Abstract
Introduction
Anticoagulants are the mainstay for prevention and/or treatment of thrombotic disorders. Each clinically used anticoagulant is associated with significant adverse consequences, especially bleeding. Factor XIa (FXIa), a key factor involved in the amplification of procoagulation signal, has been suggested as a major target for anticoagulant drug discovery because of reduced risk of bleeding.
Areas covered
Our literature search uncovered dozens of industrial and academic patents on the discovery of novel FXIa/FXI inhibitors. Small peptidomimetics, sulfated glycosaminoglycan mimetics, polypeptides, antisense oligonucleotides, and monoclonal antibodies have been developed as inhibitors of FXIa. Although many agents are in early discovery/development phases, the activity and safety of a few have been evaluated in various animal models and in humans.
Expert opinion
FXIa is a promising drug target for development of effective anticoagulants with limited bleeding complications. Literature reveals a major trend in the number of patent applications over the last three years. These inhibitors exploit different approaches for target inhibition. Allosteric modulation of FXIa and biosynthetic inhibition of FXI are mechanistically unique. Despite initial results in patients undergoing knee anthroplasty as with antisense oligonucleotides, major advances should be realized, particularly with respect to pharmacokinetics, for FXI/FXIa inhibitors to enter the clinic.
Keywords: drug discovery, enzyme inhibition, FXI/FXIa, thrombosis, safe anticoagulant, active site inhibitors, allosteric inhibitors, polypeptides, antisense oligonucleotides, monoclonal antibodies
1. Introduction
Venous thrombosis (VT) including deep vein thrombosis (DVT) and pulmonary embolism (PE) annually affects 7–14 million people worldwide [1]. In US alone, these diseases are collectively responsible for more than 100,000 deaths annually [2]. Furthermore, arterial thrombosis including ischemic heart disease and stroke collectively causes more than 10 million deaths per year worldwide and this rate has increased by 25–35% during the past 20 years. In fact, ischemic heart disease is the leading cause of death worldwide when considering associated premature death and disability. Thrombosis is the common underlying pathology of these diseases and anticoagulants are the mainstay to prevent and/or treat thrombosis [1, 2].
Current anticoagulants used for treating these diseases fundamentally target two key serine proteases, thrombin and/or factor Xa (FXa), which belong to the common pathway of the coagulation cascade. Mechanistically, clinically used anticoagulants include antithrombin activators (heparins including unfractionated heparin (UFH), low molecular weight heparins (LMWHs), and fondaparinux), vitamin K antagonists (coumarins such as warfarin) and direct inhibitors of thrombin (hirudins, argatroban, and dabigatran etexilate) and FXa (rivaroxaban, apixaban, edoxaban, betrixaban) [3]. These agents possess high efficacy and relatively low cost to benefit ratio. Yet, UFH and warfarin suffer from many agent-specific adverse effects in addition to the patient-to-patient response variations and the need for laboratory adjusted dosing. Variations in therapeutic response may arise because of the unspecific binding of UFH or the variable hepatic metabolism of warfarin. Furthermore, UFH is generally associated with osteoporosis, thrombocytopenia, and high risk of contamination [4], whereas warfarin is associated with many drug-drug and drug-food interactions [5]. Although the safety profiles of newer oral peptidomimetic anticoagulants are much better than heparins and coumarins, the lack of effective reversal strategies remains a major challenge. Recently, the FDA approved idarucizumab as a reversal agent for dabigatran, which is expected to ease anticoagulant therapy. Yet, the limited availability of assays for measuring drugs in human plasma/blood, their short half-life, relatively high drug acquisition cost, and contraindications in patients with chronic kidney diseases represent additional challenges for their wide use [6]. Further, much remain to be learned about their effects in specific patient populations such as cancer patients [7] and pregnant women [8]. Importantly, all clinically used anticoagulants are associated with the life-threatening side effect of internal bleeding, particularly intracranial, gastrointestinal, and retroperitoneal bleeding [9, 10]. Accordingly, despite the advances made in recent years, there is an urgent need for developing new anticoagulants to prevent and/or treat thromboembolic diseases without the risk of bleeding.
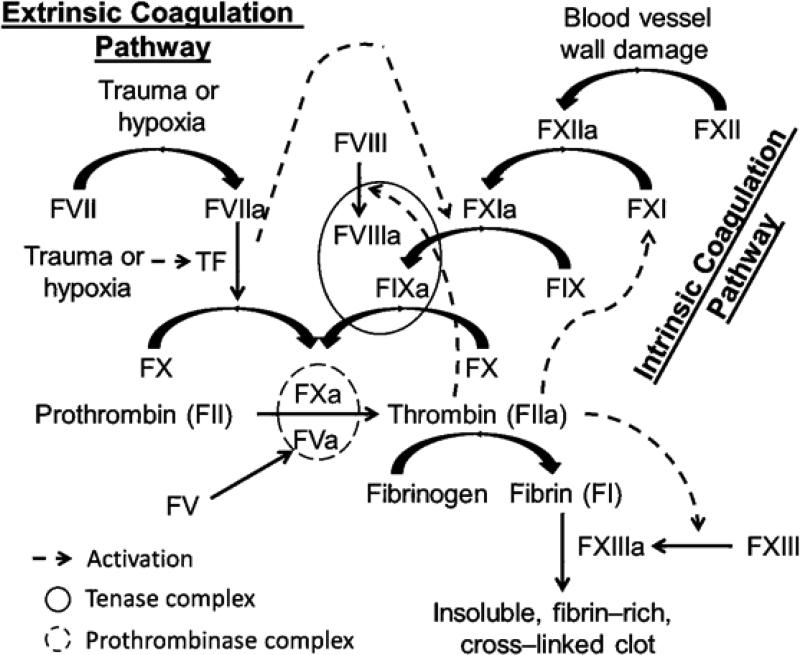
Physiologically, a fine balance is normally maintained between blood flow and blood clot, the dysfunction of which yields either hemorrhage or thrombosis. Besides endothelium, platelets, and plasmin–driven fibrinolysis, a series of proteolytic biotransformations controls the blood status. This series of enzyme–mediated biotransformations is known as the coagulation cascade, which comprises mainly two pathways: 1) the intrinsic pathway which is triggered by damage to blood vessel walls and the subsequent interactions with nonphysiological surfaces such as collagen, lipoproteins, or bacteria; and 2) the extrinsic pathway which is initiated by endothelium damage or hypoxia resulting from reduced blood flow [3] (Figure 1). Generally, proteins in the intrinsic pathway are more important for the amplification phase of coagulation, whereas those belong to the extrinsic and common coagulation pathways are more involved in the initiation and propagation phases. Therefore, it has been postulated that selective inhibition of intrinsic coagulation factors could provide antithrombotic benefits with low bleeding risk because this will keep the other pathways of coagulation intact for hemostasis. In fact, fundamental and epidemiological studies as well as observations in clinical settings suggest that factor XIa (FXIa) is a very viable drug target to develop effective and safe anticoagulants with limited risk of bleeding [9-12].
