Abstract
The genus Astragalus is a rich source of a variety of biologically active compounds including phenols, saponins, polysaccharides and essential oils. The present study was conducted to determine ontogenetic variation of the volatile organic compounds as well as total phenolic contents and antioxidant activity in leaves of A. compactus. The leaves of plant were harvested at vegetative, flowering and fructification stages and were analyzed by gas chromatography coupled with mass spectrometry (GC-MS). Total phenolic content (TPC) was determined using the Folin-Ciocalteau reagent and the antioxidant capacity was evaluated with the 1,1-diphenyl-2-picrylhydrazyl (DPPH) test. Different classes of volatile compounds were identified including alcohols, esters, hydrocarbons, sterols and terpenoides. Significant variation of these compounds was found during phenological stages of development. Sterols and hydrocarbons were the main components of essential oils at the vegetative stage. The presence of terpenoides (phytol) and alcohols (docosanol) was significant at the flowering stage. Fructification phase was characterized by the high content of sterols and hydrocarbons and absence of phytol. The antioxidant activity and phenolic content were related to the physiological stage and the highest amount detected at fructification phase. The ontogenetic variations of phenolic contents and antioxidant properties are largely contributed by climatic factors such as temperature and solar radiation.
Keywords: antioxidant, Astragalus compactus, GC-MS, phenological stages, total phenolic content, volatile compounds
Introduction
The genus Astragalus (Fabaceae) is one of the largest genera of vascular plants, comprising about 2500 species of herbs or shrubs (Lock and Simpson, 1991[12]). Astragalus is mainly distributed in cool, temperate, arid and semiarid continental region of South-Western Asia, Sino Hymalayan region, Western-North and South America, Europe, North Africa and Australia (Polhill, 1981[22]; Evans, 2002[5]). Iran, possessing nearly 800 species, is one of the most important centres of diversity for this genus (Maassoumi, 1998[13]).
Astragalus root has long been one of the most important tonic herbs used in traditional Chinese medicine and has been used to improve resistance to infections and to aid in immunological disorders and viral infections, and as hepatoprotective (Rios and Waterman, 1997[23]; Verotta and El-Sebakhy, 2001[30]). It has also been proven to be effective for clinical treatment of nephritis, cardiovascular diseases, hypertension, diabetes, and cancers (Miller, 1998[16]; Sinclair, 1998[24]). The interesting pharmacological properties of Astragalus were often associated with its chemical composition. The known beneficial components of Astragalus are mainly flavonoid, triterpene saponins, polysaccharides and essential oils (Pistelli, 2002[20]). Despite the fact that the phenolic compounds are among the most important antioxidants in plant (Choudhary and Swarnkar, 2011[1]), the information on the antioxidant activity of Astragalus is limited. On the other hand, although a great deal of research has been carried out on phenols, saponins and polysaccharides of different Astragalus species (Smee et al., 1996[25]; Nikolov and Benbassat, 1997[19]; Krasteva and Nikolov, 2000[11]; Gardner et al., 2001[6]; Johnson et al., 2001[9]), few studies have been done on the volatile compounds of the genus (Platikanov et al., 2005[21]; Teyeb et al., 2011[28]).
The volatile compounds consist of a complex mixture of chemicals with special chemical and physical properties, and are among the most valuable compounds produced by plants. It is shown that the volatile components of plant can be affected in a number of ways, especially by developmental and environmental factors (Circella et al., 1995[2]; Kokkini et al., 1997[10]; Máñez et al., 1991[14]; Taveira et al., 2003[27]). Knowledge of the factors that determine the chemical variability and yield for each species is important in particular for medicinal plants, to optimize the time of collection and to obtain higher yields of high-quality essential oils. As well as volatile compounds, the antioxidant capacity and phenolic content of plant species could be greatly affected by the environmental factors and growing season (Howard et al., 2002[8], Toor et al., 2006[29]). Such a variation is attributed to the difference in growing temperatures and light intensity. Therefore, it is hypothesized that changes in solar radiation received and temperatures in different seasons may affect the phenolic content and antioxidant components of plants.
Regarding the importance of the variability in composition of volatiles and lack of information about the effect of seasonal variation on the phenolic content and antioxidant activity of Astragalus, we studied ontogenetic variation of volatile and antioxidant activity in leaves of A. compactus Lam. A. compactus Lam. is one of the Asiatic species, which is famed for the production of gum tragacanth as an economically important natural product (Movafeghi et al., 2010[17]). The aims of the presented study were: (1) to analyze the volatile composition of A. compactus leaves; (2) to determine the link between volatile content of plant and developmental stages; and (3) to evaluate the effect of ontogenetic variation on the antioxidant activity and phenolic contents.
