Abstract
This study was conducted to examine the effects of saffron extract on preventing D-galactose and NaNO2 induced memory impairment and improving learning and memory deficits in amnestic mice. In this study, the learning and memory functions in ovariectomized mice were examined by the one way passive and active avoidance tests. In active avoidance test, training in amnestic treated (AT) and amnestic prophylaxis (AP) groups, was improved so that there was a significant difference between them and the amnestic control (AC) group. In passive avoidance test, animal's step through latency, as an index for learning, in all test groups was significantly greater than control group. Total time spent in dark room (DS), which opposes the memory retention ability, in AC was significantly greater than AT group at 1 and 2 hours after full training, while there was not any significant difference between this index in AP and AT as compared with normal control (NC) group. Our findings indicate that saffron hydro-alcoholic extract prevents and improves amnesia induced by D-galactose and NaNO2 in mice.
Keywords: learning, Alzheimer's disease, treatment, cognition, prophylaxis, herbal medicine
Introduction
Alzheimer's disease (AD) and senile dementia are the most common age-related neurodegenerative disorders which are pathologically characterized by a progressive accumulation of senile plaques and neurofibrillary tangles in cerebral tissues such as hippocampus. Their incidence increases steadily with age, and is 40-50 % in those who are over 95 years old (Ganong, 2012[10]). Oxidative stress is a key factor in progression of chronic neural degeneration in AD (Gilca et al., 2007[11]; Prigione et al., 2010[26]; Ansari and Scheff, 2010[5]). It is well established that a large number of dietaries should be regarded as an effective defense against the degenerative effects of oxidative agents (Chatterjee et al., 1995[7]; Morse and Stoner, 1993[21]; Rogers et al., 1993[27]). The dried stigmas of Crocus sativus L. (Saffron) which is a valuable spice, commonly used for flavouring and coloring food stuffs, has been prescribed for various ailments in traditional medicine (Abdullaev and Espinosa-Aguirre, 2004[2]; Srivastava et al., 2010[30]). It has been demonstrated that crude saffron stigma extract is a potent protective agent against genotoxicity and has antitumoural effects (Abdullaev and Espinosa-Aguirre, 2004[2]; Srivastava et al., 2010[30]; Abdullaev, 2002[1]; Premkumar et al., 2003[25]). The antioxidative effect of saffron is considered to be a major cause of its anticonvulsant, antidepressant and antiinflammatory properties (Hosseinzadeh and Khosravan, 2002[14]; Hosseinzadeh and Younesi, 2002[16]; Karimi et al., 2001[19]). Behavioral and electrophysiological studies have shown that saffron extract improves cognitive disorders induced by ethanol consumption and enhance the intact spatial memory and scopolamine-induced learning impairment in rats performing the Morris water maze task (Abe and Saito, 2000[3]; Sugiura, et al., 1995[33]; Hosseinzadeh and Ziaei, 2006[17]). Major well known saffron constituents considered to have pharmacological activities are safranal (a volatile agent), picrocrocin (a bitter taste compound) and crocetin and its glycoside metabolite “crocin” (dye materials) (Hosseinzadeh and Noraei, 2009[15]). Of these components crocetin and its metabolites are the most potent protective agents due to their antioxidative effects (Bouvier et al., 2003[6]). Due to the presence of protective ingredients in saffron extract, we conducted to evaluate the prophylactic and therapeutic effects of saffron crude hydro alcoholic extract on Alzheimer's disease in animal model.
Materials and Methods
Surgical procedure and amnesia induction
This study was run according to the Shahid Sadoughi Medical University Animal Ethic Committee guide for animal care which is in accordance with the internationally accepted principles for laboratory animal use and care mentioned by the European Community guidelines. 12 weeks old Balb/c mice weighting 25-30 gs were anesthetized by intraperitoneal (i.p.) injection of 45 mg/kg Thiopental sodium (Rotexmedica, trittau, Germany, Batch no. S 10318) and surgically undergone a bilateral ovariectomy. After 1 week full recovery, 40 ovariectomized mice were divided randomly and equally into 4 groups (normal control/NC, amnestic treated/AC, amnestic prophylaxis/AP and amnestic treated/AT) for each test. Amnesia was induced by intraperitoneal injection of 120 mg/kg D-galactose (Sinopharm Chemical Reagent Co. China) and 90 mg/kg NaNO2 (Merck, Germany) for 60 consecutive days (Hua et al., 2007[18]; Zhang et al., 2006[35]).