Figure 1.
An overview of the waterfall model coagulation cascade. Coagulation is triggered by either the extrinsic pathway or the intrinsic pathway, both lead into the common pathway that results in thrombin generation and the subsequent formation of insoluble, fibrin-rich, cross-linked clot. Natural anticoagulant proteins (not shown) include antithrombin (AT) which mainly inhibits thrombin and FXa, activated protein C (APC) which inhibits FVa and FVIIIa, and tissue factor pathway inhibitor (TFPI) which mainly inhibits FXa. FXIa is a serine protease in the intrinsic pathway which gets activated by FXIIa and thrombin (in addition to auto-activation) to physiologically activates FIX to FIXa.
1.1 FXIa: Structure, function, and a drug target
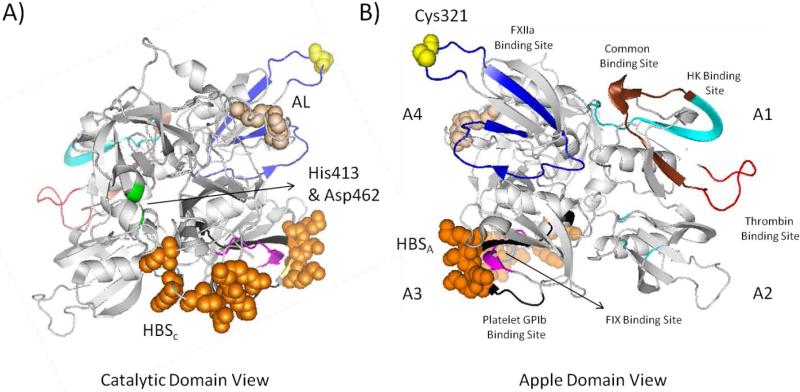
Human FXIa is a plasma serine protease that is considerably different from other coagulation proteases. It is primarily synthesized by hepatocytes and circulates in a zymogen form (FXI) with a plasma level of about 30 nM [13]. It is a 160 kDa disulfide-linked homodimer in which each monomer consists of 607 amino acid residues (Figure 2). Each monomer contains a light chain (C-terminal domain) and heavy chain (N-terminal domain). The former is a trypsin-like catalytic domain whereas the latter has four 90– or 91–amino acid repeats known as apple domains labeled as A1 through A4. The catalytic domain has the active site involving the trypsin-like catalytic triad of His413, Asp462, and Ser557 (The chymotrypsin residue numbering: His57, Asp102 and Ser195). The active site of FXIa contains several subsites with characteristic features for substrate selectivity (S4-S3-S2-S1-S1’-S2’-S3’-S4’). The S1 specificity pocket, for example, is known to bind to electropositive arginine residue on physiological substrate, FIX, and contains an aspartate residue (Asp189) at the base of the cleft. In addition, the catalytic domain also has an anion binding site (or heparin binding site) [13-15].
Figure 2.
Cartoon depiction of monomeric human FXI structure (PDB ID: 2F83). A) Front view showing the catalytic domain involving the two residues of the catalytic triad (His413 and Asp462; green), activation loop (AL) cleavage site (Arg360-Ile370; tint), and heparin binding site (HBSc) (Lys529, Arg530, Arg532, Lys536, and Lys540; orange spheres). B) Back view showing the four apple domains: A1 involving thrombin binding site (45–70; red and brown), high molecular weight kininogen (HK) binding site (56–86; cyan and brown); A2 with some residues important for HK binding; A3 involving FIX binding site (183 –191; pink), platelet GPIb binding site (248–263; black), and heparin binding site (HBSA) (Lys252, Lys253, and Lys 255; orange spheres); and A4 involving FXIIa binding site (317–350; blue) and Cys321 (yellow spheres) for dimerization. The figure was generated in PyMOL (http://www.pymol.org/).
The apple domains contain the FXI/FXIa binding sites for other macromolacules including thrombin in A1, high molecular weight kininogen (HK) in A2, FIX, platelet glycoprotein GPIb, and heparin/heparan sulfate in A3, FXIIa in A4. Domain A4 also contains the interface between the 2 monomers. The apple domains form a disk structure that interfaces with the base of the catalytic domain creating a “cup and saucer” configuration that facilitates the physiologic function of FXIa. The FXI dimer is linked by Cys321 (A4 domain) inter-chain disulfide bond between the 2 monomers. Hydrophobic residues including Leu284, Ile290, and Tyr329 of the A4 domain interface and the salt bridges between Lys331 and Glu287 from the two monomers are also required for formation of the dimer [13–15].
FXI is physiologically activated by FXIIa, thrombin, and may undergo auto-activation in a presence of polyanions, e.g., inorganic polyphosphate polymers. The FXIa so formed from FXI is physiologically inhibited mainly by plasma serpins including antithrombin and C1-inhibitor among others [13]. Heparin can either increase serpin–mediated FXIa inhibition by a template mechanism in which the FXI A3 domain (Lys252, Lys253, and Lys255) and serpin bind to heparin or directly inhibits FXIa by charge neutralization or allosteric effect through binding to its catalytic domain (Lys529, Arg530, Arg532, Lys535, and Lys539) [16,17].
In the classic waterfall model of the coagulation cascade, coagulation is triggered by either the extrinsic or the intrinsic pathway leading into the common pathway that results in thrombin generation and the subsequent formation of insoluble, fibrin-rich, cross-linked clot (Figure 1). The extrinsic pathway is initiated upon formation of tissue factor–factor VIIa (TF–FVIIa) complex, which directly or indirectly (through activation of factor IXa (FIXa)) activates FX to FXa. In contrast, the intrinsic pathway is initiated by negatively charged surfaces–mediated activation of factor XII (FXII). Such contact activation further propagates thrombin generation by sequential activation of FXI, FIX, FX, and prothrombin. Importantly, thrombin can further activate FXI in a feedback mechanism. Thrombin also activates platelets, which can subsequently support FXI activation. FXI-dependent amplification of thrombin generation can indirectly regulate fibrinolysis by activating thrombin-activatable fibrinolysis inhibitor (TAFI) [18-20]. Therefore, FXI has a significant role in blood coagulation and thrombosis by interaction with multiple elements in the hemostatic system.
