Abstract
Background Currently, Asian lineage highly pathogenic avian influenza (HPAI) H5N1 has become widespread across continents. These viruses are persistently circulating among poultry populations in endemic regions, causing huge economic losses, and raising concerns about an H5N1 pandemic. To control HPAI H5N1, effective vaccines for poultry are urgently needed.
Objective In this study, we developed HPAI virus‐like particle (VLP) vaccine as a candidate poultry vaccine and evaluated its protective efficacy and possible application for differentiating infected from vaccinated animals (DIVA).
Methods Specific pathogen‐free chickens received a single injection of HPAI H5N1 VLP vaccine generated using baculovirus expression vector system. Immunogenicity of VLP vaccines was determined using hemagglutination inhibition (HI), neuraminidase inhibition (NI), and ELISA test. Challenge study was performed to evaluate efficacy of VLP vaccines.
Results and Conclusions A single immunization with HPAI H5N1 VLP vaccine induced high levels of HI and NI antibodies and protected chickens from a lethal challenge of wild‐type HPAI H5N1 virus. Viral excretion from the vaccinated and challenged group was strongly reduced compared with a mock‐vaccinated control group. Furthermore, we were able to differentiate VLP‐vaccinated chickens from vaccinated and then infected chickens with a commercial ELISA test kit, which offers a promising strategy for the application of DIVA concept.
Keywords: Chicken, DIVA, HPAI, vaccine, VLP
Introduction
Highly pathogenic H5N1 avian influenza (HPAI), defined as a “notifiable” disease by the World Organization for Animal Health (OIE) 1 , has caused fatal infections in poultry with severe economic impact worldwide 2 . Especially, Asian lineage HPAI H5N1 has become widespread across continents, including Eurasia and Africa, and has become endemic in Southeast Asia in poultry since it was first identified in China in 1996 3 . Furthermore, to date, HPAI H5N1 has resulted in over 500 confirmed human cases with an approximately 60% fatality rate 4 , raising global public health concerns about its pandemic potential.
To control HPAI H5N1 outbreaks, several strategies have been applied including a high level of biosafety, movement control, stamping out, and vaccination. Although vaccination against HPAI in poultry is still a controversial topic and has been discouraged in the past, vaccination has also been recommended as an alternative HPAI control strategy 5 , 6 , 7 , 8 , since mass culling that is frequently used to control outbreaks in poultry has not proved successful in HPAI H5N1 endemic regions 9 . To be used as part of effective HPAI control strategies, vaccination should prevent clinical disease and death, induce resistance to infection, decrease viral excretion from infected birds, and essentially, facilitate the differentiation of infected from vaccinated animals (DIVA) 10 , which has been a major inhibitor for the use of inactivated vaccine in AIV control 11 .
Virus‐like particles (VLPs), which resemble infectious virus particles in structure and morphology with multiple antigenic epitopes, have been suggested as a new generation of vaccine candidates against various viral infections with a solid safety profile. 12 VLP vaccines were shown to be highly immunogenic probably due to its ability to stimulate a diverse set of host immune responses. 13 . As protective immune responses of influenza A VLPs against A/Udorn/72 (H3N2) have been described for the first time by Galarza et al. 14 , influenza A VLPs have been produced in different expression systems 15 , and the safety, immunogenicity, and protectivity have been studied in various animal models 15 , 16 , 17 . Recently, several studies have demonstrated efficacy and safety of VLP vaccines against HPAI H5N1 using mouse 18 , 19 and ferret 20 , 21 models for the development of human vaccine. However, VLP vaccines against HPAI H5N1 for poultry species, in which HPAI H5N1 viruses are currently circulating and threatening public health and poultry industry, have not been studied.
Previously, a few studies demonstrated immunogenicity 22 and protective efficacy 23 of LPAI VLP vaccine in ducks and chickens, respectively, that provided possibilities for the application of VLP vaccines against HPAI in poultry species, which play a critical role in the maintenance and spread of HPAI H5N1 24 . In this study, for veterinary use, we developed a HPAI H5N1 VLP vaccine and evaluated its immunogenicity and protective efficacy against HPAI in specific pathogen‐free (SPF) chickens for the first time. Furthermore, VLP vaccines were assessed for differentiating VLP‐vaccinated chickens from vaccinated and then infected chickens, which may provide a useful method for serosurveillance in vaccinated flocks.
Materials and methods
Cloning of HA, NA, and M1 genes
Viral RNA was extracted from HPAI H5N1 A/chicken/Korea/ES/2003 (GenBank AY676035) using an RNeasy® Mini Kit (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instruction. For cDNA synthesis, reverse transcription (RT) was performed on extracted viral RNA using the Omniscript™ RT Kit (QIAGEN) with Uni12 primer as previously described. 25 The following primer pairs were used for the polymerase chain reaction (PCR) amplification of the HA, NA, and M1 genes, respectively: HA_ES03 (F) 5′‐TGGATCCatgGAGAAAATAGTGC‐3′ and (R) 5′‐TAAGCTTAGTAGAAACAAGG‐3′; NA_ES03 (F) 5′‐AGAATTCatgAATCCAAATCAGAAG‐3′ and (R) 5′‐TAAGCTTAGTAGAAACAAGG‐3′; M1_ES03 (F) 5′‐AGAATTCatgAGTCTTCTAACCGAGG‐3′ and (R) 5′‐TAAGCTTTCACTTGAATCGCTGC‐3′ (ATG codons shown in lower case letters). PCR‐amplified HA, NA, and M1 genes were cloned into TA cloning vector pGEM®‐T (Promega, Madison, WI, USA), and each gene sequence was determined by DNA sequencing. The three resulting plasmid vectors containing influenza virus genes were designated as vHA, vNA, and vM1.
