Abstract
Neuroimaging has suggested that amygdala reactivity to emotional facial expressions is associated with antisocial behavior (AB), particularly among those high on callous-unemotional (CU) traits. To investigate this association and potential moderators of this relationship, including task/stimuli effects, subregional anatomy of the amygdala, and participant race, we used fMRI in a sample of 167 racially diverse, 20 year-old men from low-income families. We found that AB, but not CU traits, was negatively related to amygdala reactivity to fearful faces. This result was specific to fearful faces and strongest in the centro-medial subregion of the amygdala. Arrest record was positively related to basolateral amygdala reactivity to fearful and angry faces. Results were strongest among those identified as African American and not present in those identified as European American. Our findings suggest substantial complexity in the relationship between amygdala function and AB reflecting moderating effects of task stimulus, subregional anatomy, and race.
Keywords: psychopathy, conduct problems, externalizing, violence, neuroimaging
Antisocial behavior (AB), which includes aggression and rule breaking, is a major public health concern because of its high prevalence and the extended negative financial, social, and emotional impact on perpetrators, victims, and their families (Foster & Jones, 2005; Nock, Kazdin, Hiripi, & Kessler, 2006; Odgers et al., 2007). AB, captured by the DSM-5 diagnoses of conduct disorder in youth and antisocial personality disorder in adults (American Psychiatric Association, 2013), typically peaks in late adolescence and early adulthood, making this developmental period an important one for understanding the persistence or desistence of AB. When these behaviors begin early and persist, there is a characteristic escalation into more violent and dangerous behaviors that can continue through adulthood and lead to the onset of other psychiatric disorders such as addiction and depression (Hyde, Burt, Shaw, Donnellan, & Forbes, 2015; Loeber & Hay, 1997). Thus, there is a critical need to develop effective strategies for early identification of individuals at greater risk for developing AB and subsequently implement effective interventions. Towards this end, many researchers have begun to emphasize the need to better account for the significant heterogeneity in specific behavioral and clinical symptoms across individuals generally high in AB, which can lead towards more effective and personalized treatments (Frick & Nigg, 2012; Kahn, Frick, Youngstrom, Findling, & Youngstrom, 2012; Moffitt et al., 2008).
Dimensions and subtypes of AB
One prominent subtyping approach, recently added to the DSM-5 (American Psychiatric Association, 2013), is to identify the relative expression of callous-unemotional (CU) traits in those high on AB. CU traits are defined by a lack of empathy and guilt, as well as shallow affect. CU traits have been shown to identify a subgroup of youth with more heritable, severe, and persistent AB (Frick & White, 2008). In adults, a similar distinction has been made between AB present in antisocial personality and intermittent explosive disorder diagnoses versus AB present in those with high psychopathic traits (Patrick, 2007). Just as with CU traits, psychopathy is associated with more persistent and severe AB and a host of maladaptive personality traits, including callousness, low empathy and guilt, and shallow affect (Patrick, 2007).
Another subtyping approach is to identify the age of onset of AB. A wealth of studies have shown that early starting AB (before age 10) is associated with greater antecedent risk and more persistent and violent outcomes (Moffitt, 1993; Moffitt, Caspi, Harrington, & Milne, 2002; Patterson, Reid, & Dishion, 1992). CU traits and adult psychopathy have both been linked to significantly attenuated fear responses (Lykken, 1957), which may reflect dysfunction of the amygdala (Blair, 2007; Blair, Peschardt, Budhani, & Pine, 2006). Additionally, early starting AB has been linked to early neurocognitive deficits also hypothesized to reflect neural dysfunction (Moffitt, 1993). Thus, recent studies have used functional neuroimaging to better understand the pathophysiology of these subtypes of individuals high on AB.
Neural correlates of AB
Youth high on AB and CU traits, as well as adults high on psychopathic traits, have been the main focus of neuroimaging studies of AB. These studies have generally found that those with CU traits demonstrate decreased amygdala reactivity to emotional stimuli, particularly social signals of interpersonal distress, such as fearful facial expressions with a direct eye gaze (Jones, Laurens, Herba, Gareth, & Viding, 2009; Kiehl et al., 2001; Marsh et al., 2008). However, when CU traits are not assessed or study participants exhibit high levels of impulsive aggression, increased amygdala reactivity to fearful faces, as well as social signals of interpersonal threat such as angry facial expressions, have been observed (Beaver, Lawrence, Passamonti, & Calder, 2008; Carré, Fisher, Manuck, & Hariri, 2012; Coccaro, McCloskey, Fitzgerald, & Phan, 2007; Decety, Michalska, Akitsuki, & Lahey, 2009; Sterzer, Stadler, Krebs, Kleinschmidt, & Poustka, 2005). This subtype divergence in expression-specific amygdala reactivity has led to prominent dual pathway models based on the presence or absence of CU traits. Youth demonstrating high AB and low levels of CU traits (AB+/CU−) are posited to be emotionally dysregulated, with hyper-reactive threat responses associated with increased amygdala reactivity (Blair, Leibenluft, & Pine, 2014; Hyde, Shaw, & Hariri, 2013; Viding, Fontaine, & McCrory, 2012). Dual pathway models are consistent with wealth of behavioral studies on AB and reactive aggression (e.g., Dodge, Bates, & Pettit, 1990). In contrast, those demonstrating high levels of AB and high levels of CU traits (AB+/CU+) are posited to have relatively decreased amygdala reactivity to other’s distress, potentially associated with impaired fear learning. Developmentally, impairments in this learning process posited to emerge from amygdala dysfunction are thought to impair the development of empathy and lead to CU traits (Blair et al., 2014).
Bridging these findings, several recent studies in youth and adults have shown that when a range of both CU traits and AB are measured within the same study, AB and CU traits show divergent relationships with amygdala reactivity to angry and fearful facial expressions (Carré, Hyde, Neumann, Viding, & Hariri, 2012; Hyde, Byrd, Votruba-Drzal, Hariri, & Manuck, 2014; Lozier, Cardinale, VanMeter, & Marsh, 2014; Sebastian et al., 2012). For example, this model is nicely illustrated by a study showing that AB+CU− youth showed high amygdala reactivity, whereas AB+CU+ youth showed low amygdala reactivity, to fearful expressions (Viding, Sebastian, et al., 2012). Although this theory is gaining empirical support, only a few studies have decoupled the potential effects of AB versus CU traits on amygdala reactivity, as much of this work has been conducted with small samples of extreme clinical or forensic participants categorized dichotomously on both AB and CU traits (Kiehl et al., 2001; Viding, Sebastian, et al., 2012). In most of these studies, participants are high on both AB and CU traits (e.g., Jones et al., 2009; Marsh et al., 2008), which precludes the examination of whether amygdala reactivity is related specifically to level of CU traits or potentially to the severity of AB.
Moreover, as evidence continues to accumulate emphasizing the dimensional nature of AB (Blonigen, Hicks, Krueger, Patrick, & Iacono, 2006; Krueger, Markon, Patrick, Benning, & Kramer, 2007), studies are needed that examine AB and CU traits dimensionally, allowing for the examination of the specific contribution of AB versus CU traits on amygdala reactivity, particularly in samples that have a range of AB and CU traits (i.e., the only studies that have examined these questions dimensionally have been conducted with very healthy community samples: Carré, Hyde, et al., 2012; Hyde et al., 2014). Thus, the first goal of the current study was to examine the relationship between amygdala reactivity to angry and fearful facial expressions and dimensions of AB and CU traits in a large sample of young men in which a wide range of AB and CU traits are present.
Other important considerations
Within our goal of examining the relationship between dimensions of AB and amygdala reactivity, we also aimed to explore several sources of variance that have been theoretically linked to the direction and strength of this relationship (Hyde et al., 2013). First, most work examining amygdala reactivity and AB has contrasted fearful and angry expressions with neutral expressions to isolate the effects to emotional features (e.g., wide eyes of a fearful expression) (Jones et al., 2009; Marsh et al., 2008). However, any resulting differential response could reflect less reactivity to fearful expressions or more reactivity to neutral expressions (e.g., Somerville, Kim, Johnstone, Alexander, & Whalen, 2004). As neutral expressions elicit amygdala reactivity (Ahs, Davis, Gorka, & Hariri, 2014; Arloth et al., 2015; Fitzgerald, Angstadt, Jelsone, Nathan, & Phan, 2006) and neutral faces with directed eye gaze may be perceived differently (i.e., as more threatening) by those who are aggressive (Coccaro et al., 2007; Crick & Dodge, 1996; Dannlowski et al., 2007), previous findings may be as much due to differential response to neutral, as to fearful, expressions. Thus, we examined this relationship with a task that allowed a decoupling of amygdala reactivity to fearful, angry, and neutral expressions, as well as the differential reactivity between expressions.
A second important issue is that the amygdala is not structurally or functionally homogenous, but a heterogeneous collection of subnuclei, many with very different putative roles in threat, emotion, attention, and learning (LeDoux, 2000; LeDoux & Sciller, 2009). Recent neuroimaging studies and a wealth of animal studies have begun to systematically distinguish reactivity of two major amygdala subregions critically important for fear learning (Amunts et al., 2005) and hypothesized to have different roles in psychopathy (Moul, Killcross, & Dadds, 2012). The centromedial (CM) subregion encompasses the central nucleus, which serve as principal output structures driving innate and acquired threat responses via projections to the hypothalamus and brainstem (Duvarci & Pare, 2014). In contrast, the basolateral (BL) subregion encompasses the lateral, basal and accessory basal nuclei important for associative learning and through which prefrontal and hippocampal inputs can guide the appropriate expression of fear (Davis & Whalen, 2001; Duvarci & Pare, 2014). Though these subregions play different roles in emotion and fear behavior, little empirical work has examined how subregional reactivity may be differentially related to AB (e.g., Carré, Fisher, et al., 2012; Hyde et al., 2014).
