1. West Nile virus infection in North America
West Nile virus (WNV) is a mosquito-borne enveloped positive-strand RNA virus belonging to the family Flaviviridae, which includes Yellow fever, hepatitis C, and Dengue viruses.1 WNV was first isolated in Uganda in 1937, and emerged into the United States in 1999. From 1999–2014, WNV spread across North America, South America, and the Caribbean leading to > 41,000 cases, including 1,753 fatalities. While the majority of WNV infections are asymptomatic (~80%), some infected patients develop mild symptoms of West Nile fever (~20%), and a small subset (<1%) develop severe neuroinvasive disease, including meningitis, encephalitis, and acute flaccid paralysis.2 Currently, no vaccine or specific antiviral treatments against WNV are available. Notably, advanced age remains a dominant risk factor for WNV infection and elderly individuals are more susceptible to severe infection with neurological involvement.3,4 Among patients over 70 years of age, the case-fatality rate ranges from 15% to 29%.5
The world’s population is aging and the global human population over age 60 is predicted to increase to over 2 billion by 2050.6 With aging, elderly individuals are increasingly susceptible to infectious diseases and have reduced efficiency of responses to vaccination. While individuals over age of 65 currently constitute approximately 15% of the population in the US, the aged population accounts for a disproportionate use of medical resources. Age related changes in both innate and adaptive immune responses, termed immunosenescence, lead to inappropriate elevations, decreases, and dysregulated immune responses.7 Here, we will review age-related immune dysregulation relevant to host susceptibility to WNV infection. We will also highlight novel areas for investigation and emerging technical approaches (e.g., mass cytometry and miRNA profiling) that promise to advance our understanding of the complexity of aging and foster discovery of novel therapeutic approaches.
2. Effects of aging on innate immune responses to WNV infection
Numerous studies in elderly humans have revealed that aging has a profound impact on the phenotype and functions of innate immune cells7,8 and these cell types-neutrophils, monocytes/macrophages, and dendritic cells- have central roles in initiating immune responses to control WNV replication.9–11 Dysregulation of two other innate immune cell types, natural killer (NK) and γδ T cells, although studied in aging, have not been examined for their role in immune susceptibility to WNV in elderly individuals. Here, we will summarize recent findings on age-dependent innate immune dysregulation of neutrophils, macrophages, dendritic cells in response to WNV infection, as well as age-related alterations in NK cells and γδ T cells that may contribute to WNV susceptibility in the elderly.
2.1 Impaired neutrophil function in aging
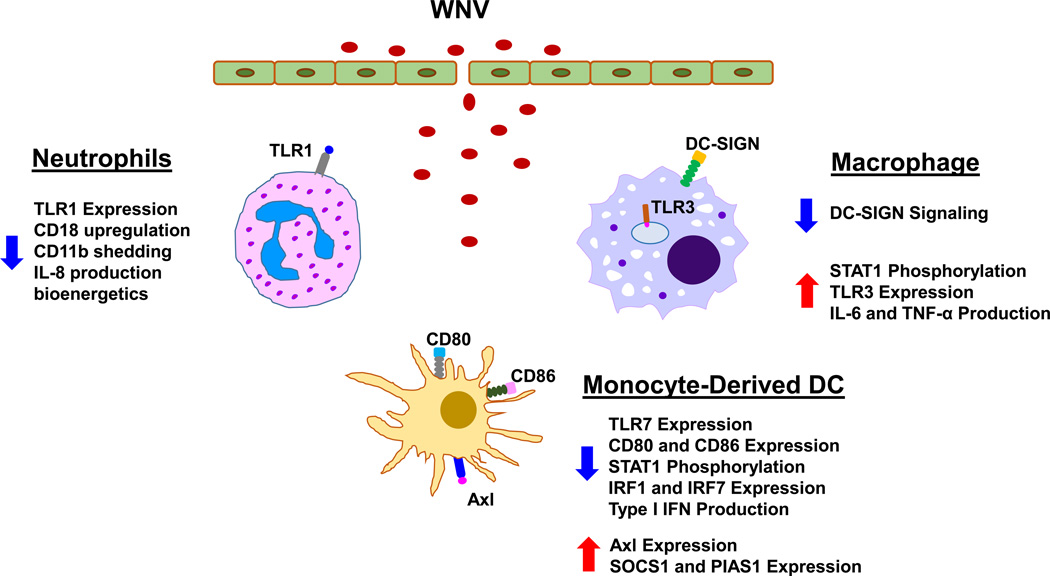
Neutrophils are the most abundant leukocytes in human blood circulation and the first immune cells to arrive at the sites of inflammation.12 At the inflamed sites, neutrophils exhibit potent antimicrobial activities by engulfing pathogens, generating reactive oxygen and nitrogen species, releasing granules containing proteolytic enzymes and antimicrobial peptides, and extruding neutrophil extracellular traps.13–15 Once the invading pathogens are cleared, neutrophils undergo apoptosis.16 A variety of neutrophil functions are impaired during aging, including chemotaxis, phagocytosis, superoxide production, NET formation, and apoptosis.17–20 Alterations of neutrophil signaling pathways and receptors have also been observed in aged individuals. Prominent affected pathways are the MAP kinases, the Jak/STAT and the PI3K-Akt pathways, which are important regulators of neutrophil functions.21,22 The decline of signal transduction in these pathways contributes to age-associated neutrophil dysfunction such as directional chemotaxis. Moreover, neutrophils in older adults have reduced bioenergetics, and lower expression of TLR1, leading to impairment of various neutrophil functions, including activation of integrins (CD18 and CD11b), and production of IL-8.22