Construction of transfer vector
Plasmid vector vHA was digested by the restriction enzyme BamHI/HindIII, and the resulting HA DNA fragment was ligated into a BamHI‐/HindIII‐digested pFastBacT1 bacmid transfer vector (Invitrogen, Carlsbad, CA, USA). Similarly, plasmid vectors vNA and vM1 were digested by the restriction enzyme EcoRI/HindIII, and the resulting DNA fragments were ligated into an EcoRI‐/HindIII‐digested pFastBacT1 bacmid transfer vector. The three resulting transfer vectors containing influenza virus genes were designated as pHA, pNA, and pM1. A bacmid transfer vector encoding both NA and M1 genes, designated as pNAM1, was constructed by cloning a SnaBI‐/HpaI‐digested fragment from pM1 into the HpaI site of pNA. Finally, a bacmid transfer vector encoding three influenza genes, pNAM1HA, was prepared by cloning a SnaBI‐/HpaI‐digested fragment from pHA into the HpaI site of pNAM1. As a result, a transfer vector, pNAM1HA, encoding HA, NA, and M1 genes included within its own polyhedrin promoter and transcription termination sequences was constructed.
Generation of recombinant baculovirus
MAX Efficiency® DH10Bac™ competent Escherichia coli cells (Invitrogen) were transformed with the constructed transfer vector plasmids, pNAM1HA, to generate recombinant bacmids according to the instructions recommended by the manufacturer. The recombinant bacmid DNA was transfected into Sf9 cells seeded in 6‐well plates, at a density of 8 × 105 cells/well, using Cellfectin® Reagent (Invitrogen) resulting in the release of recombinant baculovirus (rBV) into the culture medium. At 72 hours post‐transfection, culture medium was harvested and inoculated into Sf9 cells for generation of high‐titer rBV stock. Baculovirus titration was performed by plaque assay on Sf9 cells. 26
Production of HPAI H5N1 VLP and preparation of VLP vaccines
For VLP production, Sf9 cells, grown in Sf900III medium (GIBCO BRL, Grand Island, NY, USA), were infected with rBV expressing H5N1 A/chicken/Korea/ES/2003 HA, NA, and M1 proteins at a multiplicity of infection (MOI) of 3 for 72 hours. The culture medium containing VLPs was collected and clarified by low‐speed centrifugation (2000 × g, 30 minutes) to remove large cell debris, and culture supernatants were chemically treated with formalin at a final concentration of 0·2% for inactivation of baculovirus. After incubation for 48 hours at 37°C, formalin‐treated culture supernatant was concentrated using Vivaspin 20 (100 000 MWCO PES; Sartorius, Göttingen, Germany) protein concentrator without further purification. As VLP antigen used in this study was not sucrose‐purified to reduce production cost for veterinary use, the VLP antigen contents were quantified by hemagglutination units (HAU) of concentrated culture medium as described in the OIE manual 1 , rather than measuring total protein concentration. 23
Expression of influenza proteins in VLPs was confirmed by coomassie‐stained sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) as well as Western blotting using mouse anti‐H5 monoclonal antibody (Bionote, Inc., Hwaseong, Korea), rabbit anti‐N1 antibodies (Immune Technology, NY, USA), and rabbit anti‐M1 antibodies (Immune Technology) followed by the detection with horseradish peroxidase (HRP)‐conjugated goat anti‐mouse or anti‐rabbit IgG (AbD Serotec, UK). H5N1 influenza VLP vaccine doses in this study were determined based on hemagglutination units (HAU) because of crude VLP vaccine preparations. VLP vaccines were prepared by emulsifying the escalating VLP concentration (28, 29, and 210 HAU) of culture supernatants with Montanide ISA70 adjuvant (SEPPIC, France), which provided significant dose‐sparing effect compared with the non‐adjuvanted vaccine in our previous LPAI VLP study 23 , at a ratio of 30:70 (w/w).
Immunization of animals and virus challenge
A total of 32 five‐week‐old SPF white leghorn chickens (Namduck Sanitec, Korea) were divided into four groups (8 chickens per group). Three groups of chickens were immunized with escalating dose (28, 29, and 210 HAU) of HPAI H5N1 VLP vaccines with ISA70 adjuvant. As LPAI VLP vaccine with ISA70 adjuvant, which injected via intramuscular route, elicited high levels of HI antibody in chicken in our previous study 23 , immunization was performed intramuscularly. Injection dose was 0·5 ml per chicken. As a mock‐vaccinated control group, another eight SPF chickens were injected with an emulsified solution of conditioned SF900III medium with ISA70 in the same ratio as VLP vaccines. Three weeks after a single immunization, chickens were intranasally challenged with 100 μl of 106·0 EID50/ml of A/chicken/Korea/ES/2003 (H5N1). The challenge study and all experiments with live viruses were conducted in a biosafety level 3 (BSL3) facility in Konkuk University. All animal procedures performed in this study were reviewed, approved, and supervised by the Institutional Animal Care and Use Committee (IACUC) of Konkuk University.
