Abstract
BACKGROUND
The cardiac natriuretic peptides (NPs), atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP), have central roles in sodium and blood pressure regulation. Extracardiac factors (e.g., obesity and diabetes) influence NP production, potentially altering cardiovascular responses to volume and pressure stress.
OBJECTIVES
This study examined the effects of acute carbohydrate intake on the NP system in humans, and investigated underlying mechanisms.
METHODS
Normotensive subjects (N = 33) were given a high-carbohydrate shake. Venous blood was sampled to measure N-terminal (NT)-proANP and NT-proBNP levels. Human embryonic stem cell–derived cardiomyocytes (hESC-CMs) and HepG2 cells were treated with glucose, and expression levels of NPs and micro ribonucleic acid 425 (miR-425), a negative regulator of ANP, were examined. The role of nuclear factor kappa B (NF-κB) in the glucose-mediated effects was investigated using a NF-κB inhibitor and expression plasmids encoding NF-κB subunits.
RESULTS
We observed a 27% reduction in the levels of circulating NT-proANP (p < 0.001, maximal at 6 h) after carbohydrate challenge, with no effect on NT-proBNP levels in our human subjects. Glucose treatment of hESC-CMs for 6 h and 24 h increased levels of the primary transcript of miR-425 (pri-miR-425) and mature miR-425. A corresponding decrease in NPPA messenger RNA levels was also observed at both time points. Overexpression of NF-κB subunits in H9c2 cardiomyocytes increased miR-425 levels, whereas inhibition of NF-κB abrogated the glucose-mediated increase in pri-miR-425 levels in HepG2 cells.
CONCLUSIONS
Acute carbohydrate challenge is associated with a reduction in ANP production. The mechanism appears to involve a glucose-induced increase in the expression of miR-425, mediated by NF-κB signaling.
Keywords: cardiomyocyte, glucose, micro ribonucleic acid, obesity
The heart synthesizes a family of hormones known as natriuretic peptides (NPs), which includes atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP). These molecules promote renal sodium excretion and arterial vasodilation, and inhibit cardiac hypertrophy (1–3). Recent studies suggest that NPs have a wide range of favorable metabolic effects as well, including activation of brown fat (4), lipolysis, and improvement in skeletal muscle oxidative capacity (5).
Obese individuals have unexpectedly low NP levels (6). It has been proposed that a “relative NP deficiency” predisposes obese individuals to salt retention, hypertension, and glucose intolerance. Thus, understanding the chronic and acute factors that contribute to reduced NP levels in obese individuals holds important implications (7–9). Because the reduction in NP levels is particularly prominent among those with insulin resistance and hyperinsulinemia, we investigated whether a high dietary glucose load can acutely suppress circulating NP concentrations. Such a response would be a disadvantage in obese individuals who have lower NP levels to begin with, thereby contributing to a “vicious cycle” of impaired fat and glucose metabolism.
The objectives of this study were to: 1) assess the acute effects of a carbohydrate challenge on NP levels in healthy subjects; 2) determine whether there is a difference in the magnitude of change in NPs in lean versus obese individuals; and 3) investigate potential mechanisms linking hyperglycemia and reduced NP levels.
As part of the third objective, we investigated the role of micro ribonucleic acid (miRNA)-425 (miR-425), a recently discovered negative regulator of ANP (10). miRNAs are noncoding RNAs, which repress messenger RNA (mRNA) translation and/or stability by binding to partially complementary sequences in target mRNAs, typically in the 3′ untranslated region (3′UTR) (11). We have shown that miR-425 interacts with the 3′UTR of NPPA to repress NPPA mRNA levels and the secretion of N-terminal proANP (NT-proANP) from human cardiomyocytes (10). The regulation of miR-425 expression is largely unexplored; however, Ma and colleagues reported that in human cancer cells, the expression of miR-425 is up-regulated by the transcription factor nuclear factor kappa B (NF-κB) (12). In addition, the expression and activity of NF-κB are increased in vascular smooth muscle cells and endothelial cells in the presence of high glucose (13–15). We tested the hypothesis that NF-κB activation, induced by glucose, up-regulates miR-425 expression, leading to downstream effects on NPPA expression.
METHODS
All participants provided written informed consent, and the hospital institutional review board approved the study. Housing and procedures involving experimental animals (mice) were approved by the Institutional Animal Care and Use Committees of Massachusetts General Hospital (Subcommittee on Research Animal Care). A detailed description of the methods is provided in the Online Appendix.
STATISTICAL ANALYSIS
Plasma glucose, insulin, and natriuretic peptide measurements at baseline and after carbohydrate challenge were logarithmically transformed for analysis given their skewed distributions. Mixed effect models incorporating all non-missing data from 33 study subjects were used to assess the effects of time and carbohydrate challenge and their interaction on plasma NT-proANP and NT-proBNP levels. For mRNA, the primary transcript of miRNA (pri-miRNA), and miRNA levels, we performed 2-sample or paired Student t tests, as appropriate. All analyses were conducted using SAS Version 9.4 software (SAS Institute, Cary, North Carolina). A 2-sided p < 0.05 was considered statistically significant. Student t tests are reported without adjustments for multiple comparisons.