Third, to our knowledge, almost all studies examining this topic have been primarily, if not entirely, composed of participants that identify as White, European, or European American. This issue is consistent with a broader lack of representative and ethnically/racially diverse samples in neuroscience (Falk et al., 2013). However, the inclusion of under-represented minorities, particularly African Americans, is important based on African Americans’ disproportionate exposure to low-income, dangerous neighborhoods, which are potent predictors of AB (Leventhal & Brooks-Gunn, 2000). Moreover, children living in such communities are exposed to these stressors beginning in early childhood (e.g., harsh parenting, material hardship), with others increasing in intensity as children spend more time outside of the home during middle childhood and adolescence (e.g., exposure to neighborhood and school violence, exposure to deviant peers and adults in the neighborhood, discrimination based on race and income) (Shaw & Shelleby, 2014). Chronic exposure to these stressful contexts within and outside of the home may contribute to differences in neural reactivity (e.g., Burghy et al., 2012; Gianaros & Manuck, 2010; Mays, Cochran, & Barnes, 2007), which could lead to different neurodevelopmental trajectories towards AB in those of different racial, ethnic, or socioeconomic background. Moreover, behavioral studies of psychopathy have emphasized that many “well-established” findings, including the fear deficit hypothesized to emerge from amygdala hypo-reactivity, do not replicate in samples of African Americans (e.g., Baskin-Sommers, Newman, Sathasivam, & Curtin, 2011; Kosson, Smith, & Newman, 1990; Lorenz & Newman, 2002; Newman, Schmitt, & Voss, 1997; Newman & Schmitt, 1998), indicating a pressing need to examine these questions in racially diverse samples and to examine the extent to which race may moderate findings.
Thus, the second major aim of our study was to examine the relationship between dimensions of AB and subregional amygdala reactivity to facial expressions (i.e., fearful, angry, and neutral expressions). Moreover, we examined these aims in a racially diverse sample, where we could examine these questions separately for those identified as European American versus African American.
Age of Onset
Finally, as most research in this area has focused either on AB broadly or on AB in the context of CU and psychopathic traits, our final aim was to examine whether another dominant approach to subtyping AB (i.e., age of onset) was related to amygdala reactivity. Early starting youth are posited to have a neurodevelopmental basis for their AB, as evidenced by verbal and executive function deficits and differences in temperament and emotional reactivity (Moffitt, 1993; Passamonti et al., 2010). Moreover, early starters have also been shown to have the greatest history of harmful contextual risk factors (Moffitt, Caspi, Dickson, Silva, & Stanton, 1996; Shaw, Bell, & Gilliom, 2000; Shaw, Hyde, & Brennan, 2012) and these risk factors (e.g., poverty, maltreatment) are beginning to be linked to differences in brain structure and function (Burghy et al., 2012; Hackman, Farah, & Meaney, 2010; McCrory et al., 2013), further suggesting that early starters should have unique neural correlates. The single fMRI study to examine age of onset of AB as a predictor of amygdala reactivity in adolescence (age 16 – 21) found a robust negative relationship between AB and amygdala reactivity to angry facial expressions (Passamonti et al., 2010). However, few differences were found between early and late starting AB, and CU traits were not related to amygdala reactivity. As this study is the largest of its kind (N = 75) with a sample high on AB, the results emphasize the need for more research in larger samples that assess multiple subtypes/dimensions of AB, including age of onset and CU traits to rule out the potential conclusion that many findings in the literature are simply due to a relationship between the severity of AB and amygdala reactivity, rather than to a different etiology connected to age of onset or CU traits.
The current study
Therefore, the goal of the current study is to examine the relationship between amygdala reactivity and AB dimensionally with attention to the extent to which stimulus type and contrast, amygdala subregion, and participant race could affect findings, with additional analyses examining age of onset of AB. We examined these questions in a relatively large (N = 167) prospective longitudinal study of young men who are at higher risk for AB based on gender, low familial socioeconomic status, and urbanicity. Moreover, within this sample we were able to explore the relative contribution of multiple measures of AB (e.g., self-report, clinical interview, official records), CU traits, and age of onset to amygdala reactivity to emotional faces. We used an fMRI task that allowed for comparisons of amygdala reactivity as a function of differential processing of emotional facial expressions including angry and fearful facial expressions in comparison with neutral facial expressions, as well as a low-level non-face control condition. We also examined these questions at the transition to adulthood when violence and other forms of serious AB peaks, and when both child and adult measures of AB may be applied. We examined behaviors dimensionally to address the increasing conceptualization of psychopathology as dimensional (Krueger & Markon, 2011; Markon & Krueger, 2005) and to address recent studies showing a suppressor effect, wherein only dimensional models examining the unique effects of AB versus CU traits (i.e., those partialling the constructs’ overlapping variance) predict neural reactivity (Hyde et al., 2014; Lozier et al., 2014; Viding, Sebastian, et al., 2012). Moreover, we examined these important questions in a community sample where comorbidity between AB and CU traits is lower and a wide range of AB is present from normative to clinical levels.
Consistent with past research, we hypothesized that AB would be positively correlated, and CU traits negatively correlated, with amygdala reactivity to angry and fearful facial expressions respectively. We expected that those with early starting AB would have the highest amygdala reactivity to angry expressions (though see Passamonti et al., 2010), consistent with a literature suggesting neurocognitive and emotion regulation deficits to be primary in this group (Moffitt, 1993). Consistent with past studies, we also hypothesized that results in those high on CU traits would be specific to fearful facial expressions, whereas AB itself would be correlated with amygdala reactivity to both fearful and angry expressions. We expected these findings to be consistent whether we examined a contrast of angry or fearful versus neutral expressions or versus a non-face control condition. We expected that findings would be the strongest in the CM subregion of the amygdala based on previously studies in our group linking CM functioning to AB (Carré, Fisher, et al., 2012; Carré, Hyde, et al., 2012) and the subregion’s role in driving behavioral and physiological responsiveness to threat, which are disrupted in various forms of AB (Patrick, 2007). Finally, we did not make specific hypotheses regarding the moderating role of race of the participants, but wanted to test if findings would be stronger in the young men who were identified as European-American, as most previous research has focused on this group and several studies of psychopathy have shown a lack of replication in African American samples.
Method
Participants
Participants in this study are part of the Pitt Mother & Child Project (PMCP), an ongoing longitudinal study of 310 low-income boys and their families recruited in 1991 and 1992 from Allegheny County Women, Infant and Children (WIC) Nutritional Supplement Clinics when boys were between 6 and 17 months old (Shaw, Gilliom, Ingoldsby, & Nagin, 2003; Shaw et al., 2012). Target children and their mothers were seen almost yearly from age 1.5 – 20 in the laboratory and/or home with assessments that included questionnaires, a psychiatric interview, and at age 20, an fMRI scanning session. At the time of recruitment, 53% of the target children in the sample were European-American, 36% were African-American, 5% were biracial, and 6% were of other races (e.g., Hispanic-American or Asian-American). Two-thirds of mothers in the sample had 12 years of education or less and the mean per capita income was $241 per month ($2,892 per year) with a mean Hollingshead SES score of 24.5, indicative of a working class to impoverished sample. Thus, a large proportion of the boys/men in this study could be considered at high risk for antisocial outcomes because of their familial socioeconomic standing and urban residence. Retention rates have generally been high at each of the time points from age 1.5- to 20-years old, with sufficient data from age 10 – 17 for trajectory analyses (used for age of onset) on 268 participants (Shaw et al., 2012). For the fMRI component of the study, data were available on 167 participants. Although attrition to the age 20 visit was quite low for such a long-term study (i.e., 256 young men participated at age 20; 83% retention across 19 years), the fMRI component introduced several sources of data loss because of participants who did not want to take part in the MRI portion, had a history of head injury, had bullet or metal fragments in their body, or whose fMRI data did not meet quality standards (see Supplemental Table 1). Of the 167 participants included in the present analyses, 87 (52%) were reported to be European-American 66 (40%) were reported to be African American and were reported to be 14 (8%) “other” (including biracial) by their mothers at age 1.5. When compared with those who dropped out at earlier time points, participants who were included in imaging analyses did not differ on the CBCL externalizing scores at ages 2, or 3.5, maternal age, income or educational attainment (ps = 0.1 to .8). Participants were reimbursed for their time at the end of each assessment and all procedures have been approved by the IRB of the University of Pittsburgh.
Procedures
Amygdala reactivity paradigm
The experimental fMRI paradigm consisted of four blocks of a perceptual face processing task interleaved with five blocks of a sensorimotor control (Carré, Hyde, et al., 2012; Hyde et al., 2014; Manuck, Brown, Forbes, & Hariri, 2007). During the face processing task, subjects viewed a trio of faces and selected one of two faces (bottom) identical to a target face (top; see Figure 1). Each face processing block consisted of six images, balanced for sex, all derived from a standard set of pictures of facial affect (i.e., “Ekman faces”; Ekman & Friesen, 1976). Each of the four face processing blocks consisted of a different emotional facial expression (i.e., anger, fear, surprise, neutral), and participants were randomly assigned to one of four different orders of block presentation. During the sensorimotor control blocks, participants viewed a trio of simple geometric shapes (circles, vertical and horizontal ellipses) and selected one of two shapes (bottom) identical to a target shape (top). All blocks are preceded by brief instructions (“Match Faces” or “Match Shapes”) lasting 2 s. In the face processing blocks, each of the six face trios was presented for 4s with a variable interstimulus interval of 2—6s (mean = 4 s) for a total block length of 48s. A variable interstimulus interval was used to minimize expectancy effects and resulting habituation, as well as to maximize amygdala reactivity throughout the paradigm. In the sensorimotor control blocks, each of the six shape trios was presented for 4s with a fixed inter-stimulus interval of 2s for a total block length of 36s. Total task time was 390s. Subject performance (accuracy and reaction time) was monitored during all scans.