Genetic FXI deficiency in humans (hemophilia C) is generally associated with a relatively mild–to–moderate bleeding phenotype compared with FVIII deficiency or FIX deficiency (hemophilia A or B, respectively) [9,10]. In fact, FXI-deficient individuals rarely suffer from spontaneous bleeding, and many do not significantly bleed even if challenged with surgery. These individuals are characterized by a prolonged activated partial thromboplastin time (APTT) and normal prothrombin time (PT). Excessive bleeding in severe FXI deficiency typically occurs with surgery or trauma to tissues having high fibrinolytic activity [10]. Literature also suggests that severe FXI deficiency confers reduced risk of ischemic stroke and DVT. Epidemiological studies suggest that increased levels of FXI confer higher risk for DVT, myocardial infarction, stroke, and cardiovascular diseases in women. These studies indicate that FXIa may play a greater role in thrombosis than in hemostasis [9-12]. FXI knockout mice were first described by Gailani et al. and exhibited prolonged APTT, but normal PT, and did not cause excessive bleeding [21]. The FXI knockout mice displayed significant antithrombotic activity in several venous and arterial thrombosis animal models [22-27].
In combination, the rationale for targeting FXIa/FXI is more based on observations than truly known fundamental mechanisms. FXIa appears to be a powerful amplifier of pro-coagulant signal as far as thrombosis is concerned but appears to contribute less to the hemostatic process. Thus, targeting FXIa is expected to inhibit thrombosis but only depress, at best, hemostasis, thereby preventing bleeding consequences. Thus, these fundamental and epidemiological studies as well as the clinical observations lead to a paradigm that is beginning to shape the field of anticoagulants. Targeting proteases of the intrinsic pathway, especially FXIa, may serve as a powerful route to antithrombotics that are safer than those that inhibit FXa and thrombin.
2. Inhibitors of FXIa
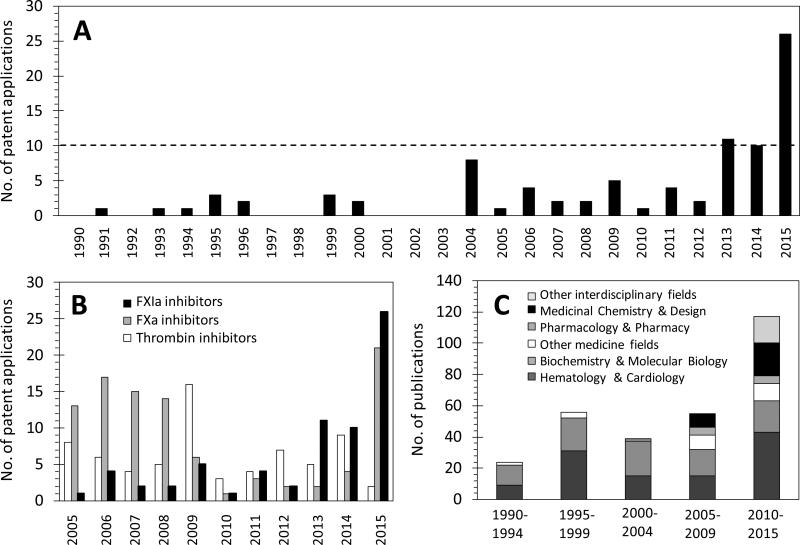
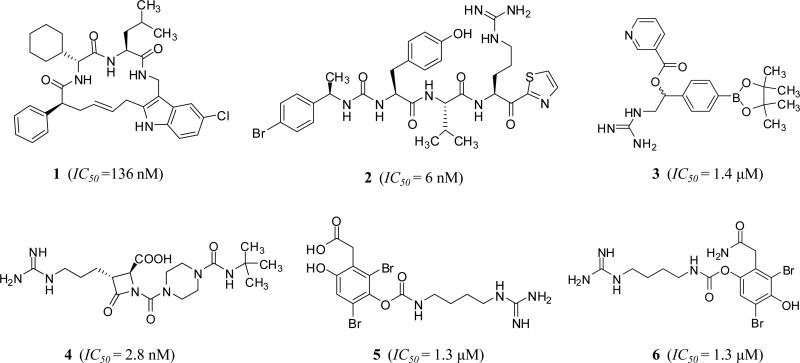
Encouraged by the above findings and results, at least five different inhibitor classes have been exploited by drug discovery programs at both academia and industry to discover, design, and develop a potentially unique generation of effective and safe anticoagulants/antithrombotics by inhibiting FXI/FXIa system so as to address deficiencies of currently available therapies. This is clearly indicated by the surge in the number of patents and patent applications for FXIa inhibitors, particularly over the last three years (Figure 3A). Availability of several X-ray crystal structures of the catalytic domain of FXIa has significantly contributed to the ligand–based and structure–based drug design efforts [28, 29]. Earlier, small molecule inhibitors have been reported demonstrating feasibility of FXIa active site inhibition by cyclic neutral peptidomimetics 1 [30], acyclic arginine–containing ketothiazole peptidomimetics 2 [31], aryl boronic acids 3 [32], β-lactams 4 [33, 34], and naturally occurring bromophenolic carbamates (clavatadines) 5 and 6 [35] (Figure 4). This report highlights more recent serious efforts toward this end by reviewing FXI/FXIa inhibitors which fall into the following categories: 1) small peptidomimetics targeting the active site; 2) sulfated glycosaminoglycan mimetics targeting the heparin allosteric site; 3) polypeptides; 4) antisense oligonucleotides (ASOs); and 5) monoclonal antibodies. Importantly, about 50% of these applications have been granted/filed only in the last three years (2013 –2015) and about 80% of these applications have been for small molecule active site or allosteric site inhibitors. These inhibitors belong to polypeptides class and represent about 15% of all patents and patent applications. The number of patents and patent applications for FXIa inhibitors was similar or exceeded those filed for thrombin or FXa inhibitors only starting 2010 (Figure 3B). Furthermore, distribution of FXIa inhibition/inhibitors-related publications among different research areas starting 1990 clearly indicated that the predominant research areas over the last 25 years are related to hematology and cardiovascular aspects in addition to biochemical and molecular biology aspects. Interestingly, scientific reporting on medicinal chemistry and design efforts toward FXIa inhibitors started only a decade ago.
Figure 3.