Treatments
Animals allocated for AP survey received 30 mg/kg/day saffron extract (i.p.) along with the amnesia induction protocol. AT group received the same amount of saffron extract for (i.p.) 15 consecutive days after amnesia induction. Animals in NC and AC groups received equivalent volume (10 ml/kg, i.p.) of distilled water.
Extraction
To yield the hydro alcoholic extract, 12 g grounded saffron (kindly supplied by Novin Saffron co, Mashhad, Iran) were mixed with 180 ml 80 % ethanol in a percolator and the extraction followed after 48 h, then the extract was dried using a rotary evaporator device (Samsam-Shariat, 2002[28]).
Apparatus
The learning and memory functions in mice were examined by the "one way active & passive avoidance learning and memory" tests in a Shuttle box apparatus (Galindo et al., 2008[9]; Pitsikas and Sakellaridis, 2006[23]). The apparatus is a rectangular box with two distinct compartments measuring 22 x 21 x 22 cm and separated by a sliding door. The walls and lid of safe compartment was made of white-colored acrylic plates while the shock compartment with the dark ones. Two stainless steel plates (22*20 cm) were obliquely embedded in the side walls of each compartment, apart from each other by 20 cm at the top and 1 cm at the bottom. A 10-W red light bulb and a buzzer (70 dB, 760 Hz) were located in the top of front wall of the safe room. The stainless steel plates in the dark compartment were connected to a square-pulse stimulator (Grass model no.S-48) in series with a constant current unit (Grass model no. CCU-1A) by which a reproducible, aversive electric foot shock of 1 mA and 1 second duration could be delivered when the mice are in contact with both plates.
Experimental procedures
Active avoidance test: One-way active avoidance test was performed basically as described by Galindo et al. (2008[9]). In training or learning session, each animal was placed in the dark room with its head towards the wall opposite to the sliding door and the lid was closed. Immediately the door between the compartments was opened and two initial conditioned stimuli (CS) sound and light flash were applied simultaneously. If the animal crossed to the white room during 10 s, a "shock free" trial was scored (avoidance). If the animal failed to cross to the white room within 10 s, an electric foot shock ( 0.4 mA), as an unconditioned stimulus (US), was continually applied until leaving the dark room and a "shock" trial was assigned (escape). Following the entrance of animal into the safe room, the sliding door was closed and after 30 s rest in this room the next trial was started. Maximum duration for electric shock delivery was 10 s, so if the animal avoids leaving the shock compartment during this period, stimulation was stopped and the animal was manually conducted to the white room to follow a new trial. In this experiment 90 trials per animal were performed in 3 successive days (3 sessions and 10 trials in each session per day) and the mean number of "shock free" trials was considered as an index for learning. In 4th day, all animals were trained to acquire at least 70 % of full learning (i.e. for each 10 trials at list in 7 trials the animal would leave the dark side without receiving any foot shock). From this point, evaluation of memory retention followed for 3 successive days, by the same procedure used in the training session without any foot shock, after 1 and 3 weeks. In these sessions also the mean number of "avoidance" trials was considered as the index of memory consolidation.