RESULTS
Characteristics of the study sample are shown in Table 1. The mean ± SD systolic blood pressure (BP) for our study participants was 112 ± 8 mm Hg and mean diastolic BP was 71 ± 7 mm Hg. Body mass index (BMI) ranged from 19 to 41 kg/m2.
TABLE 1.
Baseline Characteristics of the Study Sample
| Lean (n = 19) | Obese (n = 14) | |
|---|---|---|
| Age, yrs | 28 ± 6 | 31 ± 5 |
| Women | 40 | 38 |
| Body mass index, kg/m2 | 22 ± 2.1 | 32 ± 4.4 |
| Systolic blood pressure, mm Hg | 111 ± 7 | 114 ± 7 |
| Diastolic blood pressure, mm Hg | 71 ± 6 | 72 ± 7 |
| Sodium, mmol/l | 140 ± 1.5 | 140 ± 2.5 |
| Potassium, mmol/l | 4.4 ± 0.5 | 4.0 ± 0.3 |
| Creatinine, mg/dl | 0.94 ± 0.1 | 0.93 ± 0.2 |
| Serum glucose, mg/dl | 83 ± 8.6 | 85 ± 8.8 |
Values are mean ± SD or %.
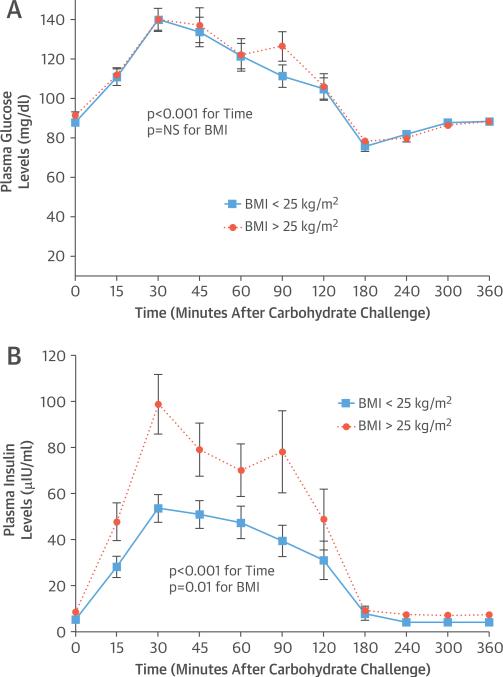
The mean fasting plasma glucose level for the overall group was 90 ± 7 mg/dl. After carbohydrate challenge (Online Table 1), plasma glucose peaked at 140 ± 23 mg/dl (p < 0.001), with no difference between the lean and overweight or obese individuals (Figure 1A). At baseline, mean plasma insulin levels were 5 ± 2 μIU/ml in lean versus 9 ± 4 μIU/ml in overweight or obese individuals. After the carbohydrate challenge, peak insulin levels increased to 54 ± 26 μIU/ml in lean individuals and to 99 ± 49 μIU/ml in overweight or obese individuals (Figure 1B).
FIGURE 1. Carbohydrate Challenge: Plasma Glucose and Insulin Levels.
Plasma glucose (A) and insulin (B) levels are shown at baseline and in response to the carbohydrate challenge in lean (n = 19) (solid lines with squares) and overweight or obese (n = 14) (dotted lines with circles) individuals. Data are expressed as mean ± SEM.
BMI = body mass index.
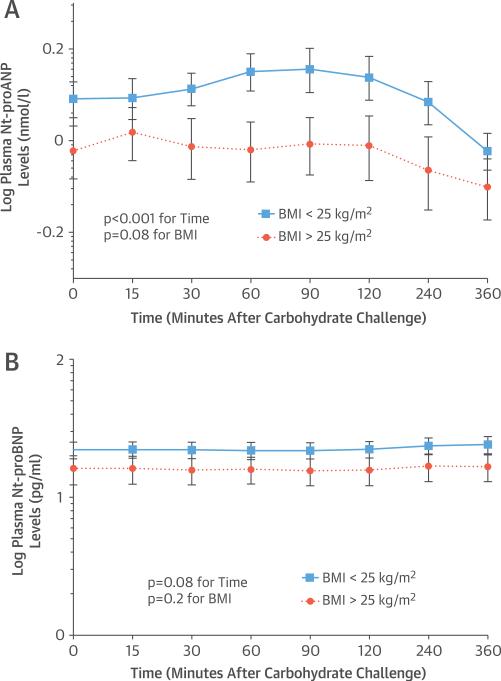
Plasma NT-proANP concentrations after overnight fasting are shown according to BMI in Figure 2A. Lean individuals had 28% higher plasma levels of NT-proANP at baseline than did overweight or obese individuals. With the carbohydrate challenge, we observed a 27% reduction in mean levels of circulating NT-proANP in the overall group (p < 0.001), with a 30% reduction in lean individuals and a 20% reduction in overweight or obese individuals (Figure 2A). There was no evidence of a time-by-BMI interaction.