A) Number of patents and patent applications reported by SciFinder®, Espacenet, and Google Patent Search over the period of 1990 – present having human FXIa as the main druggable target or one of the potential targets for the claimed technology. The search was performed using the key words “Factor XIa Inhibitors” and “FXIa Inhibitors” to uncover about 85 patents and patent applications. B) Number of patents of FXIa inhibitors relative to those filed for FXa and thrombin over the last decade, as reported by SciFinder® using the corresponding key words. Number of patents and patent applications for FXIa inhibitors was similar or exceeded those filed for thrombin or FXa inhibitors only starting 2010. C) Distribution of FXIa inhibition/inhibitors related publications (articles, reviews, letters, editorials, abstracts, chapters, proceedings, notes, but not patents) among different research areas starting 1990 as reported by Web of Science using the above key words. It is clearly indicated that the predominant research areas over the last 25 years are related to hematology and cardiovascular aspects in addition to biochemical and molecular biology aspects, and that reporting on medicinal chemistry and design efforts toward FXIa inhibitors started only a decade ago.
Figure 4.
Chemical structures of reported FXIa inhibitors in literature before the surge in number of patents and patent applications over the last three years.
2.1 Small peptidomimetics targeting the active site
Molecules in this class can be classified into several categories (acyclic, monocyclic, bicyclic, and macrocyclic derivatives) and subcategories, primarily based on the chemistry of the central domain. Representative examples are summarized in table 1.
Table 1.
Representative small peptidomimetic inhibitors of FXIa with their inhibitory profiles.
| Class | Inhibitor | Inhibition measures | APTT×2 | Positive results in models | Ref. |
|---|---|---|---|---|---|
| Acyclic | |||||
| Phenylalanines | 18 | FXIa Ki = 0.3 nM P.K. Ki = 5 nM |
1.0 μMh 2.0 μMrab |
1) Arteriovenous-shunt thrombosis model (rabbit) (ID50 = 0.6 mg/kg + 1 mg/kg/h) | 48 |
| α-Ketothiazoles | 28 | FXIa IC50 = 6 nM P.K. IC50= 10 nM |
2.4 μMh | 1) Venous thrombosis model (rat) (0.25 mg/kg) | 52 |
| Moncyclic | |||||
| Azetidinones | 30 | FXIa IC50 = 2.8 nM P.K. IC50= 550 nM |
0.14 μMh 2.2 μMrat 10.6 μMrab |
1) FeCl3-induced thrombosis in both the vena cava and carotid artery models (rat) (0.2 mg/kg+ 0.2 mg/kg/h) 2) Several bleeding tests (rat) 3) Arteriovenous-shunt thrombosis, venous thrombosis, electrolytic-mediated carotid arterial thrombosis models (rabbit) (ED50 = 0.4 – 1.5 mg/kg/h) 4) Cuticle bleeding test (rabbit) |
33&34 |
| Bicyclic | |||||
| Tetrahydroqunolines | 47 | FXIa Ki = 0.2 nM P.K. Ki = 5 nM |
2.2 μMrat 2.4 μMrat |
1) Arteriovenous shunt thrombosis model (rabbit) (ID50 = 0.95 mg/kg + 0.6 mg/kg/h) 2) Electrolytic-induced carotid arterial thrombosis (rabbit) (0.37 mg/kg + 0.27 mg/kg/h) 3) Cuticle bleeding test (rabbit) |
72 |
Acyclic derivatives
Phenylalanine derivatives
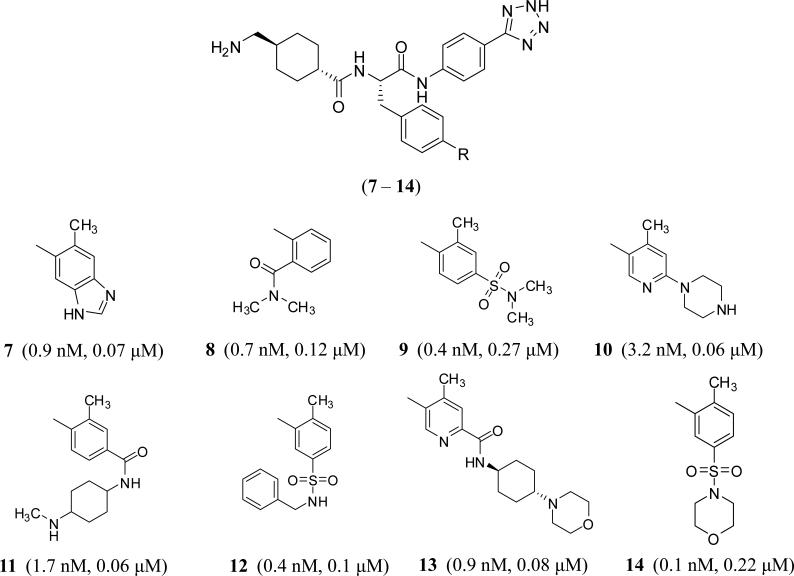
Several patent applications were filed by Bayer Pharma, Germany in 2015 introducing substituted phenylalanine derivatives as FXIa inhibitors for the treatment and/or prophylaxis of diseases, in particular cardiovascular diseases, preferably thrombotic or thromembolic diseases, and/or perioperative heavy blood loss [36]. The molecules have tripeptide-like structures in which the N-terminal residue is tranexamic acid which likely fits into S1 pocket of FXIa, the central residue is para-substituted phenylalanine which likely fits into S2 or S1’ pocket, and the C-terminal residue is 5-phenyl-2H-tetrazole, a carboxylic acid bioisoster, which seems to fit into S2’pocket. These inhibitors possess IC50 values in the nanomolar range and submicromolar activity in APTT assay. Due to the presence of the tranexamic acid moiety, some of these inhibitors may potently inhibit plasmin which represents the rationale behind their potential use to treat postoperative heavy blood loss. Inhibitors 7 – 14 in figure 5 are representative examples. Inhibitor 7 also inhibits plasmin with an IC50 of 22 nM. It is important to recognize how structurally variable the p-substituent of the phenylalanine moiety can be.
Figure 5.
Chemical structures of substituted phenylalanine-based inhibitors of FXIa (7– 14) filed by Bayer Pharma. R is represented by one of the moieties listed from 7 through 14. Reported in brackets are the FXIa IC50 values (nM) and the concentrations required to double clotting time in APTT assay (μM).