Passive avoidance test: One-way passive avoidance test was essentially carried out according to Saxena et al. (2008[29]). The test was initiated by acclimatization to the shuttle box by placing each animal to the light room with its head toward the wall opposite to the interconnected door. When the animal turned toward the dark room the door was opened in coincidence with sound and light flash display. Upon complete entrance to the dark room, the door was closed and after 30 s the animal was returned to its home cage. Adaptation trial was repeated 3 times with 30 minutes inter trial interval. 30 minutes after the last adaptation trial, the training trial was run as the same as previous except that a mild foot shock (0.5 mA; for 10 s) was delivered immediately after the animal arrived to the dark room with closed door. After applying the aversive stimulus the door was opened in order the harassed animal could escape the electrified compartment and brought to its home cage. Two minutes after the training trial learning test was carried out by introducing each animal to light room and the initial time spend to completely enter the dark room (step through latency/STL) within 2 min was recorded. Following this session, another training session was repeated while the animals remained in the apparatus and received a foot-shock upon re-entrance to the dark room. Full training trial was terminated when the mouse achieved full acquisition (i.e. when the mouse stayed in the light room for 2 consecutive minutes). The retention assay was performed 1 h, 2 h, 24 h, 1 week and 1 month after the full training session similar to the learning session. In these sessions, the initial time spend by each animal to completely enter the dark room (Retention Time/RT), number of relocating (RLN) and total time spent in dark (DS) and light (LS) rooms within 5 min were recorded. All behavioral tests were performed by blinded experimenters about the animal treatments.
Statistical analysis
In this study shock free/avoidance responses in active avoidance test were expressed as mean ± standard error of mean. These data were analyzed by two way analysis of variance (2 way ANOVA) followed by Bonferroni post test, repeated measures analysis of variance (ANOVA) followed by Tukey's multiple comparisons tests and Kruskal-Wallis followed by Dunn's multiple comparison test. In passive avoidance test, all data are presented as mean ± SEM and were statistically analyzed by Kruskal-Wallis followed by Dunn's multiple comparison test. All statistical tests were two-tailed and P < 0.05 was considered as the statistically significant level.
Results
Active avoidance test
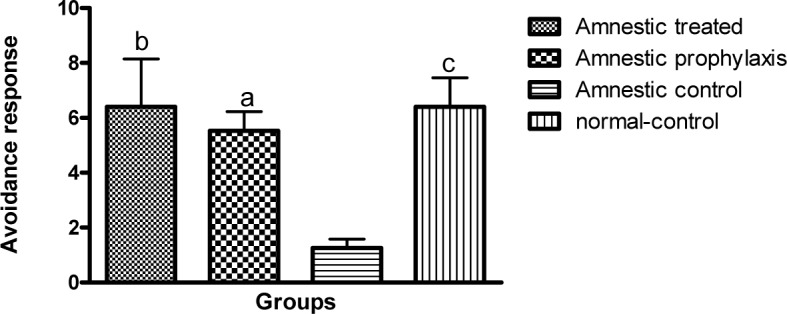
In active avoidance test, the avoidance index in all groups was progressively increased during 3 successive days of training session, except for the AC group which exhibited a decrease in avoidance response during this session (Table 1(Tab. 1)). Globally in training session, the avoidance response in AC group significantly diminished as compared to the NC group (1.933 ± 0.542 vs. 13.93 ± 1.887, p ≤ 0.001, Figure 1(Fig. 1)), while in AT and AP groups, training was improved so that there was a significant difference between them and the AC group (17.47 ± 1.693 and 8.333 ± 1.485 vs. 1.933 ± 0.542, p ≤ 0.001 and p ≤ 0.05 respectively, Figure 1(Fig. 1)). Although the training avoidance response in AT and AP groups was the same as the NC group, mean avoidance in AP group was significantly lower than the AT group (8.333 ± 1.485 vs. 17.47 ± 1.693, p ≤ 0.01, Figure 1(Fig. 1)). There was no difference in avoidance responses between AT, AP and NC groups as compared to each other, 1 and 3 weeks after the training session, but the memory retention in AC groups was significantly less than other groups (p ≤ 0.05, Figures 2(Fig. 2) and 3(Fig. 3)).
Table 1. The avoidance index (Mean ± SEM) in different groups during 3 successive days of active avoidance training session (n=10).
