Abstract
The essential oil compound, fatty acid composition and the in vitro antioxidant activity of the root and aerial of Glycyrrhiza echinata L., a medicinal plant growing in Turkey, have been studied. The antioxidant capacity tests were designed to evaluate the antioxidant activities of methanol extracts. Total phenolic and flavonoid concentrations of each extract were also determined by using both Folin-Ciocalteu reagent and aluminum chloride. The aerial part was found to possess the highest total phenolic content (146.30 ± 4.58 mg GAE/g) and total antioxidant capacity (175.33 ± 3.98 mg AE/g). The essential oil from root and aerial parts was analyzed by gas chromatography mass spectroscopy (GC-MS) systems. The major components identified were n-hexadecanoic acid, hexahydro farnesyl acetone, α-caryophyllen, hexanal and phytol. In fatty acid profiles of plant, palmitic, stearic, oleic and linoleic acid were detected as the main components. The results of this study have shown that the extracts G. echinata are suitable as a natural antioxidant and food supplement source for pharmacological and food industries due to their beneficial chemical composition and antioxidant capacity.
Keywords: Glycyrrhiza echinata, antioxidant activity, fatty acid, essential oil
Introduction
The genus Glycyrrhiza L. (Fabaceae) is represented by 8 taxa in Turkey, 4 of which (G. iconica Hub.-Mor., G. flavescens subsp. flavescens Boiss., G. flavescens subsp. antalyensis Sümbül, O. Tufan, O.D. Düşen and R.S. Göktürk, and G. asymmetrica Hub.-Mor.) are endemic (Chamberlain, 1970[5]; Davis et al, 1988[6]; Sümbül et al., 2003[27]). The roots of plant are used in traditional medicine due to antimicrobial effects (Haraguchi et al., 1998[8]), antitumor activity (Nishino et al., 1986[18]), antimutagenic activity (Zani et al., 1993[34]) and anti-ulcer effects (van Marle et al., 1981[31]).
Superoxide anion (O2·), hydrogen peroxide (H2O2) and hydroxyl radical (HO·), which are the reactive oxygenic species (ROS), comprise as being by-products of organism. ROS are dangerous, when present in excess, and can attack biological molecules including lipids, proteins, enzymes, DNA and RNA, leading to cell or tissue damage (Jung et al., 1999[12]; Valentão et al., 2002[30]), which causes several chronic diseases such as diabetes mellitus, cancer, atherosclerosis, arthritis, neurodegenerative diseases and also in the ageing process (Hogg, 1998[9]; Pong, 2003[21]). Antioxidants are components that suppress these harmful effects. When antioxidants added to foods, minimize rancidity, retard the formation of toxic oxidation products, maintain nutritional quality, and increase shelf life (Jadhav et al., 1996[11]). Plant-derived antioxidants are natural antioxidants and occur in all parts of plants. They include carotenoids, vitamins, phenols, flavonoids, dietary glutathione, and endogenous metabolites (Larson, 1988[15]).
Essential oils and their chemical constituents are widely used in the manufacturing of medicinal products, cosmetics fragrances and as food flavoring additives (Shahat et al., 2008[24]). In nature, essential oils play an important role in the protection of the plants as antibacterials, antivirals, antifungals, insecticides and also against herbivores by reducing their appetite for such plants (Bakkali et al., 2008[4]).
To the best of our knowledge, there are no such reports concerning chemical composition and antioxidant activity of G. echinata, so the current study has focused to determine the antioxidant activity, the essential oil compound and the fatty acid composition. Data obtained from this study could be assumed as the first report for this species.
Materials and Methods
Plant materials and chemicals
The root and aerial parts of G. echinata L. were collected from Antalya (Serik), Turkey. Identification of the plant material was performed by botanist Professor Dr. A. Duran. Voucher specimens (Ö.Çetin-1043) were deposited in Konya University Education Faculty Herbarium, Konya.
Potassium ferricyanide, ferric chloride, Folin-Ciocalteu's reagent, trichloroacetic acid, methanol, BHT, BHA, ascorbic acid and methanol were purchased from Merck (Darmstadt, Germany). 2,2-diphenyl-1-picrylhydrazyl (DPPH), β-carotene, linoleic acid, Tween 40 and troloks were purchased from Sigma Chemical Co. (Sigma-Aldrich GmbH, Sternheim, Germany). All other chemicals and solvents were of analytical grade.