Assessment of protection
As prevention of clinical signs (morbidity) and death (mortality) and reduction of viral shedding after HPAI challenge have been the most frequently used criteria to assess protective efficacy of HPAI vaccine 27 , 28 , 29 , 30 , 31 , we observed mortality and clinical signs daily for 10 days post‐challenge (dpc) and determined viral shedding using real‐time reverse transcriptase polymerase chain reaction (rRT‐PCR).
To determine the viral shedding, oropharyngeal and cloacal swab samples were collected at 2, 3, 5, and 7 dpc and suspended in 1 ml of phosphate‐buffered saline (PBS) supplemented with gentamycin (400 μg/ml). Of this suspension, 200 μl was used for RNA extraction using RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instruction. The content of AIV RNA was quantified by cycle threshold (Ct) value using M gene‐based rRT‐PCR as previously described 32 .
For extrapolation of the Ct values to infectious units, known titers of A/chicken/Korea/ES/2003 (H5N1) viruses from egg alantoic fluid, measured in EID50, were serially 10‐fold diluted. Viral RNA was extracted from these dilutions and quantified by rRT‐PCR as described above. For generating a standard curve, Ct values of each viral dilution were plotted against viral titers. The resulting standard curve was highly correlated (r 2 > 0·99) and was used to convert Ct values to EID50.
Serology
To determine the immunogenicity of VLP vaccines, serum samples were collected prior to vaccination and 3 weeks after vaccination for hemagglutination inhibition (HI) and neuraminidase inhibition (NI) tests. Reverse genetics–derived influenza A/chicken/Korea/ES/2003 (clade 2·5), A/Indonesia/5/2005 (clade 2·1), and A/Vietnam/1194/2004 (clade 1) were formalin‐inactivated and used as homologous or heterologous antigens. HI titers against homologous and heterologous strains were measured according to the OIE standard HI method 1 .
The anti‐NA antibody titers were measured by NI assay using the substrate 2′‐(4‐Methylumbelliferyl)‐α‐d‐N‐acetylneuraminic acid (4‐MU‐NANA; Sigma‐Aldrich, St. Louis, MO, USA) and formalin‐inactivated homologous antigen as enzyme source. Serum samples were twofold diluted with sodium acetate buffer (150 mm sodium acetate, 1 mm calcium chloride, pH 7·0), and 25 μl of diluted samples was mixed with an equal volume of predetermined amount of inactivated homologous antigen (27 HAU). After incubation for 1 hour at 37°C, 50 μl of substrate (100 μm 4‐MU‐NANA in sodium acetate buffer) was added to each well. After additional 30 minutes incubation at 37°C, the reaction was stopped by addition of 100 μl of stop solution (0·1 m glycine, 25% ethanol, pH 10·7). The fluorescence intensity was measured using SpectraMax Gemini EM fluorescent plate reader with excitation and emission wavelengths of 360 nm and 465 nm, respectively. Antibody‐positive cut‐off values were set as the mean value ‐ 2x standard deviation (mean‐2SD) of the fluorescence intensity in mock‐vaccinated chickens. NI titers were expressed as the highest serum dilution factors that resulted fluorescence intensity lower than cut‐off values.
For differentiating vaccinated chickens from vaccinated and then infected chickens, which may provide a useful method for serosurveillance in vaccinated flocks, serum samples were collected 3 weeks post‐vaccination (wpv) and 10 dpc from 28 and 210 VLP‐vaccinated chickens. Collected samples were analyzed for AIV NP‐specific antibody levels using a commercially available multispecies competitive NP‐cELISA Kit (Bionote), which is pre‐coated with AIV NP antigen into the wells of the ELISA plate. ELISA test was performed according to the manufacturer’s instruction. Sample values were calculated [sample value = 1−(OD450 sample/mean OD450 negative)], and sample value <0·5 was considered negative for the presence of antibodies to NP. To compare changes in HI titers following infection with the results from NP‐cELISA, 10 dpc HI titers from 28 and 210 VLP‐vaccinated chickens were measured using homologous antigen as described above.
Statistical analysis
Analysis of variance (anova) with a Tukey–Kramer post‐test was performed for serum HI antibody titers. Statistical analysis of differences between VLP‐vaccinated groups was performed using Fisher’s exact test. Results with P values <0·05 were considered to be statistically significant.
Results
Immune responses to VLP vaccines
HPAI H5N1 VLPs containing HA, NA, and M1 protein derived from A/Chicken/Korea/ES/2003 (H5N1) virus were produced in Sf9 cells using the baculovirus expression vector system (BEVS). After formalin treatment, unconcentrated VLP‐containing culture supernatant was found to have 27 HAU and concentrated to a final concentration of 28, 29, and 210 HAU. Influenza proteins (HA, NA, and M1) of VLPs were detected by coomassie‐stained SDS‐PAGE (Figure 1A) and Western blotting (Figure 1B).
Figure 1.
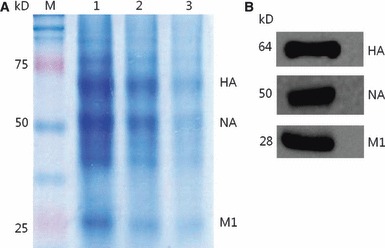
Identification of proteins in A/chicken/Korea/ES/2003(H5N1) VLPs. (A) Coomassie‐stained SDS‐PAGE analysis of VLPs expressing HA, NA, and M1. M, a standard molecular size marker (in kilodaltons); Lane 1, 210 HAU of VLP protein; Lane 2, 29 HAU of VLP protein; Lane 3, 28 HAU of VLP protein. (B) Analysis of VLPs by Western blot using mouse anti‐H5 monoclonal antibody and rabbit polyclonal antibodies for N1 or M1. Molecular weights of expressed HA, NA, and M1 are indicated on the left.