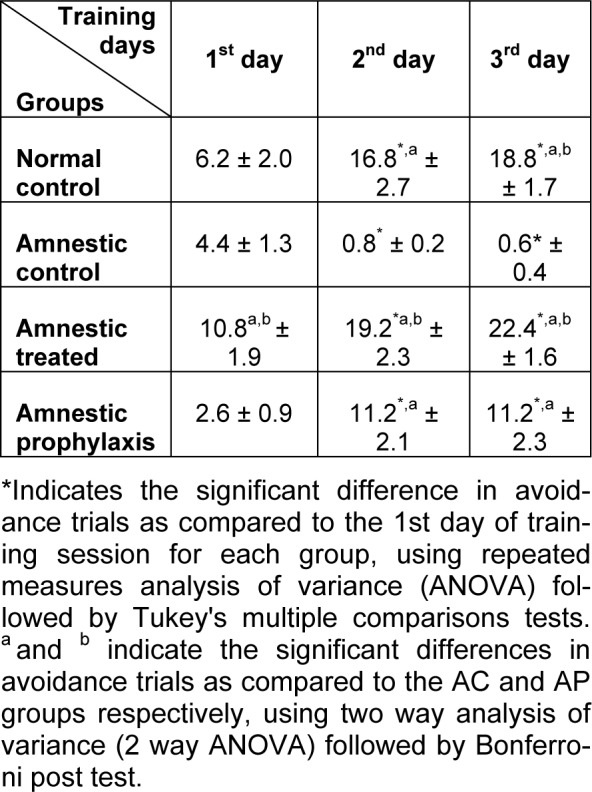
Figure 1. Figure 1: Avoidance responses of different groups in active avoidance training session (n=10). a and b indicate the significant differences in avoidance trials as compared to the AC and AP groups respectively, using repeated measures analysis of variance (ANOVA) followed by Tukey's multiple comparisons tests.
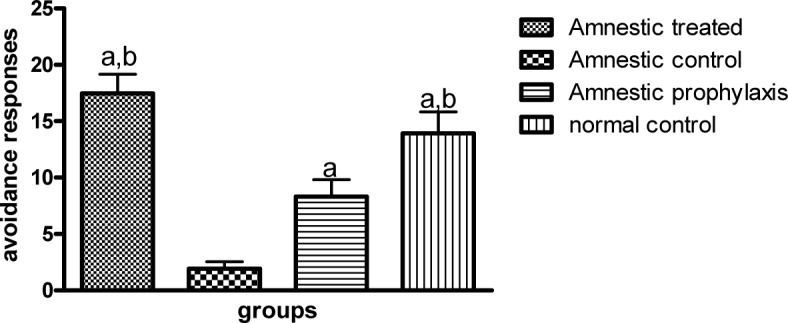
Figure 2. A comparison between the active avoidance responses in different groups during 3 successive days of short term memory session (n=10). a and b indicate the significant difference by p < 0.01 and p < 0.001 respectively, as compared with the AC group according to Kruskal-Wallis and Dunn's multiple comparison tests.
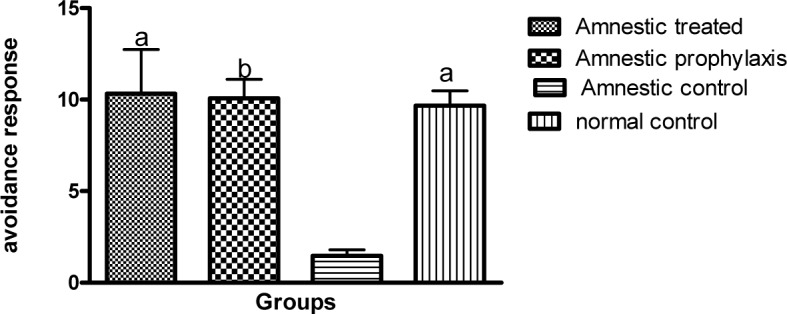
Figure 3. A comparison between the active avoidance responses in different groups during 3 successive days of long term memory session (n=10). a, b and c are indicative of significant difference by p < 0.05, p < 0.01 and p < 0.001 respectively, as compared with the AC group according to Kruskal-Wallis and Dunn's multiple comparison tests.