Extraction of essential oil
The air-dried root and aerial parts of the plant were hydrodistilled for 3 h using a Clevenger type apparatus to extract the essential oils, which were trapped in n-hexane. The obtained essential oils were stored at +4 ºC until use.
Gas chromatography-mass spectrometry
The GC-MS analyses were carried out with an Agilent 7890 GC-MS system. A HP-INNOWAX column (60 m length, 0.25 mm i.d. and 0.25 µm film thickness) was used with He as the carrier gas (1.2 ml min-1). GC oven was programmed at an initial temperature of 60 ºC for 10 min. Thereafter, the temperature was increased up to 220 ºC at the rate 4 ºC min-1, kept constant at 220 ºC for 10 min, and then increased up to 240 ºC at the rate 1 ºC min-1. Total run time was 80 min. Both injector and detector temperatures were 250 ºC. Mass spectra were recorded at 70 eV. The relative percentages of the separated compounds were calculated from total ion chromatograms. The identification of the oil components was based on the Wiley and Nist mass spectral library. Retention indices (RI) of the compounds were determined relative to the retention times of a series of n-alkanes.
Preparation of methanolic extract
The root and aerial plant materials were dried at room temperature. Dried plant materials were ground to a fine powder using a laboratory mill. Fifteen grams of powdered plant were mixed with 250 ml methanol and extracted in a Soxhlet apparatus for 6-8 h. The extracts were filtered and methanol was evaporated at 40 ºC in a rotary evaporator. Extracts were stored at +4 ºC in the dark until use.
Assays for total phenolic and flavonoid content
The phenolic content of the extracts was determined using Folin-Ciocalteu reagent (Slinkard and Singleton, 1977[25]); 0.2 ml of extract solution (2 mg ml-1) was mixed with 1 ml Folin-Ciocalteu reagent and 2 ml Na2CO3 (7.5 %). The final volume was brought up to 7 ml with deionised water. The mixture was allowed to stand for 2 h at room temperature and absorbance was measured at 765 nm with a spectrophotometer (Shimadzu, UV-1800). Gallic acid was used as a standard for calibration curve. The total phenolic content of extracts was determined as gallic acid equivalent (mg GAE g-1 extract).
The total flavonoid content in extracts was determined spectrophotometrically according to Arvouet-Grand et al. (1994[3]). Briefly, 1 ml of 2 % aluminum trichloride (AlCl3) methanolic solution was mixed with the same volume of extract solution (at 2 mg ml-1 concentration). The absorbance values of the reaction mixtures were determined at 415 nm after 10 min duration against a blank. Rutin was used as the standard and the total flavonoids content of the extracts was expressed as mg rutin equivalents per gram of extract (mg RE g-1 extract).
Total antioxidant capacity
The total antioxidant capacity of extracts was evaluated by phosphomolybdenum method according to Prieto et al. (1999[22]); 0.3 ml of extract solution (1 mg ml-1) was mixed with 3 ml of reagent solution (6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The reaction mixture was incubated at 95 ºC for 90 min. Then, the absorbance of the solution was measured at 695 nm against blank. The antioxidant capacity of extracts was evaluated as equivalent of ascorbic acid (mg AAE g-1).
Free radical scavenging activity (DPPH, 2,2-diphenyl-1-picrylhydrazyl)
The free radical scavenging activity of plant extracts was determined by slight modifications of the method described by Kirby and Schmidt (1997[13]); 0.5 ml of various concentrations of the extracts in methanol were added to 3 ml of 6.10-5 M of a methanol solution of DPPH. This solution was incubated for 30 min in the dark at room temperature. After the incubation, the mixture absorbance was measured at 517 nm. Inhibition activity was calculated in the following way:
I(%) = (A0-A1)/A0x100
where, A0 is the absorbance of the control, A1 the absorbance of the extract/standard. In the test, BHT was used as a positive control. Free radical inhibition (IC50) of extracts was calculated. The lower the IC50 value indicates high antioxidant capacity.