FIGURE 2. Carbohydrate Challenge: Plasma NT-proANP and NT-proBNP Levels.
Concentrations of plasma N-terminal pro-atrial natriuretic peptide (NT-proANP) levels (A) and plasma N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels (B) at baseline and at 15, 30, 60, 90, 120, 240, and 360 min after the carbohydrate challenge. The solid line with squares represents lean subjects, and the dotted line with circles represents overweight or obese subjects. Data are expressed as mean ± SEM.
BMI = body mass index.
In contrast to NT-proANP, plasma NT-proBNP levels did not change after the carbohydrate challenge (Figure 2B).
GLUCOSE EFFECTS ON miR-425 AND NPPA mRNA LEVELS
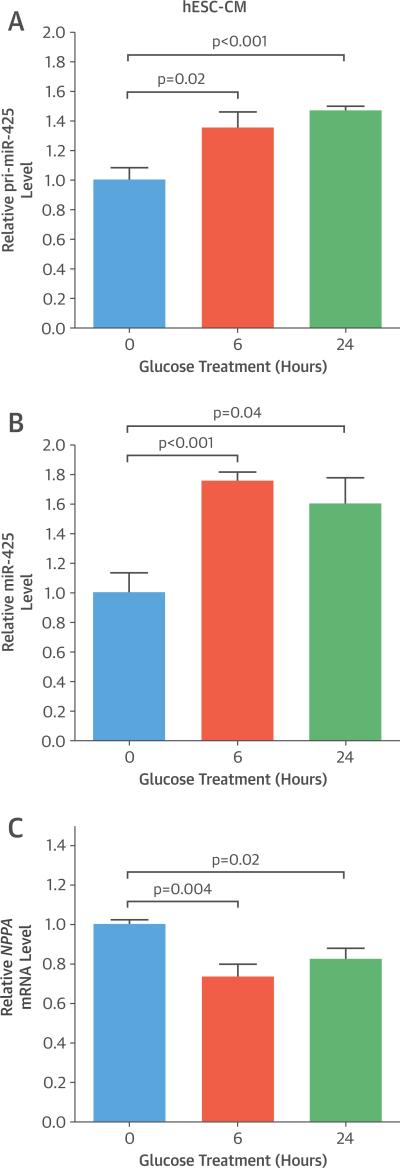
As the acute change in plasma NP levels appeared to be restricted to ANP, we performed further studies with a focus on miR-425, a negative regulator of ANP production (10). First, we investigated whether glucose treatment of human cardiomyocytes increased miR-425 expression levels. Human embryonic stem cell–derived cardiomyocytes (hESC-CMs) were cultured overnight in medium containing 1 mmol/l glucose and then treated with 5 mmol/l glucose for 6 h and 24 h. There was a 35% increase in the levels of the primary transcript of miR-425 (pri-miR-425) at 6 h (p = 0.02) and a 47% increase at 24 h (p < 0.001) of glucose treatment (Figure 3A).
FIGURE 3. Effect of Glucose Treatment on Cardiomyocytes.
Human embryonic stem cell–derived cardiomyocytes (hESC-CMs) were cultured overnight in medium containing 1 mmol/l glucose and then treated with 5 mmol/ glucose for 6 h and 24 h. Primary transcript of micro ribonucleic acid 425 (pri-miR-425) levels (A), mature miR-425 levels (B), and NPPA messenger ribonucleic acid (mRNA) levels (C) are relative to those in untreated cells. Data are expressed as mean ± SEM (n = 6).
miR-425 is an intronic miRNA, encoded in the first intron of the DALR anticodon binding domain containing 3 (DALRD3) gene. There was no increase in the mRNA levels of DALRD3 in response to either 6 h or 24 h of glucose treatment in hESC-CMs (data not shown).
In line with the increase in the levels of pri-miR-425, we observed a 76% increase in the levels of mature miR-425 at 6 h (p < 0.001) and a 60% increase in mature miR-425 levels at 24 h (p = 0.04) of glucose treatment in hESC-CMs (Figure 3B). A corresponding decrease in NPPA mRNA levels was also observed in the hESC-CMs at both time points of glucose treatment (27% decrease at 6 h, p = 0.004; and 18% decrease at 24 h, p = 0.02) (Figure 3C). These results suggest that glucose increases miR-425 at the level of transcription. There was no change in the mRNA levels of NPPB in response to either 6 h or 24 h of glucose treatment in hESC-CMs (data not shown).