Material and Methods
Plant material
Leaves of A. compactus Lam. were harvested at three phenological phases (vegetative, flowering and fructification stages) from the Payam Mountains at the east Azerbaijan province of Iran, at altitudes above 2200 m and transferred into the laboratory. Voucher specimens were deposited in the herbarium of the Tabriz University of Medical Science. The samples were dried for 10 days at room temperature and then were powdered.
Extraction
A quantity of 100 grams of powdered roots was extracted with n-hexane using a Soxhlet apparatus for 8 h. The extract was concentrated to ~1 ml under reduced pressure on a rotary evaporator. The extract was stored in sealed vials at 4 °C until GC-MS analysis.
Analytical procedure
Recognition of compounds was carried out by a Shimadzu GC-MS-QP 5050A gas chromatograph (Shimadzu Corporation, Kyoto, Japan). The column used for the analysis was a 60 m × 0.25 mm i.d. DB1 capillary column coated with a film of dimethylpolysiloxane (J&W Scientific, Folsom, CA, USA). An aliquot of 1 µl of the n-hexane extract was injected into the GC-MS system in the split mode (split ratio 1:42). Helium was used as the carrier gas with a flow rate of 1 ml/min. The column temperature was maintained at 100 °C for 2 min. Then, it was programmed to 300 °C at a rate of 5 °C/min and the final temperature was held for 38 min. Injector temperature and detector temperature were optimised at 270 °C and 310 °C, respectively. The MS operating parameters were as follows: ionization energy, 70 eV; ion source temperature, 280 °C; quadrupole, 100 °C; solvent delay, 3.0 min; scan speed 2000 u/s and scan range 30-600 u, EV voltage 3000 volt.
Identification of components
The components were identified on the basis of comparison of their retention indices and mass spectra with those for standard compounds and by matching with the Wiley 229, Nist 107 and Nist 21 libraries.
DPPH radical-scavenging activity
The DPPH quenching ability of plant methanolic extracts was measured according to Hanato et al. (1988[7]). A quantity of 5 ml of the extract at different concentrations was added to 5 ml of a DPPH methanolic solution. The mixture was shaken vigorously and left standing at room temperature for 30 min in the dark. The absorbance of the resulting solution was then measured at 517 nm. The same procedure was applied for the quercetin as a positive control. The antiradical activity was expressed as IC50 (μg/ml), the antiradical concentration required to cause a 50 % inhibition. A lower IC50 value corresponds to a higher antioxidant activity of plant extracts. The ability to scavenge the DPPH radical was calculated using the following equation:
DPPH scavenging effect (%) = (A0 − A1)/A0×100
where A0 is the absorbance of the control at 30 min, and A1 is the absorbance of the sample at 30 min.
Determination of TPC
TPC was estimated using the Folin-Ciocalteau colorimetric method described previously (Meda et al., 2005[15]) with a little modification. Briefly, 5 g of powdered samples were individually dissolved in 50 ml of aceton-water (in 4-6 ratio). After 30 min, 1 ml of these solutions were mixed with 0.2 ml of Folin-Ciocalteau reagent, and 1 ml of 2 % sodium carbonate (Na2CO3). After incubation at 40 °C for 30 min, the absorbance of the reaction mixtures were measured at 760 nm by using a spectrophotometer (Shimadzu, Kyoto, Japan). Quantification was done on the basis of the standard curve of gallic acid. Results were expressed as μg of gallic acid equivalent (GAE) per mg of dry weight (DW).
Statistical analysis
The experiment was a completely randomized design with three replications. Data were subjected to analysis of variance (ANOVA) and means were separated by Duncan multiple range test at P <0.05 significant level.