Neutrophils play a dual functional role in response to WNV infection. Neutrophils serve as reservoirs for WNV replication and dissemination in the early stages of infection, but contribute to WNV clearance later in the infection process.9 The shift in neutrophils from early pro-viral state to later anti-viral state may result from the effects of cellular context such as the robust production of type I interferon by macrophages in the context of WNV infection. In vitro pretreatment of neutrophils with type I interferon significantly reduced their WNV viral load.9 In spite of the supporting evidence in the role of neutrophils in WNV infection, the effects of aging on neutrophil functions in response to WNV remain unknown. Age-associated alterations in chemotaxis, phagocytosis, signal transduction and expression of TLR receptors likely contribute to the reduced clearance of WNV infection in older subjects.
2.2 Reduced Macrophage function in aging
Macrophages are professional phagocytes and antigen-presenting cells and many of their functions become compromised in aged individuals, including chemotaxis, phagocytosis, intracellular killing, production of reactive oxygen species and cytokines (e.g., TNF-α and IL-12), as well as expression of MHC class II and co-stimulatory molecules (Table 1).7,8 In addition, production of prostaglandin E2 is increased in activated macrophages from aged human and mice, which suppresses MHC class II expression and IL-12 production, leading to impaired antigen presentation associated with age.8 Alterations in TLR expression have been found in aged macrophages. The baseline level of TLR3 is lower in macrophages from elderly individuals.23 A few studies have also shown an age-dependent reduction in the levels of p38 MAPK, NF-κB, and MyD88, as well as in the phosphorylation capacity of STAT-1α.24 The changes of these key signaling molecules are critical factors in the decrease in macrophage activation and cytokine responses in aging.
Table 1.
Effects of aging on innate immune cells.
| Functional activity |
Cell types | ||||
|---|---|---|---|---|---|
| Neutrophils | Macrophages | Dendritic cells | NK cells | Gamma-delta T cells | |
| Reduced | Chemotaxis Phagocytosis Superoxide production NET formation Apoptosis Signal transduction TLR1 expression TLR1-induced activation |
Chemotaxis Phagocytosis Intracellular killing Reactive oxygen species Expression of MHC and co- stimulatory molecules DC-SIGN signaling Cytokine production Antigen presentation |
Chemotaxis Endocytosis TLR 1,3,5,7, 8 expression TLR-induced cytokines type I IFN production PI3-K activity Antigen presentation |
CD56bright subset frequency NCR expression DNAM-1 expression Granzyme A production Cytotoxicity per cell |
Cell frequency and absolute number In vitro expansion capacity of Vδ2 T cells by IPP stimulation |
| Increased | STAT1 phosphorylation TLR3 expression PGE2 production |
Basal cytokine expressionD86 Basal NK-kB activity LPS, ssRNA-induced cytokines |
CD56−CD16+ subset frequency CD57 expression |
Apoptosis susceptibility of in vitro expanded Vδ2 T cells |
|
Following mosquito inoculation of WNV in skin, macrophages are early responders from the innate immune system to control initial WNV replication5. They efficiently ingest WNV through receptor-mediated endocytosis, and become activated to produce a large amount of proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β, as well as type I interferons. These cytokines are critical for restriction of WNV replication and spread and for recruitment of more innate immune cells into the site of infection25–27. However, excessive inflammation and cytokine production upon WNV infection can increase permeability of blood-brain barrier, leading to viral infection of the central nervous system, and severe neurological disease.26 Our recent studies indicate some interesting clues in this regard. In contrast to WNV-induced downregulation of TLR3 expression in macrophages from young donors, in elderly donors the expression of TLR3 remains elevated in WNV-infected macrophages and leads to elevated production of proinflammatory cytokines.23 This TLR3 dysregulation results from impaired signaling between DC-SIGN and STAT1, which also leads to an early and sustained elevation of IL-6 and IFN-β1 in the elderly23. This alteration of the macrophage response with aging detected in vitro may be relevant to cytokine-mediated elevated permeability of blood-brain barrier and increased severity of WNV infection in older individuals.26
