Abstract
Background
The American Joint Committee on Cancer (AJCC) stage III classification of oral cavity squamous cell carcinoma (OCSCC) represents a heterogeneous group of patients with early local disease with regional metastases (T1N1 and T2N1) and advanced local disease with or without regional metastasis (T3N0 and T3N1).
Objective
To evaluate the prognostic heterogeneity in the stage III category
Methods and patients
An international retrospective multi-center study of 1815 patients who were treated for OCSCC from 2003 to 2011.
Results
Kaplan-Meier survival analysis and multivariate models of stage III patients revealed better overall survival (OS, p=.01, HR- 2.12, 95% CI 1.03–4.15) and disease specific survival (DSS, p=.04, HR- 1.7, 95% CI 1.16–4.12) rates for patients with T1-2N1/T3N0 than for patients with T3N1. The outcomes of patients with T3N1 and stage IVa disease were similar (p=.89 and p= .78, OS and DSS respectively). Modifying stage classification by transferring the T3N1 category to the stage VIa group resulted in a better prognostic performance (Harrell’s concordance index, C index: 0.76; Akaike’s Information Criterion, AIC: 4131.6) compared to the AJCC seventh edition staging system (C index: 0.65; AIC: 4144.9) for OS. When DSS was assessed the suggested staging system remained the best performing model (C index: 0.71; AIC: 1061.3) compared to the current AJCC7 staging (C index: 0.64; AIC: 1066.2).
Conclusions
The prognosis of T3N1 and stage IVa disease are similar in OCSCC, suggesting that these categories could be combined in future revisions of the nodal staging system, to enhance prognostic accuracy.
Keywords: oral cavity, survival, staging, AJCC, squamous
INTRODUCTION
Oral cavity squamous cell carcinoma (OCSCC) is the eighth most common cancer worldwide.1 The American Joint Committee on Cancer (AJCC) Staging Manual categorizes early OCSCC disease as stage I or II, and locally advanced disease or distance metastasis as stage IV. Stage III disease represents a heterogeneous group, composed of patients with early local disease with regional metastases (T1N1 and T2N1) and patients with T3 disease with (N1) or without (N0) regional metastasis.2 Although the simplicity and consistency across subsites of the current AJCC staging for head and neck cancer promotes clinical utility, it is widely acknowledged that the prognostic performance is suboptimal in selected subgroups.3–6 Ideally, a staging system distinguishes prognostic categories that are internally homogeneous. However, our clinical experience suggests that the stage III classification for oral cancer encompasses a wide spectrum of disease severity with variable prognoses.
In this international multicenter pooled study, we aimed to evaluate the prognostic heterogeneity in the stage III category of OCSCC. We show an improvement in patient outcome stratification based on a novel categorization of stage III compared to the previous one.
MATERIALS AND METHODS
Patient Population
Our study cohort comprised 1815 patients who were treated for OCSCC from 2003 to 2011 in 7 cancer centers worldwide. Patients with oropharyngeal disease were excluded. Relevant demographic and clinicopathological details are summarized in Table 1. The study was approved by the local institutional review board (IRB) committees. The patients ranged in age from 15 to 93 years, with a median of 54 years. The follow-up ranged from 6–116 months, with a median of 35 months.
Table 1.
Baseline clinicopathological data (N=1,810).
