Abstract
STUDY QUESTION
What is the role of microRNAs (miRs) in antiphospholipid antibody (aPL)-induced trophoblast inflammation?
SUMMARY ANSWER
aPL-induced up-regulation of trophoblast miR-146a-3p is mediated by Toll-like receptor 4 (TLR4), and miR-146a-3p in turn drives the cells to secrete interleukin (IL)-8 by activating the RNA sensor, TLR8.
WHAT IS KNOWN ALREADY
Obstetric antiphospholipid syndrome (APS) is an autoimmune disorder characterized by circulating aPL and an increased risk of pregnancy complications. We previously showed that aPL recognizing beta2 glycoprotein I (β2GPI) elicit human first trimester trophoblast secretion of IL-8 by activating TLR4. Since some miRs control TLR responses, their regulation in trophoblast cells by aPL and functional role in the aPL-mediated inflammatory response was investigated. miRs can be released from cells via exosomes, and therefore, miR exosome expression was also examined. A panel of miRs was selected based on their involvement with TLR signaling: miR-9; miR-146a-5p and its isomiR, miR-146a-3p; miR-155, miR-210; and Let-7c. Since certain miRs can activate the RNA sensor, TLR8, this was also investigated.
STUDY DESIGN, SIZE, DURATION
For in vitro studies, the human first trimester extravillous trophoblast cell line, HTR8 was studied. HTR8 cells transfected to express a TLR8 dominant negative (DN) were also used. Plasma was evaluated from pregnant women who have aPL, either with or without systemic lupus erythematous (SLE) (n = 39); SLE patients without aPL (n = 30); and healthy pregnant controls (n = 20).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Trophoblast HTR8 wildtype and TLR8-DN cells were incubated with or without aPL (mouse anti-human β2GPI mAb) for 48–72 h. HTR8 cells were also treated with or without aPL in the presence and the absence of a TLR4 antagonist (lipopolysaccharide from Rhodobacter sphaeroides; LPS-RS), specific miR inhibitors or specific miR mimics. miR expression levels in trophoblast cells, trophoblast-derived exosomes and exosomes isolated from patient plasma were measured by qPCR. Trophoblast IL-8 secretion was measured by ELISA.
MAIN RESULTS AND THE ROLE OF CHANCE
aPL significantly increased trophoblast cellular and exosome expression of miR-146a-5p, miR-146a-3p, miR-155 and miR-210. aPL-induced up-regulation of trophoblast miR-146a-5p, miR-146a-3p and miR-210, but not miR-155, was inhibited by the TLR4 antagonist, LPS-RS. While inhibition or overexpression of miR-146a-5p had no effect on aPL-induced trophoblast IL-8 secretion, miR-146a-3p inhibition significantly reduced this response. aPL-induced trophoblast IL-8 secretion was inhibited by the presence of the TLR8-DN. In the absence of aPL, transfection of trophoblast cells with a miR-146a-3p mimic significantly increased IL-8 secretion and this was inhibited by the presence of the TLR8-DN. Patients with aPL and adverse pregnancy outcomes (APOs) expressed significantly higher levels of circulating miR-146a-3p compared with healthy pregnant controls with no pregnancy complications (P < 0.05).
LIMITATIONS, REASONS FOR CAUTION
While the enrichment of miR-146a-3p in trophoblast-derived exosomes support the role of this miR acting in a paracrine or endocrine manner through exosome delivery, this has not been demonstrated. However, miR-146a-3p may also exert its pro-inflammatory effect intracellularly within the same trophoblast cell targeted by aPL.
WIDER IMPLICATIONS OF THE FINDINGS
These findings provide a novel mechanism of trophoblast inflammation through miRs activating RNA-sensing receptors. Furthermore, circulating exosomal-associated miR-146a-3p in APS patients may serve clinically as a biomarker for related APOs.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported in part by grants from the American Heart Association (#10GRNT3640032 to V.M.A.), the March of Dimes Foundation (Gene Discovery and Translational Research Grant #6-FY12-255 to V.M.A.), NICHD, NIH (R01HD049446 to V.M.A.), the Gina M. Finzi Memorial Student Summer Fellowship from the Lupus Foundation of America (to S.M.G.), and the Yale University School of Medicine Medical Student Fellowship (to S.M.G.). The authors declare no competing financial interests.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: antiphospholipid antibody, antiphospholipid syndrome, exosome, inflammation, lupus, MicroRNA, placenta, pregnancy, Toll-like receptor, trophoblast
Introduction
Antiphospholipid syndrome (APS) is a systemic autoimmune disorder characterized by a pro-thrombotic state and pregnancy morbidity. Pregnant APS patients have an increased risk for recurrent pregnancy loss and late gestational complications, such as pre-eclampsia and intrauterine fetal growth restriction (IUGR) (Valesini and Alessandri, 2005). Diagnosis of APS requires the presence of persistent circulating antiphospholipid antibodies (aPL) that may present in isolation or in the context of another autoimmune disease, most commonly systemic lupus erythematous (SLE) (Valesini and Alessandri, 2005). aPL are a heterogeneous population of autoantibodies that recognize anionic phospholipid-binding proteins. aPL targeting β2 glycoprotein I (β2GPI) are particularly pathologic in obstetric APS (Meroni et al., 2012) since the antigen is constitutively expressed by the placental trophoblast; in particular the extravillous trophoblast population that invades the decidua and remodels the uterine spiral arteries (Chamley et al., 1997; Quenby et al., 2005). β2GPI has been shown to be required for the development of aPL-mediated pregnancy loss in a murine model of APS (Robertson et al., 2004).
Given the pro-thrombotic nature of the disease, adverse pregnancy outcomes (APOs) associated with APS were initially attributed to clotting at the maternal-fetal interface, a notion supported by the apparent beneficial use of heparin in reducing fetal loss (de Jesús et al., 2014). However, evidence of placental thrombosis is uncommon. More frequently, disruptions in placentation are identified, such as reduced trophoblast invasion and impaired uterine spiral artery transformation (Sebire et al., 2002; Viall and Chamley, 2015). Inflammation is also a common and important mediator of aPL-induced pregnancy loss (Girardi et al., 2003; Van Horn et al., 2004; Berman et al., 2005; Mulla et al., 2009; Viall and Chamley, 2015).
Studies from our group have illustrated the direct impact of aPL recognizing β2GPI on human first trimester trophoblast cells. Through the Toll-like receptor 4 (TLR4) and Nalp3-inflammasome pathways, aPL induce an inflammatory response in the trophoblast (Mulla et al., 2009, 2013). In parallel, and independently of TLR4, aPL mediate dysregulation of trophoblast angiogenic factor production and inhibit cell migration (Mulla et al., 2010; Carroll et al., 2011). Specifically, anti-β2GPI antibodies elicit TLR4-dependent trophoblast secretion of interleukin (IL)-8 and IL-1β (Mulla et al., 2009). The IL-1β, but not the IL-8, response is driven by TLR4-mediated uric acid production, which in turn activates the Nalp3-inflammasome, leading to IL-1β processing and release (Mulla et al., 2013). However, it is unclear how aPL-induced IL-8 secretion is regulated downstream of TLR4.