β-Carotene/linoleic acid bleaching assay
β-carotene bleaching assay is based on rapid discoloration in the absence of an antioxidant (Kulisic et al., 2004[14]). In this assay, antioxidant activity of extracts was determined by slight modifications of the procedure described by Sokmen et al. (2004[26]). A stock solution of β-carotene-linoleic acid mixture was prepared as follows: 0.5 mg β-carotene was dissolved in 1 ml chloroform and 25 mL linoleic acid and 200 mg Tween 40 were added.
Chloroform was completely evaporated and 100 ml distilled water saturated with oxygen was added with vigorous shaking. Also, 2.5 ml of this reaction mixture dispensed into test tubes and 350 ml portion (1 mg ml-1 concentration) of the extracts were added. The reaction mixture was incubated at 50 ºC for 2 h. The same procedure was repeated with BHT and BHA, as positive control and a blank.
After this incubation period, absorbance of the mixtures was measured at 490 nm and inhibition ratio was calculated.
Ferric ion reducing power
The ferric reducing power method was applied with slight modifications of the method of Oyaizu (1986[19]). Different concentrations of extracts were mixed with 2.5 ml of 0.2 M phosphate buffer and potassium ferricyanide and 2.5 ml of 1 % mixture was incubated at 50 ºC for 20 min. After incubation, 2.5 ml of 10 % trichloroacetic acid was added. Then, 2.5 ml of the reaction mixture was mixed with 2.5 ml of distilled water and 0.5 ml of 0.1 % ferric chloride. The solution absorbance was measured at 700 nm. The reducing power of samples increased with the absorbance value. The same procedure was applied with BHA and BHT. The EC50 value (the effective concentration at which the absorbance was 0.5) was calculated for extract, BHA and BHT.
CUPRAC assay
The cupric ion reducing capacity of extracts of G. echinata were determined according to the method of Apak et al. (2006[2]); 1 ml each of 10 mM CuCl2, 7.5 mM neocuproine and NH4Ac buffer (1 M, pH 7.0) solutions were added into a test tube. Then, 0.5 ml of different concentrations of extracts were mixed and total volume was brought up to 4.1 ml with deionised water. The mixture absorbance was recorded against a blank at 450 nm after 30 min incubation at room temperature.
Extraction of oils
The oil extraction of dried and powdered plant materials (10 g) was carried with diethyl ether out at boiling point (34 ºC) for 6 h with a Soxhlet extractor using diethyl ether as a solvent. The solvent was evaporated with a rotary evaporator.
Fatty Acids Methyl Esters (FAMEs) preparation
The fatty acids in the oil were esterified into methyl esters by saponification with 0.5 N methanolic NaOH and transesterified with 14 % BF3 (v/v) in methanol (IUPAC, 1979[10]).
Gas chromatographic analysis
FAMEs were analyzed on a HP (Hewlett Packard) Agilent 6890 N model gas chromatograph (GC), equipped with a flame ionization detector (FID) and fitted to a HP-88 capillary column (100 m length, 0.25 mm i.d. and 0.2 µm film thickness). Injector and detector temperatures were set at 240 and 250 ºC, respectively. The oven was held at 160 ºC for 2 min. Thereafter the temperature was increased up to 185 ºC at rate of 4 ºC min-1, then increased at up to 200 ºC at rate of 1 ºC min-1 and held at 200 ºC for 46.75 min. Total run time was 70 min. Helium was used as carrier gas (1 ml min-1).
Identification of fatty acids was carried out by comparing sample FAME peak relative retention times with those obtained for Alltech and Accu standards. Results were expressed as FID response area in relative percentages. Each reported result is given in the average value of three GC analyses. The results are offered as means ± S.D. Atherogenic index (AI) and thrombogenicity index (TI) were calculated according to Ulbricht and Southgate (1991[29]). AI = [12:0 + (4 x 14:0) + 16:0]/[(ω6 + ω3) PUFA + 18:1 + other MUFA]; TI = [14:0 + 16:0 + 18:0]/[0.5 x 18:1 + 0.5 x other MUFA + 0.5 x ω6 PUFA + 3 x ω3 PUFA + (ω3 PUFA/ ω6 PUFA)].