Results and Discussion
GC-MS analysis
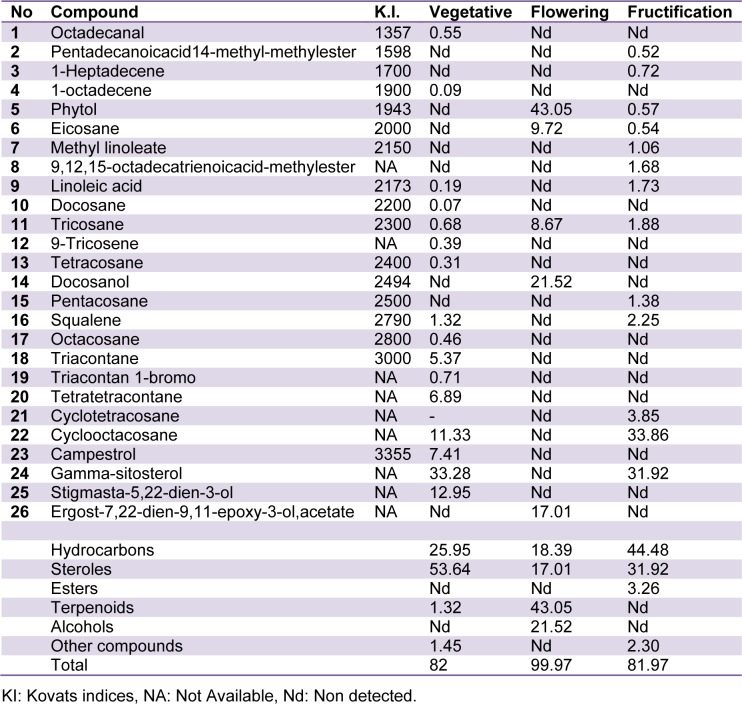
The obtained results showed the presence of different groups of compounds including alcohols, fatty acids, hydrocarbons, sterols, terpenoides and aldehydes in A. compactus leaves. The identified compounds and relative quantitative distribution are shown in Table 1(Tab. 1). Significant differences in the composition of the volatiles were found during different phenological stages (Figure 1(Fig. 1)).
Table 1. Chemical composition (%) of volatiles from the leaves of Astragalus compactus in three developmental phases.
Figure 1. Comparison of the average percentages of hydrocarbons, sterols, terpenoides and alcohols at different developmental stages.
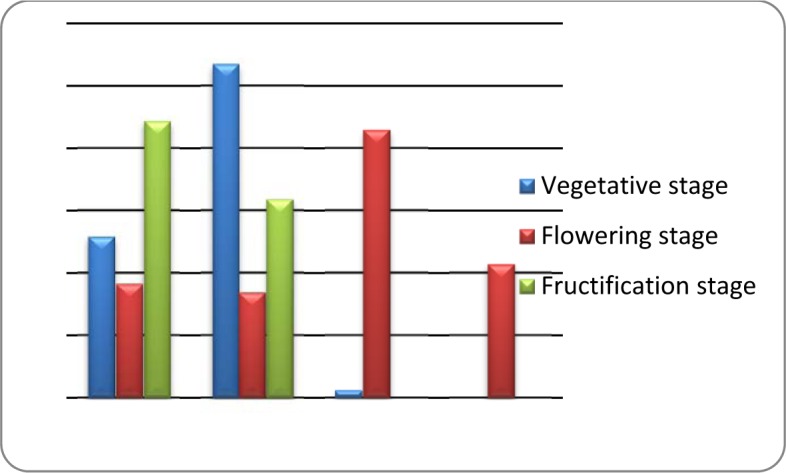
The major constituents detected in vegetative stage were sterols amounting to 53.64 %. Gamma sitosterol, campestrol and stigmasta-5,22-dien-3-ol were the predominant identified sterols. This is in agreement with the descriptions of Engvild (1995[4]) and Ebrahimzadeh et al. (2001[3]) that reported campestrol, gamma-sitosterol, and stigmasterol as the predominant sterols in the aerial parts and roots of the genus. Hydrocarbons (25.59 %) were the other main functional group identified in the vegetative stage. An almost complete series of n-alkanes have previously been identified in leaf materials of the different Astragalus species (Platikanov et al., 2005[21]; Teyeb et al., 2011[28]) and it is suggested that they probably function as a component part of the waxes. However, the presence of tetratetracontane and cyclooctacosane in the volatile composition of the genus has not yet been reported. Low amounts of linoleic acid and one aldehyde (octadecanal) were also detected in the vegetative stage.
The composition of the volatiles strongly changes at the stage of flowering. The main component in this stage of leaf development was phytol. Phytol protects leaves from the loss of water and parasites. This compound has also been detected in the leaf of other studied Astragalus species. In A. gombiformis, phytol is the main component of leaf volatiles at flowering stage, while in other species including A. cicer, A. spruneri and A. glycyphyllos hydrocarbons were the main identified volatiles at flowering (Platikanov et al., 2005[21]; Teyeb et al., 2011[28]). Considerable amount of docosanol was detected at flowering stage. Alcoholic compounds could have important ecological functions in plants as allelochemicals. There are reports about the role of docosanol as a male pheromone in some insects, indicating the change in the plant-insect interactions at flowering stage (Müller and Buchbauer, 2011[18]). Docosanol also could have medicinal properties as antiviral compound. Relatively high concentration of hydrocarbons and sterols also were detected in flowering stage.