Passive avoidance test
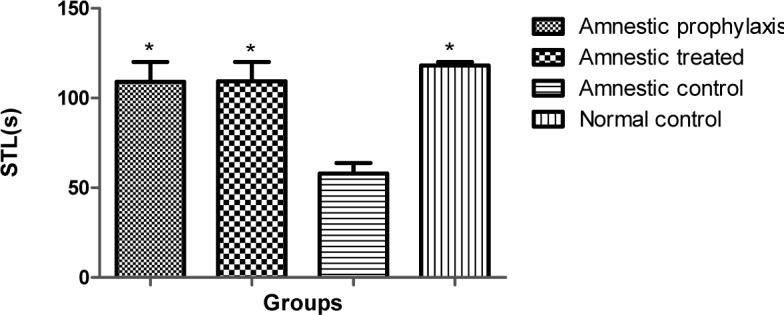
In passive avoidance test, animal's STL, as an index for learning, in NC, AC, AP and AT groups were 118.2 ± 1.8, 57.9 ± 5.9, 109 ± 11 and 109.3 ± 10.7 respectively. STL in all test groups was significantly greater than control group (p < 0.05, Figure 4(Fig. 4)).
Figure 4. Step through latency (STL) in different groups during passive avoidance learning session (n=10). * indicates the significant difference (p < 0.05) as compared with the AC group according to Kruskal-Wallis and Dunn's multiple comparison tests.
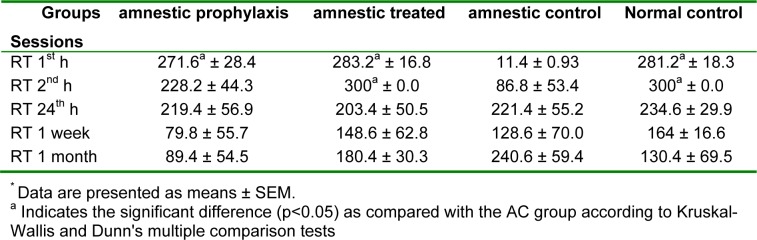
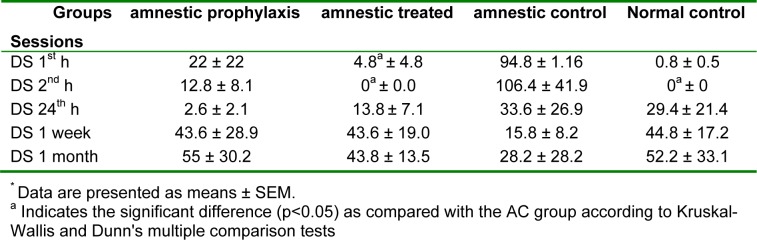
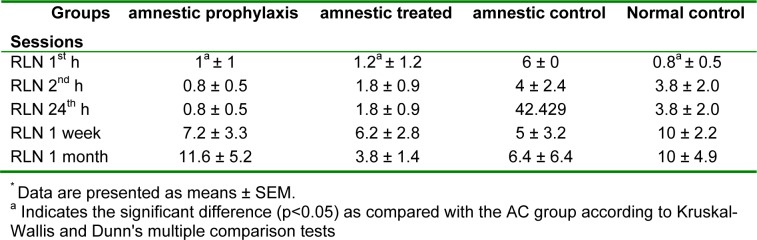
The retention time (RT) as the major index for memory consolidation at 1 h, 2 h, 24 h, 1 week and 1 month after full training session for different groups is shown in table 2(Tab. 2). There wasn't any significant difference in memory retention among different groups 1 week after training, but RT in AT and NC groups was significantly greater than AC group (300 ± 0.0 vs. 86.8 ± 53.44, p < 0.05). 1 h fallowing full training session, RT in AC was significantly less than other 3 groups (Table 2(Tab. 2), p < 0.05). Total time spent in dark room (DS), which opposes the memory retention ability, in AC was significantly greater than AT group at 1 and 2 hours after full training (p < 0.05), while there was not any significant difference between this index in AP and AT as compared with NC group (Table 3(Tab. 3), p < 0.05). Another index which evaluated in passive avoidance test was the number of relocating between light and dark rooms, which in AC was significantly greater than the AT and NC groups 1 h after the full training session (Table 4(Tab. 4), p < 0.05).
Table 2. A comparison between the retention time* (RT) in different groups 1 h, 2 h, 24 h, 1 week and 1 month after full training session of passive avoidance test (n=10).