2.3 Dendritic cell function is diminished in aging
Dendritic cells (DCs) are potent antigen presenting cells which act as a bridge between the innate and the adaptive immune systems.28 Studies have shown dysregulation of several functions in both myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) with aging. DCs display an age-related decline in chemotaxis, endocytosis, and global reduction in expression of expression of Toll-like receptors (TLR) 1, 3, 5, 7, and 8, production of IL-12, and antigen presentation, leading to impaired activation of naïve T cells (Table 1).8,29 Paradoxically, DCs from elderly individuals produce a higher basal level of cytokines (e.g., TNF-α, IL-6, and IL-23). In pDCs, reduced expression of TLR7, decreased production of IFN-α by TLR stimulation, and impaired phosphorylation of IRF-7 have been shown in older individuals.30,31 DCs from aged adults have reduced expression of co-stimulatory molecules CD80 and CD86, diminished induction of TLR7 expression, as well as decreased production of IFN-α and IFN-β following WNV infection. This dysregulation is suggested to result from impaired STAT-1 phosphorylation, diminished induction of IRF-1 and IRF-7, and enhanced expression of negative signaling molecules Axl, SOCS1 and PIAS1 in DCs from elderly subjects.32 These deficits in critical signaling pathways in DC antiviral responses may contribute to the increased susceptibility to WNV infections in the elderly.
2.4 Natural Killer cell anti-viral activity wanes with aging
Natural Killer (NK) cells are large granular lymphocytes, 10–15% of the circulating lymphocyte pool, that specialize in early defense against virus infections and tumor cells.33 NK cells recognize abnormal or infected cells through a complex recognition pathway involving both MHC and a repertoire of invariant activating and inhibitory NK receptors. NK cells maintain extraordinary their functional diversity determined from combinatorial expression of multiple activating and inhibitory receptors.33,34 NK cells are classically divided into two major functional subsets (immature and mature) based on the differential expression of surface markers CD56 and CD16: CD56brightCD16− (immature) and CD56dimCD16+ (mature).35,36 Immature NK subsets secrete cytokines and chemokines on activation and following maturation exhibit high cytotoxic capacity.37 NK cells control viral replication by killing infected cells during the earliest stage of infection, and shape adaptive immune responses through cytokine release or by direct interaction with DCs.38–40 An important role for NK cells has been noted previously in many viral infections, such as HIV-1, influenza virus, cytomegalovirus, and hepatitis C virus.41–43 In aging, frequency of the immature CD56bright NK cell subset is reduced (Table 1) which may contribute to the impaired production of cytokines and chemokines observed in NK cells of aged subjects.44 NK cells from older subjects show upregulation of the maturation marker CD57, reduced expression of activating receptors DNAM-1 and NKp30 and NKp46, as well as impaired cytotoxicity and decreased production of granzyme A.24,45
Primary human NK cell responses to WNV include activation following interaction of NKp44 receptor with WNV envelope protein46,47; however deficiency of NK cells did not change morbidity in the murine model.48,49 It has been challenging to identify precise changes within the NK cell population in humans since current platforms of flow cytometry are limiting for interrogation of the more than 20 NK receptors expressed per cell. However, the recent development of mass cytometry (CyTOF) provided the first opportunity to simultaneously evaluate NK cell phenotype and function within the context of the overall immune response. Recent studies have used high-dimensional single-cell data to highlight the extreme diversity of the NK cell repertoire as well as to discover the functional significance of NK cell diversity in viral infection.50 Indeed, the diversity of the NK repertoire increases following infection with either HIV or WNV, leading to terminal differentiation and reduced degranulation and an increased risk of viral acquisition.51 Thus NK cell diversity may serve as a measure of immunological age and susceptibility, which may precede chronological aging.