TRANSCRIPTIONAL REGULATION OF miR-425
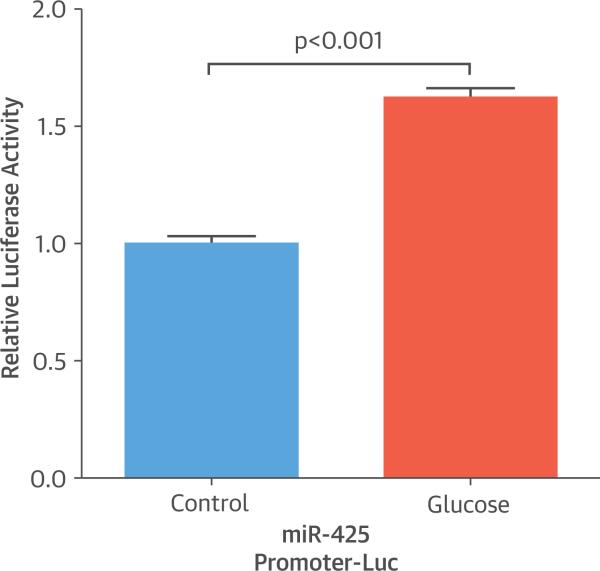
To further examine whether glucose transcriptionally regulates miR-425 expression, we cloned the miR-425 promoter sequence (from –2,000 base pairs [bp] to –1 bp from the transcription start site) in front of a firefly luciferase reporter gene, generating the miR-425 promoter-Luc construct, and evaluated reporter activity in HepG2 cells treated with or without glucose. Glucose treatment for 6 h resulted in a 62% increase in luciferase activity in cells containing the miR-425 promoter-Luc construct compared with cells without glucose treatment (p < 0.001) (Figure 4). These results suggest that glucose treatment induces miR-425 promoter activity and provide support for a potential transcriptional mechanism underlying the glucose-mediated increase in miR-425 expression levels.
FIGURE 4. Luciferase Activity.
Glucose treatment increased the luciferase activity encoded by the micro ribonucleic acid 425 (miR-425) promoter-luciferase construct (miR-425 promoter-Luc). The ratio of firefly luciferase activity to renilla luciferase activity was normalized to that in HepG2 cells transfected with a plasmid directing expression of luciferase without the miR-425 promoter sequence (relative luciferase activity). Data are expressed as mean ± SEM (n = 6).
To identify transcription factors that could interact with the promoter of the miR-425 gene and regulate its transcription, we used the Encyclopedia of DNA Elements (ENCODE) (16) databases. From the transcription factor ChIP-seq track (17), we identified binding sites for NF-κB, a transcription factor known to be induced by glucose (14,18), in the promoter region of the gene encoding miR-425 (Online Figure 1). In addition, we manually screened the miR-425 promoter region (from –2,000 bp to –1 bp from the transcription start site), and identified a putative NF-κB binding site located at –651 bp, with the NF-κB consensus sequence 5′-GGGAGGCCCC-3′. These analyses led us to hypothesize that glucose could activate NF-κB to up-regulate miR-425 expression, leading to a reduction in ANP production.
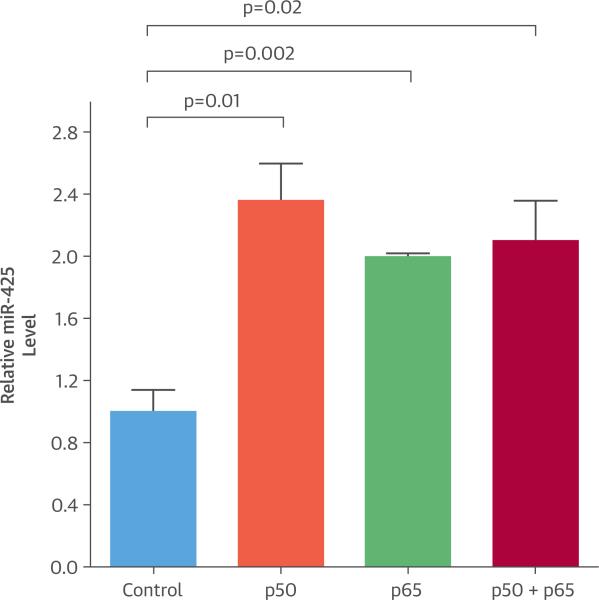
We transfected plasmids encoding NF-κB subunits p50 and/or p65 into H9c2 rat cardiomyocytes and examined the expression levels of mature miR-425. Overexpression of NF-κB subunits p50 and/or p65 in H9c2 rat cardiomyocytes resulted in a 2-fold increase in mature miR-425 levels (p50, p = 0.01; p65, p = 0.002; p50 + p65, p = 0.02) (Figure 5), suggesting that NF-κB activation up-regulates miR-425 levels in cardiomyocytes. Similar results were seen in HEK 293 cells (Online Figure 2).
FIGURE 5. Effect of NF-κB on miR-425.