Results and Discussion
In the root and aerial parts, thirty-three and twenty-four known compounds have been detected, accounting for 95.73 % and 96.075 % of the total mass, respectively (Table 1(Tab. 1)). The most abundant compound was n-hexadecanoic acid, which accounted for approximately 78.271 % and 72.946 % in the root and aerial parts, respectively. The other main components were characterized as hexanal (2.790 %) and phytol (2.783 %) in the root, hexahydro farnesyl acetone (9.339 %) and α-caryophyllen (6.590 %) in the aerial parts.
Table 1. Essential oil composition of the different parts of Glycyrrhiza echinata.
Our results differed from those reported for G. pallidiflora, which was reported to be dominated by 5-(2-propenyl)-1,3-benzodioxole (19.02 %), 3-7dimethyl-1,6-octadien-3-ol (17.70 %), [1R-(1R*.4Z.9S*)]-4.11.11-trimethyl-8-methylene-bicyclo[7.2.0]undec-4-ene (11.53 %), 2.3.6-trimethyl-1.6-heptadiene (8.36 %), 2-undecanone (4.24 %), coumari-7.8-diol (3.81 %), 2-methyl-6-methylene-7-octen-2-ol (3.47 %) and 3.7.11.15-tetramethyl-2-hexadecen-1-ol (3.43 %) (Zhang et al., 2004[35]). Miyazawa and Kameoka (1990[17]) reported that the major components of the essential oil of G. glabra var. glandulifera from China were octanoic acid (11.4 %), paenol (8.9 %), octadecane (8.6 %), benzaldehyde (7.5 %), α-terpineol (7.5 %) and 4-terpineol (7.2 %).
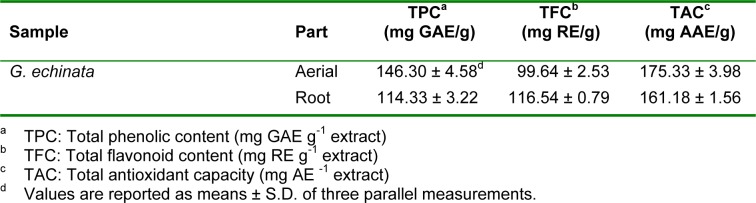
The root of Glycyrrhiza species is one of the richest sources of biological active compounds such as phenolic and flavanoid compounds (Roth, 2004[23]). The total phenolic contents in extracts from the root and aerial parts were 114.33 and 146.30 mg GAE g-1, respectively (Table 2(Tab. 2)). Tohma and Gulçin (2010[28]) observed significantly lower phenolic concentration in aqueous extracts than our results. However, in their investigation the level of phenolic content in ethanol extracts was higher than our study.
Table 2. Total phenolics, flavonoid contents and antioxidant capacities of methanolic extracts obtained from Glycyrrhiza echinata.
The contents of total flavonoid were 116.54 mg RE g-1 in the root and 99.64 mg RE g-1 in the aerial part (Table 2(Tab. 2)). Li et al. (2011[16]) studied the flavonoid content of different solvent extracts (hexan, chloroform, ethyl acetate, n-butanol and water) of G. uralensis root and found that it varied from 3.601 to 66.546 mg RE g-1.
In phosphomolybdenum method, Mo (VI) is reduced to form a green phosphate/Mo (V) complex. Total antioxidant capacities of root and aerial part were 175.33 and 161.18 mg AAE g-1, respectively (Table 2(Tab. 2)).