The fructification stage is characterized by the presence of high concentrations of hydrocarbons and sterols and by the reduction or absence of alcohols in leaves. Except for cyclotetracosane and cyclooctacosane, other hydrocarbons have been previously reported in other species of Astragalus. Gamma-sitosterol was the only identified sterol in fructification stage. The distinctive features of this stage were the disappearance of alcohols and strong reduction of phytol. Most of other studied species of Astragalus also show a low concentration or absence of phytol at fructification phase. The reason could be the use of this component in the synthesis of lignin and/or by further oxidation of phenol compounds catalysed by the increase of polyphenoloxidase and peroxidase activity. According to Máñez et al. (1991[14]) the changes in the composition of the volatiles with the maturation of the organ are directly related to higher rates of cyclization and dehydration of the compounds.
Total phenolic content
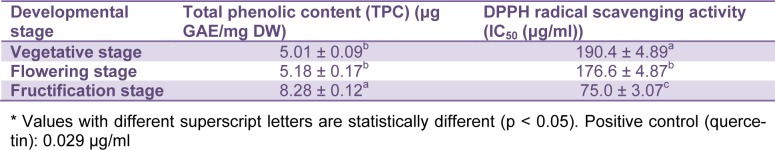
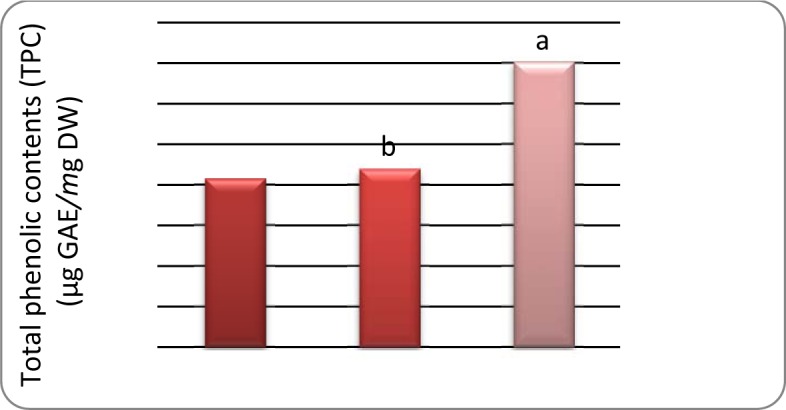
Based on obtained results, the TPC ranged from 5.01 to 8.28 μg GAE/mg DW (Table 2(Tab. 2)). The TPC (Figure 2(Fig. 2)) was significantly higher at the fructification stage than the vegetative and flowering stage. It has been shown that the mean temperature and solar radiation are significantly higher in summer months than the other months (Toor et al., 2006[29]). Therefore, increase of TPC during fructification could be due to an increase in the amount of UV radiation associated with an increase in solar radiation received by the plants. The increased temperature and increase in the age of plants may have also caused an accumulation of phenolics in plants. Light increases the rate of biosynthesis of phenolics in plants by increasing the activities of enzymes, especially phenylalanine ammonia-lyase (PAL) which plays an important role in converting phenylalanine into coumaric acid, which are the initial precursor molecules involved in the synthesis of phenolic components in plants (Smith, 1973[26]).
Table 2. The antioxidant activity and Total phenolic contents (TPC) in leaves of A. compactus during development.
Figure 2. Variation of the Total phenolic contents (TPC) in leaves of A. compactus during development.
Antioxidant activity
A developmental variation in antioxidant activity of A. compactus was observed with the IC50 ranging from 75 to 190.4 μg/ml (Table 2(Tab. 2)). The capacity to quench free radical seemed to be related to the physiological stage. Figure 3(Fig. 3) shows that the fructification stage exhibited the highest antioxidant activity (corresponds with the lowest IC50), whilst flowering and vegetative stages showed the less antioxidant activity (corresponds with higher IC50).
Figure 3. Variation of the antioxidant activity (IC50 (μg/ml)) in leaves of A. compactus during development.