One mechanism of TLR regulation is through microRNAs (miRs); small noncoding RNAs that regulate protein expression through post-transcriptional interactions with mRNA, either by suppressing translation, or reducing levels of target mRNA (O'Neill et al., 2011). A number of miRs can be induced by TLRs and can subsequently regulate TLR activity by targeting elements of the signaling cascade (O'Neill et al., 2011). These include miR-9, which targets nuclear factor (NF)κB (Bazzoni et al., 2009); miR-146a-5p (formerly designated miR-146a), which targets interleukin-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6) (Taganov et al., 2006); and miR-155, which targets myeloid differentiation primary response 88 (MyD88) (Tang et al., 2010). Altered miR expression has been reported in trophoblast cells exposed to hypoxia, environmental toxins or infectious components (Donker et al., 2007; Maccani et al., 2010; Dai et al., 2011; Garg et al., 2013), and in placentas from pathological pregnancies (Pineles et al., 2007; Mayor-Lynn et al., 2011). However, knowledge about the role of miRs in trophoblast function is still limited, and no information exists on whether aPL-induced trophoblast responses may be regulated by miRs.
The objective of this study was to determine the impact of aPL on human trophoblast miRs and their functional role in these cells. Since the trophoblast constitutively releases microparticles, such as exosomes (Mincheva-Nilsson and Baranov, 2010), and these contain miRs that can be transferred from cell to cell and remain functional (Delorme-Axford et al., 2013), exosome miR expression was also examined. aPL induce trophoblast IL-8 secretion via TLR4 (Mulla et al., 2009) and, therefore, a panel of miRs involved in TLR signaling was selected: miR-9, miR-146a-5p and miR-155 (O'Neill et al., 2011). The panel also included two miRs known to affect trophoblast IL-6 production and migration similarly to aPL: miR-210, which inhibits trophoblast migration and invasion, and is induced by TLR3 and TLR4 (Qi et al., 2012; Zhang et al., 2012; Anton et al., 2013; Kopriva et al., 2013); and Let-7c, which inhibits trophoblast IL-6 secretion and is induced by TLR2 (Garg et al., 2013). Despite expression levels often lower than their miR counterparts, isomiRs can associate with the RNA-induced silencing complex and interact with mRNA, suggesting that they may have functional roles (Cloonan et al., 2011). Coupled with this and that miR-146a-5p targets TLR4 signaling relatively upstream, miR-146a-3p was also analyzed. IsomiRs can have widely varying targets and both synergistic and antagonistic functions within the same cell (Zhou et al., 2010; Cloonan et al., 2011). Thus, we postulated in the trophoblast that miR-146a-5p and miR-146a-3p could act on different targets. Furthermore, some miRs act outside of the canonical role of mRNA degradation and translational repression by directly interacting with proteins to alter function (Eiring et al., 2010). Indeed, certain miRs act non-classically by binding and activating protein receptors. One receptor capable of binding miRs, such as miR-21 and miR-29a, is TLR8 (Fabbri et al., 2012). Since we previously reported that TLR8 is expressed by the trophoblast, and mediates IL-8 production in response to viral ssRNA (Potter et al., 2015), we considered its role in response to endogenous miRs in the aPL-exposed trophoblast.
Materials and Methods
Trophoblast cell lines
The human first trimester extravillous trophoblast cell line, HTR8, was used in these studies and were a kind gift from Dr Charles Graham (Queens University, Kingston, ON, Canada) (Graham et al., 1993). The TLR8 dominant negative (TLR8-DN) HTR8 trophoblast cells have been previously characterized (Potter et al., 2015).
Isolation of primary trophoblast from first trimester placenta
First trimester placentas (7–12 weeks gestation) were obtained from elective terminations of normal pregnancies performed at Yale-New Haven Hospital. The use of patient samples was approved by Yale University's Human Research Protection Program. Tissue specimens were washed with cold Hanks Balanced Salt Solution (Gibco, Waltham, MA, USA) to remove excess blood, minced, transferred to trypsin-EDTA (Invitrogen, Carlsbad, CA, USA) digestion buffer and incubated at 37°C for 40 min with shaking. The mixture was then passed through a nylon strainer and then layered over Lymphocyte Separation Media (ICN Biomedicals, Inc., Aurora, OH, USA) and centrifuged at 400× g for 25 min. The cellular interface containing the trophoblast cells was collected and resuspended in Dulbeccos minimum essential medium (D-MEM) with D-valine (Caisson Labs, North Logan, UT, USA) supplemented with 10% (v/v) normal human serum (Gemini Bio-Products, Woodland, CA, USA) and cultured at 37°C/5% CO2 (in air).
Antiphospholipid antibodies
This study used the aPL, IIC5, which is a mouse IgG1 anti-human β2GP1 monoclonal antibody (mAb). This aPL has been previously characterized. Like patient-derived polyclonal aPL, IIC5 binds β2GPI when it is immobilized on a negatively charged surface such as phospholipids, cardiolipin, phosphatidyl serine or irradiated polystyrene (Chamley et al., 2001). IIC5 reacts specifically with an epitope within domain V of β2GPI, which may be more important than domain I binding aPL for pregnancy morbidity in APS patients (Albert et al., 2014). Moreover, IIC5 binds to human first trimester extravillous trophoblast cells (Chamley et al., 1997; Quenby et al., 2005; Mulla et al., 2009), and alters their function in a similar fashion to patient-derived polyclonal aPL-IgG (Mulla et al., 2013), and polyclonal IgG aPL recognizing β2GPI (Mulla et al., 2009; Carroll et al., 2011). Mouse IgG1 clone 107.3 (BD Biosciences) was used as an isotype control.
Patient samples
Plasma was collected as part of The PROMISSE Study (Predictors of pRegnancy Outcome: bioMarkers In antiphospholipid antibody Syndrome and Systemic lupus Erythematosus), a multicenter National Institutes of Health-funded prospective observational study of pregnancies of women with aPL, SLE or both, as well as healthy pregnant controls. Details about this cohort of patients as well as inclusion and exclusion criteria have been previously published (Salmon et al., 2011; Lockshin et al., 2012; Mulla et al., 2013; Andrade et al., 2015; Buyon et al., 2015). This paper concerns a subset of pregnant participants who have aPL (aPL+) either with or without SLE (n = 39), and healthy pregnant controls (n = 20). We also studied SLE patients (SLE+) without aPL (aPL−) (n = 30). The Institutional Review Board at each of the PROMISSE Study sites approved participation of patients. Written informed consent was obtained from all participants. Primary study outcomes were defined as the occurrence of one or more of the following: (i) otherwise unexplained fetal death; (ii) neonatal death prior to hospital discharge and due to complications of prematurity; (iii) indicated preterm delivery prior to 37 weeks' gestation because of gestational hypertension, pre-eclampsia or placental insufficiency; and (iv) birthweight <5th percentile and/or delivery before 37 weeks because of IUGR and confirmed by birthweight <10th percentile. A list of the APOs are shown in Supplementary data, Table SI. From the aPL+ (SLE+ or SLE−) group, 21 patients were APO+, and from the aPL− SLE+ group, 11 patients were APO+. Plasma collected during the second trimester (18–27 weeks gestation) was analyzed for exosome-associated miR-136a-3p expression.