| Variable | No. | % |
|---|---|---|
| Age, years | ||
| Mean ± SD | 55 ± 13 | |
| Median (range) | 54 (15–93) | |
| Sex | ||
| Male | 1158 | 64 |
| Female | 652 | 36 |
| Pathological T stage | ||
| T1 | 341 | 19 |
| T2 | 564 | 31 |
| T3 | 205 | 12 |
| T4 | 700 | 38 |
| Pathological N stage | ||
| N0 | 1001 | 55 |
| N1 | 259 | 14 |
| N2a | 35 | 2 |
| N2b | 404 | 22 |
| N2c | 99 | 6 |
| N3 | 12 | 1 |
| TNM stage | ||
| I | 268 | 15 |
| II | 333 | 18 |
| III | 236 | 13 |
| IV | 973 | 54 |
| Adjuvant treatment | ||
| No | 504 | 28 |
| Radiotherapy | 795 | 44 |
| Chemoradiotherapy | 370 | 20 |
| Chemoradiotherapy & cetuximab | 141 | 8 |
| Extent of neck dissection | ||
| Selective (I–III or I–IV) | 1093 | 62 |
| Comprehensive (I–V) | 253 | 14 |
| Bilateral | 434 | 24 |
| Follow up (months) | ||
| Median (range) | 35 (6–116) | |
Treatment
Treatment modalities included surgery alone (61 patients, 26%), surgery and adjuvant radiotherapy (101 patients, 43%), or surgery followed by chemoradiotherapy (74 patients, 31%). Adjuvant cetuximab was administered to 28 patients (11%). All patients underwent a standardized neck dissection involving levels I–III, I–IV, or I–V, as described by the American Head and Neck Society.7 The type of neck dissection was pre-specified in all patients prior to the operation. Elective neck dissection was performed in 147 patients (62%), while 89 patients (38%) underwent therapeutic neck dissection.
Data Entry, Patient Exclusions, and Statistical Methods
Data were entered into a commercially available spreadsheet (Microsoft Excel 2000, Microsoft Corporation, Seattle, WA), and statistical analysis was performed using a computerized software package (JMP version 4.0, SAS Institute Inc., Cary, NC and SPSS, SPSS Inc. Chicago, IL). The follow-up interval was calculated in months from the date of surgery to the date of last follow-up or death. Overall survival (OS) and disease-specific survival (DSS) rates were calculated using the Kaplan-Meier method, and univariate comparisons between groups were performed using the log-rank test. Overall survival was calculated from the date of surgery to the date of death or last follow-up. For DSS, patients who died from causes other than OCSCC were censored at the time of death. Patients not experiencing these end points were censored at last follow-up. Other covariates of interest included age, clinical nodal status, surgical margin status (clear, close [<5mm], involved), extracapsular nodal spread (ECS) (absent, present), depth of invasion (<5mm), and treatment group (surgery alone, postoperative radiotherapy, or postoperative chemoradiotherapy). Multivariable analyses were performed using Cox proportional hazards regression, stratified by study center. The additional prognostic value of covariates of interest was determined by: a) tests of statistical significance in multivariable analyses; b) Akaike’s Information Criterion (AIC) and the Harrell’s concordance index (c statistic), a generalization of the area under the receiver operating characteristic curve that quantifies the proportion of all patient pairs for whom the predicted and observed survival outcomes are concordant.8 A value of c = .5 indicates no predictive ability as compared with chance alone and a value of 1 indicates perfect discrimination. In general, a predictive model with a low AIC indicates a better model fit and a high c statistic represents a better discrimination ability;9 and c) comparison to multivariable models with and without the covariate of interest, using a likelihood ratio test to determine whether model fit was significantly improved. A P value of ≤ .05 was considered significant, and significant factors were entered into multivariate analysis using the Cox proportional hazards model.
RESULTS
To assess differences in outcome, patients with stage III OCSCC were divided according to the primary tumor (T) and regional lymph node (N) classification into 4 groups: T1N1 (n = 29 patients, 12%), T2N1 (n = 80 patients, 34%), T3N0 (n = 91 patients, 39%) and T3N1 (n = 36 patients, 15%). Table 2 presents the clinical and demographical data of patients with OCSCC stage III (n = 236). We first investigated the differences in demographic and clinical characteristics between the 4 groups. Male gender was more prevalent in patients with T3 than combined T1 and T2 classification (82% versus 65% P = .02). Patients in the T2N1 group were younger (mean age 50.8 ± 1.4 versus 55.8 ± 2.3 years, P = .03) than in the other classification groups combined. There were no differences between the groups in the rates of depth of invasion or positive surgical margins.