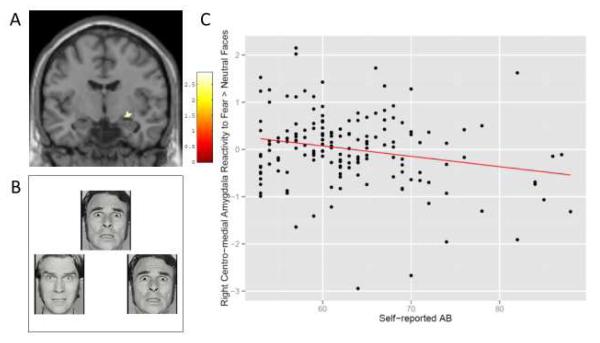
Figure 1.
Self-reported antisocial behavior predicts less centro-medial amygdala reactivity to fearful versus neutral faces.
Caption: (A) Self-report AB was is negatively correlated with amygdala reactivity to fearful faces versus neutral faces in the right centro-medial amygdala. The colored area in the right amygdala identified the region in which a negative correlation exists with self-reported AB (centered at the peak voxel, MNI: 28, −8, −8; t = 2.89, k = 23). (B) An example of the fMRI stimuli used. Participants are asked which face on the bottom matches the face on the top (fearful faces shown). (C) (D) Scatter plot of the relationship between reactivity to fearful versus neutral faces in the left centro-medial amygdala (cluster shown in Panel A) versus scores on the self-report of delinquency.
BOLD fMRI acquisition parameters
Each participant was scanned with a research-dedicated Siemens 3-T Tim Trio. Blood oxygen level–dependent (BOLD) functional images were acquired with a gradient-echo echoplanar imaging (EPI) sequence (repetition time/echo time=2000/29 milliseconds, field of view=200×200), which covered 34 interleaved axial slices (3-mm slice thickness) aligned with the AC-PC plane and encompassing the entire cerebrum and most of the cerebellum to maximum coverage of limbic structures. All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before collecting fMRI data for each participant, a reference echoplanar imaging scan was acquired and visually inspected for artifacts (e.g., ghosting) and good signal across the entire volume of acquisition, including the amygdala. Additionally, an autoshimming procedure was conducted before the acquisition of BOLD data in each participant to minimize field inhomogeneities. Higher-order shimming also was implemented as needed.
Image processing and analysis
Whole-brain image analysis was completed using the general linear model of SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Structural images for each participant were grey matter segmented and functional images were realigned to the mean volume in the time series and unwarped to correct for head motion, co-registered to high resolution structural scans (MPRAGE), spatially normalized into a standard stereotactic space (MNI template) using a 12-parameter affine model, and smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter set at 6 mm FWHM. Voxelwise signal intensities were ratio-normalized to the whole-brain global mean. After preprocessing, the Artifact detection Tools (ART) software package (http://www.nitrc.org/projects/artifact_detect/) was used to detect global mean intensity and translation or rotational motion outlier volumes (>4.5 SD from the mean global brain activation, >2mm movement or 2° translation in any direction) within each participant’s data and to create a regressor accounting for the possible confounding effects of volumes as outliers. Additionally, because of the relatively extensive signal loss typically observed in the amygdala, single-subject BOLD fMRI data were only included in subsequent analyses if there was a minimum of 90% signal coverage in the amygdala bilaterally using our amygdala ROI (Carré, Hyde, et al., 2012).
BOLD fMRI data analysis
The general linear model (GLM) of SPM8 was used to conduct fMRI data analyses. Linear contrasts employing canonical hemodynamic response functions were used to estimate condition-specific (i.e., fear > shapes) BOLD activation for each individual and scan. These individual contrast images (i.e., weighted sum of the beta images) were then used in second-level random effects models that account for both scan-to-scan and participant-to-participant variability to determine mean expression-specific reactivity using one-sample t-tests. The main goal of this study was to examine amygdala reactivity to emotional faces relative to “neutral” faces, with a focus on fearful and angry expressions as has been examined in previous research (e.g., Jones et al., 2009; Marsh et al., 2008). Thus, we present results first with the contrasts of fear>neutral and anger>neutral. Next, as our goal was additionally to explore expression-specific amygdala reactivity in which we could separate out the unique effect of each face type, we also report findings from following contrasts to confirm that any findings from our fear>neutral or anger>neutral are not being driven by differential response to neutral: 1) fear > shapes, 2) anger > shapes, and 3) neutral > shapes (e.g., see Arloth et al., 2015).
As our focus was on subregions of the amygdala, we used two regions of interest (ROI). We defined amygdala centromedial (CM) and basolateral (BL) ROIs using maximum probability maps of cytoarchitectonic boundaries developed by Amunts et al (2005) and implemented through the SPM Anatomy Toolbox (Eickhoff et al., 2005). We then examined reactivity within these specific ROIs. We conducted all amygdala analyses using a small volume correction via the 3DClustSim program which uses a Monte Carlo simulation to provide thresholds that will achieve a Family-Wise Error (FWE) correction for multiple comparisons of p < .05 within each ROI. We used a voxel-wise threshold of p < .01 which resulted in cluster thresholds of k = 7 - 20 contiguous voxels depending on the contrast and resulting smoothness estimate. Note that although these cluster thresholds may seem small, the ROIs are relatively small and using a voxel-level threshold of p < .01 decreased the size of the cluster needed to achieve a FWE correction of p < .05.
Measures
Self-reported Antisocial Behavior
To assess self-reported AB, the Self-Report of Delinquency Questionnaire (SRD) (Elliott, Huizinga, & Ageton, 1985) was administered from age 10 – 20 containing age-appropriate items (33 items from ages 10–12, 62 items from ages 15–17, and 53 at age 20) (see Hyde et al., 2015). At age 20, these items were summed to form a dimensional measure of AB and demonstrated good internal consistency (α = .87 in the imaging subsample).
Age of Onset of Antisocial Behavior (age 10 – 17)
Age of onset of AB was assessed using previously constructed trajectory groups (see Shaw et al., 2012). Within these previous analyses, SRD scores at ages 10, 11, 12, 15, 16, and 17 were used to create trajectory groups using Nagin’s semi-parametric group-based mixture modeling in Proc Traj in SAS 9.2 (Nagin, 2005). Trajectory group models were evaluated using the following criteria: BIC scores, no groups smaller than 4% of the sample, and high posterior probability of group membership. These analyses yielded 4 distinct groups: a low group (n = 171; 63%), a late-starting moderate group (n = 54; 20%), an early high/late desisting group (n = 15; 6%), and an increasing high group (n = 28; 10%). These groups were previously shown to discriminate both court involvement and clinical diagnoses of conduct disorder and oppositional defiant disorder at age 17, as well as antisocial personality disorder and substance use at age 20 (Shaw et al., 2012). As the size of the “early high/late desisting” group was relatively small, especially in the smaller imaging subsample, and as these youth also demonstrated early starting behaviors, this group was combined with the “increasing high” group to create an early starting group. Moreover, even though the “early high/late desisting” group appeared to be “desisting,” they were rated as higher on “lying” at age 10 by their parent, and had antecedent risk and outcomes commensurate with the other early starting group as shown in several recent reports on these trajectories (Hyde et al., 2015; Shaw et al., 2012), leading us to believe that this group may be more accurately characterized as “early starting.” These trajectory analyses were used to create 3 groups in participants with overlapping trajectory and fMRI data (n =157): never antisocial (n = 97; 62%), late starting (n = 30; 19%), and a group that combined two small early starting groups (n = 20; 13%). The proportion of youth in each trajectory group in the imaging subsample was similar to that in the full sample (i.e., 62%, 22%, and 15% respectively).
Antisocial Personality Disorder Symptoms
To assess AB using clinical diagnostic criteria, symptoms of antisocial personality disorder (APD) were made using the SCID-II (First & Gibbon, 1997). The SCID-II is a structured interview that assess DSM-IV adult Axis-II psychiatric symptoms across the lifetime. To establish reliability, clinical interviewers participated in an intensive training program by the training director of the onsite PhD clinical psychology training center who is a licensed clinical psychologist and teaches assessment courses to clinical psychology PhD students. This training was augmented by further supervision and training from advanced doctoral-level clinical psychology students. All examiners were tested for agreement on clinical training video tapes and observed multiple times by experienced examiners before administering the interview. Additionally, every case in which a participant approached or met diagnostic criteria was discussed at regularly held consensus diagnosis meetings, which included all interviewers and the second and last authors, who are licensed clinical psychologists with decades of combined experience in structured clinical interviews. Of the 167 participants with fMRI data available, 16 (9.6%) met criteria for antisocial personality disorder.