Trophoblast treatments and transfections
HTR8 cells were treated with or without the aPL, IIC5 (20 µg/ml) or the IgG1 isotype control (20 µg/ml) in serum-free OptiMEM (Gibco). For TLR4 inhibition, cells were pretreated for 1 h with or without the TLR4 antagonist, lipopolysaccharide from R. sphaeroides (LPS-RS; Invivogen) at 10 µg/ml (Mulla et al., 2013). To assess miR function, HTR8 cells were transfected with 100 nM of either an anti-miR scramble sequence control (AM17010) or specific inhibitors of miR-146a-5p (AM10722), miR-146a-3p (AM13059), miR-155 (AM12601) or miR-210 (AM10516) (Life Technologies, Waltham, MA, USA), using siPORT NeoFX transfection reagent (Invitrogen). Similarly, cells were transfected with a specific miR-146a-5p mimic (PM10722), miR-146a-3p mimic (PM13059) or the scramble sequence control at 200 nM (Life Technologies). Transfection efficiency in excess of 99% was determined using Cy-3 labeled anti-miR scramble sequence control and direct visualization under fluorescence microscopy (data not shown). At 72 h, supernatants were collected and measured for IL-8 by ELISA (Enzo Life Sciences). Trophoblast cell migration was measured using a two-chamber colormetric assay as previously described (Mulla et al., 2010).
Exosome isolation
Trophoblast-derived exosomes were isolated from 800 µl of cell-free supernatants using the ExoQuick-TC precipitation solution according to the manufacturer's instructions (System Biosciences, Palo Alto, CA, USA). Exosomes were also isolated from 50 µl of patient plasma using the Exoquick solution for biofluids. Isolated exosomes were immediately processed for RNA or protein isolation. Although Exoquick can also isolate microvesicles, the presence of isolated exosomes was confirmed by western blot for the marker CD63 (Kshirsagar et al., 2012) using the rabbit antibody sc-15363 from Santa Cruz Biotechnology (Sana Cruz, CA, USA).
RNA isolation and qRT-PCR
Trophoblast cellular RNA was extracted using TRIzol as described (Potter et al., 2015). Exosomal RNA was isolated using miRCURY RNA Cell and Plant isolation kit following the manufacturer's protocol (Exiqon, Woburn, MA, USA). Trophoblast cellular and exosomal expression of miR-9, miR-146a-5p, miR-146a-3p, miR-155, miR-210 and let-7c were measured using the Taqman MicroRNA Assay (Life Technologies) and normalized to the housekeeping gene, U6 for trophoblast cells and trophoblast-derived exosomes (Kumar et al., 2013; Ge et al., 2014; Rice et al., 2015). miR-16 was selected as the housekeeping gene for the patient exosome studies since it has been reported to be the most stable in this type of sample (Ge et al., 2014), and U6 had very low expression in these exosomes (data not shown). For miR-146a-3p, a pre-amplification step was included prior to performing PCR using the Taqman PreAmp master mix kit (Life Technologies). Data were analyzed using the Δ-Δ CT method and plotted as fold change in the miR expression normalized to the endogenous control. For plasma exosomal miR-146a-3p data were plotted as relative abundance after normalization to miR-16.
Statistical analysis
Experiments were performed at least three times and assayed in duplicate. Data were pooled and expressed as mean ± SEM. Statistical significance (P< 0.05) was determined by Student's t-test or analysis of variance followed by correction for multiple comparisons using Prism software (Graphpad Software, Inc.).
Results
aPL up-regulate trophoblast cellular expression of miR-146a-5p, miR-146a-3p, miR-155 and miR-210
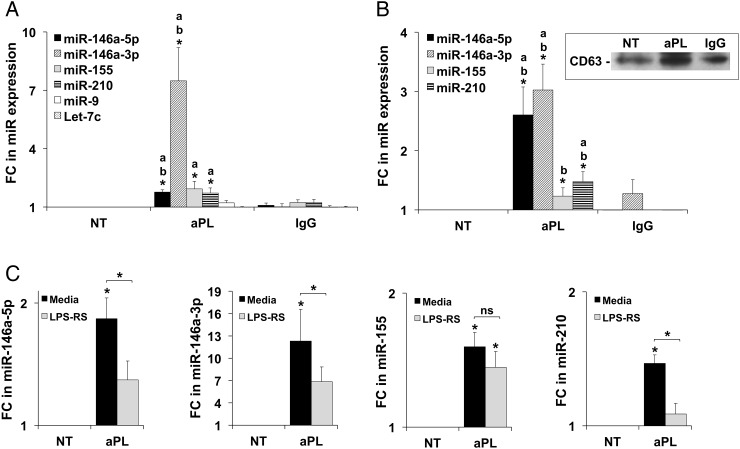
At 48 h, aPL induced a significant up-regulation of trophoblast cellular expression of miR-146a-5p, miR-146a-3p, miR-155 and miR-210 when compared with the no treatment (NT) control. The up-regulation of miR-146a-5p and miR-146a-3p was also significant when compared with the IgG control (Fig. 1A). No statistically significant changes in miR expression were observed at earlier time points (data not shown). aPL had no significant effect on miR-9 or Let-7c expression compared with controls (Fig. 1A). No significant alterations in expression levels of any of the tested miRs was observed in response to the mouse IgG1 isotype control compared with the NT, indicating that the responses were specific to aPL (Fig. 1A). Of note, after aPL treatment, cellular expression of miR-146a-3p was significantly higher than the other tested miRs (P< 0.05), suggesting that aPL preferentially increase cellular miR-146a-3p expression in the trophoblast. This was confirmed using primary human first trimester trophoblast cells; aPL increased cellular expression of miR-146a-3p by 2.4-fold compared with controls (Supplementary data, Fig. S1).
Figure 1.
Antiphospholipid antibodies (aPL) increase trophoblast cellular and exosomal microRNA (miR) expression in a Toll-like receptor 4 (TLR4)-dependent and independent manner. (A and B) The human first trimester trophoblast cell line, HTR8, was incubated with no treatment (NT), aPL or IgG control for 48 h. (A) cellular (n = 8–11) and (B) exosomal (n = 4–8) RNA was isolated and analyzed for miR expression by qPCR using U6 as an internal control. Data are expressed as fold change (FC) relative to the NT control. *P< 0.05; a versus NT and b versus IgG. Insert in Fig. 1B shows CD63 protein expression in isolated exosomes under the different treatment conditions. (C) HTR8 cells were treated with NT or aPL in the presence of media or lipopolysaccharide from Rhodobacter sphaeroides (LPS-RS) after which cellular expression of (i) miR-146a-5p (n = 7); (ii) miR-146a-3p (n = 7); (iii) miR-155 (n-13) and (iv) miR-210 (n = 9) was measured by qPCR. Data are expressed as fold change (FC) in miR expression relative to the controls. *P< 0.05 versus NT unless otherwise indicated; ns, not significant.
aPL up-regulate trophoblast exosome expression of miR-146a-5p, miR-146a-3p and miR-210
Isolation of trophoblast-derived exosomes was confirmed by CD63 expression (Fig. 1B, insert). Treatment of trophoblast with aPL significantly increased exosomal expression of miR-146a-5p, miR-146a-3p and miR-210 compared with the NT and IgG controls (Fig. 1B). While exosomal miR-155 was significantly up-regulated by aPL when compared with the IgG control, compared with the NT this was not significant (Fig. 1B). After aPL treatment, the expression levels of exosomal miR-146a-3p and miR-146a-5p were significantly higher than miR-155 and miR-210 (P< 0.05), suggesting that aPL preferentially increase miR-146a-5p and its isomiR, miR-146a-3p packaging into and release via trophoblast exosomes (Fig. 1B). Furthermore, based on their fold increase in response to aPL, miR-146a-5p appears enriched in exosomes while miR-146a-3p is enriched in the cellular compartment (Fig. 1A and B). Thus, miR-146a-5p may be preferentially expressed in trophoblast-derived exosomes after aPL treatment.
aPL-induced trophoblast expression of miR-146a-5p, miR-146a-3p and miR-210 is TLR4-dependent
The TLR4 antagonist, LPS-RS, significantly attenuated the aPL-induction of cellular miR-146a-5p by 24.9 ± 8.3%, miR-146a-3p by 42.8 ± 10.7% and miR-210 by 24.9 ± 5.9%, respectively (Fig. 1C). However, the aPL-induced cellular expression of miR-155 was not significantly altered by the presence of LPS-RS (Fig. 1C).