Table 2.
Demographics and Clinical Characteristics of 236 Patients with stage III OCSCC
| Variable | T1N1 No. of Patients (%) 29 (12) |
T2N1 No. of Patients (%) 80 (34) |
T3N0 No. of Patients (%) 91 (39) |
T3N1 No. of Patients (%) 36 (15) |
P |
|---|---|---|---|---|---|
| Age, y | |||||
| <65 | 20 (69) | 66 (83) | 66 (73) | 31 (86) | .15 |
| ≥65 | 9 (31) | 14 (17) | 25 (27) | 5 (14) | |
| Gender | |||||
| Male | 17 (58) | 54 (67) | 75 (82) | 29 (80) | .02 |
| Female | 12 (42) | 26 (33) | 16 (18) | 7 (20) | |
| Clinical N classification | |||||
| N0 | 15 (51) | 41 (51) | 76 (84) | 15 (41) | < .001 |
| N+ | 14 (49) | 39 (49) | 15 (16) | 21 (59) | |
| Extent of neck dissection | |||||
| I–III or I–IV | 26 (89) | 70 (88) | 71 (78) | 26 (72) | .04 |
| I–V | 0 | 1 (1) | 2 (2) | 0 | |
| Bilateral | 3 (11) | 9 (11) | 18 (20) | 10 (28) | |
| Nodal yield | |||||
| <18 | 6 (21) | 11 (14) | 13 (14) | 3 (8) | .55 |
| ≥18 | 23 (79) | 69 (86) | 78 (86) | 33 (92) | |
| Surgical Margins | |||||
| Negative | 23 (79) | 61 (76) | 74 (81) | 25 (69) | .67 |
| Close (<5mm) | 5 (17) | 13 (16) | 10 (11) | 6 (17) | |
| Positive | 1 (4) | 6 (8) | 7 (8) | 5 (14) | |
| Depth of invasion | |||||
| <5 | 7 (24) | 8 (10) | 17 (19) | 1 (2) | .12 |
| ≥5 | 22 (76) | 72 (90) | 74 (81) | 35 (98) | |
| Extracapsular spread | |||||
| Yes | 6 (21) | 22 (27) | 0 | 12 (33) | < .001 |
| No | 23 (79) | 59 (73) | 81 (100) | 24 (67) | |
| Treatment | |||||
| Surgery | 8 (28) | 16 (20) | 36 (40) | 1 (2) | |
| Surgery +RT | 15 (51) | 34 (42) | 36 (40) | 16 (44) | < .001 |
| Surgery+ CRT | 6 (21) | 30 (38) | 19 (20) | 19 (52) |
Abbreviations: RT, radiotherapy; CRT, chemoradiation, OCSCC, oral cavity squamous cell carcinoma.
We identified differences in treatment regimens between classification groups: T3N1 patients underwent postoperative radiotherapy with or without chemotherapy more frequently than did patients in the other stage III groups combined (98% and 70%, respectively, P < .001).