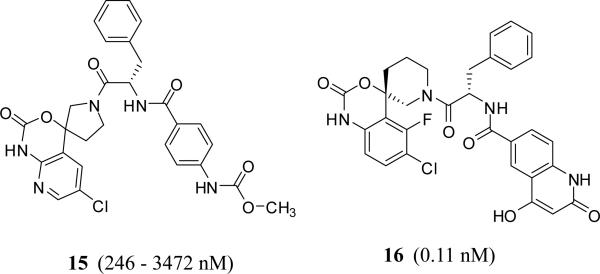
Very recently, Merck &Sharp Co. claimed tripeptide-like phenylalnine derivatives in which the carboxylic end of phenylalanine is coupled with a spiro[benzo]oxazine-piperidine or pyrrolidine and the amino end could be part of acyclic or cyclic moiety [37]. Phenylalanine moiety can also be replaced with a variety of groups including fluoroethyl, difluoroazetidine ethanone, methoxycyclopropylmethyl, N-ethylcyclobutanecarboxamide, and cyclopropyl-methylisoxazole resulting in active site inhibitors of FXIa with Ki values of 0.1 – 5000 nM. Particularly, inhibitor 15 inhibited FXIa with Ki values of 246 – 3472 nM (mixture), whereas inhibitor 16 inhibited FXIa with an Ki value of 0.11 nM. The latter also inhibited human plasma kallikrein with a Ki value of 0.33 nM (Figure 6).
Figure 6.
Chemical structures of phenylalanine-based FXIa inhibitors claimed by Merck with spiro-structures. Numbers in brackets represent the Ki values toward FXIa.
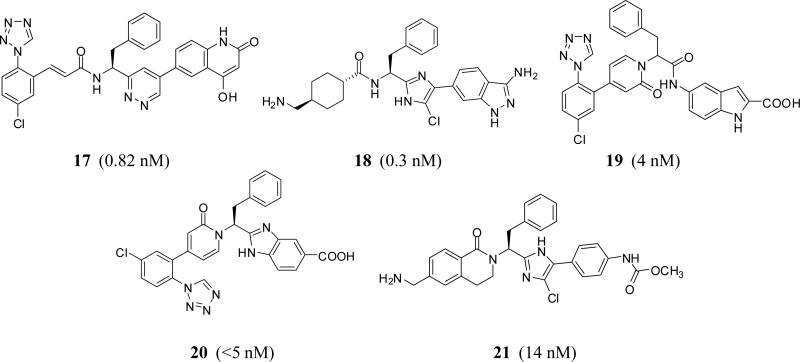
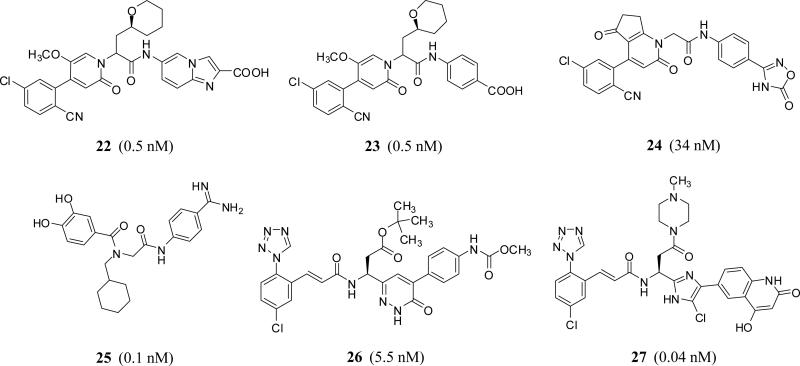
In fact starting 2005 through 2015, the concept of tripeptide-like phenylalanine inhibitors has been claimed by many other companies. Generally speaking, the tripeptide-like phenylalanine inhibitors could be further modified by recruiting different tactics including 1) engaging the carbonyl moiety in cyclic structures such as pyridazine as with inhibitor 17 (C=O is mimicked by C=N1 in pyridazine) [38] or imidazole as with inhibitor 18 (C=O is mimicked by C=N3 in imidazole moiety) [39] (Bristol Myers Squibb); 2) engaging the amino group in a cyclic moiety such as oxopyridine as with inhibitor 19 [40] (Sichuan Haisco); or 3) a combination of these two tactics as with inhibitors 20 [41] and 21 [42] (Merck) (Figure 7). The phenylalanine could be itself replaced by tetrahydropyran as with inhibitors 22 [43] and 23 [44] (Bayer), glycine as with inhibitor 24 [45] (Bayer), N-cyclohexyl glycine as with inhibitor 25 [46] (LegoChem Bioscience), protected aspartate as with inhibitor 26 [38] (Bristol Myers Squibb), or N-methylpiperazine carbonylmethylene as with inhibitor 27 [47] (Bristol Myers Squibb) (Figure 8).
Figure 7.
Chemical structures of modified phenylalanine-based FXIa inhibitors claimed by different pharmaceutical companies. Numbers in brackets represent Ki values toward FXIa.
Figure 8.
Chemical structures of FXIa inhibitors with tetrahydropyran-3-methylene, glycine, and N-cyclohexylglycine, O-Boc-aspartate, and N-methylpiperazine-4-carbonylmethylene groups as substitutes of phenylalanine. Numbers in brackets represent IC50 values (22–24) Ki values (25–27) toward FXIa.
More specifically, phenylimidazole inhibitor 18 (P1–P1’–P2’) was reported by Bristol Myers Squibb to inhibit human FXIa with a Ki value of 0.3 nM and demonstrated a minimum selectivity of at least 10,000-fold over FXa, FVIIa, FXIIa, FIXa, thrombin, and chymotrypsin. Yet, selectivity was compromised against plasma kallikrein (Ki =5 nM) and trypsin (Ki = 23 nM) (Table 1). It did also prolong clotting time in APTT assay (ECx2 = 1 μM) but not in PT assay (ECx2 > 40 μM). The inhibitor also demonstrated in vivo dose-dependent antithrombotic efficacy in the rabbit arteriovenous-shunt thrombosis model (ID50 = 0.6 mg/kg + 1 mg/kg/hr) [48]. Bristol Myers Squibb also reported in more details about inhibitor 27 which had a FXIa Ki of 0.04 nM and an APTT ECX2 of 1.0 μM (Table 1). The inhibitor was highly selective (>100,000-fold) over FVIIa, FXa, FIXa, FXIIa, thrombin, trypsin, tissue kallikrein, chymotrypsin, tPA, urokinase, and APC. Yet, the inhibitor was not selective against plasma kallikrein (Ki = 7 nM). It is important to mention here that the new moiety of N-methylpiperazine fits into S2 pocket of human FXIa rather than S1’ pocket. The inhibitor was evaluated in the rabbit electrically-induced carotid arterial thrombosis model. It produced a dose-dependent increase in integrated blood flow of the injured artery. A dose-dependent increase in potency of thrombus reduction was evident with an EC50 of 0.53 μM. In the rabbit cuticle bleeding time model, only minimal bleeding time prolongation was observed for the inhibitor even at the highest dose studied of 10 μM. At the highest dose tested, inhibitor 27 prolonged APTT by 3.2-fold, but not PT or TT, as expected with the FXIa inhibition mechanism [47].