Cardiomyocytes were transfected with constructs expressing either nuclear factor kappa B (NF-κB) p50 or p65. After 24 h, cells were harvested. Overexpression of the NF-κB p50 subunit alone or together with the p65 subunit increased mature micro ribonucleic acid 425 (miR-425) levels in H9c2 cardiomyocytes. Mature miR-425 levels are relative to those in cells transfected with an empty construct (Control). Data are expressed as mean ± SEM (n = 3).
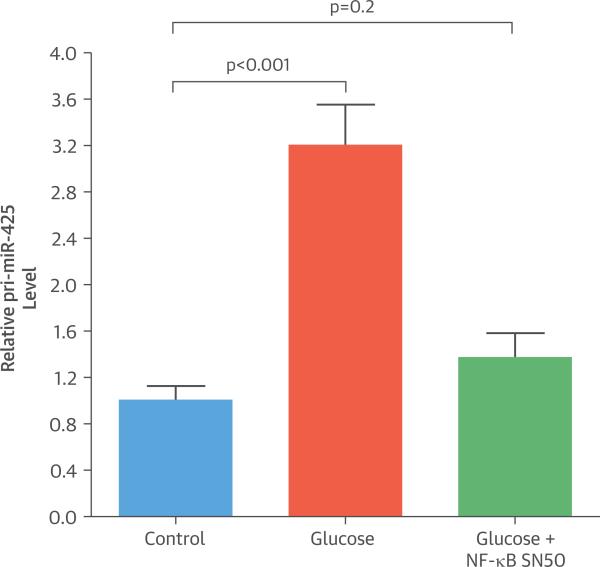
To confirm a role for NF-κB in the glucose-mediated increase in miR-425, we treated HepG2 cells with 30 mmol/l glucose for 6 h in the presence or absence of SN50, an NF-κB inhibitor. Similar to our results in hESC-CMs, HepG2 cells treated with glucose showed a 3-fold increase in the levels of primiR-425 (p < 0.001) (Figure 6) and a 92% increase in the levels of mature miR-425 (p = 0.03) compared with untreated cells. The increase in pri-miR-425 levels in response to glucose was abrogated by SN50 (Figure 6). These results suggest that NF-κB has a key role in elevating miR-425 levels in the presence of glucose by enhancing the transcription of the miR-425 gene.
FIGURE 6. Effect of SN50 on pri-miR-425.
HepG2 cells were cultured overnight in medium containing 1 mmol/l glucose and 50 μg/ml SN50, an NF-κB inhibitor, followed by treatment with 30 mmol/l glucose for 6 h. Glucose-mediated increase in pri-miR-425 levels is abrogated in the presence of SN50. pri-miR-425 levels are relative to those in untreated cells (Control). Data are expressed as mean ± SEM (n = 6).
Abbreviations as in Figure 5.
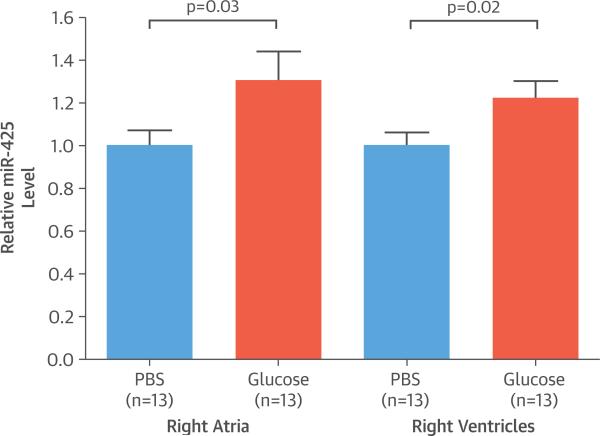
Next, we investigated whether high blood glucose concentrations in vivo were associated with increased miR-425 expression. Although the mature miR-425 sequence is conserved between human and mouse, the miR-425-binding site in NPPA is primate-specific and not present in the mouse Nppa 3′UTR. Thus, we used transgenic mice carrying the human NPPA gene on a C57BL/6J background (NPPAtg/+ mice). Following a 16-h fasting period, NPPAtg/+ mice (n = 13) were gavaged with either phosphate-buffered saline or 2 g/kg glucose and then sacrificed 6 h later. The NPPAtg/+ mice lost 18% of body weight after overnight fasting at the time of harvest (p < 0.001). Blood glucose levels increased from a mean 66 ± 6 mg/dl to a peak of 241 ± 56 mg/dl 30 min after glucose administration via gavage (Online Figure 3). The right atria and right ventricles of mice administered glucose exhibited a 30% (p = 0.03) and 22% (p = 0.02) increase in miR-425 expression levels, respectively (Figure 7). No effect on miR-425 levels was seen in the left atria or ventricles (data not shown). A corresponding decrease in human NPPA mRNA levels (p = 0.03) was observed in the right ventricles of mice administered glucose. Additionally, we noted a 19% increase in miR-425 expression levels in the pancreas of mice administered glucose (p = 0.01).
FIGURE 7. Effect of Glucose on Transgenic Mice.