2.5 Gamma-Delta T cells in aging
γδ T Cells an intriguing and enigmatic T cell subset, are present in humans as less than 10% of lymphocytes in the peripheral blood (Vδ1 subset) and in diverse tissues, such as skin, liver, gut epithelial tissue and bronchial epithelia (Vδ2 subset).52 γδ T cells respond rapidly to antigens from bacteria, parasites and viruses, do not require antigen processing and MHC presentation of peptide epitopes, and produce pro-inflammatory cytokines IFN-γ, TNF-α, and IL-17.53–55 Numbers of γδ T cells in the blood increase in patients with viral infections and potent anti-viral responses include IFN-γ production and CCR5-mediated migration.53,56–58 In mouse models of WNV infections, although γδ T cells produce cytokines involved in inflammation and pathogenesis (IL-17, IL-10 and TGF-β), deficient mice (TCRδ−/−) are nevertheless more susceptible, showing elevated viremia and more severe encephalitis. This suggests an important role for γδ T cells in resistance to WNV infection which remains incompletely understood.54 In aging, both the frequency and absolute number of γδ T cells are reduced (Table 1), stimulated expansion is reduced, and apoptosis is increased, which may contribute to increased susceptibility of older people to WNV infection.59–61
3. Adaptive immunity shows decreased responses to WNV in aging
Decline of the adaptive immunity with age has been well established. These changes include decreased pools of naïve T and B cells accompanied by increased memory and effector T and B cells, decreased diversity of antigen receptor repertoire, defective signal transduction in T cells with dysregulated cytokine production pattern, reduced class switching of B cells, and decreased clonal expansion and function of antigen-specific T and B cells.62,63 The age-associated deficits in the CD4 and CD8 T cell response against WNV including impaired production of cytokines and lytic granules, contributing to increased WNV viral titers in the brain of aged mice.64 Moreover, aged mice show lower levels of primary and memory T and B cell responses induced by vaccination with West Nile encephalitis vaccine, and repeated in vivo restimulation is needed to generate protective cellular and humoral immunity in older populations.65 Collectively, these observations suggest age-related alterations of adaptive immunity are also relevant for increased WNV susceptibility in the elderly.
4. New directions for aging-related investigation
Recent advances in technology hold the promise for increasing our understanding of essential changes in immune cells associated with aging, and fostering new discoveries for prevention and therapeutic approaches to improve health. In particular, we highlight mass cytometry to characterize in depth phenotypic and functional changes in multiple cell types simultaneously; and micro RNA (miRNA) profiling to identify miRNAs that regulate expression of pivotal genes relevant to aging-associated conditions.66,67
4.1 Mass cytometry (CyTOF): novel multidimensional single cell phenotyping
Mass cytometry, or cytometry by time-of-flight (CyTOF), is a novel technology for multiparametric single cell analysis based on detection of metal-conjugated antibodies.68 CyTOF improves on fluorescence flow cytometry and has greater dimensionality (40 parameters vs 8–10 by flow cytometry) and resolution of compensation issues. Furthermore, CyTOF can efficiently detect as few as 10,000 cells, which supports investigation from limited samples available through translational and clinical studies.69 High dimensional data generated from CyTOF requires specialized computational methods for dimensionality reduction, clustering, visualization, and single cell resolution.70–72 CyTOF technology is leading to advances in biology and medicine, such as cancer, autoimmune diseases, and infectious diseases.73–76 Emerging studies have employed CyTOF to characterize single cell immune responses to viral infections and vaccination,77–79 and promising results in studies of aging advance our understanding of age-associated changes in immune responses.80,81
4.2 microRNA regulation of gene expression
Recent studies have identified an important role for non-coding short microRNAs (miRNAs, ~22 nucleotides) in posttranscriptional regulation of gene expression by binding to specific mRNA targets and facilitating their degradation and/or translational inhibition. The human genome is believed to encode ~1,000 miRNAs, each of which may regulate expression of hundreds of genes.67 Emerging evidence has shown that expression of dozens of miRNAs are altered with aging in different tissues and organisms, which may be associated with age-dependent diseases and disorders.82,83 Interestingly, several key immune-regulated miRNAs such as miR-21, -146a, and -155, also show alterations during aging, suggesting that miRNAs may contribute to the age-associated basal inflammation.82,84–86 Cellular miRNAs have also been implicated in restriction or promotion of infection of various viruses, including Hepatitis C virus (miR-122) and retrovirus primate foamy virus type 1 (miR-32).87,88 Several miRNAs including miR-196a, -202-3p, -449c, and -125a-3p have been shown to be differentially expressed following WNV infection, suggesting their potential role in WNV resistance and pathogenesis.89,90 miRNA profiling will lead us to a better understanding of miRNA regulation in aging and viral infections as well as new discoveries for miRNA-based therapeutic intervention.