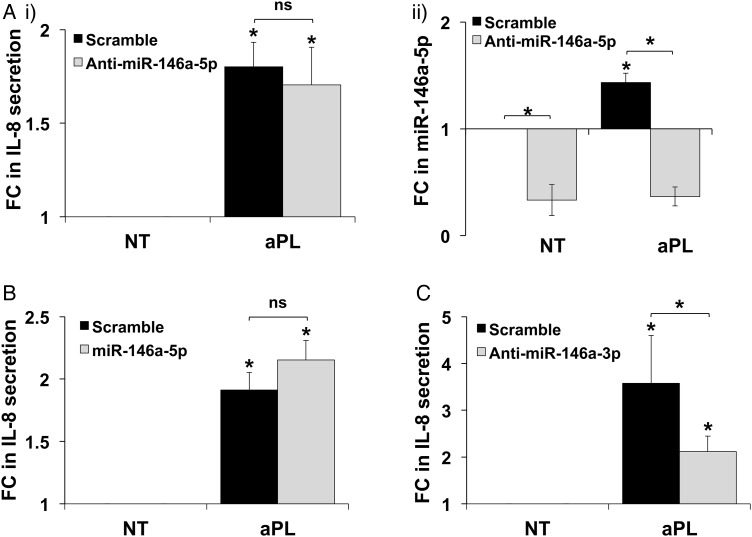
aPL-induced trophoblast IL-8 secretion is mediated by miR-146a-3p
Since miR-146a-5p inhibits IRAK1 and TRAF6, we postulated that inhibition of aPL-induced miR-146a-5p in the trophoblast would result in unrestrained TLR4 signaling and a further increase in end-function responses to aPL. Despite in silico identification of potential target mRNAs (Jazdzewski et al., 2009), no definitive information exists on the functional role of miR-146a-3p. A specific anti-miR-146a-5p inhibitor had no significant effect on aPL-induced trophoblast IL-8 secretion (Fig. 2A, i), in spite of basal and aPL-induced miR-146a-5p expression being significantly inhibited (Fig. 2A, ii). This suggested that in trophoblast cells exposed to aPL, miR-146a-5p is not acting classically to inhibit TLR4 signaling. Similarly, overexpression of a miR-146a-5p mimic had no significant effect on aPL-induced IL-8 secretion (Fig. 2B). In contrast, a specific anti-miR-146a-3p inhibitor significantly reduced aPL-induced trophoblast IL-8 secretion from 3.6 ± 1.0-fold to 2.1 ± 0.3-fold, when compared with the scramble control (Fig. 2C). This indicated that miR-146a-3p was mediating the aPL-induced IL-8 secretion by the trophoblast. Of note, there were no significant differences in basal IL-8 secretion in the presence of the anti-miR-146a5p, the miR-146a-5p mimic or the anti-miR-146a-3p when compared with the scramble control (data not shown).
Figure 2.
Inhibition of miR-146a-3p reduces antiphospholipid antibody (aPL)-induced trophoblast interleukin 8 (IL-8) secretion. Trophoblast HTR8 cells were transfected with a microRNA (miR) scramble control or either: (A) an anti-miR-146a-5p inhibitor (n = 10); (B) a miR-146a-5p mimic (n = 6) or (C) an anti-miR-146a-3p inhibitor (n = 4). Following transfection, cells were treated with no treatment (NT) or aPL. (A: i), (B) and (C) At 72 h supernatants were collected and measured for IL-8. Data are expressed as fold change (FC) in IL-8 secretion relative to the NT controls. (A: ii) At 48 h cellular RNA was isolated and analyzed for miR-146a-5p expression by qPCR using U6 as an internal control (n = 3). Data are expressed as fold change (FC) in miR-146a-5p expression relative to the NT scramble control. *P< 0.05 versus NT/scramble unless otherwise specified. *P< 0.05 versus the scramble control unless otherwise indicated; ns, not significant.
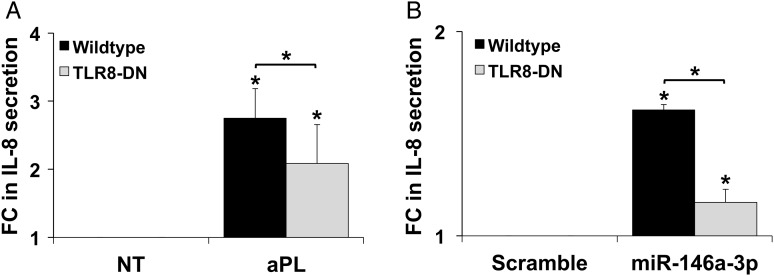
aPL-induced miR-146a-3p drives trophoblast IL-8 secretion through activation of TLR8
Given our observation that miR-146a-3p inhibition reduced trophoblast IL-8 secretion in response to aPL, we postulated that aPL trigger this inflammatory response through the up-regulation of miR-146a-3p and subsequent activation of the RNA sensor, TLR8, within the same or neighboring cells; the latter via exosomes. To test this, we compared the IL-8 secretory responses of wildtype and TLR8-DN expressing HTR8 trophoblast cells (Potter et al., 2015) to either aPL or exogenous miR-146a-3p. Treatment of wild-type cells with aPL significantly increased IL-8 secretion by 2.7 ± 0.4-fold when compared with the NT control, and this was significantly inhibited 29.3 ± 8.0% by the presence of the TLR8-DN (Fig. 3A). Similarly, in the absence of aPL, transfection of wild-type cells with a miR-146a-3p mimic resulted in a significant 1.6 ± 0.0-fold increase in IL-8 secretion compared with the miR scramble control (Fig. 3B), and this was significantly inhibited 27.8 ± 3.7% by the presence of the TLR8-DN (Fig. 3B). Thus, aPL induce trophoblast IL-8 secretion, at least in part, through miR-146a-3p-dependent activation of TLR8.
Figure 3.
Antiphospholipid antibodies (aPl) and miR-146a-3p induce trophoblast interleukin 8 (IL-8) secretion in a Toll-like receptor 8 (TLR8)-dependent manner. Wildtype and TLR8-dominant negative (TLR8-DN)-expressing HTR8 cells were: (A) treated with no treatment (NT) or aPL (n = 5); or (B) transfected with either a microRNA (miR) scramble control or a miR-146a-3p mimic (n = 4). At 72 h supernatants were collected and measured for IL-8. Data are expressed as fold change (FC) in IL-8 secretion relative to the (A) NT or (B) scramble controls *P< 0.05 versus (A) NT or (B) scramble control, unless otherwise indicated.