Table 3. A comparison between the time spent in dark room* (DS), in different groups 1 h, 2 h, 24 h, 1 week and 1 month after full training session of passive avoidance test (n=10).
Table 4. A comparison between the number of relocating* (RLN), between light and dark rooms, in different groups 1 h, 2 h, 24 h, 1 week and 1 month after full training session of passive avoidance test (n=10).
Discussion
Our findings indicated that chronic systemic administration of hydro alcoholic extract of Crocus sativus L. in both active and passive avoidance tests could prevent and treat the learning impairment and memory deficit induced by D-galactose and NaNO2 in adult male mice. The enhancement of memory retention in active avoidance test was more persistent than in passive avoidance. This may be due to the greater frequency of foot shock delivery in training session of active avoidance test which could be as much as 90 shock/day and for 3 successive days instead of only 1 shock in passive avoidance test. Although, based on our literature review this is for the first time that the prolonged systemic administration of saffron extract is used to evaluate its prophylaxis and therapeutic effect on a model of chemically induced cognitive deficit, its findings globally supports previous studies regarding enhancing effect of saffron and its major constituents on learning and memory consolidation processes.
According to the literature review, Zhang et al. (1994[36]), for the first time reported that the alcohol extract of pistils of Crocus sativus L. improves learning and memory in mice. This finding was further supported by Sugiura et al. (1995[33]), who reported that oral administration of saffron extract and its major constituent, crocin, alone had no effect on learning behavior of mice in passive avoidance test, but significantly improved ethanol-induced impairment of memory acquisition. They also suggested that, in vivo oral or Intracerebroventricular administration of crocin or its in vitro application on hippocampal brain slices, significantly antagonized the inhibitory effect of ethanol on long-term potentiation (LTP) which is a mechanism underlying memory formation (Sugiura et al., 1995[31][32]). It has been also suggested that alcohol extract of Crocus sativus L. can prevent aversive effects induced by ethanol and its metabolite on neural activity in the dentate gyrus of anesthetized rats (Abe et al., 1999[4]).
Hosseinzadeh and Ziaei (2006[17]) demonstrated that, the Crocus sativus stigma aqueous extract, and crocin inhibited the hyoscine impaired acquisition in rats performing the Morris water maze task but did not affect the animal's intact memory. In the same way, the extract could inhibit morphine-induced impairments on spatial learning and memory in rats (Haghighizad et al., 2008[12]). In a research report the antagonizing effect of saffron extract on memory impairments, in the object recognition and the step-through passive avoidance task, was demonstrated in the rat (Pitsikas and Sakellaridis, 2006[23]). In this study Pitsikas and Sakellaridis (2006[23]), used a single injection of 3 low doses of saffron aqueous extract and the animal's memory was also destroyed by a single dose of scopolamine administration, while in our study we used a low dose of hydro alcoholic extract (30 mg/kg) for a long period of 60 days in the case of prevention and 15 days for therapeutic evaluation. Pitsikas et al. (2007[24]), proposed that the memory enhancing effect of saffron extract is due to the crocins which are water soluble carotenoid constituents of this plant. Khalili et al. (2009[20]) also have shown that aqueous Crocus sativus L. extract administration significantly inhibited learning and memory deficit in streptozotocin-induced dementia in passive avoidance test.
Recently it has been proposed that the positive effect of saffron on learning and memory processing is due to the antioxidant activity of its active components (Papandreou et al., 2011[22]). It has also been suggested that crocins derived from Crocus sativus L. significantly increase the retinal and choroidal blood flow and improve age related macular degeneration (Xuan et al., 1999[34]). In the present study the amnesia induction was performed by prolonged intraperitoneal administration of D-galactose and NaNO2 in ovariectomized mice. NaNO2 causes hypoxia and disability of consciousness in mice while D-galactose leads to an increase in free radicals and the combination of these 2 drugs induces a significant neuronal damage and atrophy in the mice brain hippocampus (Fang and Liu 2007[8]). Therefore the protective effects of saffron extract against this model of learning and memory impairment can be due to its oxidative scavenger and/or increasing the cerebral blood flow which needs to be further investigated.