Adult Arrest Record
To assess AB from official records, each young man’s involvement with the legal system during early adulthood was assessed as an outcome of interest. We used the Pennsylvania state public court records website to search for the young man’s name and date of birth. These records were last checked in February of 2014 when almost all boys were at least 21 years old (and up to 24 years old). Of the full cohort that was searched, 91 young men (29%) had at least one arrest (44 of 167 in the current subsample). Arrest record was dichotomized (0 = absent; 1 = present/any arrest).
Callous/Unemotional Traits
To assess CU traits, we used a sum of five of six items from the CU factor from self-report on the Antisocial Process Screening Device (APSD) (Frick, Bodin, & Barry, 2000) These 5 items from the APSD assess lack of empathy and affect, and callousness (e.g., you are concerned about the feelings of others) on a 3-point rating scale (1 = not at all true, 2 = sometimes true, 3 = definitely true). At age 20, exploratory and confirmatory factor analyses with MLR estimation in Mplus 5 supported the 6 original CU items loading on one factor. However, because one item (‘you hide your emotions from others’) had a poor loading (−.11) and consistent with our previous work with this measure in this sample (Hyde et al., 2015), this item was excluded, leading to the use of a 5-item factor with modest internal consistency (α = .60), consistent with internal consistency of this measure in past studies (see Dillard, Salekin, Barker, & Grimes, 2013).
Covariates
To assess the extent to which any relationships between AB and amygdala reactivity were unique to AB and not linked to other comorbid psychopathology, we used lifetime symptom counts from the Structured Clinical Interview for the DSM-I (SCID-I) (First, Spitzer, Gibbon, & Williams, 1996) for the diagnoses of Generalized Anxiety Disorder, Social Anxiety Disorder, and Substance Use Disorders, as well as any present symptoms of a Major Depressive Episode. Consistent with DSM-IV criteria, Substance Use Disorders were controlled for by using 4 covariates: Alcohol Abuse symptoms, Alcohol Dependence symptoms, Substance Abuse symptoms, and Substance Dependence symptoms, with all symptoms referring to lifetime diagnostic criteria (see Hyde et al., 2015).
Results
Behavioral results
Zero-order correlations between behavioral variables are reported in Supplemental Table 2. As expected, different measures of AB were significantly related to each other. Self-reported AB correlated moderately with APD symptom counts (r = .41), but more modestly with arrest record (r = .19). CU traits demonstrated positive but unexpectedly small, non-significant relationships with most measures of AB (self-report AB, r = .12, APD symptoms), and a more modest correlation with arrest record (r = .22).
Neural correlates of AB
As shown in Table 1, greater AB was negatively correlated with right CM reactivity for the contrast of fear greater than neutral expressions (see Figure 1). This result extended across both self-report of AB and clinically assessed APD symptoms. Moreover, this result was consistent when examining reactivity from the contrast of fearful expressions versus shapes. This fear versus shapes contrast further revealed two clusters in the left CM in which reactivity was negatively correlated with AB. There was little evidence that response to neutral faces affected the findings, as there were no relationships between AB or CU traits and amygdala reactivity to neutral faces versus shapes. AB was not related to amygdala reactivity to angry faces versus neutral faces, nor when decoupling the contrasts and examining amygdala reactivity to angry or neutral faces versus shapes.
Table 1.
Dimensions of antisocial behavior as they relate to amygdala reactivity
| Measure | Direction of relationship |
Basolateral ROI (MNI) t and voxel extent (k) |
Centro-medial ROI (MNI) t and voxel extent (k) |
|---|---|---|---|
| Fear > Neutral | |||
| Self-report AB | - | Right: 28, −8, −8 t = 2.89, k = 23+* |
|
| Suppression (−) |
Left: −20, −6, −14 t = 3.38, k = 17+ |
Left: −22, −4, −10 t = 3.11, k = 9+ |
|
| APD symptoms | - | Right: 28, −10, −10 t = 2.74, k = 9+* |
|
| CU Traits | ns | ||
| Anger > Neutral | |||
| CU Traits | Suppression (−) |
Right: 36, −4, −20 t = 2.78, k = 12+* |
|
| No relationships with measures of AB found | |||
| Fear > Shapes | |||
| Self-reported AB | - | Left: −22 −4 −12+* t = 4.06; k=19 |
Left: −22 −4 −10+* t = 4.44, k = 12 Left: −30 −12 −10+* t = 3.33, k= 7 Right: 28 −8 −8+* t = 4.65, k = 49 |
| APD symptoms | - | Right: 28 −8 −10+* t = 2.55; k = 7 |
|
| CU traits | ns | ||
| Anger > Shapes | |||
| No significant relationships with AB or CU traits found | |||
| Neutral > Shapes | |||
| No significant relationships with AB or CU traits found | |||
Note: indicated clusters that survived control for CU traits or self-reported AB;
indicated clusters that survived control for CU traits/self-reported AB as well as other psychiatric diagnostic covariates (depression, generalized anxiety disorder, social anxiety disorder, alcohol and substance abuse and dependence symptoms). Suppression indicates clusters that emerged only when controlling for the overlap of self-reported AB and CU traits.
MNI = coordinates in the atlas by the Montreal Neurological Institute. AB = Antisocial Behavior. APD = Antisocial Personality Disorder. CU = Callous Unemotional.
Thus, in contrast to our hypothesis, there was consistent evidence that AB was negatively correlated with CM reactivity to fearful faces (regardless of comparison condition) and this relationship did not extend to angry faces1. Most of the results linking AB to amygdala reactivity to fearful faces, particularly when contrasted to shapes, continued to be significant even after controlling for other possible comorbidities, including substance use, anxiety, and depression, as well as when accounting for overlapping variance with CU traits. Interestingly, there was some evidence of suppression: When controlling for CU traits, new clusters emerged in the left CM and BL ROIs for the contrast of fearful versus neutral faces that were negatively correlated with self-reported AB (see Table 1).
In sum, in contrast to our hypotheses that AB would be related to greater amygdala reactivity to fearful and angry faces, AB was related to less amygdala reactivity and this result was specific to fearful faces. Moreover, these results extended across both self- and clinician-rated AB with some indication of suppression effects when including CU traits in the model. These results were mostly found within the CM subregion of the amygdala.
Neural correlates of CU traits
CU traits were not related to CM or BL reactivity to fearful or angry faces when contrasted to neutral faces in zero-order analyses. However, there was evidence of suppression in that a negative relationship between CU traits and BL reactivity to angry versus neutral faces emerged when controlling for the overlap with AB. CU traits were not related to reactivity of the amygdala to any of the facial emotions when contrasted with shapes. Thus, in contrast to our hypothesis, there was little evidence of a negative relationship between CU traits and amygdala reactivity to fearful faces. Moreover, there was an unexpected suppression relationship in which CU traits predicted less BL reactivity to angry faces when controlling for self-reported AB, suggesting that only the variance unique to CU traits (and not overlapping with AB) was related to BL reactivity.
Neural correlates of age of onset
Age of onset was not related to CM or BL reactivity to fearful faces when contrasted to neutral faces or shapes (see Table 2). Age of onset trajectory groups were related to reactivity to angry faces versus neutral faces. In this case, early and late starters had more CM reactivity than those in the low AB group, but early and late starters did not differ from each other. However, this result appeared to be related to differences in reactivity to neutral rather than angry faces. That is, there was not a relationship between age of onset and reactivity to angry faces versus shapes, but early and late starters had less CM reactivity to neutral faces than those low on AB (and thus more relative reactivity to anger > neutral in this contrast). Overall, however, there was little evidence that amygdala reactivity was different between early versus late starters, and rather that only those in an AB group (early or late starting) were lower on CM reactivity to angry faces than those in the low AB group.
Table 2.
Neural reactivity in relationship to person-centered measures of antisocial behavior
| Measure | Direction of relationship |
Basolateral ROI (MNI) t and voxel extent (k) |
Centro-medial ROI (MNI) t and voxel extent (k) |
|---|---|---|---|
| Fear > Neutral | |||
| No significant relationships with age of onset or arrest record found | |||
| Anger > Neutral | |||
| No significant relationships with age of onset or arrest record found | |||
| Age of onset | Low AB < Early + Late Starters |
Right: 32, −10, −12 t = 2.84, k = 6+* |
|
| Fear > Shapes | |||
| Adult arrest | + | Left: −20 −4 −20+* t = 4.12, k = 104 Right: 24 −4 −18+* t = 4.01, k = 44 |
|
| Age of onset | ns | ||
| Anger > Shapes | |||
| Adult arrest | + | Left: −22, −4, −16+* t = 3.03, k = 28 Right: 24, −6, −12+* t = 3.64, k = 20 |
|
| Age of onset | ns | ||
| Neutral > Shapes | |||
| Adult arrest | + | ||
| Age of onset | Low AB > Early + Late starters |
Right: 32, −10, −10 t= 2.93, k = 6 |
|
| Suppression: Low AB > Early+ Late starters |
Right: 32, −12, −12+* t = 3.23, k = 13 |
||
Note: indicates clusters that survived controlling for CU traits;
indicated clusters that survived control for CU traits as well as other psychiatric diagnostic covariates (depression, generalized anxiety disorder, social anxiety disorder, alcohol and substance abuse and dependence symptoms) AB = Antisocial Behaviors; MNI = coordinates in the atlas by the Montreal Neurological Institute.