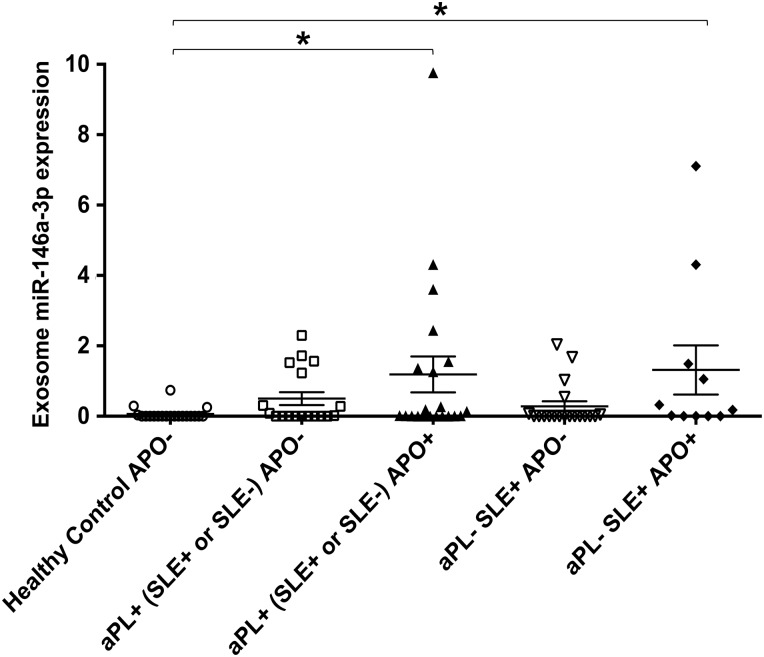
Women with aPL and APO have elevated circulating exosomal miR-146a-3p
Women with aPL and patients with SLE (a multisystem autoimmune disease) are at increased risk of APOs (Lockshin et al., 2012). Twenty-five percent of SLE patients have aPL, and the presence of both risk factors dramatically increases the frequency of APOs (Buyon et al., 2015). To validate our in vitro findings, we analyzed the plasma collected at 18–27 weeks gestation from pregnant women with aPL and without SLE for exosomal miR-146a-3p expression and compared the levels in those with and without an APO. To determine whether APOs in autoimmune patients without detectable aPL have aberrant expression of miR-146a-3p, we also analyzed plasma from SLE patients with and without APOs. Plasma exosome miR-146a-3p expression in patients who had aPL, either with or without SLE, and an APO [aPL+ (SLE+ or SLE−) APO+] was significantly higher when compared with plasma exosomes from healthy controls. Interestingly, plasma exosomes from patients without aPL but with SLE and an APO [aPL− SLE+ APO+] also expressed significantly higher miR-146a-3p compared with healthy controls (Fig. 4). There was no significant difference in plasma exosomal miR-146a-3p expression levels between the healthy controls and patients with aPL (either with or without SLE) and no APO (Fig. 4).
Figure 4.
miR-146a-3p is up-regulated in serum from patients with adverse pregnancy outcomes. Exosomes were isolated from plasma from the following patients: healthy adverse pregnancy outcome negative (APO−) (n = 20); antiphospholipid antibody positive (aPL) + (systemic lupus erythematous positive (SLE+) or SLE−) APO− (n = 18); aPL+ (SLE+ or SLE−) APO+ (n = 21); aPL− SLE+ APO− (n = 19); and aPL− SLE+ APO+ (n = 11). Total RNA was extracted and qPCR performed for miR-146a-3p using miR-16 as an internal control. Data are expressed as relative abundance of miR-146a-3p after normalization to miR-16. Chart shows miR-146a-3p expression for each patient plotted (*P< 0.05).
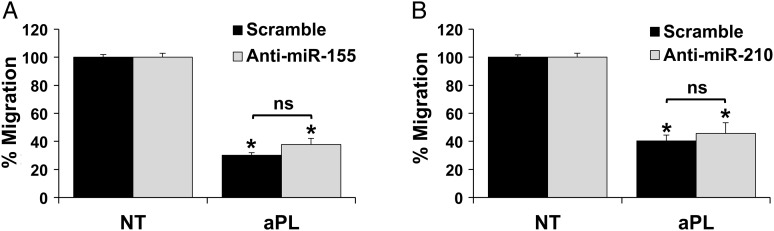
aPL-mediated inhibition of trophoblast migration is independent of miR-155 and miR-210
miR-155 and miR-210 were up-regulated in the trophoblast by aPL, independently and dependently of TLR4 (Fig. 1), and both have been linked to the inhibition of trophoblast migration and invasion (Dai et al., 2012; Zhang et al., 2012; Anton et al., 2013). Therefore, we sought to evaluate their role in the aPL-mediated inhibition of trophoblast migration, which is TLR4-independent (Mulla et al., 2010). The presence of specific inhibitors to miR-155 or miR-210 either alone (Fig. 5) or in combination (Supplementary data, Fig. S2) had no effect on aPL-mediated inhibition of migration when compared with the miR scramble control.
Figure 5.
Antiphospholipid antibody (aPL)-inhibition of trophoblast migration is independent of miR-155 and miR-210. Trophoblast HTR8 cells were transfected with a microRNA (miR) scramble control or either an (A) anti-miR-155 inhibitor (n = 3) or (B) anti-miR-210 inhibitor (n = 4). Cells were then placed in the upper chamber of a two-chamber migration assay and were treated with either no treatment (NT) or aPL. At 48 h, migration was measured. Data are expressed as % migration normalized to the NT control, which was then set to 100%. *P< 0.05 versus NT (ns, not significant).
Discussion
Women with APS are at increased risk of pregnancy complications (Valesini and Alessandri, 2005; Meroni et al., 2012). Clinical and experimental studies have established that these are caused by inflammatory processes at the maternal-fetal interface and altered trophoblast function (Sebire et al., 2002; Girardi et al., 2003; Van Horn et al., 2004; Berman et al., 2005; Mulla et al., 2009; Viall and Chamley, 2015). Anti-β2GPI antibodies bind human first trimester extravillous trophoblast (Chamley et al., 1997; Quenby et al., 2005; Mulla et al., 2009), and induce functional changes through TLR4-dependent and TLR4-independent mechanisms (Mulla et al., 2009, 2010, 2013; Carroll et al., 2011). Despite recent advances, little is known about the mechanisms involved, and current therapies remain unable to prevent obstetric APS.
Since aPL induce an inflammatory IL-8 and IL-1β response in human first trimester trophoblast cells through activation of TLR4 (Mulla et al., 2009, 2013), this study examined a small panel of miRs known to be induced by, and involved in the regulation of, TLR signaling. Trophoblast cellular expression of miR-146a-5p, miR-155 and miR-210 were all significantly up-regulated in response to aPL. miR-146a-3p, the less understood and thus understudied isomiR of miR-146a, was also up-regulated by aPL. The most significant up-regulation of these miRs occurred at 48 h. While this is late in comparison with miR responses in some other systems (Ceppi et al., 2009), trophoblast responses to aPL are typically slow, with end function changes being seen 48–72 h (Mulla et al., 2009, 2010, 2013; Carroll et al., 2011). Similarly, slow changes in miR expression have also been reported in trophoblast (Maccani et al., 2010; Dai et al., 2011) and other cells (Hou et al., 2009). aPL up-regulation of trophoblast miR-146a-5p and miR-146a-3p was TLR4-mediated, in keeping with current literature (Taganov et al., 2006; O'Neill et al., 2011). miR-210 up-regulation was also TLR4-mediated in keeping with previous reports (Qi et al., 2012). miR-146a-5p, miR-146a-3p and miR-210 were all increased in exosomes, and potentially contaminating microvesicles, released from the aPL-treated trophoblast.