Conclusion
According to our findings and previous evidences, it can be postulated that saffron and its active constituents can be potentially new drugs in the treatment of cognitive dysfunctions such as Alzheimer's disease and should be further studied through clinical trial investigations.
Acknowledgements
The authors would like to express their gratitude to the research deputy of Shahid Sadoughi University of Medical Sciences Yazd, Iran for granting this project. We are also grateful the biological lab staffs in research and clinical center for infertility and clinical sciences for providing ovariectomized mice. We have furthermore to thank the Novin Saffron co, Mashhad, Iran for kindly supplementation of pure saffron.
References
- 1.Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) Exp Biol Med. 2002;227:20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 2.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Abe K, Saito H. Effects of saffron extract and its constituent crocin on learning behaviour and long term potentiation. Phytother Res. 2000;14:149–152. doi: 10.1002/(sici)1099-1573(200005)14:3<149::aid-ptr665>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Abe K, Sugiura M, Yamaguchi S, Shoyama Y, Saito H. Saffron extract prevents acetaldehyde-induced inhibition of long-term potentiation in the rat dentate gyrus in vivo. Brain Res. 1999;851:287–289. doi: 10.1016/s0006-8993(99)02174-5. [DOI] [PubMed] [Google Scholar]
- 5.Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropath Exp Neur. 2010;69:155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouvier F, Suire C, Mutterer J, Camara B. Oxidative remodeling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis. The Plant Cell Online. 2003;15:47–62. doi: 10.1105/tpc.006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee IB, Mukhopadhyay CK, Ghosh MK. Vitamin C: a potential saviour against free radical-induced oxidative damage. Curr Sci. 1995;69:747–751. [Google Scholar]
- 8.Fang F, Liu G. A novel cyclic squamosalmide analogue compound FLZ improves memory impairment in artificial senescence mice induced by chronic injection of D galactose and NaNO2. Basic Clin Pharmacol. 2007;101:447–454. doi: 10.1111/j.1742-7843.2007.00138.x. [DOI] [PubMed] [Google Scholar]
- 9.Galindo LE, Garin-Aguilar ME, Medina AC, Serafín N, Quirarte GL, Prado-Alcalá RA. Acquisition and retention of enhanced active avoidance are unaffected by interference with serotonergic activity. Behav Brain Res. 2008;195:153–158. doi: 10.1016/j.bbr.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Ganong WF. Review of medical physiology. Connecticut: Appleton and Lange; 2012. pp. 289–296. [Google Scholar]
- 11.Gilca M, Stoian I, Atanasiu V, Virgolici B. The oxidative hypothesis of senescence. J Postgrad Med. 2007;53:207–213. doi: 10.4103/0022-3859.33869. [DOI] [PubMed] [Google Scholar]
- 12.Haghighizad H, Pourmotabbed A, Sahraei H, Ghadami MR, Ghadami S, Kamalinejad M. Protective effect of Saffron extract on morphine-induced inhibition of spatial learning and memory in rat. Physiol Pharmacol. 2008;12:170–179. [Google Scholar]
- 13.Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effect of Crocus sativus L. stigma extracts and their constituents, crocin and safranal, in mice. Acta Hort (ISHS) 2004;650:435–445. [Google Scholar]
- 14.Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of crocus sativus L. Stigmas in mice. Arch Irn Med. 2002;5(1):44–47. [Google Scholar]
- 15.Hosseinzadeh H, Noraei NB. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother Res. 2009;23:768–774. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- 16.Hosseinzadeh H, Younesi H. Petal and stigma extracts of Crocus sativus L. have antinociceptive and anti-inflammatory effects in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosseinzadeh H, Ziaei T. Effects of Crocus sativus stigma extract and its constituents, crocin and safranal, on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. J Med Plants. 2006;5:40–50. [Google Scholar]