To evaluate the immunogenicity of HPAI H5N1 VLP vaccines, seroconversion was determined 3 weeks after a single immunization with a range of 28 to 210 HAU of VLP vaccines. Throughout the experiment, none of the VLP‐vaccinated chickens showed any signs of adverse effect (e.g. unusual local or systemic response) after administration of the crude VLP vaccine. As shown in Figure 2, significantly increased levels of HI antibody titers against homologous or heterologous antigens were induced in VLP‐vaccinated chickens. As expected, both the heterologous HI titers (clades 2·1 and 1) were significantly lower than those against the homologous strain (clade 2·5). Additionally, heterologous HI titers against the clade 2·1 were observed at higher levels than the clade 1, reflecting the antigenic closeness. As shown in Figure 3, significantly increased levels of NI antibody titers against homologous antigen were induced in all VLP‐vaccinated chickens. NI antibodies elicited by H5N1 VLP vaccine are expected to confer additional protective effect against HPAI challenge, because antibodies to the NA protein thought to reduce the amount of virus released from infected cells by aggregating influenza virus on the cell surface 33 . Groups with higher antigen doses, 29 or 210 HAU VLP vaccines, showed moderately higher levels of HI and NI antibody titers against homologous antigen than the 28 HAU VLP vaccine group although there were no statistical differences among vaccinated groups. However, no detectable levels of antibody responses were found in the mock‐vaccinated control group that received an emulsified solution of conditioned SF900III cell culture medium with ISA70 adjuvant. These results suggest that crude HPAI H5N1 VLP vaccination is safe and can induce anti‐H5 and anti‐N1 functional antibody responses in chickens.
Figure 2.
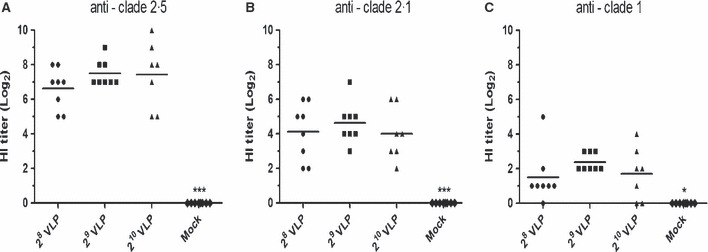
Mean serum hemagglutination inhibition (HI) titers (log2) induced in specific pathogen‐free (SPF) chickens after a single dose of virus‐like particle (VLP) vaccine with ISA70 adjuvant. A total of 32 five‐week‐old SPF chickens (8 per group) were intramuscularly immunized with HPAI H5N1 VLP vaccines. HI titers against the homologous antigen (A) A/chicken/Korea/ES/2003 (clade 2·5) and heterologous antigen (B) A/Indonesia/5/2005 (clade 2·1) and (C) A/Vietnam/1194/2004 (clade 1) were determined 3 weeks after vaccination. Mock – emulsified solution of conditioned SF900III medium with ISA70. *P < 0·05 and ***P < 0·001 by anova with Tukey–Kramer post‐test compared with other groups.
Figure 3.
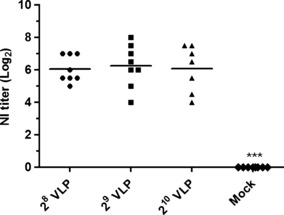
Mean serum neuraminidase inhibition (NI) titers (log2) induced in specific pathogen‐free (SPF) chickens after a single dose of virus‐like particle (VLP) vaccine with ISA70 adjuvant. A total of 32 five‐week‐old SPF chickens (8 per group) were intramuscularly immunized with HPAI H5N1 VLP vaccines. NI titers against the homologous antigen were determined 3 weeks after vaccination. Mock – emulsified solution of conditioned SF900III medium with ISA70. ***P < 0·001 by anova with Tukey–Kramer post‐test compared with other groups.
Protection against HPAI H5N1 and reduced viral shedding in VLP‐vaccinated chickens
To examine the protectivity and efficacy of H5N1 VLP vaccines, a challenge infection with a high dose of HPAI A/chicken/Korea/ES/2003 (H5N1) was performed 3 weeks after a single immunization. Mock‐vaccinated chickens showed severe clinical signs and 100% mortality within 3 days after challenge [mean death time (MDT) = 2·4], which insured that proper challenge was accomplished. However, 100% of the VLP‐vaccinated chickens were protected from mortality, and only mild clinical signs, including swollen head and diarrhea, were observed in two chickens each in 28 and 29 VLP‐vaccinated groups (Table 1). No clinical signs were observed in 210 VLP‐vaccinated chickens throughout the study.
Table 1.
Morbidity and mortality in VLP‐ and mock‐vaccinated chickens intranasally challenged with 105EID50 of A/chicken/Korea/ES/2003 (H5N1) highly pathogenic avian influenza (HPAI) virus
| Group* | Mortality [number dead/total (MDT**)] | Morbidity (number ill/total) |
|---|---|---|
| 28 VLP | 0/8 | 1/8 |
| 29 VLP | 0/8 | 1/8 |
| 210 VLP | 0/7*** | 0/7 |
| Mock | 8/8 (2·4) | 6/8 |
*A dose of VLP vaccine as indicated by units of hemagglutination activity.