Since miR-146a-5p targets IRAK1 and TRAF6, two adapter proteins critical to TLR signaling (Taganov et al., 2006), the role of miR-146a-5p and miR-146a-3p in the regulation of aPL-induced trophoblast IL-8 secretion was investigated (Mulla et al., 2009, 2010). Specific inhibition or overexpression of miR-146a-5p had no effect on aPL-induced trophoblast IL-8 secretion, indicating that miR-146a-5p was not functioning classically in the trophoblast, in the context of aPL, to restrain TLR4 activity.
An alternative explanation might be that aPL-induced TLR4 activation exceeds the ability of endogenous miR-146a-5p to negatively regulate the pathway, such that further, inhibition of miR-146a-5p activity is not detectable. In striking contrast, the reduction in aPL-induced IL-8 secretion by miR-146a-3p inhibition provides strong evidence that this isomiR is functionally active in the trophoblast as part of a pro-inflammatory mechanism, clearly distinct from the canonical role of miR-146a-5p in the negative feedback of TLR4 signaling (Taganov et al., 2006; O'Neill et al., 2011).
MicroRNA expression and function can be highly tissue-specific (Krol et al., 2010); miRs and their respective isomiRs can have opposing effects on TLR-induced inflammation (Zhou et al., 2010). Some miRs induce inflammation through binding to, and activating, TLRs themselves. Endosomal TLR8 binds miR-21 and miR-29a, carried in exosomes, resulting in receptor activation and inflammation (Fabbri et al., 2012). Given that miR-146a-3p was up-regulated in trophoblast-derived exosomes as well as the cellular compartment in response to aPL, we postulated that miR-146a-3p may be a TLR8-activating miR capable of carrying out its function both endogenously, and through exosome delivery, locally and/or systemically. Using wildtype and TLR8-DN expressing trophoblast cells, we demonstrated that aPL-mediated trophoblast IL-8 secretion is dependent on TLR8. Moreover, miR-146a-3p overexpression, in the absence of aPL, also induced trophoblast IL-8 secretion and this too was mediated by TLR8. Thus, aPL induce human trophoblast IL-8 secretion by up-regulating miR-146a-3p through TLR4, which subsequently binds and activates TLR8. This is in keeping with a study reporting aPL-induced inflammation in monocytes is TLR8-dependent (Doring et al., 2010). Furthermore, Prinz et al. (2011) demonstrated that aPL-induced monocyte cytokine production is dependent on endogenous RNAs. Since aPL and miR-146a-3p induced IL-8 were only partially inhibited by the TLR8-DN, it is possible that aPL may induce other TLR-activating miRs, and TLR8 may not be the only mediator of aPL-induced IL-8 production.
Having demonstrated a functional role for miR-146a-3p in driving trophoblast inflammation and its elevated expression in trophoblast-derived exosomes, we sought to validate our in vitro findings in vivo. aPL elevate trophoblast shedding of microparticles (Chen et al., 2009; Viall et al., 2013), and elevated circulating placental microparticles are associated with pre-eclampsia (Mincheva-Nilsson and Baranov, 2010). Therefore, plasma-derived exosomal miR-146a-3p levels in aPL− and aPL+ patients enrolled in the PROMISSE study were measured (Salmon et al., 2011; Lockshin et al., 2012; Mulla et al., 2013; Andrade et al., 2015; Buyon et al., 2015). Patients with aPL, either with or without SLE, who presented with an APO had significantly higher circulating exosomal miR-146a-3p levels than healthy controls with normal pregnancies; an elevation not observed in aPL+ patients (with or without SLE) with no APO. Interestingly patients without aPL who had SLE and an APO also expressed significantly higher circulating exosomal miR-146a-3p compared with healthy controls, while this was not the case for aPL−/SLE+ patients with normal pregnancies. This suggests that both APS and SLE are associated with this miR response during pregnancy, or vice versa. Prior to this study, little was known about miR expression and function in either APS or SLE, and nothing was known about miRs in pregnant women with these diseases. One study reported that monocytes from patients with either APS or SLE express lower levels of miR-19b and miR-20a than controls (Teruel et al., 2011); however, there are no reports on miR-146a in APS patients. In SLE patients, plasma and PBMC expression of miR-146a-5p has been reported to be lower than in healthy controls; however, miR146a-3p expression was not established (Tang et al., 2009; Wang et al., 2010). In the context of pregnancy, one small study reported that circulating levels of miR-146a were unchanged in women with pre-eclampsia (Campos et al., 2014), whereas maternal cigarette smoking during pregnancy is associated with reduced placental miR-146a expression (Maccani et al., 2010). However, again, miR-146a-3p was not studied. Thus, our findings constitute the first report of elevated miR-146a-3p in adverse outcomes in pregnancies complicated by APS and SLE. Although we cannot determine whether elevated miR-146a-3p in aPL+ patients with pregnancy complications is a cause or consequence of placental dysfunction from our data, this observation strongly supports our in vitro studies and the notion that the source as well as the target of excess miR-146a-3p in aPL-associated adverse pregnancies may be the placenta. That aPL−/SLE+ pregnancies also displayed elevated miR-146a-3p suggests in these patients there is also an induction of placental dysfunction (Andrade et al., 2015), potentially via this isomiR.
Trophoblast migration and invasion are impaired in pregnancies complicated by APS (Sebire et al., 2002; Viall and Chamley, 2015). miR-155 and miR-210 are dysregulated in placentae from pathologic pregnancies (Zhang et al., 2010; Mayor-Lynn et al., 2011), and negatively regulate trophoblast migration and invasion (Dai et al., 2012; Zhang et al., 2012; Anton et al., 2013; Li et al., 2014). Since aPL inhibit human first trimester trophoblast migration independently of TLR4 (Mulla et al., 2010), and therefore, independently of IL-8, in contrast to other reports (Jovanovic et al., 2010); and we found miR-155 and miR-210 were up-regulated in the trophoblast by aPL, we questioned their role in regulating cell migration. However, there was no rescue of migration by inhibition of either miR-155 or miR-210 either alone or in combination, indicating that aPL impair trophoblast migration independently of these miRs, through alternate mechanisms (Mulla et al., 2010; Albert et al., 2014; Ulrich et al., 2016), although it should be noted that efficiency of miR-155 and miR-210 knockdown was not assessed.
In summary, this study demonstrates a novel mechanism of aPL-induced trophoblast inflammation, and further supports the concept that certain miRs can induce positive functional changes through endogenous activation of RNA-sensing receptors, such as TLR8. We demonstrate for the first time a functional role of the isomiR miR-146a-3p, reinforcing the need to address both mature forms of a miR, regardless of abundance, when studying miRs in both physiological and pathophysiological contexts. The expression of miR-146a-3p in trophoblast-derived exosomes supports the idea of miRs acting in a paracrine or endocrine manner through exosome delivery. However, miR-146a-3p may also exert its pro-inflammatory effect intracellularly within the same trophoblast cell targeted by aPL. Finally, our findings have exciting translational potential as circulating exosomal-associated miR-146a-3p in APS patients may serve clinically as a biomarker for related APOs.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/ online.
Authors' roles
S.M.G. and V.M.A. designed the research and drafted the paper; S.M.G. and M.J.M. performed the experiments; S.M.G. and V.M.A. analyzed the results and made the figures; J.E.S., M.G. and L.W.C. provided antibodies and patient samples and information; S.M.G., V.M.A., J.J.B., J.E.S. and L.W.C. revised the paper for important intellectual content.