After a 16-h fast, C57BL/6J mice expressing a human NPPA transgene (NPPAtg/+ mice) were administered glucose at 2 g/kg body weight or phosphate-buffered saline (PBS) as a control. Oral administration of glucose increased cardiac miR-425 levels in NPPAtg/+ mice compared with controls. Data are expressed as mean ± SEM (n = 13). miR-425, micro ribonucleic acid 425.
DISCUSSION
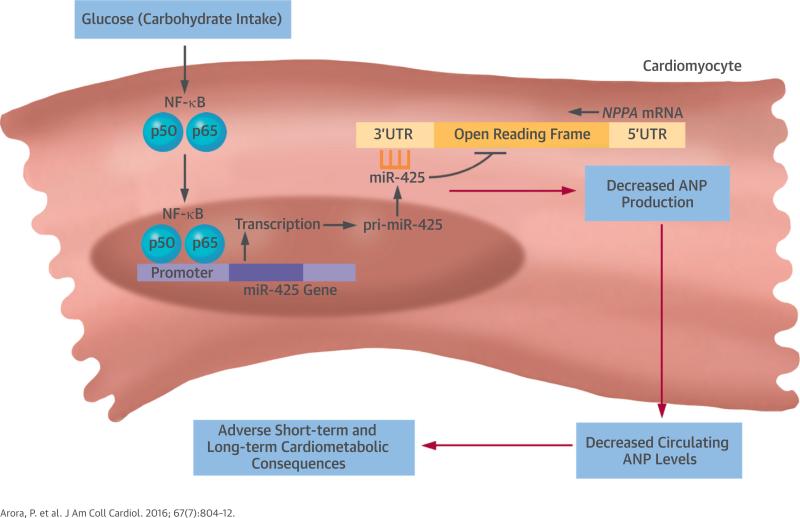
In this study, we demonstrated that a high carbohydrate intake acutely decreases circulating NT-proANP levels in humans. We provided evidence that the underlying mechanism appears to involve the induction of NF-κB by glucose, leading to increased expression of miR-425, a negative regulator of NPPA (Central Illustration). These clinical and experimental findings elucidate a novel mechanism for acute metabolic regulation of the NP axis, which may have important physiologic implications given the role of the NP system in cardiovascular homeostasis.
CENTRAL ILLUSTRATION. Metabolic Influences on NPs: Model of a High-Carbohydrate Challenge in Humans.
A high glucose load stimulates nuclear factor kappa B (NF-κB) signaling, which induces the expression of micro ribonucleic acid 425 (miR-425), a negative regulator of atrial natriuretic peptide (ANP) production, in cardiomyocytes. Suppression of ANP production could have adverse short- and long-term cardiometabolic consequences, because ANP plays a role in blood pressure reduction, salt excretion, activation of brown fat, and increased energy expenditure. mRNA = messenger ribonucleic acid; NP = natriuretic peptide; pri-miR-425 = primary transcript of miR-425; UTR = untranslated region.
We and others have previously reported that low NP levels are particularly prevalent among individuals with hyperinsulinemia (19–21). This raises the possibility that hyperinsulinemia and/or reduced insulin sensitivity may contribute to the low NP levels found in obese individuals compared with lean individuals. In our study, plasma NT-proANP levels were lower in obese individuals compared with lean individuals at all time points.
Although an abundance of clinical data demonstrates that obesity and glucose intolerance are associated with lower NP concentrations, there are few data regarding the response of the NP system to acute metabolic shifts. Previously, Goetze (22) examined the effect of a carbohydrate challenge on circulating NP concentrations in 6 individuals. As in our study, Goetze found reduced plasma NT-proANP levels after a high-carbohydrate meal; however, the underlying mechanism for this phenomenon was not investigated. Similar to Goetze (22), we did not observe a significant change in plasma NT-proBNP levels over the course of 6 h after ingestion of the high-carbohydrate shake. If anything, we noted a trend towards a slight increase in plasma NT-proBNP levels at 4 to 6 h after the carbohydrate challenge in both groups (p = 0.08) (Figure 2B). These data suggested that it is unlikely that BNP would have subsequently decreased over longer periods of observation, although we cannot exclude that possibility.
The isolated reduction in NT-proANP levels observed in our human study raised the possibility of an NPPA-specific mechanism, because ANP and BNP appear to use common pathways for clearance. We previously reported that miR-425 is a negative regulator of ANP production (10). Thus, we hypothesized that up-regulation of miR-425 expression is involved in the reduction in NT-proANP levels. Experiments in 2 different human cell types (hESC-CMs and HepG2) supported the hypothesis that glucose up-regulates miR-425 expression. In human cardiomyocytes, glucose treatment also resulted in a corresponding decrease in NPPA mRNA levels. Transcriptional regulation of miR-425 expression by glucose was supported by a glucose-mediated increase in pri-miR-425 levels in the human cardiomyocytes, as well as by our luciferase reporter assays in HepG2 cells, in which glucose treatment increased the luciferase activity of a miR-425 promoter-Luc construct. Interestingly, although miR-425 is encoded in the intron of the host gene DALRD3, glucose treatment of hESC-CMs did not increase DALRD3 mRNA levels. Our finding is in accordance with previous studies (23,24) that have reported a low correlation between coexpression of miR-425 and DALRD3 mRNA levels in different tissues, indicating that the miR-425 gene may be regulated by a different promoter.