Neural correlates of history of arrest
We present correlations with history of arrest separately. Although arrest records are an “objective” measure of AB, many other factors contribute to who is and is not arrested (e.g., income, race, neighborhood). Interestingly, results for arrest record were quite divergent relative to findings using clinical and self-reported AB (see Table 2). History of arrest was not related to CM or BL reactivity to either primary contrast (i.e., fearful > neutral; angry > neutral facial expression), but was positively correlated with BL reactivity to fearful and angry faces when contrasted with shapes (see Supplemental Figure 1). These results are in contrast to findings using self-reported AB in which the correlation was negative and within the CM subregion. The divergence in direction and subregional location of effects between arrest record and APD symptoms are highlighted in Supplemental Figure 1.
Race-specific relationships
Overall, across all analyses, most results appeared to be largely driven by the African American participants (see Supplemental Table 3). There were no significant relationships between CM or BL reactivity to any contrast and AB or CU traits in the European American subsample. Many of the significant relationships found in the total sample were present in the African American subsample, but these relationships were not as robust in this smaller subsample. For example, within the African American subsample, self-reported AB was negatively related to CM reactivity to fearful faces (contrasted with neutral faces or shapes); however, APD symptoms were not related to CM or BL reactivity to any contrast.
Discussion
The current study sought to better understand the relationship between AB and CU traits and amygdala reactivity to angry and fearful facial expressions as indicators of interpersonal threat and distress, respectively, in an at-risk sample of urban men during the transition to adulthood. With a relatively large sample and a substantial range of AB and CU traits, we were able to examine dimensional relationships between AB and CU traits. We further considered the importance of amygdala subregion, participant race, and age of onset of AB. When focusing on the most studied and specific contrast of fearful versus neutral facial expressions, we found that multiple dimensions of AB (but not CU traits) were negatively correlated with CM reactivity. These results were specific to reactivity in the CM amygdala, to fearful expressions contrasted to neutral expressions or to a non-face baseline, and were robust to controlling for relevant psychiatric comorbidities. These results were strongest within the African American men in the sample. In fact, the associations were not significant in the European American men in the sample, thus failing to replicate previous reports.
Dimensional Analyses
Our analyses focusing on dimensional measures of AB and CU traits demonstrated that AB, but not CU traits, were negatively correlated with reactivity in the CM subregion to fearful facial expressions across different measures of AB. That these results were robust across contrasts of fearful versus neutral expressions and fearful expressions versus a control, non-face condition allays concern that results from previous studies were affected by more reactivity to neutral expressions. At the same time, our results linking AB, but not CU traits, to less amygdala reactivity to fearful expressions stand in contrast to much of the extant literature that has suggested CU traits specifically are linked to less amygdala reactivity both dimensionally (Carré, Hyde, et al., 2012; Hyde et al., 2014; Lozier et al., 2014) and categorically (Jones et al., 2009; Marsh et al., 2008; Viding, Sebastian, et al., 2012).
One major limitation in many of the studies conducted in this area is that their samples were recruited to contain groups extreme on both AB and CU traits, which may confound severity of AB with the presence of CU traits. Even within a recent study well-designed to address this confound that utilized a control group and two groups high on AB (i.e., AB+CU+ versus AB+CU−), the AB+CU+ group still had the highest levels of AB (Viding, Sebastian, et al., 2012). The current results suggest that the severity of AB, and not the presence of CU traits, underlies the negative association with amygdala reactivity to fearful facial expressions. This conclusion, if replicated in future studies, would have important implications for our understanding of the neural correlates of AB and challenge many of the current neurobiological models of CU traits and psychopathy, which assume that lower amygdala reactivity is uniquely associated with AB in the presence of high CU traits or with CU traits specifically.
At the same time, our results may not be comparable to these other studies in several ways. First, many of the studies examining this question categorically have done so in samples that compare youth high on AB and high versus low on CU traits (Jones et al., 2009; Marsh et al., 2008; Viding, Sebastian, et al., 2012). However, even within the current sample that had greater than a 30% arrest rate in adolescence and in early adulthood, few young men met criteria for APD (or even conduct disorder at age 17) with a “diagnosis” of CU traits/limited prosocial emotions (see Hyde et al., 2015). Thus, we could not examine large subgroups with clinically meaningful levels of both AB and CU traits. It may be that CU traits have a different relationship with amygdala reactivity when in the presence of very high and clinically meaningful AB, than when considered dimensionally across a range of levels of AB. To address this potential issue, we ran several regressions in which we examined the interaction of AB and CU traits dimensionally and found no evidence that this interaction term predicted amygdala reactivity to fearful or angry facial expressions (results not shown). Future studies that compare categorical versus dimensional measures of AB and CU traits in samples with a wide range of AB and CU and heavy enrichment for AB could help to clarify the extent to which the relationship between CU traits and amygdala reactivity may be dependent on the level of AB.
Second, this sample is comprised only of young men during the transition to adulthood within a narrow age range (i.e., age 20). Most of the studies in this area have focused on extreme groups of youth in early to mid-adolescence with a wider age range (e.g., Viding, Sebastian, et al., 2012), relatively healthy young adults (e.g., Carré, Hyde, et al., 2012), or incarcerated adults (Kiehl et al., 2001), leaving few studies of comparable age and of comparable distribution of AB and CU traits. The age of participants is likely important, as mean levels of amygdala reactivity to emotional facial expressions change throughout childhood, adolescence, and adulthood (Hare et al., 2008), with increased maturation of top-down prefrontal control of limbic regions (Giedd et al., 1996; Shaw et al., 2008). Thus, studies are needed that explore age as a potential moderator of the relationship between AB and amygdala reactivity (Hyde, 2015).
Third, our fMRI task is different than the one used in many prior studies. In most of these studies (e.g., Jones et al., 2009; Marsh et al., 2008), the participant is shown one face and asked to identify the face as either “male” or “female”. In our study, participants saw three faces and were asked to match two identical faces. Our task consistently elicits amygdala reactivity, however, it differs with regards to perceptual processing and attentional demands because the participant matches multiple faces based on perceptual features, rather than identifying the gender of a single face. Research has shown that the attentional load of the task may affect the relationship between AB and amygdala reactivity (White et al., 2012), that low eye contact may underlie emotional deficits in those high on CU traits (Dadds, El Masry, Wimalaweera, & Guastella, 2008; Han, Alders, Greening, Neufeld, & Mitchell, 2011), and that attention to the eye region of the face affects amygdala reactivity (Demos, Kelley, Ryan, Davis, & Whalen, 2008). If our task required more attention to the eyes than a gender decision task, then that difference could have normalized the amygdala reactivity of those higher on CU traits (Han et al., 2011). The use of eye-tracking during these common fMRI tasks will help address this question.
Fourth, within this sample we have found that our measure of CU traits does not seem to be a robust predictor of many AB outcomes. In a previous behaviorally-focused report (Hyde et al., 2015) and the current neuroimaging study, we have seen few relationships between the current measure of CU traits in this sample and expected outcomes, such as greater AB across time (for more information on this measure and its distribution compared to other samples see Hyde et al., 2015). It could be that self-reported CU trait items “mean” something different within this low-income, racially diverse sample. Alternatively, it could be that applying this measure to young adults is inappropriate or that the well-documented psychometric issues with the measure (Dillard et al., 2013) may have led to an underestimation of the effects of CU traits in this sample (i.e., our measure had a relatively low internal consistency and was not highly correlated with AB, which could lead to an underestimation of effects; see Supplemental Tables 2 and 5 for descriptive statistics and correlations). Our null findings could reflect poor measurement of the construct and analyses with a more extensive measure of CU traits (e.g., the Inventory of Callous-Unemotional traits; Kimonis et al., 2008), an age-appropriate self-report measure of psychopathy (e.g., the Self-Report of Psychopathy, see Carré, Hyde, et al., 2012), or a structured interview (e.g., the Psychopathy Checklist), may have yielded different results. We did try analyzing these data with an augmented measure of CU traits that combines the current items of the Antisocial Process Screening Device with items tapping empathy from the Child and Adolescent Disposition Scale (Lahey et al., 2008) to generate a measure of “limited prosocial emotions” (Waller, Shaw, Forbes, & Hyde, 2014). However, this measure did not yield significant relationships with amygdala reactivity either.
Suppression
We did find some evidence of suppression when examining dimensional measures of AB. These effects were not large (i.e., no evidence of cross-over suppression where the direction of the relationship changes), but controlling for the overlap of CU traits and AB, did appear to strengthen the relationship between amygdala reactivity to fearful expressions and AB and even uncovered a relationship between CU traits and amygdala reactivity to angry expressions. It should not be surprising that these suppression effects were not particularly large based on the fairly low correlation between AB and CU traits. Suppression is likely to be more pronounced when the overlap between variables is larger (Hyde et al., 2014; Paulhus, Robins, Trzesniewski, & Tracy, 2004). However, these results continue to support the notion that controlling for the overlap between AB and CU traits may uncover specific relationships with criterion variables and that variance unique to AB versus CU traits may be the most highly related to amygdala reactivity.
Age of onset
For the most part, age of onset was not predicted by amygdala reactivity to any of the stimuli. Although early and late starters differed from the low AB group in reactivity to fearful and neutral faces, there was little evidence for differences in neural reactivity between early and late starters. Our generally null findings with regard to separating early versus late starters are consistent with the one previous study examining age of onset that found a main effect for AB, but few differences between early and late starters (Passamonti et al., 2010). Interestingly, in this study the only difference found between early and late starters was in response to sad facial expressions, which our task did not contain. Thus, we may not have found differences because they are specific to sad facial expressions. On the other hand, amygdala reactivity may not the best neural measure for examining etiological differences between early and late starters. Many of the neurocognitive differences found between early and late starters have been related to executive functioning (Moffitt, 1993), and tasks that tap cortical regions subserving these functions are important for future research aiming to understand the neural differences between these groups.