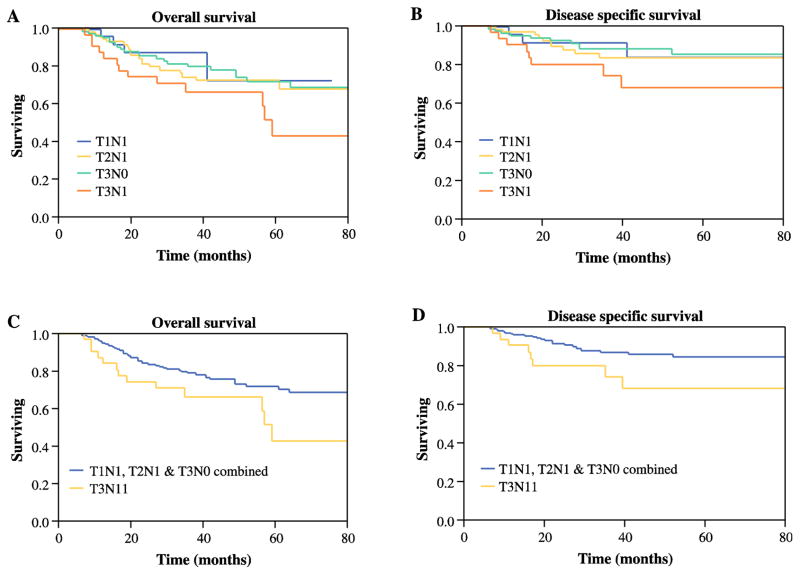
Kaplan-Meier analysis of patients with stage III disease according to TNM groups is shown in figure 1. The curves show better 5-year OS for patients with T1N1, T2N1, and T3N0 (71%, 67%, and 73%, respectively) than for those with T3N1 (52%). Similarly, stratifying stage III disease by T3N1 and T1-2N1/T3N0 revealed significantly worse outcomes for T3N1 patients: DSS (P = .037) and OS (P = .036). Most important, multivariable models for stage III patients show significantly worse outcomes for T3N1patients than for patients in the other stage III groups combined: DSS (P = .04, HR 1.7, 95% CI 1.16–4.12) and OS (P =.01, HR 2.12, 95% CI 1.03–4.15), as shown in Table 3.
Figure 1.
Kaplan-Meier analysis of stage III patients according to TNM groups (T1N1, T2N1, T3N0, and T3N1). (A) 5-year overall survival (OS) and (B) disease-specific survival (DSS). (C) 5-year OS and (D) DSS of patients with T1-2N1/T3N0 (red line) and T3N1 (blue line) disease.
Table 3.
Multivariable analysis of disease-specific and overall survival to determine prognostic value of stage III stratification a
| Overall Survival | Disease-Specific Survival | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Stage III stratification | .01 | .04 | ||
| T1N1 | Referent | Referent | ||
| T2N1 | 1.11 (0.6–1.84) | 1.16 (0.7–1.56) | ||
| T3N0 | 1.42 (0.8–2.14) | 1.43 (0.72–1.99) | ||
| T3N1 | 2.12 (1.03–4.15) | 1.7 (1.16–4.12) | ||
| Age, years | 0.99 (0.23–3.93) | .9 | 0.69 (0.09–4.44) | .7 |
| Clinical nodal status | .03 | .08 | ||
| N0 | Referent | Referent | ||
| N+ | 1.96 (1.05–3.78) | 2.17 (0.94–5.33) | ||
| Nodal yield | .04 | .01 | ||
| <18 | Referent | |||
| ≥18 | 3.79 (1.33–9.89) | |||
| ECS | .06 | .05 | ||
| Absent | Referent | Referent | ||
| Present | 2.15 (0.94–4.7) | 2.74 (0.99–7.2) | ||
| Excision margin | .06 | .1 | ||
| Clear | Referent | Referent | ||
| Close (<5mm) | 1.1 (0.41–2.5) | 1.07 (0.16–8.08) | ||
| Involved | 3.00 (1.19–6.89)* | 1.26 (0.16–4.06) | ||
| Adjuvant therapy | .53 | .44 | ||
| Nil | Referent | Referent | ||
| Adjuvant RT | 1.6 (0.69–3.90) | 0.84 (0.36–2.04) | ||
| Adjuvant CRT | 1.56 (0.60–4.21) | 1.18 (0.48–2.96) | ||
| Depth of invasion | .15 | .92 | ||
| < 5mm | Referent | Referent | ||
| ≥ 5mm | 1.98 (0.75–4.64) | 1.07 (0.16–4.12) | ||
p<.05
Abbreviations: HR, hazard ratio; CI, confidence interval; vs, versus; ECS, extracapsular spread; RT, radiotherapy.