**MDT – mean death time denoted in days.
***One bird died before challenge study because of cannibalism.
Oropharyngeal and cloacal swab samples collected at 2, 3, 5, and 7 dpc were tested for viral RNA quantification by rRT‐PCR. All swab samples from mock‐vaccinated chickens yielded positive results with Ct values ranging from 27·2 to 23·3 for oropharyngeal swabs, representing infectious titers of approximately 3·1 to 4·6 log EID50/ml, respectively, and 27·5 to 23·6 for cloacal swabs, corresponding to 3·0 to 4·5 log EID50/ml.
All groups of VLP‐vaccinated chickens not only survived, but showed significantly reduced viral excretion compared with mock‐vaccinated chickens. In oropharyngeal swabs from VLP‐vaccinated groups, samples showed reduced levels of viral excretion with the lowest mean Ct value of 32·2 (1·1 log EID50/ml). Even though there were no statistical differences between groups, oropharyngeal shedding rate of challenge virus was generally lower in 29 and 210 VLP‐vaccinated groups compared with the 28 VLP‐vaccinated group, at 2 and 7 dpc (Table 2), which is probably due to moderately higher levels of HI and NI antibody titers of 29 and 210 VLP‐vaccinated groups (2, 3). Moreover, one chicken that received a 210 VLP vaccine was negative for oropharyngeal excretion throughout the study (Table 2). The cloacal excretion of challenge virus from VLP‐vaccinated chickens was less frequent compared with oropharyngeal specimens with the lowest mean Ct value of 33·1 (0·8 log EID50/ml) (Table 3). Cloacal excretion of challenge virus was not detected at 7 dpc in all groups of VLP‐vaccinated chickens. Especially, in 2 dpc, rRT‐PCR test failed to detect AIV from oral and cloacal swab samples in a large number of VLP‐vaccinated birds, while all mock‐vaccinated chickens at 2 dpc showed positive test results with low Ct values (2, 3). Kinetic of shedding in the vaccinated group might be delayed, and/or viral shedding was suppressed probably because of effective immune responses elicited by VLP vaccine. It is believed that viral shedding level in a large number of challenged birds at 2 dpc was below the detection limit of rRT‐PCR test used in this study. Lower frequency of viral shedding in earlier days, 2 dpc in this study, could be seen in data from other study. 34 These results suggest that a single immunization of chickens with H5N1 VLP vaccines is effective in reducing viral shedding of challenge virus.
Table 2.
Challenge virus excretion from oropharyngeal swab samples
| Day post‐challenge | 28 VLP | 29 VLP | 210 VLP | Mock vaccinated | ||||
|---|---|---|---|---|---|---|---|---|
| No. of positive/total | Avg Ct (logEID50/ml*) | No. of positive/total | Avg Ct (logEID50/ml) | No. of positive/total | Avg Ct (logEID50/ml) | No. of positive/total | Avg Ct (logEID50/ml) | |
| 2 | 3/8 | 32·8 (0·88) | 1/8 | 32·2 (1·11) | 1/7 | 34·7 (0·09) | 8/8 | 24·8 (4·05) |
| 3 | 8/8 | 33·6 (0·57) | 8/8 | 33·8 (0·47) | 6/7 | 33·8 (0·47) | 3/3 | 23·8 (4·45) |
| 5 | 3/8 | 34·4 (0·25) | 3/8 | 34·0 (0·42) | 3/7 | 34·2 (0·30) | – | N/A |
| 7 | 1/8 | 34·4 (0·24) | 0/8 | – | 0/7 | – | – | N/A |
N/A, not applicable.
*log EID50 equivalents were determined with the use of real‐time reverse transcriptase polymerase chain reaction (rRT‐PCR). Numbers in parentheses are averages of viral titers shed from chickens in each group.
Table 3.
Challenge virus excretion from cloacal swab samples
| Day post‐challenge | 28 VLP | 29 VLP | 210 VLP | Mock vaccinated | ||||
|---|---|---|---|---|---|---|---|---|
| No. of positive/total | Avg Ct (logEID50/ml*) | No. of positive/total | Avg Ct (logEID50/ml) | No. of positive/total | Avg Ct (logEID50/ml) | No. of positive/total | Avg Ct (logEID50/ml) | |
| 2 | 0/8 | – | 0/8 | – | 0/7 | – | 8/8 | 25·6 (3·72) |
| 3 | 6/8 | 34·1 (0·37) | 5/8 | 34·3 (0·29) | 4/7 | 34·6 (0·15) | 3/3 | 25·4 (3·79) |
| 5 | 2/8 | 34·6 (0·15) | 2/8 | 33·1 (0·76) | 1/7 | 34·0 (0·42) | – | N/A |
| 7 | 0/8 | – | 0/8 | – | 0/7 | – | – | N/A |
NA, not applicable.
*log EID50 equivalents were determined with the use of rRT‐PCR. Numbers in parentheses are averages of viral titers shed from chickens in each group.
Differentiation of vaccinated chickens from vaccinated and then infected chickens
Serum samples were collected from VLP‐vaccinated chickens before and 10 days post‐challenge for serological testing. As expected, all pre‐challenge sera from VLP‐vaccinated chickens were negative by the NP‐cELISA (1−[S/N] < 0·5). However, as shown in Figure 4A, 6 of 8 and 1 of 7 post‐challenge sera from 28 VLP‐ and 210 VLP‐vaccinated chickens, respectively, showed a positive immune response to influenza NP (1−[S/N] ≥ 0·5). Therefore, VLP vaccination and the companion DIVA test, NP‐cELISA, could differentiate vaccinated group from vaccinated and then infected group. Interestingly, the 28 VLP‐vaccinated group, which resulted higher NP‐cELISA test positive rate, showed moderately increased mean HI titer at 10 dpc compared with pre‐challenge sera whereas the 210 VLP‐vaccinated group showed similar mean HI titers between pre‐ and post‐challenge sera (Figure 4B).