Aided by fragment–based drug design, AstraZeneca very recently reported another phenylalanine-based inhibitor, which has 6-chloro-3,4-dihydro-1H-quinolin-2-one moiety for binding to the S1 site. The inhibitor (P1= 4-(aminomethyl) -6-chloroquinolin-2(1H)-one; P1’ = phenylalanine; P2’= 4-hydroxy-2-oxo-1,2-dihydroquinoline-6-carbonyl) had potent activity against human FXIa with an IC50 of 1 nM. Although the molecule inhibited plasma kallikrein with an IC50 value of 27 nM, it had more than 100,000-fold selectivity index over thrombin, FIXa, FXa, plasmin, trypsin, and tPA. Yet, the hydrophilicity and its large polar surface area of this potent inhibitor compromised its pharmacokinetic profile [49].
In general, the most frequently used P1 groups that fit into S1 pocket of FXIa are tranexamic acid, 1-(4-chlorophenyl)-1H-tetrazole, and 4-chlorobenzonitrile, whereas S2’ pocket is frequently occupied by a weakly acidic group which could be carboxylic acid, tetrazole, 1,2,4-oxadiazol-5(4H)-one, methyl carbamate, 4-hydroxy-quinolinone, or 1H-indazol-3-amine. Furthermore, it appears that most of these inhibitors have inhibitory activities against other serine proteases, particularly plasmin and plasma kallikrein. For example, inhibitors 22 and 23 inhibited plasma kallikrein with Ki values of 4.8 and 5.9 nM, respectively. Broadly speaking, these molecules have primarily been claimed to prevent and/or treat thromboembolic diseases. Some of them (dual FXIa/kallikrein inhibitors) have also been claimed as treatment for inflammatory diseases, fibrotic changes, edema, ophthalmological diseases, and hemagglutination.
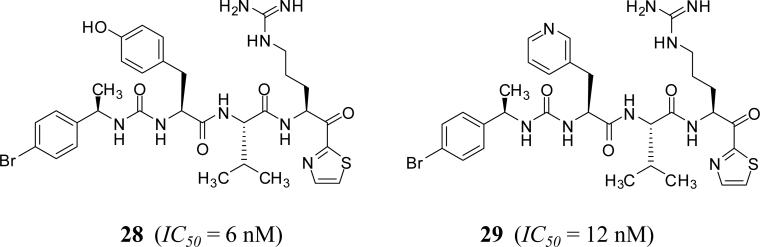
α–Ketothiazole-arginine derivatives
A series of α-ketothiazole-arginine peptidomimetics was patented by Suntory Pharmaceutical Research Laboratories, USA [50, 51]. These peptidomimetics are tetrapeptide-like structures in which the C-terminal group has α-ketothiazole moiety, which covalently binds to Ser195 in the active site of human FXIa. The most potent inhibitor of human FXIa identified in this group was inhibitor 28 which has an IC50 value of 6 nM (Figure 9). The ketothiazole moiety occupies S1’ pocket of FXIa, arginine moiety of the inhibitor fits into S1 pocket of FXIa active site, and valine into S2 pocket. Likewise, tyrosine of 28 may fit into S4 pocket and 4-bromophenyl may interact with Lys192. The inhibitor was selective over thrombin, FXa, FVIIa, and APC with IC50 values of at least 1,600 nM, but not over plasma kallikrein (IC50 = 10 nM) or trypsin (IC50 = 12 nM). The pharmacokinetic profile of inhibitor 28 was studied following IV administration to rats. The inhibitor displayed relatively high clearance (32 mL/kg/min), a short half-life (t1/2 of 45 min), and a low volume of distribution (Vdss ~236 mL/kg). The inhibitor caused a doubling of APTT measurement in human plasma at 2.4 μM and that of PT measurement at 25 μM and was efficacious in a rat model of venous thrombosis at a dose of 0.25 mg/kg (Table 1). The extent of the reduction is comparable to that obtained with heparin given as an IV bolus of 50 units/kg followed by IV infusion at a rate of 25 units/kg/h. Similar results were obtained with inhibitor 29 (IC50 of 12 nM and in vitro activity of APTTx2 = 2.4 μM and PTx2 = 31 μΜ) (Figure 9). This inhibitor did not alter the bleeding time when it was tested in a rat mesenteric arteriole bleeding model, whereas heparin, at a dose comparable to the clinically relevant dose, significantly increased bleeding [52].
Figure 9.
Chemical structures of α-ketothiazole – based FXIa inhibitors.
Monocyclic derivatives
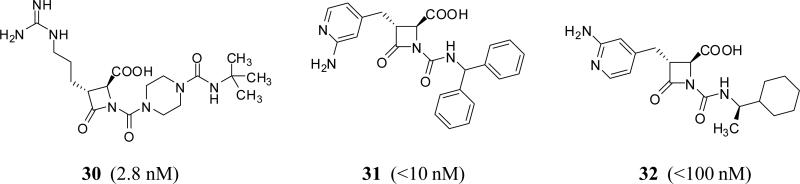
Azetidinone and β-lactam derivatives
Mechanistically, these molecules are expected to be irreversible, covalent inhibitors of FXIa through the interaction between Ser195 of FXIa and the β-lactam moiety of inhibitors. Furthermore, their guanidine group (or 2-aminopyridine group) fits into the S1 pocket of FXIa. The most studied FXIa inhibitor in this category is BMS-262084 30 (Figure 10) which was claimed by Bristol Myers Squibb in 2004 [53]. BMS-262084 is a 4-carboxy-2-azetidinone containing small molecule inhibitor of human FXIa with an IC50 of 2.8 nM (Table 1) [33].
Figure 10.
Chemical structures of β-lactam FXIa inhibitors. Numbers in brackets represent IC50 values toward FXIa.