History of arrest
Examining a categorical measure of arrest led to a more complex picture of the results. In this case, arrest was not related to the fearful or angry versus neutral expressions, but was positively related to amygdala reactivity to fearful and angry facial expressions when contrasted with the control, non-face condition. Although this positive relationship appears to contradict the negative relationship we found between other measures of AB and amygdala reactivity to fearful expressions, closer inspection suggests a potential explanation for this finding. In this case, the findings with adult arrest were found exclusively within the BL subregion, which could highlight divergence in the relationship between AB and amygdala reactivity depending on subregion (see Supplemental Figure 1 to compare these regions). In fact, if we lowered the multiple comparison statistical threshold on our analysis of dimensional measures of AB (i.e., self-reported AB, APD symptoms) correlating with amygdala reactivity, this same positive relationship with reactivity to fearful facial expressions emerged in the BL (results not shown, as these thresholds do not adequately address multiple comparison concerns). Albeit thought-provoking, we consider this explanation very tentative.
The potential explanation that AB could have a divergent relationships with amygdala reactivity in the CM versus BL subregions would be broadly consistent with the recently proposed “Differential Amygdala Activation Model” (DAAM; Moul et al., 2012). However, the DAAM posits the basolateral amygdala to be underactive and the central amygdala average or overactive in psychopathy, which is the opposite direction of our current findings. Though our findings are at odds with this model, it may be difficult for our results to address the DAAM for two reasons. First, our findings and sample are not focused on those diagnosed with psychopathy. Second, as noted above, our neuroimaging task requires matching faces which constrains eye movement. This aspect of the task may “force” attention and could normalize any potential etiological differences in the automatic allocation of attention. Thus, our task may not be ideal for testing the DAAM model. At the very least, our findings emphasize the need to examine different subregions of the amygdala.
Beyond an anatomical explanation for the positive relationship between BL reactivity and arrest, it is important to consider that arrest record is a complex, multifaceted outcome subject to many factors outside of the behaviors of the participant. For example, the type of AB, where it occurs, and the race of the participant all affect the probability of arrest (Krisberg et al., 1987; Smith, 1986). Thus, factors that contribute to arrest may have different neural correlates than those that involve ABs not resulting in arrest (Gao & Raine, 2010).
Race specific findings
Overall, many of our results appeared to be most robust among African Americans. To the extent that our findings diverge from the extant literature, our race-specific analyses may help to explain the divergence and suggest, along with studies in psychopathy (e.g., Baskin-Sommers et al., 2011; Lorenz & Newman, 2002), that building a literature on samples that are mostly or exclusively European in origin may be problematic and lead to “well-established” findings that do not replicate in minority samples (Falk et al., 2013), the latter of which also tend to have lower socioeconomic status. Within the European American subsample, the sample size (n = 87) was still much larger than most existing studies in this area, and yet, we found no significant relationships between AB or CU and amygdala reactivity to angry or fearful facial expressions. Thus, it is important to consider the subsample results as a potential failure to replicate past studies, which is important for this fast-growing field.
We should also consider an additional caveat about this task (and many other similar tasks), when interpreting the findings in the African American subsample: the faces all came from the original Ekman set (Ekman & Friesen, 1976), which were generated by actors of apparent European ancestry (i.e., White). A growing literature has shown that amygdala reactivity is modulated by the race of the model (Hart et al., 2000). The extent to which the faces use in our task are seen as in-group versus out-group members (Van Bavel, Packer, & Cunningham, 2008) may modulate the extent to which they function as a probe of “threat” (Phelps et al., 2000) or the extent to which pictures of distress in outgroup members stimulates empathy and affective response in participants (Gutsell & Inzlicht, 2012). A more balanced set of faces with multiple races represented (e.g., Tottenham et al., 2009) would allow for an examination of the extent to which the race of the face presented moderates the current findings and we are currently collecting such data to examine this question.
A final and critical consideration in interpreting our findings that are specific to African Americans (and largely in the opposite direction as past literature), is that African Americans, particularly those in this high risk, urban sample, are often exposed to a higher rate of a broad range of acute and chronic stressors, even relative to the European American men in this sample (Williams, Yu, Jackson, & Anderson, 1997). Moreover, African Americans face specific stressors including micro-aggressions, structural racism, and overt racism, not experienced by European Americans, even those of the same low SES (Mays et al., 2007). Although life stress has been linked to greater amygdala reactivity (Burghy et al., 2012; McCrory et al., 2013), these studies have been carried out with samples of youth that are composed of primarily European Americans. It could be that the broader and more chronic stressors faced by the African American men in this sample across 20 years may contribute to race-based differences in allostatic load and to differential patterns of amygdala response to fearful faces in early adulthood (e.g., a blunting over time of response to threat in a subset of participants that are at increased risk for AB) (Mays et al., 2007; Ng-Mak, Salzinger, Feldman, & Stueve, 2004). These findings are important from a measurement and theoretical standpoint emphasizing that research only focusing on those of European origin is quite limited and that those of different races, ethnicities, and economic strata may have different neurodevelopmental pathways towards AB. These findings further highlight the need to consider the context participants live and grew-up in when studying or theorizing about pathways to AB (Raine, 2002). Therefore, important next steps in this field are to leverage longitudinal data to better understand how these individual differences in amygdala reactivity emerge and if they differ across development and context.
Limitations
Whereas our study has several strengths, including a relatively large sample size, a racially diverse sample of participants, longitudinal measurement of AB trajectories, multi-method measurement of AB, a well-studied neuroimaging task, and dimensional measures of AB and CU traits, there are several limitations worth noting. First, as noted above, our sample and methodology differs in many ways from previous studies including the use of a different task and a different age group. The use of a measure of CU traits, rather than an adult measure of psychopathy, may have affected our findings among these emerging adults. Moreover, our measure of CU traits was self-reported and there have been debates in the field about validity of self-reports of CU and psychopathic traits (Lilienfeld, 1994). Second, this study involved a sample of high-risk urban men and thus may not generalize to samples of girls and women, those of higher socioeconomic status, or those outside of urban areas. Third, even though the sample was practically enriched for AB, it was a longitudinal, high-risk design, rather than a case-control design, and thus there was not a large proportion of young men meeting criteria for clinical diagnoses of APD. Moreover, several of our participants were not able to participate because of current incarceration, further limiting the representation of more severe AB. Hence, our findings are not comparable to clinical or forensic studies.
Future Directions
Our findings may offer more questions than they answer, but they also make clear the importance of attending to “nuances” in study design and analysis including the importance of amygdala subregion, and the age, SES, race, and ethnicity of participants. The next important studies in this field will need to examine these questions simultaneously in a variable and person-centered approach in large samples that contain substantial proportions of individuals high on AB, with substantial variability in CU traits, and with multiple fMRI tasks that tap different functions of the amygdala (e.g., Viding, Sebastian, et al., 2012; White et al., 2012). Moreover, longitudinal studies with diverse samples are needed with multiple neuroimaging time points to examine the impact of race and developmental stage on the direction of findings (Hyde et al., 2013). Finally, as studies begin to articulate the role amygdala reactivity plays in various forms of AB, the next important question is how these individual differences in amygdala reactivity arise, which will involve specifying the specific interactions between experience and biology that shape neurodevelopmental and behavioral trajectories across childhood and adolescence (Hyde, 2015). To accomplish this goal will require measuring and conceptualizing the complex development of AB at multiple levels of analysis within studies that consider multiple factors (e.g., parenting, genetic variation, different behavioral manifestations) over time in an interactive and mechanistic framework (e.g., Beauchaine & McNulty, 2013).
Conclusions
In sum, we found that self-reported and clinical ratings of AB were negatively related to amygdala reactivity, specifically to fearful facial expressions. These results appeared to be strongest in the CM subregion and within the African American subsample of our relatively large, diverse, and high-risk sample of young men. CU traits and age of onset showed few significant associations with amygdala reactivity, but arrest record was positively correlated with BL reactivity to both fearful and angry expressions. These findings challenge the notion that amygdala hypoactivity observed in past studies is specific to CU traits and may instead map onto the severity of AB. Moreover, our results suggest that the CM and BL subregions of the amygdala play different roles in AB and CU traits, with CM reactivity appearing most important for AB. Finally, our results call for greater attention to examining neural correlates of AB in more diverse samples, as our findings were not equivalent across those identified as European American and African American. Clearly the association between amygdala reactivity to emotional facial expressions and AB is complex and likely affected by task demands, sample demographics, developmental stage, subregional anatomy, and measurement of AB and CU traits (Hyde et al., 2013). Consideration of these nuances, however, will be necessary to fully inform our understanding of the etiology and treatment of AB.
01
Supplemental Table 1: Summary of available data for analyses
Supplemental Table 2: Zero-order correlations of main study behavioral variables
Supplemental Table 3: Neural reactivity correlates of dimensions of antisocial behavior by parentreported race
Supplemental Table 4: Neural reactivity correlates of age of onset and arrest record behavior by parentreported race
Supplemental Table 5: Descriptive statistics for measures of antisocial behavior across total and European and African American subsamples.
Supplemental Figure 1: Different measures of antisocial behavior demonstrate divergent relationships with centro-medial versus basolateral amygdala reactivity.