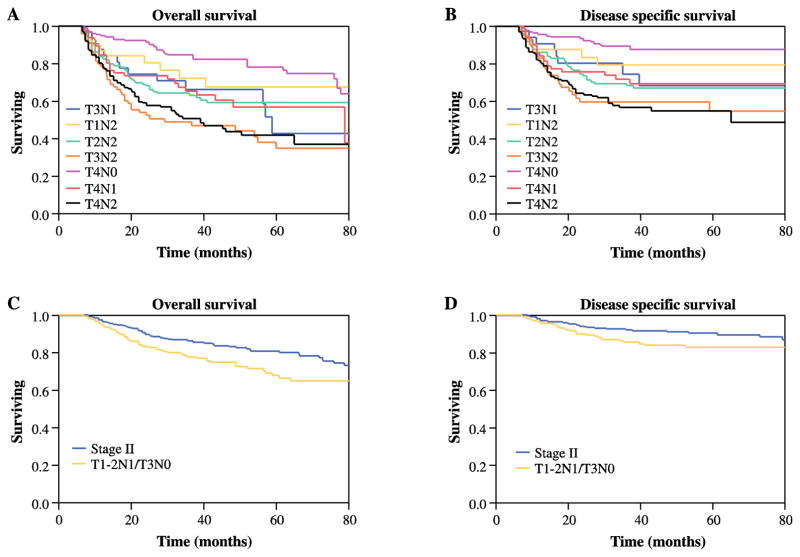
Next, we assessed whether T3N1 patients have similar prognosis as those in stage IVa (T4aN0-2, T1-3N2). Figures 2A and B compare OS and DSS of stage IVa patients with rates of the T3N1 group. Five-year OS and 5 year DSS for T3N1 patients were comparable to rates for the stage IVa subgroups (Figure 2A, P = .89 and Figure 2B, P = .78, respectively). Similarly, multivariate analysis showed no significant differences between stage IVa and T3N1 patients. Furthermore, removal of T3N1 patients from the stage III category preserved the differences in prognosis between stages II and III (OS, P=.01; DSS, P=.04 Figures 2C–D). Through regression modeling, the staging system with the best prognostic discriminatory ability was then assessed through iterative statistical models and comparison of AIC and c-statistic values. For overall survival, the suggested staging was noted to have a better prognostic performance (C index: 0.76; AIC: 4131.6) than the AJCC seventh edition (C index: 0.65; AIC: 4144.9). When DSS was assessed the suggested staging system remained the best performing model (C index: 0.71; AIC: 1061.3) compared to the current AJCC7 staging (C index: 0.64; AIC: 1066.2).
Figure 2.
Kaplan-Meier analysis of (A) 5-year overall survival (OS) and (B) disease-specific survival (DSS) of patients with T3N1 and different stage IV groups (T1N2, T2N2, T3N2, T4N0, T4N1 and, T4N2) disease. (C) 5-year OS and (D) DSS of patients with stage II (red line) and stage III after removal of T3N1 (blue line) disease.
To confirm our results, we repeated the multivariate analysis on an independent cohort of 255 pathologically staged III–IV patients who were treated in a single institution (National Cancer Center of Singapore, Singapore). Statistical analyses of this single institution cohort showed that T3N1 patients had worse OS and DSS than did other stage III patients (p=.002 and p=.001, respectively). Similar to the results of the current, larger cohort, we did not find significant differences in outcomes between T3N1 and stage IV patients.