Funding
This study was supported in part by grants from the American Heart Association (#10GRNT3640032 to V.M.A.), the March of Dimes Foundation (Gene Discovery and Translational Research Grant #6-FY12-255 to V.M.A.), NICHD, NIH (R01HD049446 to V.M.A.), the Gina M. Finzi Memorial Student Summer Fellowship from the Lupus Foundation of America (to S.M.G.), and the Yale University School of Medicine Medical Student Fellowship (to S.M.G.).
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
The authors thank Dr Nancy Stanwood and Dr Aileen Gariepy for their help with collecting patient tissues.
References
- Albert CR, Schlesinger WJ, Viall CA, Mulla MJ, Brosens JJ, Chamley LW, Abrahams VM. Effect of hydroxychloroquine on antiphospholipid antibody-induced changes in first trimester trophoblast function. Am J Reprod Immunol 2014;71:154–164. [DOI] [PubMed] [Google Scholar]
- Andrade D, Kim M, Blanco LP, Karumanchi SA, Koo GC, Redecha P, Kirou K, Alvarez AM, Mulla MJ, Crow MK et al. Interferon-alpha and angiogenic dysregulation in pregnant lupus patients who develop preeclampsia. Arthritis Rheumatol 2015;67:977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton L, Olarerin-George AO, Schwartz N, Srinivas S, Bastek J, Hogenesch JB, Elovitz MA. miR-210 inhibits trophoblast invasion and is a serum biomarker for preeclampsia. Am J Pathol 2013;183:1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA, Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci USA 2009;106:5282–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J, Girardi G, Salmon JE. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J Immunol 2005;174:485–490. [DOI] [PubMed] [Google Scholar]
- Buyon JP, Kim MY, Guerra MM, Laskin CA, Petri M, Lockshin MD, Sammaritano L, Branch DW, Porter TF, Sawitzke A et al. Predictors of pregnancy outcomes in patients with Lupus: a Cohort Study. Ann Intern Med 2015;163:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CB, Marques TM, Pereira RW, Sandrim VC. Reduced circulating miR-196b levels is associated with preeclampsia. Pregnancy Hypertens 2014;4:11–13. [DOI] [PubMed] [Google Scholar]
- Carroll TY, Mulla MJ, Han CS, Brosens JJ, Chamley LW, Giles I, Pericleous C, Rahman A, Sfakianaki AK, Paidas MJ et al. Modulation of trophoblast angiogenic factor secretion by antiphospholipid antibodies is not reversed by heparin. Am J Reprod Immunol 2011;66:286–296. [DOI] [PubMed] [Google Scholar]
- Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, Pierre P. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci USA 2009;106:2735–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley LW, Allen JL, Johnson PM. Synthesis of beta2 glycoprotein 1 by the human placenta. Placenta 1997;18:403–410. [DOI] [PubMed] [Google Scholar]
- Chamley LW, Konarkowska B, Duncalf AM, Mitchell MD, Johnson PM. Is interleukin-3 important in antiphospholipid antibody-mediated pregnancy failure? Fertil Steril 2001;76:700–706. [DOI] [PubMed] [Google Scholar]
- Chen Q, Viall C, Kang Y, Liu B, Stone P, Chamley L. Anti-phospholipid antibodies increase non-apoptotic trophoblast shedding: a contribution to the pathogenesis of pre-eclampsia in affected women? Placenta 2009;30:767–773. [DOI] [PubMed] [Google Scholar]
- Cloonan N, Wani S, Xu QY, Gu J, Lea K, Heater S, Barbacioru C, Steptoe AL, Martin HC, Nourbakhsh E et al. MicroRNAs and their isomiRs function cooperatively to target common biological pathways. Genome Biol 2011;12:R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai YM, Diao ZY, Sun HX, Li RT, Qiu ZH, Hu YL. MicroRNA-155 is involved in the remodelling of human-trophoblast-derived HTR-8/SVneo cells induced by lipopolysaccharides. Hum Reprod 2011;26:1882–1891. [DOI] [PubMed] [Google Scholar]
- Dai Y, Qiu Z, Diao Z, Shen L, Xue P, Sun H, Hu Y. MicroRNA-155 inhibits proliferation and migration of human extravillous trophoblast derived HTR-8/SVneo cells via down-regulating cyclin D1. Placenta 2012;33:824–829. [DOI] [PubMed] [Google Scholar]
- de Jesús GR, Rodrigues G, de Jesús NR, Levy RA. Pregnancy morbidity in antiphospholipid syndrome: what is the impact of treatment? Curr Rheumatol Rep 2014;16:1–9. [DOI] [PubMed] [Google Scholar]
- Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y, Wang T, Stolz DB, Sarkar SN, Morelli AE et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci USA 2013;110:12048–12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donker RB, Mouillet JF, Nelson DM, Sadovsky Y. The expression of Argonaute2 and related microRNA biogenesis proteins in normal and hypoxic trophoblasts. Mol Hum Reprod 2007;13:273–279. [DOI] [PubMed] [Google Scholar]
- Doring Y, Hurst J, Lorenz M, Prinz N, Clemens N, Drechsler MD, Bauer S, Chapman J, Shoenfeld Y, Blank M et al. Human antiphospholipid antibodies induce TNFalpha in monocytes via Toll-like receptor 8. Immunobiology 2010;215:230–241. [DOI] [PubMed] [Google Scholar]
- Eiring AM, Harb JG, Neviani P, Garton C, Oaks JJ, Spizzo R, Liu SJ, Schwind S, Santhanam R, Hickey CJ et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell 2010;140:652–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA 2012;109:E2110–E2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M, Potter JA, Abrahams VM. Identification of microRNAs that regulate TLR2-mediated trophoblast apoptosis and inhibition of IL-6 mRNA. PLoS One 2013;8:e77249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q, Zhou Y, Lu J, Bai Y, Xie X, Lu Z. miRNA in plasma exosome is stable under different storage conditions. Molecules 2014;19:1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest 2003;112:1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 1993;206:204–211. [DOI] [PubMed] [Google Scholar]
- Hou J, Wang P, Lin L, Liu X, Ma F, An H, Wang Z, Cao X. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol 2009;183:2150–2158. [DOI] [PubMed] [Google Scholar]
- Jazdzewski K, Liyanarachchi S, Swierniak M, Pachucki J, Ringel MD, Jarzab B, de la Chapelle A. Polymorphic mature microRNAs from passenger strand of pre-miR-146a contribute to thyroid cancer. Proc Natl Acad Sci USA 2009;106:1502–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic M, Stefanoska I, Radojcic L, Vicovac L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1. Reproduction 2010;139:789–798. [DOI] [PubMed] [Google Scholar]
- Kopriva SE, Chiasson VL, Mitchell BM, Chatterjee P. TLR3-induced placental miR-210 down-regulates the STAT6/interleukin-4 pathway. PLoS One 2013;8:e67760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010;11:597–610. [DOI] [PubMed] [Google Scholar]
- Kshirsagar SK, Alam SM, Jasti S, Hodes H, Nauser T, Gilliam M, Billstrand C, Hunt JS, Petroff MG. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 2012;33:982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Luo Y, Tudela C, Alexander JM, Mendelson CR. The c-Myc-regulated microRNA-17∼92 (miR-17∼92) and miR-106a∼363 clusters target hCYP19A1 and hGCM1 to inhibit human trophoblast differentiation. Mol Cell Biol 2013;33:1782–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li C, Dong X, Gou W. MicroRNA-155 inhibits migration of trophoblast cells and contributes to the pathogenesis of severe preeclampsia by regulating endothelial nitric oxide synthase. Mol Med Rep 2014;10:550–554. [DOI] [PubMed] [Google Scholar]