Caption: (A) Clinical ratings of antisocial behavior are negatively related to amygdala reactivity to fearful faces: APD symptoms are negatively related to amygdala reactivity to fearful faces versus shapes in the right centro-medial amygdala region of interest (centered on right peak voxel, MNI: 28, −10, −10, t = 2.74, k = 9). (B) Young men with a history of being arrested as an adult demonstrate greater amygdala reactivity to fearful faces: Bilateral amygdala reactivity differentiates those who have versus those who have not been arrested in the basolateral amygdala region of interest centered on the peak voxel in the left basolateral amygdala (MNI: −20, −4, −20, t = 4.12, k = 104; right cluster MNI: 24, −4, −18, t = 4.01, k = 44).
Acknowledgments
The research reported in this paper was supported by grants to D.S.S. (R01 MH50907, R01 MH01666, and K05 DA25630), D.S.S. and E.E.F (R01 DA026222), and L.W.H. (L40 DA036468) by the National Institutes of Health. A.R.H. received support by National Institutes of Health grants R01DA033369, R01DA031579, R01AG049789, and R21MH106715. We are grateful to the work of the staff of the Pitt Mother & Child Project for their many years of service, and to our study families for sharing their lives with us and making the research possible.
Footnotes
As our findings supported the specificity of AB being related to amygdala reactivity to fearful and not angry facial expressions, we ran exploratory analyses with a direct contrast of fearful versus angry facial expressions. There were no statistically significant main effects in the amygdala of this task contrast across the entire sample (likely because both expressions significantly engage the amygdala). However, activity from this contrast was significantly correlated with both self-reported and clinical symptoms of AB supporting the notion that AB is uniquely related to less reactivity to fearful facial expressions (results available upon request).
Authorship: D.S.S. designed the longitudinal study. D.S.S. and E.E.F. designed the study concept and oversaw all data collection. A.R.H. designed the neuroimaging faces protocol. D.S.S., E.E.F., and L.W.H. contributed to study design. L.W.H., L.M., and A.G. performed data analysis and interpretation under the supervision of L.W.H. and L.W.H. drafted the paper and all authors provided critical revisions of the manuscript. All authors approved the final version of the paper for submission.
References
- Ahs F, Davis CF, Gorka AX, Hariri AR. Feature-based representations of emotional facial expressions in the human amygdala. Social Cognitive and Affective Neuroscience. 2014;9:1372–1378. doi: 10.1093/scan/nst112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Arloth J, Bogdan R, Weber P, Frishman G, Menke A, Wagner KV, et al. Genetic Differences in the Immediate Transcriptome Response to Stress Predict Risk-Related Brain Function and Psychiatric Disorders. Neuron. 2015;86:1189–1202. doi: 10.1016/j.neuron.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Newman JP, Sathasivam N, Curtin JJ. Evaluating the generalizability of a fear deficit in psychopathic African American offenders. Journal of Abnormal Psychology. 2011;120:71–78. doi: 10.1037/a0021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, McNulty T. Comorbidities and Continuities as Ontogenic Processes: Toward a Developmental Spectrum Model of Externalizing Psychopathology. Development and Psychopathology. 2013;25:1505–1528. doi: 10.1017/S0954579413000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, Passamonti L, Calder AJ. Appetitive motivation predicts the neural response to facial signals of aggression. The Journal of Neuroscience. 2008;28:2719–2725. doi: 10.1523/JNEUROSCI.0033-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Leibenluft E, Pine DS. Conduct Disorder and Callous–Unemotional Traits in Youth. New England Journal of Medicine. 2014;371:2207–2216. doi: 10.1056/NEJMra1315612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Peschardt KS, Budhani S, Pine DS. The development of psychopathy. Journal of Child Psychology and Psychiatry. 2006;47:262–276. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, Iacono WG. Continuity and change in psychopathic traits as measured via normal-range personality: a longitudinal-biometric study. Journal of Abnormal Psychology. 2006;115:85–95. doi: 10.1037/0021-843X.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Social Cognitive and Affective Neuroscience. 2012;7:213–221. doi: 10.1093/scan/nsq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré JM, Hyde LW, Neumann CS, Viding E, Hariri AR. The neural signature of distinct psychopathic traits. Social neuroscience. 2012;8:122–135. doi: 10.1080/17470919.2012.703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Crick NR, Dodge KA. Social information processing mechanisms in reactive and proactive aggression. Child Development. 1996;67:993–1002. [PubMed] [Google Scholar]
- Dadds MR, El Masry Y, Wimalaweera S, Guastella AJ. Reduced eye gaze explains “fear blindness” in childhood psychopathic traits. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:455–463. doi: 10.1097/CHI.0b013e31816407f1. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, et al. Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Research: Neuroimaging. 2007;154:13–20. doi: 10.1016/j.pscychresns.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y, Lahey BB. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biological Psychology. 2009;80:203–211. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Kelley WM, Ryan SL, Davis FC, Whalen PJ. Human amygdala sensitivity to the pupil size of others. Cerebral Cortex. 2008;18:2–29. doi: 10.1093/cercor/bhn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard CL, Salekin RT, Barker ED, Grimes RD. Psychopathy in Adolescent Offenders: An Item Response Theory Study of the Antisocial Process Screening Device–Self Report and the Psychopathy Checklist: Youth Version. Personality Disorders: Theory, Research, and Treatment. 2013;4:101–120. doi: 10.1037/a0028439. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Bates JE, Pettit GS. Mechanisms in the cycle of violence. Science. 1990;250:1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen W. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Elliott D, Huizinga D, Ageton S. Explaining delinquency and drug use. Sage Publications; Beverly Hills, CA: 1985. [Google Scholar]
- Falk EB, Hyde LW, Mitchell C, Faul J, Gonzalez R, Heitzeg MM, et al. Neuroscience meets Population Science: What is a representative brain? Proceedings of the National Academy of Sciences. 2013;110:17615–17622. doi: 10.1073/pnas.1310134110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders Research Version (SCID-I) New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- First MB, Gibbon M. User's guide for the structured clinical interview for DSM-IV axis II personality disorders: SCID-II. American Psychiatric Pub; 1997. [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Foster EM, Jones DE. The high costs of aggression: Public expenditures resulting from conduct disorder. American Journal of Public Health. 2005;95:1767–1772. doi: 10.2105/AJPH.2004.061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, Bodin SD, Barry CT. Psychopathic traits and conduct problems in community and clinic-referred samples of children: Further development of the Psychopathy Screening Device. Psychological assessment. 2000;12:382–393. [PubMed] [Google Scholar]
- Frick PJ, Nigg JT. Current issues in the diagnosis of attention deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder. Annual Review of Clinical Psychology. 2012;8:77–107. doi: 10.1146/annurev-clinpsy-032511-143150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, White SF. Research review: The importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology and Psychiatry. 2008;49:359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raine A. Successful and unsuccessful psychopaths: A neurobiological model. Behavioral Sciences and the Law. 2010;28:194–210. doi: 10.1002/bsl.924. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Manuck SB. Neurobiological pathways linking socioeconomic position and health. Psychosomatic Medicine. 2010;72:450–461. doi: 10.1097/PSY.0b013e3181e1a23c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey B, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996;6:551–559. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gutsell JN, Inzlicht M. Intergroup differences in the sharing of emotive states: neural evidence of an empathy gap. Social Cognitive and Affective Neuroscience. 2012;7:596–603. doi: 10.1093/scan/nsr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature Reviews Neuroscience. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T, Alders GL, Greening SG, Neufeld RWJ, Mitchell DGV. Do fearful eyes activate empathy-related brain regions in individuals with callous traits? Social Cognitive and Affective Neuroscience. 2011 doi: 10.1093/scan/nsr068. epub online 10/22/11; doi: 10.1093/scan/nsr1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey B. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Neuroreport. 2000;11:2351–2354. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Hyde LW. Developmental psychopathology in an era of molecular genetics and neuroimaging: A developmental neurogenetics approach. Development and Psychopathology. 2015;27:587–613. doi: 10.1017/S0954579415000188. [DOI] [PubMed] [Google Scholar]
- Hyde LW, Burt SA, Shaw DS, Donnellan MB, Forbes EE. Early starting, aggressive and callous-unemotional? Examining overlap and predictive utility of antisocial behavior subtypes. Journal of Abnormal Psychology. 2015;124:329–342. doi: 10.1037/abn0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Byrd AL, Votruba-Drzal E, Hariri AR, Manuck SB. Antisocial behavior and amygdala reactivity: Divergent correlates of antisocial personality and psychopathy traits in a community sample. Journal of Abnormal Psychology. 2014;123:214–224. doi: 10.1037/a0035467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, Hariri AR. Neuroscience, developmental psychopathology and youth antisocial behavior: Review, integration, and directions for research. Developmental Review. 2013;33:168–223. doi: 10.1016/j.dr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Gareth JB, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kahn RE, Frick PJ, Youngstrom E, Findling RL, Youngstrom JK. The effects of including a callous–unemotional specifier for the diagnosis of conduct disorder. Journal of Child Psychology and Psychiatry. 2012;53:271–282. doi: 10.1111/j.1469-7610.2011.02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, et al. Limbic abnormalities in affective processing by criminal psychpaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Skeem JL, Marsee MA, Cruise K, Munoz LC, et al. Assessing callous-unemotional traits in adolescent offenders: Validation of the Inventory of Callous-Unemotional Traits. International Journal of Law and Psychiatry. 2008;31:241–252. doi: 10.1016/j.ijlp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Smith SS, Newman JP. Evaluating the construct validity of psychopathy in black and white male inmates: three preliminary studies. Journal of Abnormal Psychology. 1990;99:250–259. doi: 10.1037//0021-843x.99.3.250. [DOI] [PubMed] [Google Scholar]