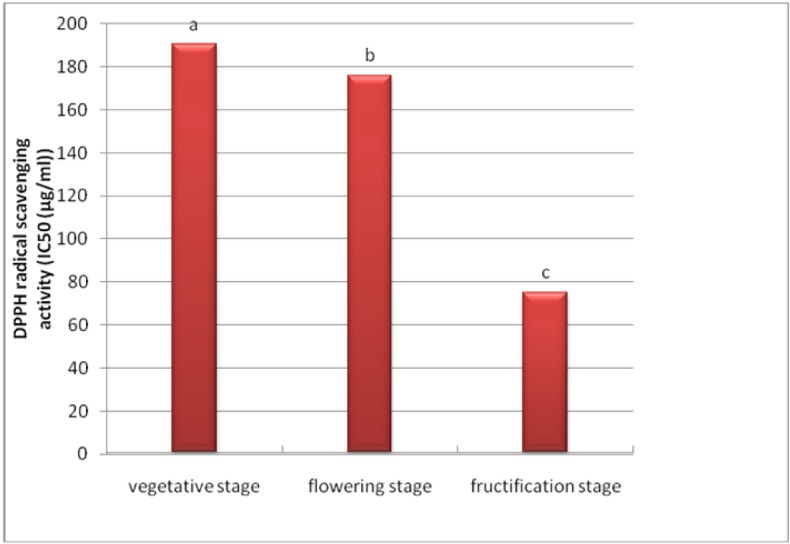
The higher antioxidant activity at fructification stage is related to the higher total phenolic content. Therefore, a positive relationship was observed between the antioxidant activity and the total phenolic content. The increase in the antioxidant activity and total phenolics at the fructification stage, which is characterized by the high temperature and a high exposure to solar radiation, leads us to conclude that A. compactus accumulates antioxidant compounds specially phenolic contents before and during this period probably to protect itself against solar radiations and/or grazers.
These results show that the antioxidant activity of A. compactus is influenced by the environmental conditions and the high solar radiation has a positive effect on the accumulation of major antioxidant components. The results suggest that A. compactus methanolic extracts may serve as potential sources of natural antioxidants and that the fructification phase could be considered as the best stage for the harvesting of the leaves of this plant species.
Acknowledgements
We thank the Research Office of the University of Tabriz, Iran for financial support.
References
- 1.Choudhary RK, Swarnkar PL. Antioxidant activity of phenolic and flavonoid compounds in some medicinal plants of India. Nat Prod Res. 2011;25:1101–1109. doi: 10.1080/14786419.2010.498372. [DOI] [PubMed] [Google Scholar]
- 2.Circella G, Franz CH, Novak J, Resch H. Influence of day length and leaf insertion on the composition of marjoram essential oil. Flav Fragr J. 1995;10:371–4. [Google Scholar]
- 3.Ebrahimzadeh H, Niknam V, Maassoumi AA. The sterols of Astragalus species from Iran: GLC separation and quantification. Biochem Syst Ecol. 2001;29:393–404. doi: 10.1016/s0305-1978(00)00065-x. [DOI] [PubMed] [Google Scholar]
- 4.Engvild KC. The natural chlorinated plant hormone of pea, 4-chioroindole-3-acetic acid, an endogenous herbicide? In: Grimvall A, de Leer EWB, editors. Naturally-produced organohalogens. Dordrecht: Kluwer Academic Publishers; 1995. pp. 227–234. [Google Scholar]
- 5.Evans WC. Trease and Evans Pharmacognosy. 15th. London: Elsevier Science Ltd; 2002. [Google Scholar]
- 6.Gardner DR, Molyneux RJ, Ralphs MH. Analysis of swaisonine: extraction methods, detection and measurement in populations of locoweeds (Oxytropis spp.) J Agric Food Chem. 2001;49:4573–4580. doi: 10.1021/jf010596p. [DOI] [PubMed] [Google Scholar]
- 7.Hanato T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull. 1988;36:2090–7. doi: 10.1248/cpb.36.2090. [DOI] [PubMed] [Google Scholar]
- 8.Howard LR, Pandjaitan N, Morelock T, Gil MI. Antioxidant capacity and phenolic content of spinach as affected by genetics and growing season. J Agr Food Chem. 2002;50:5891–6. doi: 10.1021/jf020507o. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DL, Majak W, Benn MH. Excretion of miserotoxin and detoxification of the aglycone by grasshoppers (Orthoptera: Acrididae) Phytochemistry. 2001;58:739–742. doi: 10.1016/s0031-9422(01)00310-7. [DOI] [PubMed] [Google Scholar]
- 10.Kokkini S, Karousou R, Dardioti A, Krigas N, Lanaras T. Autumn essential oils of Greek oregano. Phytochemistry. 1997;44:883–6. [Google Scholar]
- 11.Krasteva I, Nikolov S. Flavonoids from genus Astragalus L. Pharmacia. 2000;34:34–48. [Google Scholar]