- Lockshin MD, Kim M, Laskin CA, Guerra M, Branch DW, Merrill J, Petri M, Porter TF, Sammaritano L, Stephenson MD et al. Prediction of adverse pregnancy outcome by the presence of lupus anticoagulant, but not anticardiolipin antibody, in patients with antiphospholipid antibodies. Arthritis Rheum 2012;64:2311–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani MA, Avissar-Whiting M, Banister CE, McGonnigal B, Padbury JF, Marsit CJ. Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics 2010;5:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor-Lynn K, Toloubeydokhti T, Cruz AC, Chegini N. Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. Reprod Sci 2011;18:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni PL, Raschi E, Grossi C, Pregnolato F, Trespidi L, Acaia B, Borghi MO. Obstetric and vascular APS: same autoantibodies but different diseases? Lupus 2012;21:708–710. [DOI] [PubMed] [Google Scholar]
- Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol 2010;63:520–533. [DOI] [PubMed] [Google Scholar]
- Mulla MJ, Brosens JJ, Chamley LW, Giles I, Pericleous C, Rahman A, Joyce SK, Panda B, Paidas MJ, Abrahams VM. Antiphospholipid antibodies induce a pro-inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am J Reprod Immunol 2009;62:96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulla MJ, Myrtolli K, Brosens JJ, Chamley LW, Kwak-Kim JY, Paidas MJ, Abrahams VM. Antiphospholipid antibodies limit trophoblast migration by reducing IL-6 production and STAT3 activity. Am J Reprod Immunol 2010;63:339–348. [DOI] [PubMed] [Google Scholar]
- Mulla MJ, Salmon JE, Chamley LW, Brosens JJ, Boeras CM, Kavathas PB, Abrahams VM. A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody-induced IL-1beta production by human first trimester trophoblast. PLoS One 2013;8:e65237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol 2011;11:163–175. [DOI] [PubMed] [Google Scholar]
- Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, Draghici S, Espinoza J, Kusanovic JP, Mittal P et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol 2007;196:261.e1–261.e6. [DOI] [PubMed] [Google Scholar]
- Potter JA, Garg M, Girard S, Abrahams VM. Viral single stranded RNA induces a trophoblast pro-inflammatory and antiviral response in a TLR8-dependent and -independent manner. Biol Reprod 2015;92:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz N, Clemens N, Strand D, Putz I, Lorenz M, Daiber A, Stein P, Degreif A, Radsak M, Schild H et al. Antiphospholipid antibodies induce translocation of TLR7 and TLR8 to the endosome in human monocytes and plasmacytoid dendritic cells. Blood 2011;118:2322–2332. [DOI] [PubMed] [Google Scholar]
- Qi J, Qiao Y, Wang P, Li S, Zhao W, Gao C. microRNA-210 negatively regulates LPS-induced production of proinflammatory cytokines by targeting NF-kappaB1 in murine macrophages. FEBS Lett 2012;586:1201–1207. [DOI] [PubMed] [Google Scholar]
- Quenby S, Mountfield S, Cartwright JE, Whitley GS, Chamley L, Vince G. Antiphospholipid antibodies prevent extravillous trophoblast differentiation. Fertil Steril 2005;83:691–698. [DOI] [PubMed] [Google Scholar]
- Rice J, Roberts H, Rai SN, Galandiuk S. Housekeeping genes for studies of plasma microRNA: A need for more precise standardization. Surgery 2015;158:1345–1351. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Roberts CT, van Beijering E, Pensa K, Sheng Y, Shi T, Krilis SA. Effect of beta2-glycoprotein I null mutation on reproductive outcome and antiphospholipid antibody-mediated pregnancy pathology in mice. Mol Hum Reprod 2004;10:409–416. [DOI] [PubMed] [Google Scholar]
- Salmon JE, Heuser C, Triebwasser M, Liszewski MK, Kavanagh D, Roumenina L, Branch DW, Goodship T, Fremeaux-Bacchi V, Atkinson JP. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med 2011;8:e1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebire NJ, Fox H, Backos M, Rai R, Paterson C, Regan L. Defective endovascular trophoblast invasion in primary antiphospholipid antibody syndrome-associated early pregnancy failure. Hum Reprod 2002;17:1067–1071. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 2006;103:12481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y, Huang X, Zhou H, de Vries N, Tak PP et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum 2009;60:1065–1075. [DOI] [PubMed] [Google Scholar]
- Tang B, Xiao B, Liu Z, Li N, Zhu ED, Li BS, Xie QH, Zhuang Y, Zou QM, Mao XH. Identification of MyD88 as a novel target of miR-155, involved in negative regulation of Helicobacter pylori-induced inflammation. FEBS Lett 2010;584:1481–1486. [DOI] [PubMed] [Google Scholar]
- Teruel R, Perez-Sanchez C, Corral J, Herranz MT, Perez-Andreu V, Saiz E, Garcia-Barbera N, Martinez-Martinez I, Roldan V, Vicente V et al. Identification of miRNAs as potential modulators of tissue factor expression in patients with systemic lupus erythematosus and antiphospholipid syndrome. J Thromb Haemost 2011;9:1985–1992. [DOI] [PubMed] [Google Scholar]
- Ulrich V, Gelber SE, Vukelic M, Sacharidou A, Herz J, Urbanus RT, de Groot PG, Natale DR, Harihara A, Redecha P et al. ApoE receptor 2 mediation of trophoblast dysfunction and pregnancy complications induced by antiphospholipid antibodies in mice. Arthritis Rheumatol 2016;68:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valesini G, Alessandri C. New facet of antiphospholipid antibodies. Ann N Y Acad Sci 2005;1051:487–497. [DOI] [PubMed] [Google Scholar]
- Van Horn JT, Craven C, Ward K, Branch DW, Silver RM. Histologic features of placentas and abortion specimens from women with antiphospholipid and antiphospholipid-like syndromes. Placenta 2004;25:642–648. [DOI] [PubMed] [Google Scholar]
- Viall CA, Chamley LW. Histopathology in the placentae of women with antiphospholipid antibodies: a systematic review of the literature. Autoimmun Rev 2015;14:446–471. [DOI] [PubMed] [Google Scholar]
- Viall CA, Chen Q, Liu B, Hickey A, Snowise S, Salmon JE, Stone PR, Chamley LW. Antiphospholipid antibodies internalised by human syncytiotrophoblast cause aberrant cell death and the release of necrotic trophoblast debris. J Autoimmun 2013;47:45–57. [DOI] [PubMed] [Google Scholar]
- Wang G, Tam LS, Li EK, Kwan BC, Chow KM, Luk CC, Li PK, Szeto CC. Serum and urinary cell-free MiR-146a and MiR-155 in patients with systemic lupus erythematosus. J Rheumatol 2010;37:2516–2522. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Diao Z, Su L, Sun H, Li R, Cui H, Hu Y. MicroRNA-155 contributes to preeclampsia by down-regulating CYR61. Am J Obstet Gynecol 2010;202:466.e1–466.e7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Fei M, Xue G, Zhou Q, Jia Y, Li L, Xin H, Sun S. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: new insights into molecular mechanisms for the disease. J Cell Mol Med 2012;16:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Huang X, Cui H, Luo X, Tang Y, Chen S, Wu L, Shen N. miR-155 and its star-form partner miR-155* cooperatively regulate type I interferon production by human plasmacytoid dendritic cells. Blood 2010;116:5885–5894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.