The effect of inhibiting FXIa was determined in rat models of thrombosis and hemostasis. BMS-262084 doubled the clotting time in APTT assay in human and rat plasma at 0.14 and 2.2 μM, respectively, whereas the PT was unaffected at up to 100 μM. IV administration of BMS-262084 was found to be effective against FeCl3-induced thrombosis in both the vena cava and carotid artery (97% and 73% maximum reduction, respectively) at a pretreatment dose of 12 mg/kg + 12 mg/kg/h, which increased the ex vivo APTT measurement 3-fold. This dose level also arrested growth of venous and arterial thrombi when administered after partial thrombus formation. BMS-262084 was most potent in FeCl3-induced venous thrombosis, decreasing thrombus weight 38% at a threshold dose of 0.2mg/kg + 0.2mg/kg/h. In contrast, doses of up to 24 mg/kg+24 mg/kg/h had no effect on either TF-induced venous thrombosis or the ex vivo PT measurement. The inhibitor did not significantly prolong bleeding time provoked by either cuticle incision, template incision of the renal cortex, or puncture of small mesenteric blood vessels using doses up to 24 mg/kg + 24 mg/kg/h [33].
The in vitro and in vivo properties of BMS-262084 were also evaluated in rabbits. Studies were conducted in arteriovenous-shunt thrombosis (AVST), venous thrombosis (VT), electrolytic-mediated carotid arterial thrombosis (ECAT) and cuticle bleeding time models. In vitro, IV administered BMS-262084 doubled the APTT measurement at a concentration of 10.6 μM in rabbit plasma, and did not prolong the PT, TT, and HepTest measurement. In vivo, BMS-262084 produced dose-dependent antithrombotic effects in rabbits with antithrombotic ED50 in AVST, VT and ECAT of 0.4, 0.7 and 1.5 mg/kg/h IV, respectively. BMS-262084 enhanced ex vivo APTT dose-dependently without changes in PT and TT. BMS-262084 did not alter ex vivo rabbit platelet aggregation to ADP and collagen. Increase in bleeding time determined at 3 and 10 mg/kg/h of BMS-262084 were 1.17 ± 0.04 fold and 1.52 ± 0.07 fold, respectively, suggesting a minimal impact on hemostasis [34].
Very recently, Exithera Pharmaceuticals, USA has claimed the preparation of β-lactam derivatives as inhibitors of FXIa or kallikrein [54]. The molecules have been claimed to be useful for reducing the risk of stroke as well as non-CNS systemic embolism, treating deep vein thrombosis, reducing the risk of recurrence of deep vein thrombosis or pulmonary embolism, treating nonvalvular atrial fibrillation in a mammal including human, or treating the following risk factors for stroke including a prior stroke, transient ischemic attack, non-CNS systemic embolism, 75 years or older of age, hypertension, heart failure or left ventricular ejection fraction, or diabetes mellitus. Inhibitor 31 is a representative structure which showed human FXIa IC50 of <10 nM and 1- to 500-fold selectivity over factor Xa, thrombin, and trypsin (Figure 10) [54]. Likewise, in 2006, Daiamed, USA has introduced the preparation of substituted azetidinones as inhibitors of tryptase, thrombin, trypsin and factors Xa, VIIa and XIa. It was claimed that this class of molecules may be employed in preventing and/or treating asthma, chronic asthma, allergic rhinitis, and thrombotic disorders. Particularly, inhibitor 32 inhibited FXIa and tryptase with IC50 values of <100 nM (Figure 10) [55]. Structurally, inhibitors 31 and 32 have 2-aminopyridine as the positively charged moiety that fits into S1 pocket of FXIa.
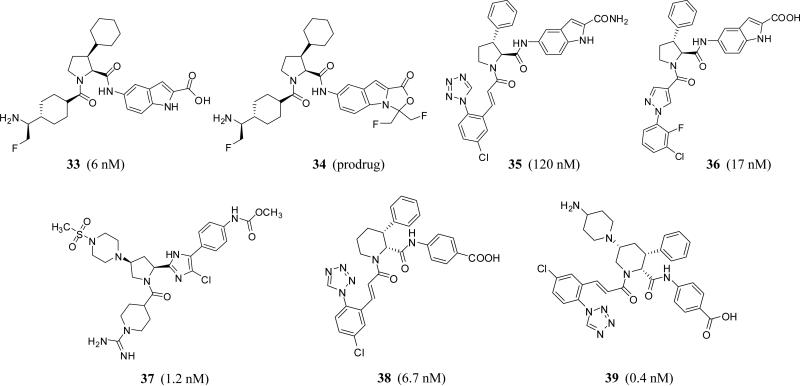
Proline, pyrrolidine, and piperidine-2-carboxylic acid derivatives
Several new FXIa inhibitors with a highly substituted, saturated monocyclic central domain have been recently claimed for the treatment and/or prevention of thrombosis without the risk of internal bleeding. The Japanese Dainippon Sumitomo Pharma Co. patented a series of 1,2,3-trisubstituted proline derivatives (inhibitors 33–36) (Figure 11) which demonstrated nanomolar inhibition potency against human FXIa (6 –4100 nM) in vitro and inhibited thrombus formation in rabbits. Some of these molecules inhibited different other serine proteases with variable potencies, particularly plasma kallikrein [56–60]. Along those lines another Japanese company, Ono Pharmaceutical Co., has claimed a series of 1,2,4-trisubstituted pyrrolidine derivatives (inhibitor 37) (Figure 11) as a new class of active site inhibitors of human FXIa [61, 62]. Very recently, Merck Co. also introduced highly substituted piperidine derivatives as selective FXIa inhibitors or dual inhibitors of FXIa and plasma kallikrein for treating or preventing thromboses, embolisms, hypercoagulability or fibrotic changes. I,2,3- and 1,2,4-trisubstituted piperidine-carboxylic acid derivatives including inhibitor 38 and inhibitor 39 exhibited IC50 values of 6.7 and 0.4 nM, respectively (Figure 11) [63, 64].
Figure 11.
Chemical structures of proline (33–36), pyrrolidine (37), and piperidine-2-carboxylic acid derivatives (38, 39). Numbers in brackets represent IC50 values toward FXIa.
In more details, inhibitor 33 has IC50 values of 6 nM against FXIa and 430 nM against plasma kallikrein. The inhibitor was selective over thrombin, TF/FVIIa, FIXa, FXa, FXIIa, plasmin, urokinase, t-PA, tryptase, and trypsin with IC50 values of >10000 nM. The inhibitor demonstrated excellent antithrombotic activity in venous thrombosis rabbit model and could be prepared as condensed 5-oxazolidinone oral prodrug 34. In fact, the class of such oral prodrugs demonstrated very significant antithrombotic activity in venous thrombosis rabbit model, limited effect in rabbit nail bleeding model, good rat and dog pharmacokinetic profiles, and excellent effect on cynomolgus monkey APTT measurements [56-60].