Figure 4.
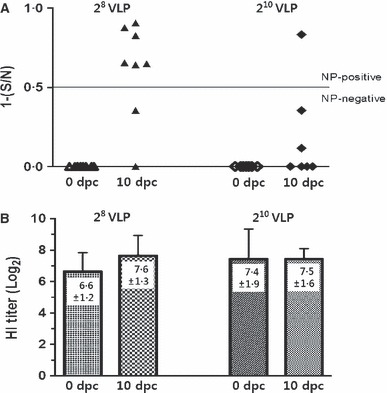
Differentiation of vaccinated chickens from vaccinated and then infected chickens and changes in hemagglutination inhibition (HI) titers following infection. Serum samples were taken 3 weeks post‐vaccination from 28 and 210 VLP‐vaccinated chickens. 10 days post‐challenge (dpc) infection, serum samples were taken from 28 and 210 VLP‐vaccinated and then infected chickens. (A) NP antibody levels from each serum samples were tested with commercially available NP‐cELISA Kit. Each dot represents the NP‐specific antibody value of each chicken. (B) Hemagglutination inhibition (HI) titers (log2) against homologous antigen at 10 dpc were compared with pre‐challenge sera. Data shown are the meant titers of each group ± standard deviation.
Discussion
In the present study, even a single immunization with HPAI H5N1 VLP vaccine elicited high levels of functional antibodies and fully protected chickens from a lethal HPAI challenge with a very small number of chickens showing minor clinical signs. Serology tests that revealed the lack of detecting seroconversions against AIV NP in 8 of 15 chickens indicated that virus replication was strongly suppressed, especially in the 210 VLP‐vaccinated group. These results are in accordance with other studies 27 , 28 , 35 showing missing seroconversion after HPAI challenge in vaccinated chickens, which could possibly confirm protective efficacy of the HPAI VLP vaccines developed in this study.
For effective vaccination programs, adequate serological or virological surveillance is essential to determine whether the field virus is circulating in vaccinated flocks. 36 Moreover, for both trade and surveillance purposes, it is important not only to differentiate naturally infected and vaccinated birds, but also to identify vaccinated birds that become infected with field virus. 11 As expected, the HPAI VLP vaccine developed in this study did not induce antibodies against AIV NP. However, after H5N1 challenge, antibodies against AIV NP were detected using NP‐cELISA in VLP‐vaccinated and then infected groups, which allowed us to differentiate VLP‐vaccinated chickens from VLP‐vaccinated and then infected chickens. Classical agar gel immunodiffusion (AGID) test that targets AIV NP is inexpensive, simple, and most widely used diagnostic tool to detect influenza infection. As VLP vaccine does not induce antibodies to NP, VLP vaccination accompanied by AGID test is expected to allow DIVA strategy without unusual supplies or expensive equipment. Interestingly, in the previous 27 , 28 , 35 and present studies, antibody responses were not detectable in some vaccinated and then HPAI H5N1‐infected chickens. Lack of seroconversion in some chickens, occurring as a result of high levels of protection, is a potential issue for all DIVA vaccination strategies except for the use of unvaccinated sentinel birds in vaccinated flocks. Therefore, DIVA strategies based on serosurveillance should only be utilized on a flock basis and not for individual birds.
In terms of safety and antigenic similarity, VLP vaccine technology against HPAI has potential advantages over egg‐based inactivated influenza vaccines, because VLPs can be rapidly produced from regional HPAI strains without the need for high biocontainment facilities, which is essentially required for the use of HPAI strains for vaccine production. Although inactivated vaccines prepared from LPAI H5 strains have been frequently used in HPAI H5N1 vaccination programs and have been shown to confer resistance to HPAI H5N1 infection in vaccinated birds 28 , 31 , these vaccines could be less effective for control of HPAI because of antigenically distinct properties. 37 , 38 Recently, reverse genetics–derived inactivated vaccine, allowing high antigenic similarity, has been reported to efficiently protect chickens against HPAI challenge. 39 However, these vaccines could have limitations on DIVA strategy, which highlights advantage of VLP‐based vaccine use for HPAI H5N1 control in endemic region. Currently, HPAI H5N1 viruses undergo continuous antigenic mutations that allow viruses to drift away from current vaccine strains. Because VLP vaccine technology allows rapid vaccine production, it could provide easy updates against mutated strains. Moreover, VLP antigens can be modified easily to construct so‐called chimeric VLPs that make VLP technology attractive for novel vaccine design and development. In this study, HPAI H5N1 VLP vaccine elicited significantly lower HI titers against different clades of HPAI H5N1 antigen than the HI titers against homologous antigen. Broadened immune responses of chimeric VLPs that include molecular adjuvant 40 or multiple subtypes of HA 41 have been studied previously. Using similar techniques, including incorporation of various Toll‐like receptor (TLR) ligands into VLPs or co‐expression of HA proteins from multiple clades of H5N1 within a VLP, HPAI H5N1 VLP vaccines providing high level of cross‐clade protection with increased vaccine efficacy might be designed for HPAI control in different endemic regions and need to be further developed. Additionally, VLP manufacturing facilities for poultry vaccine could be rapidly used for human vaccine production in case of H5N1 pandemic outbreak.