- 18.Hua X, Lei M, Zhang Y, Ding J, Han Q, Hu G, et al. Long-term d-galactose injection combined with ovariectomy serves as a new rodent model for Alzheimer's disease. Life Sci. 2007;80:1897–1905. doi: 10.1016/j.lfs.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Karimi GHR, Hosseinzadeh H, Khaleghpanah P. Study of antidepressant effect of aqueous and ethanolic extract of Crocus sativus in mice. I J B M S. 2001;4:11–15. [Google Scholar]
- 20.Khalili M, Roghani M, Ekhlasi M. The effect of aqueous Crocus sativus L. extract on intracerebroventricular streptozotocin-induced cognitive deficits in rat: a behavioral analysis. Iran J Pharm Res. 2009;8:185–191. [Google Scholar]
- 21.Morse MA, Stoner GD. Cancer chemoprevention: principles and prospects. Carcinogenesis. 1993;14:1737–1746. doi: 10.1093/carcin/14.9.1737. [DOI] [PubMed] [Google Scholar]
- 22.Papandreou MA, Tsachaki M, Efthimiopoulos S, Cordopatis P, Lamari FN, Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav Brain Res. 2011;219:197–204. doi: 10.1016/j.bbr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Pitsikas N, Sakellaridis N. Crocus sativus L. extracts antagonize memory impairments in different behavioural tasks in the rat. Behav Brain Res. 2006;173:112–115. doi: 10.1016/j.bbr.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Pitsikas N, Zisopoulou S, Tarantilis PA, Kanakis CD, Polissiou MG, Sakellaridis N. Effects of the active constituents of Crocus sativus L., crocins on recognition and spatial rats' memory. Behav Brain Res. 2007;183:141–146. doi: 10.1016/j.bbr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Premkumar K, Abraham SK, Santhiya ST, Ramesh A. Protective effects of saffron (Crocus sativus Linn.) on genotoxins induced oxidative stress in Swiss albino mice. Phytother Res. 2003;17:614–617. doi: 10.1002/ptr.1209. [DOI] [PubMed] [Google Scholar]
- 26.Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28:721–733. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- 27.Rogers AE, Zeisel SH, Groopman J. Diet and carcinogenesis. Carcinogenesis. 1993;14:2205–2217. doi: 10.1093/carcin/14.11.2205. [DOI] [PubMed] [Google Scholar]
- 28.Samsam-Shariat SH. The extraction of the effective agents in medicinal plant and diagnosis and evaluation methods of them. Vol. 2002. Tehran: Mani Publ.; pp. 14–16. [Google Scholar]
- 29.Saxena G, Singh SP, Agrawal R, Nath C. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. Eur J Pharmacol. 2008;581:283–289. doi: 10.1016/j.ejphar.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava R, Ahmed H, Dixit RK. Crocus sativus L.: a comprehensive review. Pharmacognosy Rev. 2010;4:200–208. doi: 10.4103/0973-7847.70919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugiura M, Saito H, Abe K, Shoyama Y. Ethanol extract of Crocus sativus L. Antagonizes the inhibitory action of ethanol on hippocampal long term potentiation in vivo. Phytother Res. 1995;9:100–104. [Google Scholar]
- 32.Sugiura M, Shoyama Y, Saito H, Abe K. The effects of ethanol and crocin on the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Jpn J Pharmacol. 1995;67:395–397. doi: 10.1254/jjp.67.395. [DOI] [PubMed] [Google Scholar]
- 33.Sugiura M, Shoyama Y, Saito H, Nishiyama N. Crocin improves the ethanol-induced impairment of learning behaviors of mice in passive avoidance tasks. P Jpn Acad B-Phys. 1995;71:319–324. [Google Scholar]
- 34.Xuan B, Zhou YH, Li N, Min ZD, Chiou GCY. Effects of crocin analogs on ocular blood flow and retinal function. J Ocul Pharmacol Ther. 1999;15:143–152. doi: 10.1089/jop.1999.15.143. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Liu G, Shi J, Zhang J. Coeloglossum viride var. bracteatum extract attenuates D-galactose and NaNO2 induced memory impairment in mice. J Ethnopharmacol. 2006;104:250–256. doi: 10.1016/j.jep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Shoyama Y, Sugiura M, Saito H. Effects of Crocus sativus L. on the ethanol-induced impairment of passive avoidance performances in mice. Biol Pharm Bull. 1994;17:217–221. doi: 10.1248/bpb.17.217. [DOI] [PubMed] [Google Scholar]