DISCUSSION
The mainstay of treatment in oral cavity cancer is surgery.10 Adjuvant treatment is given to patients with early disease (staged as I–II) only in the presence of adverse pathological features. In contrast, in patients with advanced disease (staged as III–IV) or in the presence of positive lymph nodes, adjuvant treatment is usually indicated. Early disease is well defined by limited local disease or the absence of metastatic lymph nodes (T1-2, N0). Nevertheless, stages III and IV entail heterogeneous groups, which may be characterized by: limited local disease with extensive nodal metastases, locally advanced disease and no lymph node metastases, or locally advanced disease with nodal metastases.11
Improvements in the sensitivity of imaging techniques, computerized tomography (CT), positron emission tomography (PET) and ultrasound, have augmented our ability to identify metastatic lymph nodes as small as 3 mm in size.12–14 The result is an increase in the number of patients presenting with a single nodal metastasis. The presence of single lymph node metastases in patients with early local disease (T1-2) has been associated with poor outcome.15–19 In the current study we show for the first time that the presence of both single lymph node metastasis and a tumor larger than 4 cm (i.e. T3N1) predicts worse outcome than do the other risk groups within the stage III category. Furthermore, our multivariate analysis shows that this group of patients has similar prognosis to that of patients staged as IVa, and that removal of T3N1 patients from the stage III category improved the statistical coherence of this group, while preserving the prognostic characteristics of stages II and IV.
Current NCCN guidelines indicate adjuvant radiotherapy in patients with pT3, regardless of their nodal status. The addition of concurrent chemotherapy in such cases, in the absence of extracapsular spread or positive margins, is still equivocal and not under uniform consensus. Compared to radiotherapy alone, slight improvement in five-year survival rates have been demonstrated after adjuvant concurrent chemoradiation therapy for advanced head and neck SCC. 20 However, due to the significant morbidity associated with intensification of adjuvant treatment i.e. adding chemotherapy to radiotherapy, there is still considerable controversy over the pathological characteristics of the tumor that predict the need for more aggressive adjuvant treatment. 19
Re-stratifying patients with T3N1 classification to stage IVa can potentially assist in identifying patients with poor outcomes, and for whom concomitant adjuvant treatment may be needed. Further studies are required to determine whether these patients will benefit from concurrent chemoradiation therapy.
We realize that limitations of this study are the potential inconsistencies in surgical techniques and in the processing of pathological specimens, and the differences in treatment regimens. To address the matter of heterogeneity we performed an external validation using data from a single institute not included in our cohort. In that analysis we found that T3N1 classification remained a significant independent predictor of outcome and that T3N1 patients had worse OS and DSS compared to other stage III patients. Conversely, the significance of our heterogeneous cohort across multiple countries assures the broad applicability of our research findings worldwide and might facilitate the upstaging of T3N1 into stage IVa in diverse patient populations.21 Due to the retrospective nature of the study, data regarding primary tumor site, smoking status, and alcohol exposure were not consistently available, and were therefore not included in the analysis.
In conclusion, our data derived from a multi-institutional international study that represents the largest and most detailed cohort of OSCC to date, suggest the reclassification of T3N1 patients as stage IVa. We show that T3N1 classification represents patients at high risk of treatment failure similar to those in stage IVa and therefore for whom concurrent adjuvant treatment may be considered.
SYNOPSIS.
The prognosis of T3N1 and stage IVa disease are similar in OCSCC, suggesting that these categories could be combined in future revisions of the nodal staging system, to enhance prognostic accuracy.
Acknowledgments
Cindy Cohen is thanked for editorial assistance.