Cost of vaccine is one of the main concerns that might seriously impede the use of vaccination in poultry species. In our previous report 23 , as performed by other VLP studies 18 , 19 , 20 , VLP antigens were purified by sucrose density gradient purification requiring expensive equipment (e.g. ultracentrifuge) and time‐consuming process, which would potentially increase the vaccine production cost. In this study, however, we prepared VLP antigen without further purification except for low centrifugation to remove large cell debris. As we prepared VLP antigen without expensive and time‐consuming purification process, it is evident that VLP vaccine used in this study costs less to produce compared with highly purified VLP vaccines reported in previous studies 18 , 19 , 20 , 23 . Although VLP antigen was prepared without sucrose gradient purification, all groups of VLP‐vaccinated chickens showed high levels of HI antibody titers without any signs or symptoms attributable to crude VLP antigen. These results provide method for decreasing production cost of VLP vaccines in poultry use.
In conclusion, HPAI H5N1 VLP vaccine developed in this study was safe, immunogenic, and fully protected SPF chickens from lethal HPAI infection with strongly reduced viral shedding, even without costly purification. Furthermore, we could differentiate VLP‐vaccinated chickens from vaccinated and then infected chickens, supporting the use of VLP vaccine as an effective DIVA vaccination strategy. The results in this study demonstrate that VLP vaccination in poultry species is a promising strategy for the control of HPAI H5N1.
Acknowledgements
This work was supported by Grant no. 610001‐03‐1‐SU000 from the Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea, and in part by NIH/NIAID grant AI093772 (S.M.K.). We are grateful to Hyo‐Seon Joo, Kyung‐Min Kim, Soo‐Won Choi, and Jin‐Yong Noh for their technical support.
References
- 1. World‐Organization‐for‐Animal‐Health . OIE Terrestrial Manual, CHAPTER 2.3.4, AVIAN INFLUENZA. Available at http://www.oie.int/international‐standard‐setting/terrestrial‐manual/access‐online/ (Accessed 1 December 2011).
- 2. Swayne DE. Avian influenza vaccines and therapies for poultry. Comp Immunol Microbiol Infect Dis 2009; 32:351–363. [DOI] [PubMed] [Google Scholar]
- 3. Xu X, Subbarao K, Cox NJ, Guo Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 1999; 261:15–19. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. Available at http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html.
- 5. Butler D. Vaccination will work better than culling, say bird flu experts. Nature 2005; 434:810. [DOI] [PubMed] [Google Scholar]
- 6. Food‐and‐Agriculture‐Organization . OIE/FAO International Scientific Conference on Avian Influenza, OIE Paris, France. Available at http://www.fao.org/avianflu/documents/OIE_FAO_Recom_05.pdf (Accessed 1 December 2011).
- 7. World‐Organization‐for‐Animal‐Health . AVIAN INFLUENZA VACCINATION. Available at http://www.oie.int/eng/info_ev/Other%20Files/A_Guidelines%20on%20AI%20vaccination.pdf (Accessed 1 December 2011).
- 8. Food‐and‐Agriculture‐Organization . FAO Recommendations on the Prevention, Control and Eradication of Highly Pathogenic Avian Influenza (HPAI) in Asia. Available at http://www.fao.org/docs/eims/upload/165186/FAOrecommendationsonHPAI.pdf (Accessed 1 December 2011).
- 9. Abbott A, Pearson H. Fear of human pandemic grows as bird flu sweeps through Asia. Nature 2004; 427:472–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Capua I, Terregino C, Cattoli G, Mutinelli F, Rodriguez JF. Development of a DIVA (Differentiating Infected from Vaccinated Animals) strategy using a vaccine containing a heterologous neuraminidase for the control of avian influenza. Avian Pathol 2003; 32:47–55. [DOI] [PubMed] [Google Scholar]
- 11. Suarez DL. Overview of avian influenza DIVA test strategies. Biologicals 2005; 33:221–226. [DOI] [PubMed] [Google Scholar]
- 12. Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus‐like particles in vaccine development. Expert Rev Vaccines 2010; 9:1149–1176. [DOI] [PubMed] [Google Scholar]
- 13. Kang SM, Compans RW. Host responses from innate to adaptive immunity after vaccination: molecular and cellular events. Mol Cells 2009; 27:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galarza JM, Latham T, Cupo A. Virus‐like particle vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol 2005; 18:365–372. [DOI] [PubMed] [Google Scholar]
- 15. Kang SM, Song JM, Quan FS, Compans RW. Influenza vaccines based on virus‐like particles. Virus Res 2009; 143:140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quan FS, Huang C, Compans RW, Kang SM. Virus‐like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol 2007; 81:3514–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kang SM, Pushko P, Bright RA, Smith G, Compans RW. Influenza virus‐like particles as pandemic vaccines. Curr Top Microbiol Immunol 2009; 333:269–289. [DOI] [PubMed] [Google Scholar]