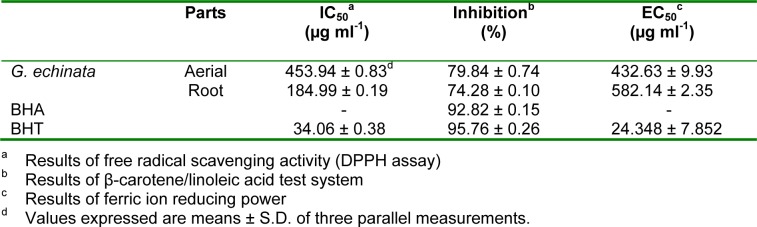
The root was characterized by lower IC50 (184.99 µg ml-1) than aerial part (453.94 µg ml-1) (Table 3(Tab. 3)). Li et al. (2011[16]) reported that DPPH radical scavenging activities of hexan, chloroform, ethyl acetate, n-butanol and water extracts from G. uralensis were 1.436 ± 0.137 µg/ml, 1.208 ± 0.129 µg/ml, 1.156 ± 0.065 µg/ml, 5.475 ± 0.015 µg/ml and 64.038 ± 4.548 µg/ml, respectively. In the other study, DPPH radical scavenging activity of G. lepidota reported as 49.7 % at a concentration of 1 mg (Amarowicz et al., 2004[1]). In another antioxidant study of G. glabra root (Visavadiya et al., 2009[32]) was demonstrated that the water (64.2 µg/ml) and ethanol (38.4 µg/ml) extracts have the high ability to DPPH radical scavenging activity compared to our study.
Table 3. Table 3: Free radical scavenging, linoleic acid inhibition, ferric ion reducing power activity of Glycyrrhiza echinata.
In β-carotene/linoleic acid bleaching assay, β-carotene undergoes rapid discoloration in the absence of an antioxidant. In Table 3(Tab. 3) are presented the inhibition of β-carotene bleaching by the root and aerial extracts of G. echinata, and by the two positive controls (BHA and BHT). In term of β-carotene bleaching effect, those samples exhibited the following order: BHT > BHT > aerial > root. Aerial and root extracts exhibited 79.84 % and 74.28 % inhibition activity.
As seen in Table 3(Tab. 3), the reducing powers of extracts are expressed as EC50. The lower the EC50 value indicates high antioxidant capacity. In ferric reducing power method, ferric-ferricyanide complex is reduced to the ferrous form depending on the presence of antioxidants (Amarowicz et al., 2004[1]). The aerial extract was clearly higher than root extracts (432.63 and 582.14 µg ml-1, respectively).
The CUPRAC assay used copper(II)-neocuproine reagent as the chromogenic oxidizing agent. The method is based on the measurement of absorbance at 450 nm by formation of stable complex between neocuproine and copper (I) (Ozyurek et al., 2011[20]). The cupric ion reducing power of extracts was dependent on the concentration of extract (Figure 1(Fig. 1)). According to CUPRAC data of G. echinata, the root showed higher reducing power activity than aerial part. The obtained results of antioxidant values of G. echinata are in agreement with the results of other authors (Tohma and Gulçin, 2010[28]).
Figure 1. Cupric reducing antioxidant capacity (CUPRAC) of the different parts of Glycyrrhiza echinata.

Twenty-two fatty acids were identified and compared among different parts. In the aerial part dominant fatty acids were detected: palmitic (C16:0) and stearic acid (C18:0) as saturated fatty acids (SFA); oleic (C18:1ω9) and eicosaenoic acid (C20:1ω9) as monounsaturated fatty acids (MUFA); linoleic (C18:2ω6) and arachidonic acid (C20:4ω6) as polyunsaturated fatty acids (PUFA) (Table 4(Tab. 4)). Major fatty acids of the root were palmitic (25.23 %), linoleic (20.32 %), oleic (20.29 %) and stearic acid (15.48 %). In previous studies, G. uralensis (Fu et al., 2007[7]) and G. glabra (Yunusova et al., 1995[33]) were characterized by high linoleic acid content. The levels of total SFA, MUFA and PUFA of aerial part and root were found as 66.90-47.34, 14.08-23.95 and 19.03-28.68, respectively. Atherogenic (AI) and thrombogenicity index (TI) indicate the dietetic quality of lipids (Ulbricht and Southgate, 1991[29]). The root oil showed the lowest AI (1.09) and TI (1.08) values.
Table 4. Fatty acid composition of the different parts of Glycyrrhiza echinata (%).

In conclusion, our results showed that G. echinata root and aerial parts contain high amounts of phenolic and flavonoid contents. Furthermore, the result of this study indicate that extracts obtained from G. echinata are effective antioxidants that exhibit high activities in vitro models of DPPH free radical scavenging activity, inhibition rate of oxidation of linoleic acid, ferric and cupric reducing power assay. Therefore, G. echinata root and aerial parts are suitable as a natural supplement source for pharmacological and food industries.