Footnotes
Conflict of interest: none for any authors
Financial disclosure. This research was supported by the ICRF Barbara S. Goodman endowed research career development award (2011-601-BGPC) and a grant from the US-Israel Binational Science Foundation. No other financial disclosures from the authors are to be made.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Edge SB, BD, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 3.Groome PA, Schulze K, Boysen M, Hall SF, Mackillop WJ. A comparison of published head and neck stage groupings in carcinomas of the oral cavity. Head & neck. 2001;23:613–624. doi: 10.1002/hed.1087. [DOI] [PubMed] [Google Scholar]
- 4.Manikantan K, Sayed SI, Syrigos KN, et al. Challenges for the future modifications of the TNM staging system for head and neck cancer: case for a new computational model? Cancer treatment reviews. 2009;35:639–644. doi: 10.1016/j.ctrv.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Hall SF, Groome PA, Irish J, O’Sullivan B. TNM-based stage groupings in head and neck cancer: application in cancer of the hypopharynx. Head & neck. 2009;31:1–8. doi: 10.1002/hed.20917. [DOI] [PubMed] [Google Scholar]
- 6.Kreppel M, Drebber U, Rothamel D, et al. Prognostic impact of different TNM-based stage groupings for oral squamous cell carcinoma. Head & neck. 2011;33:1467–1475. doi: 10.1002/hed.21630. [DOI] [PubMed] [Google Scholar]
- 7.Robbins KT, Shaha AR, Medina JE, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008;134:536–538. doi: 10.1001/archotol.134.5.536. [DOI] [PubMed] [Google Scholar]
- 8.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in medicine. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Akaike H. New look at statistical-model identification. IEEE Trans Automat Contr. 1982;19:716–23. [Google Scholar]
- 10.Shah JP, Gil Z. Current concepts in management of oral cancer--surgery. Oral oncology. 2009;45:394–401. doi: 10.1016/j.oraloncology.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao CT, Lin CY, Fan KH, et al. Identification of a high-risk subgroup of patients with resected pT3 oral cavity cancer in need of postoperative adjuvant therapy. Annals of surgical oncology. 2011;18:2569–2578. doi: 10.1245/s10434-011-1616-4. [DOI] [PubMed] [Google Scholar]
- 12.Merritt RM, Williams MF, James TH, Porubsky ES. Detection of cervical metastasis. A meta-analysis comparing computed tomography with physical examination. Arch Otolaryngol Head Neck Surg. 1997;123:149–152. doi: 10.1001/archotol.1997.01900020027004. [DOI] [PubMed] [Google Scholar]
- 13.Gil Z, Fliss DM. Contemporary management of head and neck cancers. Isr Med Assoc J. 2009;11:296–300. [PubMed] [Google Scholar]
- 14.Kyzas PA, Evangelou E, Denaxa-Kyza D, Ioannidis JP. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst. 2008;100:712–720. doi: 10.1093/jnci/djn125. [DOI] [PubMed] [Google Scholar]
- 15.Rudoltz MS, Benammar A, Mohiuddin M. Does pathologic node status affect local control in patients with carcinoma of the head and neck treated with radical surgery and postoperative radiotherapy? Int J Radiat Oncol Biol Phys. 1995;31:503–508. doi: 10.1016/0360-3016(94)00394-Z. [DOI] [PubMed] [Google Scholar]
- 16.Parsons JT, Mendenhall WM, Stringer SP, Cassisi NJ, Million RR. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys. 1997;39:137–148. doi: 10.1016/s0360-3016(97)00152-1. [DOI] [PubMed] [Google Scholar]
- 17.Gavilan J, Prim MP, De Diego JI, Hardisson D, Pozuelo A. Postoperative radiotherapy in patients with positive nodes after functional neck dissection. Ann Otol Rhinol Laryngol. 2000;109:844–848. doi: 10.1177/000348940010900911. [DOI] [PubMed] [Google Scholar]
- 18.Shingaki S, Takada M, Sasai K, et al. Impact of lymph node metastasis on the pattern of failure and survival in oral carcinomas. Am J Surg. 2003;185:278–284. doi: 10.1016/s0002-9610(02)01378-8. [DOI] [PubMed] [Google Scholar]
- 19.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 20.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. The New England journal of medicine. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 21.Trimble EL, Abrams JS, Meyer RM, et al. Improving cancer outcomes through international collaboration in academic cancer treatment trials. J Clin Oncol. 2009;27:5109–5114. doi: 10.1200/JCO.2009.22.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]