- 12.Lock JM, Simpson K. Legumes of West Asia. A check-list. London: Royal Botanic Gardens, Kew; 1991. [Google Scholar]
- 13.Maassoumi AA. Astragalus in the Old World, Check List. Tehran, Iran: Research Institute of Forests and Rangeland; 1998. [Google Scholar]
- 14.Máñez S, Jiménez A, Villar A. Volatiles of Sideritis mugronensis flower and leaf. J Essent Oil Res. 1991;3:395–7. [Google Scholar]
- 15.Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and praline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–7. [Google Scholar]
- 16.Miller AL. Botanical influences on cardiovascular disease. Altern Med Rev. 1998;3:422–31. [PubMed] [Google Scholar]
- 17.Movafeghi A, Djozan Dj, Razeghi JA, Baheri T. Identification of volatile organic compounds in leaves, roots and gum of Astragalus compactus Lam. using solid phase microextraction followed by GC-MS analysis. Nat Prod Res. 2010;24:703–709. doi: 10.1080/14786410802361446. [DOI] [PubMed] [Google Scholar]
- 18.Müller M, Buchbauer G. Essential oil components as pheromones. A review. Flavour Fragr J. 2011;26:357–77. [Google Scholar]
- 19.Nikolov S, Benbassat N. Triterpenoid saponins and sapogenins from genus Astragalus L. Pharmacia. 1997;36:20–25. [Google Scholar]
- 20.Pistelli LF. Secondary metabolites of genus Astragalus: Structure and biological activity. In: Rahman A, editor. Studies in natural product chemistry. Volume 27: Bioactive natural products, Part H. Amsterdam: Elsevier Science Publishers; 2002. pp. 443–545. [Google Scholar]
- 21.Platikanov S, Nikolov S, Pavlova D, Evstatieva L, Popov S. Volatiles from four Astragalus species: phenological changes and their chemotaxonomical application. Z Naturforsch C. 2005;60:591–599. doi: 10.1515/znc-2005-7-814. [DOI] [PubMed] [Google Scholar]
- 22.Polhill RM. Papilionoideae. In: Polhill RM, Raven PH, editors. Advances in legume systematics, Part 1. London: Royal Botanic Gardens, Kew; 1981. pp. 191–208. [Google Scholar]
- 23.Rios LJ, Waterman GP. A review of the pharmacology and toxicology of Astragalus. Phytother Res. 1997;11:411–418. [Google Scholar]
- 24.Sinclair S. Chinese herbs: a clinical review of astragalus, ligusticum, and schizandrae. Altern Med Rev. 1998;3:338–44. [PubMed] [Google Scholar]
- 25.Smee DF, Sidwell RW, Huffman JH, Huggins JW, Kende M, Verbiscar AJ. Antiviral activities of tragacanthin polysaccharides on Punta Toro virus infections in mice. Chemotherapy. 1996;42:286–293. doi: 10.1159/000239457. [DOI] [PubMed] [Google Scholar]
- 26.Smith H. Regulatory mechanisms in the photocontrol of flavonoid biosynthesis. In: Milborrow BV, editor. Biosynthesis and its control in plants. New York: Academic Press; 1973. pp. 303–320. [Google Scholar]
- 27.Taveira FSN, de Lima WN, Andrade EHA, Maia JGS. Seasonal essential oil variation of Aniba canelilla. Biochem Syst Ecol. 2003;31:69–75. [Google Scholar]
- 28.Teyeb H, Zouari S, Douki W, Najjar MF, Neffati M. Variation in volatiles of Astragalus gombiformis Pomel. Z Naturforsch C. 2011;66:1–6. [PubMed] [Google Scholar]
- 29.Toor RK, Savage GP, Lister CE. Seasonal variations in the antioxidant composition of greenhouse grown tomatoes. J Food Comp Anal. 2006;19:1–10. [Google Scholar]
- 30.Verotta L, El-Sebakhy NA. Cycloartane and oleanane saponins from Astragalus sp. In: Rahman A, editor. Studies in natural products chemistry, Vol. 25. Amsterdam: Elsevier Science Publishers; 2001. pp. 179–234. [Google Scholar]