- 18. Bright RA, Carter DM, Crevar CJ et al. Cross‐clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus‐like particle. PLoS ONE 2008; 3:e1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kang SM, Yoo DG, Lipatov AS et al. Induction of long‐term protective immune responses by influenza H5N1 virus‐like particles. PLoS ONE 2009; 4:e4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mahmood K, Bright RA, Mytle N et al. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine 2008; 26:5393–5399. [DOI] [PubMed] [Google Scholar]
- 21. Perrone LA, Ahmad A, Veguilla V et al. Intranasal vaccination with 1918 influenza virus‐like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol 2009; 83:5726–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prel A, Le Gall‐Recule G, Jestin V. Achievement of avian influenza virus‐like particles that could be used as a subunit vaccine against low‐pathogenic avian influenza strains in ducks. Avian Pathol 2008; 37:513–520. [DOI] [PubMed] [Google Scholar]
- 23. Lee DH, Park JK, Lee YN et al. H9N2 avian influenza virus‐like particle vaccine provides protective immunity and a strategy for the differentiation of infected from vaccinated animals. Vaccine 2011; 29:4003–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suarez DL. Avian influenza: our current understanding. Anim Health Res Rev 2010; 11:19–33. [DOI] [PubMed] [Google Scholar]
- 25. Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full‐length amplification of all influenza A viruses. Arch Virol 2001; 146:2275–2289. [DOI] [PubMed] [Google Scholar]
- 26. King LA, Hitchman R, Possee RD. Recombinant baculovirus isolation. Methods Mol Biol 2007; 388:77–94. [DOI] [PubMed] [Google Scholar]
- 27. Kalthoff D, Giritch A, Geisler K et al. Immunization with plant‐expressed hemagglutinin protects chickens from lethal highly pathogenic avian influenza virus H5N1 challenge infection. J Virol 2010; 84:12002–12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Terregino C, Toffan A, Cilloni F et al. Evaluation of the protection induced by avian influenza vaccines containing a 1994 Mexican H5N2 LPAI seed strain against a 2008 Egyptian H5N1 HPAI virus belonging to clade 2.2.1 by means of serological and in vivo tests. Avian Pathol 2010; 39:215–222. [DOI] [PubMed] [Google Scholar]
- 29. Bublot M, Le Gros FX, Nieddu D, Pritchard N, Mickle TR, Swayne DE. Efficacy of two H5N9‐inactivated vaccines against challenge with a recent H5N1 highly pathogenic avian influenza isolate from a chicken in Thailand. Avian Dis 2007; 51:332–337. [DOI] [PubMed] [Google Scholar]
- 30. Lee YJ, Sung HW, Choi JG et al. Effects of homologous and heterologous neuraminidase vaccines in chickens against H5N1 highly pathogenic avian influenza. Avian Dis 2007; 51:476–478. [DOI] [PubMed] [Google Scholar]
- 31. Terregino C, Toffan A, Beato MS, De Nardi R, Drago A, Capua I. Conventional H5N9 vaccine suppresses shedding in specific‐pathogen‐free birds challenged with HPAI H5N1 A/chicken/Yamaguchi/7/2004. Avian Dis 2007; 51:495–497. [DOI] [PubMed] [Google Scholar]
- 32. Spackman E, Senne DA, Bulaga LL et al. Development of real‐time RT‐PCR for the detection of avian influenza virus. Avian Dis 2003; 47:1079–1082. [DOI] [PubMed] [Google Scholar]
- 33. Sylte MJ, Suarez DL. Influenza neuraminidase as a vaccine antigen. Curr Top Microbiol Immunol 2009; 333:227–241. [DOI] [PubMed] [Google Scholar]
- 34. Pfeiffer J, Suarez DL, Sarmento L, To TL, Nguyen T, Pantin‐Jackwood MJ. Efficacy of commercial vaccines in protecting chickens and ducks against H5N1 highly pathogenic avian influenza viruses from Vietnam. Avian Dis 2010; 54:262–271. [DOI] [PubMed] [Google Scholar]
- 35. Terregino C, De Nardi R, Guberti V et al. Active surveillance for avian influenza viruses in wild birds and backyard flocks in Northern Italy during 2004 to 2006. Avian Pathol 2007; 36:337–344. [DOI] [PubMed] [Google Scholar]
- 36. Swayne DE. Principles for vaccine protection in chickens and domestic waterfowl against avian influenza: emphasis on Asian H5N1 high pathogenicity avian influenza. Ann N Y Acad Sci 2006; 1081:174–181. [DOI] [PubMed] [Google Scholar]
- 37. Soda K, Sakoda Y, Isoda N et al. Development of vaccine strains of H5 and H7 influenza viruses. Jpn J Vet Res 2008; 55:93–98. [PubMed] [Google Scholar]
- 38. Sasaki T, Kokumai N, Ohgitani T et al. Long lasting immunity in chickens induced by a single shot of influenza vaccine prepared from inactivated non‐pathogenic H5N1 virus particles against challenge with a highly pathogenic avian influenza virus. Vaccine 2009; 27:5174–5177. [DOI] [PubMed] [Google Scholar]
- 39. Hwang SD, Kim HS, Cho SW, Seo SH. Single dose of oil‐adjuvanted inactivated vaccine protects chickens from lethal infections of highly pathogenic H5N1 influenza virus. Vaccine 2011; 29:2178–2186. [DOI] [PubMed] [Google Scholar]
- 40. Wang BZ, Quan FS, Kang SM, Bozja J, Skountzou I, Compans RW. Incorporation of membrane‐anchored flagellin into influenza virus‐like particles enhances the breadth of immune responses. J Virol 2008; 82:11813–11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pushko P, Pearce MB, Ahmad A et al. Influenza virus‐like particle can accommodate multiple subtypes of hemagglutinin and protect from multiple influenza types and subtypes. Vaccine 2011; 29:5911–5918. [DOI] [PubMed] [Google Scholar]