5. Concluding remarks
Aging remains a dominant risk factor for susceptibility to infection with WNV4, and aging-associated changes in innate and adaptive immunity may contribute to increased illness among the elderly. As reviewed here, dysregulation of TLR pathways in macrophages23, reduced production of IFN by dendritic cells32, and reduced efficiency of PMN clearance of virus9 may contribute to the increased susceptibility to WNV infection in elderly individuals. In addition, in-depth investigations are needed to identify whether age-related differences in NK cells and γδ T cells may also be relevant to control of WNV infection in humans. Emerging technologies including single cell CyTOF and miRNA profiling provide multidimensional, high-throughput, genome- and proteome-wide analysis of age-associated changes in cell function and may offer new insights into pathogenesis of age-related diseases or disorders for development of promising preventive and therapeutic approaches.
Figure 1.
References
- 1.Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45(8):1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 2.Lindsey NP, Lehman JA, Staples JE, Fischer M Division of Vector-Borne Diseases, N.C.f.E., and Zoonotic Infectious Diseases, C.D.C. West nile virus and other arboviral diseases - United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(24):521–526. [PMC free article] [PubMed] [Google Scholar]
- 3.Qian F, Goel G, Meng H, Wang X, You F, Devine L, Raddassi K, Garcia MN, Murray KO, Bolen CR, Gaujoux R, Shen-Orr SS, Hafler D, Fikrig E, Xavier R, Kleinstein SH, Montgomery RR. Systems immunology reveals markers of susceptibility to west nile virus infection. Clin Vaccine Immunol. 2015;22(1):6–16. doi: 10.1128/CVI.00508-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery RR, Murray KO. Risk factors for West Nile virus infection and disease in populations and individuals. Expert Rev Anti Infect Ther. 2015;13(3):317–325. doi: 10.1586/14787210.2015.1007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colpitts TM, Conway MJ, Montgomery RR, Fikrig E. West Nile Virus: biology, transmission, and human infection. Clin Microbiol Rev. 2012;25(4):635–648. doi: 10.1128/CMR.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United Nations. World population ageing, 1950–2050. New York: United Nations; 2002. Department of Economic and Social Affairs. Population Division; p. xlix.p. 483. [Google Scholar]
- 7.Montgomery RR, Shaw AC. Paradoxical changes in innate immunity in aging: recent progress and new directions. J Leukoc Biol. 2015 doi: 10.1189/jlb.5MR0315-104R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai F, Kong KF, Dai J, Qian F, Zhang L, Brown CR, Fikrig E, Montgomery RR. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J Infect Dis. 2010;202(12):1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Nathan D, Huitinga I, Lustig S, van Rooijen N, Kobiler D. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch Virol. 1996;141(3–4):459–469. doi: 10.1007/BF01718310. [DOI] [PubMed] [Google Scholar]
- 11.Silva MC, Guerrero-Plata A, Gilfoy FD, Garofalo RP, Mason PW. Differential activation of human monocyte-derived and plasmacytoid dendritic cells by West Nile virus generated in different host cells. J Virol. 2007;81(24):13640–13648. doi: 10.1128/JVI.00857-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008;181(8):5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 14.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15(7):602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 15.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30(11):513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Peters T, Weiss JM, Sindrilaru A, Wang H, Oreshkova T, Wlaschek M, Maity P, Reimann J, Scharffetter-Kochanek K. Reactive oxygen intermediate-induced pathomechanisms contribute to immunosenescence, chronic inflammation and autoimmunity. Mech Ageing Dev. 2009;130(9):564–587. doi: 10.1016/j.mad.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Butcher SK, Chahal H, Nayak L, Sinclair A, Henriquez NV, Sapey E, O'Mahony D, Lord JM. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol. 2001;70(6):881–886. [PubMed] [Google Scholar]
- 19.Fulop T, Jr, Fouquet C, Allaire P, Perrin N, Lacombe G, Stankova J, Rola-Pleszczynski M, Gagne D, Wagner JR, Khalil A, Dupuis G. Changes in apoptosis of human polymorphonuclear granulocytes with aging. Mech Ageing Dev. 1997;96(1–3):15–34. doi: 10.1016/s0047-6374(96)01881-7. [DOI] [PubMed] [Google Scholar]