NF-κB emerged as a potential mediator of the glucose-induced up-regulation of miR-425 expression because we observed that the promoter of the miR-425 gene contains binding sites for NF-κB. Also, pre-incubation of HepG2 cells with SN50, an inhibitor of NF-κB, abrogated the glucose-mediated increase in pri-miR-425 levels. Transcriptional activation of miR-425 expression by NF-κB has previously been reported in human gastric adenocarcinoma cells lines (12). Our findings were the first to demonstrate that NF-κB–mediated induction of miR-425 can be regulated by glucose and hyperglycemic conditions. In addition, we observed that in NPPAtg/+ mice, glucose administration elevated cardiac miR-425 levels, particularly in the right atria and right ventricles, and decreased human NPPA mRNA levels in the right ventricles. Moreover, it is important to note that glucose administration increased miR-425 levels in the pancreas, a tissue that is sensitive to changes in blood glucose levels. Taken together, our in vitro and in vivo results support the hypothesis that the carbohydrate-induced reduction in plasma NT-proANP levels observed in humans is NF-κB– and miR-425–dependent.
STUDY LIMITATIONS
We studied young normotensive volunteers in order to minimize interference from comorbidities or medication use. The clinical findings of NPPA suppression after acute carbohydrate challenge warrant replication in different populations, such as individuals of older age with hypertension or heart failure. Our study protocol required 3 days of standardized diets, followed by overnight fasting and ingestion of a high-carbohydrate meal, thus limiting the sample size. However, providing study subjects with a standardized diet for 3 days allowed us to reduce some of the random variation in NP measurements that can be observed in the general population.
Although we had adequate power for detecting the main effect of ANP and BNP responses to carbohydrate ingestion, larger sample sizes may have been needed to detect subgroup interactions, such as the difference in response between lean and overweight or obese individuals. The chamber specificity of the miR-425 and NPPA response to glucose in the NPPAtg/+ mice is unclear, but a possible explanation is that there could be chamber-specific differences in the expression levels of GLUT4 (glucose transporter type 4), the major glucose transporter expressed in the heart (25).
In our in vivo studies, we fasted the NPPAtg/+ mice for 16 h before glucose administration, rather than for shorter periods of time, to reduce competition for glucose utilization from long-chain fatty acids, the main source of energy in the heart (26). Glucose and fatty acids compete for usage by the heart, depending on the concentrations of either substrate in the body, and reciprocally inhibit the usage of the other. A 16-h fast increased the probability that fat stores in the mice would have been depleted, resulting in significantly diminished levels of circulating fatty acids.
CONCLUSIONS
We identified a novel mechanism whereby diet may influence the cardiovascular system in an acute manner. Our findings suggest that the induction of miR-425 levels in response to glucose leads to reduced ANP levels after a carbohydrate challenge. The NPs exert a range of beneficial actions, including reduction in BP, salt excretion, activation of brown fat, and increased energy expenditure. Thus, suppression of NP production could have adverse short-term and long-term cardiometabolic consequences. Additional work is warranted to investigate whether interrupting NF-κB or miR-425 signaling could prevent these acute changes in ANP production and have favorable effects on cardiometabolic health.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Acute increases in blood glucose decrease circulating levels of NT-proANP in both lean and overweight or obese individuals, though obese people generally have lower ANP levels at baseline. The beneficial effects of ANP, which include reducing blood pressure and preventing myocardial hypertrophy, may explain the advantages to over-weight and obese patients of avoiding foods high in carbohydrates.
TRANSLATIONAL OUTLOOK
More work is needed to develop methods that augment ANP production under hyperglycemic conditions and evaluate the utility of this strategy to prevent the cardiovascular problems associated with obesity and diabetes.
ACKNOWLEDGMENTS
The authors thank the Massachusetts General Hospital Clinical Research Center staff and the Massachusetts General Hospital Center for Comparative Medicine for animal care.
This study was supported by the following grants: Harvard Catalyst 1 UL1 RR025758; R01-HL-086875; R01-HL-102780; R01-DK-082971; funds of the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital; T32HL007208 from the National Heart, Lung, And Blood Institute; and the Leducq Foundation. Drs. Arora, Bloch, Newton-Cheh, and Wang are named as co-inventors on a patent application relating to the use of miRNAs for the treatment of hypertension and other disorders.