- Krisberg B, Schwartz I, Fishman G, Eisikovits Z, Guttman E, Joe K. The incarceration of minority youth. Crime & Delinquency. 1987;33:173–205. [Google Scholar]
- Krueger RF, Markon KE. A Dimensional-Spectrum Model of Psychopathology: Progress and Opportunities. Archives of General Psychiatry. 2011;68:10. doi: 10.1001/archgenpsychiatry.2010.188. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Chronis AM, Jones HA, Williams SH, Loney J, et al. Psychometric characteristics of a measure of emotional dispositions developed to test a developmental propensity model of conduct disorder. Journal of Clinical Child & Adolescent Psychology. 2008;37:794–807. doi: 10.1080/15374410802359635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sciller D. The human amygdala: Insights from other animals. In: Whalen PJ, Phelps EA, editors. The human amygdala. Guilford Press; New York: 2009. pp. 43–60. [Google Scholar]
- Leventhal T, Brooks-Gunn J. The neighborhoods they live in: The effects of neighborhood residence on child and adolescent outcomes. Psychological Bulletin. 2000;126:309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO. Conceptual problems in the assessment of psychopathy. Clinical Psychology Review. 1994;14:17–38. [Google Scholar]
- Loeber R, Hay D. Key issues in the development of aggression and violence from childhood to early adulthood. Annual Review of Psychology. 1997;48:371–410. doi: 10.1146/annurev.psych.48.1.371. [DOI] [PubMed] [Google Scholar]
- Lorenz AR, Newman JP. Do emotion and information processing deficiencies found in Caucasian psychopaths generalize to African-American psychopaths? Personality and individual differences. 2002;32:1077–1086. [Google Scholar]
- Lozier LM, Cardinale EM, VanMeter JW, Marsh AA. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71:627–636. doi: 10.1001/jamapsychiatry.2013.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT. A study of anxiety in the sociopathic personality. The Journal of Abnormal and Social Psychology. 1957;55:6–10. doi: 10.1037/h0047232. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal stability of individual differences in amygdala reactivity. American Journal of Psychiatry. 2007;164:1613–1614. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- Markon KE, Krueger RF. Categorical and continuous models of liability to externalizing disorders: A direct comparison in NESARC. Archives of General Psychiatry. 2005;62:1352–1359. doi: 10.1001/archpsyc.62.12.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson DS, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annual Review of Psychology. 2007;58:201–225. doi: 10.1146/annurev.psych.57.102904.190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, et al. Amygdala activation in maltreated children during pre-attentive emotional processing. The British Journal of Psychiatry. 2013;4:269–276. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Jaffee SR, Kim Cohen J, Koenen KC, Odgers CL, et al. Research Review: DSM V conduct disorder: research needs for an evidence base. Journal of Child Psychology and Psychiatry. 2008;49:3–33. doi: 10.1111/j.1469-7610.2007.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Dickson N, Silva P, Stanton W. Childhood-onset versus adolescent-onset antisocial conduct problems in males: natural history from ages 3 to 18 years. Development and Psychopathology. 1996;8:399–424. [Google Scholar]
- Moffitt TE, Caspi A, Harrington H, Milne BJ. Males on the life-course-persistent and adolescence-limited antisocial pathways: follow-up at age 26 years. Development and Psychopathology. 2002;14:179–207. doi: 10.1017/s0954579402001104. [DOI] [PubMed] [Google Scholar]
- Moul C, Killcross S, Dadds MR. A Model of Differential Amygdala Activation in Psychopathy. Psychological Review. 2012;119:789–806. doi: 10.1037/a0029342. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Group-based modeling of development. Harvard Univ Press; Cambridge, MA: 2005. [Google Scholar]
- Newman J, Schmitt W, Voss W. Processing of contextual cues in psychopathic and nonpsychopathic offenders. Journal of Abnormal Psychology. 1997;106:563–575. doi: 10.1037//0021-843x.106.4.563. [DOI] [PubMed] [Google Scholar]
- Newman JP, Schmitt WA. Passive avoidance in psychopathic offenders: a replication and extension. Journal of Abnormal Psychology. 1998;107:527–532. doi: 10.1037//0021-843x.107.3.527. [DOI] [PubMed] [Google Scholar]
- Ng-Mak DS, Salzinger S, Feldman RS, Stueve CA. Pathologic adaptation to community violence among inner-city youth. American Journal of Orthopsychiatry. 2004;74:196–208. doi: 10.1037/0002-9432.74.2.196. [DOI] [PubMed] [Google Scholar]
- Nock MK, Kazdin AE, Hiripi E, Kessler RC. Prevalence, subtypes, and correlates of DSM-IV conduct disorder in the National Comorbidity Survey Replication. Psychological Medicine. 2006;36:699–710. doi: 10.1017/S0033291706007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odgers CL, Caspi A, Broadbent JM, Dickson N, Hancox RJ, Harrington HL, et al. Prediction of differential adult health burden by conduct problem subtypes in males. Archives of General Psychiatry. 2007;64:4–6. doi: 10.1001/archpsyc.64.4.476. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, Rowe JB, et al. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Archives of General Psychiatry. 2010;67:729–738. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ. Antisocial personality disorder and psychopathy. In: O'Donohue W, Fowler KA, Lilienfield SO, editors. Personality disorders: Toward the DSM-V. Sage Publications, Inc; Thousand Oaks, CA, US: 2007. [Google Scholar]
- Patterson GR, Reid JB, Dishion TJ. Antisocial boys. Castalia; Eugene, OR: 1992. [Google Scholar]
- Paulhus DL, Robins RW, Trzesniewski KH, Tracy JL. Two replicable suppressor situations in personality research. Multivariate Behavioral Research. 2004;39:303–328. doi: 10.1207/s15327906mbr3902_7. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, et al. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJP, Cecil CAM, Lockwood PL, De Brito SA, Fontaine NMG, et al. Neural Responses to Affective and Cognitive Theory of Mind in Children With Conduct Problems and Varying Levels of Callous-Unemotional Traits. Archives of General Psychiatry. 2012;69:814–822. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Bell RQ, Gilliom M. A truly early starter model of antisocial behavior revisited. Clinical child and family psychology review. 2000;3:155–172. doi: 10.1023/a:1009599208790. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Gilliom M, Ingoldsby EM, Nagin DS. Trajectories leading to school-age conduct problems. Developmental Psychology. 2003;39:189–200. doi: 10.1037//0012-1649.39.2.189. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Hyde LW, Brennan LM. Predictors of Boys’ Antisocial Trajectories from Toddlerhood through Adolescence. Development and Psychopathology. 2012;24:871–888. doi: 10.1017/S0954579412000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DS, Shelleby EC. Early-onset conduct problems: intersection of conduct problems and poverty. Annual Review of Clinical Psychology. 2014;10:503. doi: 10.1146/annurev-clinpsy-032813-153650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA. The neighborhood context of police behavior. Crime and justice. 1986;8:313–341. [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biological Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biological Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bavel JJ, Packer DJ, Cunningham WA. The neural substrates of in-group bias a functional magnetic resonance imaging investigation. Psychological Science. 2008;19:1131–1139. doi: 10.1111/j.1467-9280.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- Viding E, Fontaine NMG, McCrory EJ. Antisocial behaviour in children with and without callous-unemotional traits. Journal of the Royal Society of Medicine. 2012;105:195–200. doi: 10.1258/jrsm.2011.110223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CAM, De Brito SA, et al. Amygdala Response to Preattentive Masked Fear in Children With Conduct Problems: The Role of Callous-Unemotional Traits. American Journal of Psychiatry. 2012;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Waller R, Shaw DS, Forbes EE, Hyde LW. Understanding early contextual and parenting risk factors for the development of limited prosocial emotions. Journal of Abnormal Child Psychology. 2014 doi: 10.1007/s10802-014-9965-7. Epub ahead of print: DOI 10.1007/s10802-10014-19965-10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Marsh AA, Fowler KA, Schechter JC, Adalio C, Pope K, et al. Reduced Amygdala Response in Youths With Disruptive Behavior Disorders and Psychopathic Traits: Decreased Emotional Response Versus Increased Top-Down Attention to Nonemotional Features. American Journal of Psychiatry. 2012;169:750–758. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health socio-economic status, stress and discrimination. Journal of health psychology. 1997;2:335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Summary of available data for analyses
Supplemental Table 2: Zero-order correlations of main study behavioral variables
Supplemental Table 3: Neural reactivity correlates of dimensions of antisocial behavior by parentreported race
Supplemental Table 4: Neural reactivity correlates of age of onset and arrest record behavior by parentreported race
Supplemental Table 5: Descriptive statistics for measures of antisocial behavior across total and European and African American subsamples.
Supplemental Figure 1: Different measures of antisocial behavior demonstrate divergent relationships with centro-medial versus basolateral amygdala reactivity.
Caption: (A) Clinical ratings of antisocial behavior are negatively related to amygdala reactivity to fearful faces: APD symptoms are negatively related to amygdala reactivity to fearful faces versus shapes in the right centro-medial amygdala region of interest (centered on right peak voxel, MNI: 28, −10, −10, t = 2.74, k = 9). (B) Young men with a history of being arrested as an adult demonstrate greater amygdala reactivity to fearful faces: Bilateral amygdala reactivity differentiates those who have versus those who have not been arrested in the basolateral amygdala region of interest centered on the peak voxel in the left basolateral amygdala (MNI: −20, −4, −20, t = 4.12, k = 104; right cluster MNI: 24, −4, −18, t = 4.01, k = 44).