- 20.Hazeldine J, Harris P, Chapple IL, Grant M, Greenwood H, Livesey A, Sapey E, Lord JM. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell. 2014;13(4):690–698. doi: 10.1111/acel.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larbi A, Douziech N, Fortin C, Linteau A, Dupuis G, Fulop T., Jr The role of the MAPK pathway alterations in GM-CSF modulated human neutrophil apoptosis with aging. Immun Ageing. 2005;2(1):6. doi: 10.1186/1742-4933-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian F, Guo X, Wang X, Yuan X, Chen S, Malawista SE, Bockenstedt LK, Allore HG, Montgomery RR. Reduced bioenergetics and toll-like receptor 1 function in human polymorphonuclear leukocytes in aging. Aging (Albany NY) 2014;6(2):131–139. doi: 10.18632/aging.100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong KF, Delroux K, Wang X, Qian F, Arjona A, Malawista SE, Fikrig E, Montgomery RR. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol. 2008;82(15):7613–7623. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24(5):331–341. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Tapia D, Hassett DE, Mitchell WJ, Jr, Johnson GC, Kleiboeker SB. West Nile virus encephalitis: sequential histopathological and immunological events in a murine model of infection. J Neurovirol. 2007;13(2):130–138. doi: 10.1080/13550280601187185. [DOI] [PubMed] [Google Scholar]
- 26.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10(12):1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 27.Shrestha B, Zhang B, Purtha WE, Klein RS, Diamond MS. Tumor necrosis factor alpha protects against lethal West Nile virus infection by promoting trafficking of mononuclear leukocytes into the central nervous system. J Virol. 2008;82(18):8956–8964. doi: 10.1128/JVI.01118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 29.Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, Allore HG, Montgomery RR, Shaw AC. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184(5):2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jing Y, Shaheen E, Drake RR, Chen N, Gravenstein S, Deng Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum Immunol. 2009;70(10):777–784. doi: 10.1016/j.humimm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sridharan A, Esposo M, Kaushal K, Tay J, Osann K, Agrawal S, Gupta S, Agrawal A. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age (Dordr) 2011;33(3):363–376. doi: 10.1007/s11357-010-9191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian F, Wang X, Zhang L, Lin A, Zhao H, Fikrig E, Montgomery RR. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J Infect Dis. 2011;203(10):1415–1424. doi: 10.1093/infdis/jir048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 34.Strauss-Albee DM, Horowitz A, Parham P, Blish CA. Coordinated regulation of NK receptor expression in the maturing human immune system. J Immunol. 2014;193(10):4871–4879. doi: 10.4049/jimmunol.1401821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 36.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97(10):3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 37.Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol. 2013;132(3):536–544. doi: 10.1016/j.jaci.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zitvogel L, Terme M, Borg C, Trinchieri G. Dendritic cell-NK cell cross-talk: regulation and physiopathology. Curr Top Microbiol Immunol. 2006;298:157–174. doi: 10.1007/3-540-27743-9_8. [DOI] [PubMed] [Google Scholar]
- 41.Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–194. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- 42.Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol. 2011;11(3):176–186. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 44.Mariani E, Meneghetti A, Neri S, Ravaglia G, Forti P, Cattini L, Facchini A. Chemokine production by natural killer cells from nonagenarians. Eur J Immunol. 2002;32(6):1524–1529. doi: 10.1002/1521-4141(200206)32:6<1524::AID-IMMU1524>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 45.Solana R, Campos C, Pera A, Tarazona R. Shaping of NK cell subsets by aging. Curr Opin Immunol. 2014;29:56–61. doi: 10.1016/j.coi.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Hershkovitz O, Rosental B, Rosenberg LA, Navarro-Sanchez ME, Jivov S, Zilka A, Gershoni-Yahalom O, Brient-Litzler E, Bedouelle H, Ho JW, Campbell KS, Rager-Zisman B, Despres P, Porgador A. NKp44 receptor mediates interaction of the envelope glycoproteins from the West Nile and dengue viruses with NK cells. J Immunol. 2009;183(4):2610–2621. doi: 10.4049/jimmunol.0802806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang M, Daniel S, Huang Y, Chancey C, Huang Q, Lei YF, Grinev A, Mostowski H, Rios M, Dayton A. Anti-West Nile virus activity of in vitro expanded human primary natural killer cells. BMC Immunol. 2010;11:3. doi: 10.1186/1471-2172-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shrestha B, Samuel MA, Diamond MS. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J Virol. 2006;80(1):119–129. doi: 10.1128/JVI.80.1.119-129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samuel MA, Diamond MS. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006;80(19):9349–9360. doi: 10.1128/JVI.