar]
- 8. Campbell A, Rodin R, Kropp R et al. Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. CMAJ 2010; 182:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zarychanski R, Stuart TL, Kumar A et al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ 2010; 182:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen‐Van‐Tam JS, Openshaw PJ, Hashim A et al. Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May–September 2009). Thorax 2010; 65:645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siston AM, Rasmussen SA, Honein MA et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010; 303:1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dominguez‐Cherit G, Lapinsky SE, Macias AE et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 2009; 302:1880–1887. [DOI] [PubMed] [Google Scholar]
- 13. Kumar A, Zarychanski R, Pinto R et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302:1872–1879. [DOI] [PubMed] [Google Scholar]
- 14. Miller RR 3rd, Markewitz BA, Rolfs RT et al. Clinical findings and demographic factors associated with ICU admission in Utah due to novel 2009 influenza A(H1N1) infection. Chest 2010; 137:752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Webb SA, Pettila V, Seppelt I et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 2009; 361:1925–1934. [DOI] [PubMed] [Google Scholar]
- 16. Nicolay N, Callaghan MA, Domegan LM et al. Epidemiology, clinical characteristics and resource implications of pandemic (H1N1) 2009 in intensive care units in Ireland. Crit Care Resusc 2010; 12:255–261. [PubMed] [Google Scholar]
- 17. Jouvet P, Hutchison J, Pinto R et al. Critical illness in children with influenza A/pH1N1 2009 infection in Canada. Pediatr Crit Care Med 2010; 11:603–609. [DOI] [PubMed] [Google Scholar]
- 18. Randolph AG, Vaughn F, Sullivan R et al. Critically ill children during the 2009–2010 influenza pandemic in the United States. Pediatrics 2011; 128:e1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fiore AE, Shay DK, Broder K et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58:1–52. [PubMed] [Google Scholar]
- 20. Keren R, Zaoutis TE, Bridges CB et al. Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection. JAMA 2005; 294:2188–2194. [DOI] [PubMed] [Google Scholar]
- 21. Bhat N, Wright JG, Broder KR et al. Influenza‐associated deaths among children in the United States, 2003–2004. N Engl J Med 2009; 353:2559–2567. [DOI] [PubMed] [Google Scholar]
- 22. Reddihough D, Baikie G, Walstab JE. Cerebral palsy in Victoria, Australia: mortality and causes of death. J Paediatr Child Health 2001; 37:183–186. [DOI] [PubMed] [Google Scholar]
- 23. Forsgren L, Hauser WA, Olafsson E, Sander JWAS, Sillanpaa M, Tomson T. Mortality of epilepsy in developed countries: a review. Epilepsia 2005; 46:18–27. [DOI] [PubMed] [Google Scholar]
- 24. Haas CF, Loik PS, Gay SE. Airway clearance applications in the elderly and in patients with neurologic or neuromuscular compromise. Respiratory Care 2007; 52:1362–1381. [PubMed] [Google Scholar]
- 25. Papadopoulos NG, Christodoulou I, Rohde G et al. Viruses and bacteria in acute asthma exacerbations – A GA(2) LEN‐DARE* systematic review. Allergy 2010; 66:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morgan OW, Bramley A, Fowlkes A et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS ONE 2010; 5:e9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsatsanis C, Margioris AN, Kontoyiannis DP. Association between H1N1 infection severity and obesity – adiponectin as a potential etiologic factor. J Infect Dis 2010; 202:459–460. [DOI] [PubMed] [Google Scholar]
- 28. Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol 2010; 108:206–211. [DOI] [PubMed] [Google Scholar]
- 29. Fiore AE, Uyeki TM, Broder K et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010; 59:1–62. [PubMed] [Google Scholar]
- 30. Li G, Yilmaz M, Kojicic M et al. Outcome of critically ill patients with influenza virus infection. J Clin Virol 2009; 46:275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Itoh Y, Shinya K, Kiso M et al. In vitro and in vivo characterization of new swine‐origin H1N1 influenza viruses. Nature 2009; 460:1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maines TR, Jayaraman A, Belser JA et al. Transmission and pathogenesis of swine‐origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 2009; 325:484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mauad T, Hajjar LA, Callegari GD et al. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med 2010; 181:72–79. [DOI] [PubMed] [Google Scholar]
- 34. Shieh WJ, Blau DM, Denison AM et al. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol 2010; 177:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bautista E, Chotpitayasunondh T, Gao Z et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362:1708–1719. [DOI] [PubMed] [Google Scholar]
- 36. Lee EH, Wu C, Lee EU et al. Fatalities associated with the 2009 H1N1 influenza A virus in New York city. Clin Infect Dis 2010; 50:1498–1504. [DOI] [PubMed] [Google Scholar]
- 37. Farias JA, Fernandez A, Monteverde E et al. Critically ill infants and children with influenza A (H1N1) in pediatric intensive care units in Argentina. Intensive Care Med 2010; 36:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harper SA, Bradley JS, Englund JA et al. Seasonal influenza in adults and children – diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fiore AE, Fry A, Shay D et al. Antiviral agents for the treatment and chemoprophylaxis of influenza – recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–24. [PubMed] [Google Scholar]