Acknowledgements
This research was supported financially as a project (Project No: 111T614). We thank The Scientific and Technological Research Council of Turkey (TUBITAK) for providing financial support for this study.
References
- 1.Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84:551–562. [Google Scholar]
- 2.Apak R, Guclu K, Ozyurek M, Karademir SE, Ercag E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nutr. 2006;57:292–304. doi: 10.1080/09637480600798132. [DOI] [PubMed] [Google Scholar]
- 3.Arvouet-Grand A, Vennat B, Pourrat A, Legret P. Standardisation d’un extrait de propolis et identification des principaux constituants. J Pharm Belg. 1994;49:462–468. [PubMed] [Google Scholar]
- 4.Bakkali F, Averbeck S, Averbeck D, Waomar M. Biological effects of essential oils-A review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain DF. Glycyrrhiza. In: Davis PH, editor. Flora of Turkey and the East Aegean Islands, Vol. 3. Edinburgh: Edinburgh University Press; 1970. pp. 260–263. [Google Scholar]
- 6.Davis PH, Mill RR, Tan K. Flora of Turkey and the East Aegean Islands (Vol. 10, Suppl. 1) Edinburgh: Edinburgh University Press; 1988. [Google Scholar]
- 7.Fu YJ, Wang W, Zu YG, Suschke U, Reichling J, Schwarz G. Supercritical fluid extraction of seed oil from Chinese Licorice (Glycyrrhiza uralensis Fisch.): Chemical composition and antibacterial activity. S Afr J Chem. 2007;60:67–70. [Google Scholar]
- 8.Haraguchi H, Tanimoto K, Tamura Y, Mizutani K, Kinoshita T. Mode of antibacterial action of retrochalcones from Glycyrrhiza inflate. Phytochemistry. 1998;48:125–129. doi: 10.1016/s0031-9422(97)01105-9. [DOI] [PubMed] [Google Scholar]
- 9.Hogg N. Free radicals in disease. Semin Reprod Endocr. 1998;16:241–288. doi: 10.1055/s-2007-1016284. [DOI] [PubMed] [Google Scholar]
- 10.IUPAC. Standards methods for analysis of oils, fats and derivatives. Oxford: Oxford Pergamon Press; 1979. pp. 59–66. [Google Scholar]
- 11.Jadhav SJ, Nimbalkar SS, Kulkarni AD, Madhavi DL. Lipid oxidation in biological and food systems. In: Madhavi DL, Deshpande SS, Salunkhe DK, editors. Food antioxidants. New York: Dekker; 1996. pp. 5–63. [Google Scholar]
- 12.Jung HA, Park JC, Chung HY, Kim J, Choi JS. Antioxidant flavonoids and chlorogenic acid from the leaves of Eriobotrya japonica. Arc Pharmacal Res. 1999;22:213–218. doi: 10.1007/BF02976549. [DOI] [PubMed] [Google Scholar]
- 13.Kirby AJ, Schmidt RJ. The antioxidant activity of Chinese herbs for eczema and of placebo herbs. J Ethnopharmacol. 1997;56:103–108. doi: 10.1016/s0378-8741(97)01510-9. [DOI] [PubMed] [Google Scholar]
- 14.Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85:633–640. [Google Scholar]
- 15.Larson RA. The antioxidants of higher plants. Phytochemistry. 1988;2:969–978. [Google Scholar]
- 16.Li M, Xu Y, Yang W, Li J, Xu X, Zhang X, et al. In vitro synergistic anti-oxidant activities of solvent-extracted fractions from Astragalus membranaceus and Glycyrrhiza uralensis. LWT - Food Sci Technol. 2011;44:1745–1751. [Google Scholar]
- 17.Miyazawa M, Kameoka H. Volatile flavour components of Glycyrrhiza Radix (Glycyrrhiza glabra L. var. glandulifera Regel et Herder) from China. Flavour Frag J. 1990;5:157–160. [Google Scholar]
- 18.Nishino H, Yoshioka K, Iwashima A, Takizawa H, Konishi S, Okamoto H, et al. Glycyrrhetic-acid inhibits tumor-promoting activity of teleocidin and 12-O-tetradecanoylphorbol-13-acetate in two-stage mouse skin carcinogensis. Jpn J Cancer Res. 1986;77:33–38. [PubMed] [Google Scholar]