ABBREVIATIONS AND ACRONYMS
- 3′UTR
3′ untranslated region
- ANP
atrial natriuretic peptide
- BMI
body mass index
- BNP
B-type natriuretic peptide
- BP
blood pressure
- bp
base pair(s)
- hESC-CM
human embryonic stem cell–derived cardiomyocyte
- miRNA
micro ribonucleic acid
- miRNA-425
micro ribonucleic acid 425
- mRNA
messenger ribonucleic acid
- NF-κB
nuclear factor kappa B
- NP
natriuretic peptide
- NT-proANP
N-terminal pro-atrial natriuretic peptide
- NT-proBNP
N-terminal pro–B-type natriuretic peptide
- pri-miR-425
primary transcript of miR-425
- pri-miRNA
primary transcript of micro ribonucleic acid
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For an expanded Methods section, and a supplemental table and figures, please see the online version of this article.
REFERENCES
- 1.John SW, Krege JH, Oliver PM, et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–81. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 2.Tamura N, Ogawa Y, Chusho H, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A. 2000;97:4239–44. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver PM, Fox JE, Kim R, et al. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A. 1997;94:14730–5. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bordicchia M, Liu D, Amri EZ, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–36. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engeli S, Birkenfeld AL, Badin PM, et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122:4675–9. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 7.Pivovarova O, Gogebakan O, Kloting N, et al. Insulin up-regulates natriuretic peptide clearance receptor expression in the subcutaneous fat depot in obese subjects: a missing link between CVD risk and obesity? J Clin Endocrinol Metab. 2012;97:E731–9. doi: 10.1210/jc.2011-2839. [DOI] [PubMed] [Google Scholar]
- 8.Zois NE, Bartels ED, Hunter I, et al. Natriuretic peptides in cardiometabolic regulation and disease. Nat Rev Cardiol. 2014;11:403–12. doi: 10.1038/nrcardio.2014.64. [DOI] [PubMed] [Google Scholar]
- 9.Arora P, Reingold J, Baggish A, et al. Weight loss, saline loading, and the natriuretic peptide system. J Am Heart Assoc. 2015;4:e001265. doi: 10.1161/JAHA.114.001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora P, Wu C, Khan AM, et al. Atrial natriuretic peptide is negatively regulated by microRNA-425. J Clin Invest. 2013;123:3378–82. doi: 10.1172/JCI67383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–82. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Liu J, Wang Z, et al. NF-kappaB-dependent microRNA-425 upregulation promotes gastric cancer cell growth by targeting PTEN upon IL-1beta induction. Mol Cancer. 2014;13:40. doi: 10.1186/1476-4598-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Osta A, Brasacchio D, Yao D, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–17. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hattori Y, Hattori S, Sato N, Kasai K. High-glucose-induced nuclear factor kappaB activation in vascular smooth muscle cells. Cardiovasc Res. 2000;46:188–97. doi: 10.1016/s0008-6363(99)00425-3. [DOI] [PubMed] [Google Scholar]
- 15.Pieper GM, Riaz-ul-Haq Activation of nuclear factor-kappaB in cultured endothelial cells by increased glucose concentration: prevention by calphostin C. J Cardiovasc Pharmacol. 1997;30:528–32. doi: 10.1097/00005344-199710000-00019. [DOI] [PubMed] [Google Scholar]
- 16.ENCODE Project Consortium The ENCODE (ENCyclopedia Of DNA Elements) Project. Science. 2004;306:636–40. doi: 10.1126/science.1105136. [DOI] [PubMed] [Google Scholar]
- 17.Gerstein MB, Kundaje A, Hariharan M, et al. Architecture of the human regulatory network derived from ENCODE data. Nature. 2012;489:91–100. doi: 10.1038/nature11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yerneni KK, Bai W, Khan BV, et al. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes. 1999;48:855–64. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- 19.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112:2163–8. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 20.Wang TJ, Larson MG, Keyes MJ, et al. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation. 2007;115:1345–53. doi: 10.1161/CIRCULATIONAHA.106.655142. [DOI] [PubMed] [Google Scholar]
- 21.Khan AM, Cheng S, Magnusson M, et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. J Clin Endocrinol Metab. 2011;96:3242–9. doi: 10.1210/jc.2011-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetze JP. Plasma proANP decreases after meal intake. Clin Chem. 2013;59:1270–1. doi: 10.1373/clinchem.2012.202416. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Lu M, Miao J, et al. Cepred: predicting the co-expression patterns of the human intronic microRNAs with their host genes. PLoS One. 2009;4:e4421. doi: 10.1371/journal.pone.0004421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Leva G, Piovan C, Gasparini P, et al. Estrogen mediated-activation of miR-191/425 cluster modulates tumorigenicity of breast cancer cells depending on estrogen receptor status. PLoS Genet. 2013;9:e1003311. doi: 10.1371/journal.pgen.1003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware B, Bevier M, Nishijima Y, et al. Chronic heart failure selectively induces regional heterogeneity of insulin-responsive glucose transporters. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1300–6. doi: 10.1152/ajpregu.00822.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–88. doi: 10.1161/01.cir.99.4.578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.