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, Davis MM, Norman PJ, Guethlein LA, Desai M, Parham P, Blish CA. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. 2013;5(208):208ra145. doi: 10.1126/scitranslmed.3006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strauss-Albee DM, Fukuyama J, Liang EC, Yao Y, Jarrell JA, Drake AL, Kinuthia J, Montgomery RR, John-Stewart G, Holmes S, Blish CA. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci Transl Med. 2015;7(297):297ra115. doi: 10.1126/scitranslmed.aac5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chien YH, Bonneville M. Gamma delta T cell receptors. Cell Mol Life Sci. 2006;63(18):2089–2094. doi: 10.1007/s00018-006-6020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace M, Malkovsky M, Carding SR. Gamma/delta T lymphocytes in viral infections. J Leukoc Biol. 1995;58(3):277–283. doi: 10.1002/jlb.58.3.277. [DOI] [PubMed] [Google Scholar]
- 54.Wang T, Welte T. Role of natural killer and Gamma-delta T cells in West Nile virus infection. Viruses. 2013;5(9):2298–2310. doi: 10.3390/v5092298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Brien RL, Roark CL, Born WK. IL-17-producing gammadelta T cells. Eur J Immunol. 2009;39(3):662–666. doi: 10.1002/eji.200839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poccia F, Agrati C, Castilletti C, Bordi L, Gioia C, Horejsh D, Ippolito G, Chan PK, Hui DS, Sung JJ, Capobianchi MR, Malkovsky M. Anti-severe acute respiratory syndrome coronavirus immune responses: the role played by V gamma 9V delta 2 T cells. J Infect Dis. 2006;193(9):1244–1249. doi: 10.1086/502975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bank I, Marcu-Malina V. Quantitative peripheral blood perturbations of gammadelta T cells in human disease and their clinical implications. Clin Rev Allergy Immunol. 2014;47(3):311–333. doi: 10.1007/s12016-013-8391-x. [DOI] [PubMed] [Google Scholar]
- 58.Qin G, Liu Y, Zheng J, Ng IH, Xiang Z, Lam KT, Mao H, Li H, Peiris JS, Lau YL, Tu W. Type 1 responses of human Vgamma9Vdelta2 T cells to influenza A viruses. J Virol. 2011;85(19):10109–10116. doi: 10.1128/JVI.05341-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colonna-Romano G, Aquino A, Bulati M, Lio D, Candore G, Oddo G, Scialabba G, Vitello S, Caruso C. Impairment of gamma/delta T lymphocytes in elderly: implications for immunosenescence. Exp Gerontol. 2004;39(10):1439–1446. doi: 10.1016/j.exger.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Argentati K, Re F, Donnini A, Tucci MG, Franceschi C, Bartozzi B, Bernardini G, Provinciali M. Numerical and functional alterations of circulating gammadelta T lymphocytes in aged people and centenarians. J Leukoc Biol. 2002;72(1):65–71. [PubMed] [Google Scholar]
- 61.Colonna-Romano G, Potestio M, Aquino A, Candore G, Lio D, Caruso C. Gamma/delta T lymphocytes are affected in the elderly. Exp Gerontol. 2002;37(2–3):205–211. doi: 10.1016/s0531-5565(01)00185-1. [DOI] [PubMed] [Google Scholar]
- 62.Ludmila Muller GP. Introduction to Ageing of the Adaptive Immune System. In: Bosch JAP, AC, Lord JM, editors. Immunosenescence. New York Dordrecht Heidlberg London: Springer; 2013. [Google Scholar]
- 63.Rymkiewicz PD, Heng YX, Vasudev A, Larbi A. The immune system in the aging human. Immunol Res. 2012;53(1–3):235–250. doi: 10.1007/s12026-012-8289-3. [DOI] [PubMed] [Google Scholar]
- 64.Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Zugich J. Key role of T cell defects in age-related vulnerability to West Nile virus. J Exp Med. 2009;206(12):2735–2745. doi: 10.1084/jem.20090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uhrlaub JL, Brien JD, Widman DG, Mason PW, Nikolich-Zugich J. Repeated in vivo stimulation of T and B cell responses in old mice generates protective immunity against lethal West Nile virus encephalitis. J Immunol. 2011;186(7):3882–3891. doi: 10.4049/jimmunol.1002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 2012;36(1):142–152. doi: 10.1016/j.immuni.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13(5):358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bendall SC, Simonds EF, Qiu P, Amir el AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe'er D, Tanner SD, Nolan GP. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332(6030):687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yao Y, Liu R, Shin MS, Trentalange M, Allore H, Nassar A, Kang I, Pober JS, Montgomery RR. CyTOF supports efficient detection of immune cell subsets from small samples. J Immunol Methods. 2014;415:1–5. doi: 10.1016/j.jim.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiu P, Simonds EF, Bendall SC, Gibbs KD, Jr, Bruggner RV, Linderman MD, Sachs K, Nolan GP, Plevritis SK. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat Biotechnol. 2011;29(10):886–891. doi: 10.1038/nbt.