- 19.Oyaizu M. Studies on products of browning reactions: antioxidative activities of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. [Google Scholar]
- 20.Ozyurek M, Guclu K, Tutem E, Baskan KS, Ercag E, Celik SE, et al. A comprehensive review of CUPRAC methodology. Anal Meth. 2011;3:2439–2453. [Google Scholar]
- 21.Pong K. Oxidative stress in neurodegenerative diseases: therapeutic implications for superoxide dismutase mimetics. Expert Opin Biol Ther. 2003;3:127–139. doi: 10.1517/14712598.3.1.127. [DOI] [PubMed] [Google Scholar]
- 22.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphor molybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 23.Roth K. Licorice spirals. Chemie in unserer Zeit. 2004;38:202–207. [Google Scholar]
- 24.Shahat AA, El-Barouty G, Hassan RA, Hammouda FM, Abdel-Rahman FH, Saleh MA. Chemical composition and antimicrobial activities of the essential oil from the seeds of Enterolobium contortisiliquum (leguminosae) J Environ Sci Health Part B. 2008;43:519–525. doi: 10.1080/03601230802174714. [DOI] [PubMed] [Google Scholar]
- 25.Slinkard K, Singleton VL. Total phenol analysis: Automation and comparison with manual methods. Am J Enol Viticult. 1977;28:49–55. [Google Scholar]
- 26.Sokmen A, Gulluce M, Akpulat HA, Daferera D, Tepe B, Polissiou M, et al. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control. 2004;15:627–634. [Google Scholar]
- 27.Sümbül H, Tufan Ö, Düşen O, Göktürk RS. A new taxon Glycyrrhiza L. (Fabaceae) from southwest Anatolia. Isr J Plant Sci. 2003;51:71–74. [Google Scholar]
- 28.Tohma HŞ, Gulçin İ. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.) Int J Food Prop. 2010;13:657–671. [Google Scholar]
- 29.Ulbricht TLV, Southgate DAT. Coronary heart disease seven dietary factors. Lancet. 1991;338:985–992. doi: 10.1016/0140-6736(91)91846-m. [DOI] [PubMed] [Google Scholar]
- 30.Valentão P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, Bastos ML. Antioxidative properties of cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical, and hypochlorous acid. J Agric Food Chem. 2002;50:4989–4993. doi: 10.1021/jf020225o. [DOI] [PubMed] [Google Scholar]
- 31.van Marle J, Aarsen PN, Lind A, van Weeren-Kramer J. Deglycyrrhizinized licorice (DGL) and the renewal of rat stomach epithelium. Eur J Pharmacol. 1981;72:219–225. doi: 10.1016/0014-2999(81)90276-4. [DOI] [PubMed] [Google Scholar]
- 32.Visavadiya NP, Soni B, Dalwadi N. Evaluation of antioxidant and anti-atherogenic properties of Glycyrrhiza glabra root using in vitro models. Int J Food Sci Nutr. 2009;60:135–149. doi: 10.1080/09637480902877998. [DOI] [PubMed] [Google Scholar]
- 33.Yunusova SG, Danilov VT, Yunusov MS, Murinov YI, Tsyrlina EM, Straek R. Chemistry of natural compounds and bioorganic chemistry - Lipids of Glycyrrhiza-glabra root. Russ Chem Bull. 1995;44:359–362. [Google Scholar]
- 34.Zani F, Cuzzoni MT, Daglia M, Benvenuti S, Vampa G, Mazza P. Inhibition of mutagenicity in Salmonella typhimurium by Glycyrrhiza glabra extract, glycyrrhizinic acid, 18α- and 18β-glycyrrhetinic acids. Planta Med. 1993;59:502–507. doi: 10.1055/s-2006-959748. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Ma J, Wang Y, Yao J, Yang Y. Analysis of leaf volatile chemical components of Glycyrrhiza pallidiflora. Acta Pratacult Sin. 2004;13:103–105. [Google Scholar]