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruggner RV, Bodenmiller B, Dill DL, Tibshirani RJ, Nolan GP. Automated identification of stratifying signatures in cellular subpopulations. Proc Natl Acad Sci U S A. 2014;111(26):E2770–E2777. doi: 10.1073/pnas.1408792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amir el AD, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, Shenfeld DK, Krishnaswamy S, Nolan GP, Pe'er D. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol. 2013;31(6):545–552. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han A, Newell EW, Glanville J, Fernandez-Becker N, Khosla C, Chien YH, Davis MM. Dietary gluten triggers concomitant activation of CD4+ and CD8+ alphabeta T cells and gammadelta T cells in celiac disease. Proc Natl Acad Sci U S A. 2013;110(32):13073–13078. doi: 10.1073/pnas.1311861110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaudilliere B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M, Silva J, Ganio EA, Yeh CG, Maloney WJ, Huddleston JI, Goodman SB, Davis MM, Bendall SC, Fantl WJ, Angst MS, Nolan GP. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med. 2014;6(255):255ra131. doi: 10.1126/scitranslmed.3009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nair N, Mei HE, Chen SY, Hale M, Nolan GP, Maecker HT, Genovese M, Fathman CG, Whiting CC. Mass cytometry as a platform for the discovery of cellular biomarkers to guide effective rheumatic disease therapy. Arthritis Res Ther. 2015;17(1):127. doi: 10.1186/s13075-015-0644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong MT, Chen J, Narayanan S, Lin W, Anicete R, Kiaang HT, De Lafaille MA, Poidinger M, Newell EW. Mapping the Diversity of Follicular Helper T Cells in Human Blood and Tonsils Using High-Dimensional Mass Cytometry Analysis. Cell Rep. 2015;11(11):1822–1833. doi: 10.1016/j.celrep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 77.O'Gorman WE, Huang H, Wei YL, Davis KL, Leipold MD, Bendall SC, Kidd BA, Dekker CL, Maecker HT, Chien YH, Davis MM. The Split Virus Influenza Vaccine rapidly activates immune cells through Fcgamma receptors. Vaccine. 2014;32(45):5989–5997. doi: 10.1016/j.vaccine.2014.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swadling L, Capone S, Antrobus RD, Brown A, Richardson R, Newell EW, Halliday J, Kelly C, Bowen D, Fergusson J, Kurioka A, Ammendola V, Del Sorbo M, Grazioli F, Esposito ML, Siani L, Traboni C, Hill A, Colloca S, Davis M, Nicosia A, Cortese R, Folgori A, Klenerman P, Barnes E. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med. 2014;6(261):261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sen N, Mukherjee G, Arvin AM. Single Cell Mass Cytometry Reveals Remodeling of Human T Cell Phenotypes by Varicella Zoster Virus. Methods. 2015 doi: 10.1016/j.ymeth.2015.07.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whiting CC, Siebert J, Newman AM, Du HW, Alizadeh AA, Goronzy J, Weyand CM, Krishnan E, Fathman CG, Maecker HT. Large-Scale and Comprehensive Immune Profiling and Functional Analysis of Normal Human Aging. PLoS One. 2015;10(7):e0133627. doi: 10.1371/journal.pone.0133627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin V, Wu YC, Kipling D, Dunn-Walters DK. Age-related aspects of human IgM B cell heterogeneity. Ann N Y Acad Sci. 2015 doi: 10.1111/nyas.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Olivieri F, Procopio AD, Montgomery RR. Effect of aging on microRNAs and regulation of pathogen recognition receptors. Curr Opin Immunol. 2014;29:29–37. doi: 10.1016/j.coi.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garg D, Cohen SM. miRNAs and aging: a genetic perspective. Ageing Res Rev. 2014;17:3–8. doi: 10.1016/j.arr.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132(21):4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 85.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res. 2012;110(3):496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- 86.Chen LH, Chiou GY, Chen YW, Li HY, Chiou SH. MicroRNA and aging: a novel modulator in regulating the aging network. Ageing Res Rev. 2010;9(Suppl 1):S59–S66. doi: 10.1016/j.arr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 87.Lecellier CH, Dunoyer P, Arar K, Lehmann-Che J, Eyquem S, Himber C, Saib A, Voinnet O. A cellular microRNA mediates antiviral defense in human cells. Science. 2005;308(5721):557–560. doi: 10.1126/science.1108784. [DOI] [PubMed] [Google Scholar]
- 88.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309(5740):1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 89.Chugh PE, Damania BA, Dittmer DP. Toll-like receptor-3 is dispensable for the innate microRNA response to West Nile virus (WNV) PLoS One. 2014;9(8):e104770. doi: 10.1371/journal.pone.0104770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar M, Nerurkar VR. Integrated analysis of microRNAs and their disease related targets in the brain of mice infected with West Nile virus. Virology. 2014;452–453:143–151. doi: 10.1016/j.virol.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]