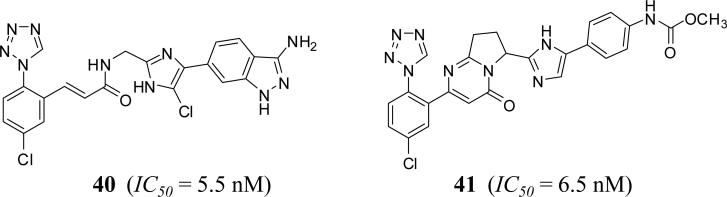
2-methylene-imidazole derivatives
Several flexible and rigid 2-methylene-imidazole were filed by Bristol Myers Squibb (2008) and Ono Pharmaceutical Company (2013) as inhibitors of FXIa claiming that they will be useful in the prevention of and/or as therapy for thromboembolic diseases. In the former case, the methylene unit bridges the imidazole to azolyl arylpropionamide, arylacrylamide, arylpropynamide, or arylmethylurea moieties, while in the latter case; it bridges the imidazole moiety to either pyridinone or pyrimidinone. Representative examples of this class are shown in figure 12. Inhibitors 40 and 41 have IC50 values of about 5 nM toward FXIa [65, 66]. Oral bioavailability and significant impact only on clotting time in APTT assay were detected for inhibitor 41 [66].
Figure 12.
Chemical structures of 2-methylene-imidazole–based FXIa inhibitors.
Bicyclic derivatives
Tetrahydroisoquinolines, tetrahydroquinolines, and other bicyclic derivatives
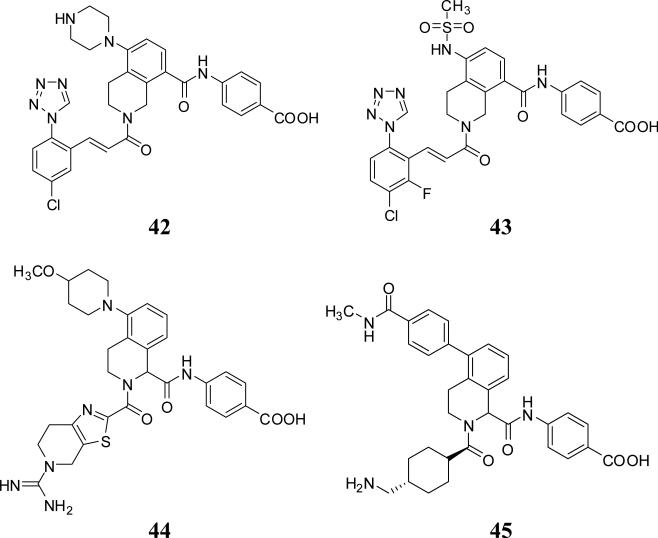
Bristol Myers Squibb has filed for a class of tetrahydrosioquinoline-2,8-diamides, exemplified by inhibitors 42 and 43 in 2013, as inhibitors of FXIa (Figure 13). These molecules were also substituted at position-5 with an amine-containing group and inhibited FXIa with Ki values of <5nM. Further modifications in this class included moving the 8-amide group to position-1 as in inhibitors 44 and 45 (Figure 13). Despite the high potency of these molecules toward FXIa, they also potently inhibited plasma kallikrein with nanomolar IC50s. Generally, position-1 bears a carboxylate group which occupies S2’ pocket of FXIa active site while position-2 may carry a guanidine, amine, or neutral group that fits into S1 pocket [67-71].
Figure 13.
Chemical structures of tetrahydroisoquinoline (THIQ)– based FXIa inhibitors (Ki <5 nM).
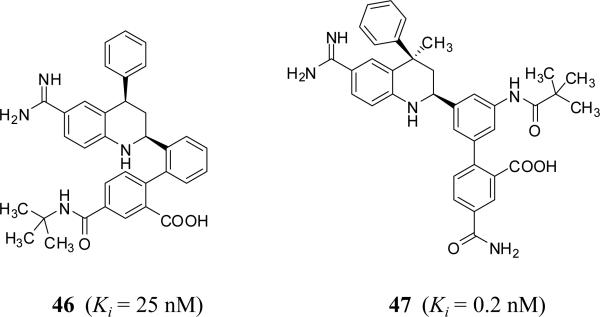
Tetrahydroquinoline derivatives were also reported as potent and selective FXIa inhibitors (Figure 14). Inhibitor 46, a 1,2-phenylene tetrahydroquinoline, was identified through screening of potential serine protease inhibitors. It has a FXIa Ki of 25 nM with about 4-fold selectivity over FVIIa and 40-fold selectivity over FXa. Extensive structure – activity relationship study led to the identification of inhibitor 47 which has a FXIa Ki of 0.20 nM (Table 1) [72,73].
Figure 14.
Chemical structures of tetrahydroquinoline (THQ)– based FXIa inhibitors.
Inhibitor 47 demonstrated >1000-fold selectivity for FXIa versus most of the enzymes tested except for plasma kallikrein (23-fold) and activated protein C (365-fold). An X-ray crystal structure of the inhibitor 47 –FXIa complex was also obtained. The benzamidine group was bound tightly in the S1 pocket by a salt bridge to Asp189 and an H-bond to the backbone carbonyl of Gly218. The NH of the THQ forms an H-bond with Ser195 side chain. A stacking interaction was observed between His57 and the inner phenyl ring. The carboxylic group forms H-bonds with His57 and the oxyanion hole. The amide nitrogen formed two H-bonds with the backbone carbonyls of residues 40 and 41. The amide carbonyl interacts with Arg39 and forms an additional H-bond with the hydroxyl of Tyr58, extending the isobutyl moiety deeper into the S2 pocket. Inhibitor 47 exhibits excellent in vitro anticoagulant activity in the APTT assay with an EC2x of 2.2 μM. Consistent with inhibition of the intrinsic coagulation system, it had no activity in the PT assay. It is competitive with small molecule FXIa substrates and shows reversible inhibition kinetics. It shows mixed type inhibition using FIX as a substrate. Inhibitor 47 was studied in the rabbit arteriovenous shunt thrombosis model by IV administration at five doses given by loading dose plus continuous infusion. A dose-dependent antithrombotic effect was observed with an ID50 of 0.95 mg/kg/hr. Similar results were obtained in the rabbit model of electrolytic-induced carotid arterial thrombosis. Bleeding time was not increased compared to vehicle-treated animals as studied in cuticle bleeding time assay [72]. Furthermore, inhibitor 47 did not alter platelet aggregation induced by ADP, arachidonic acid, or collagen.
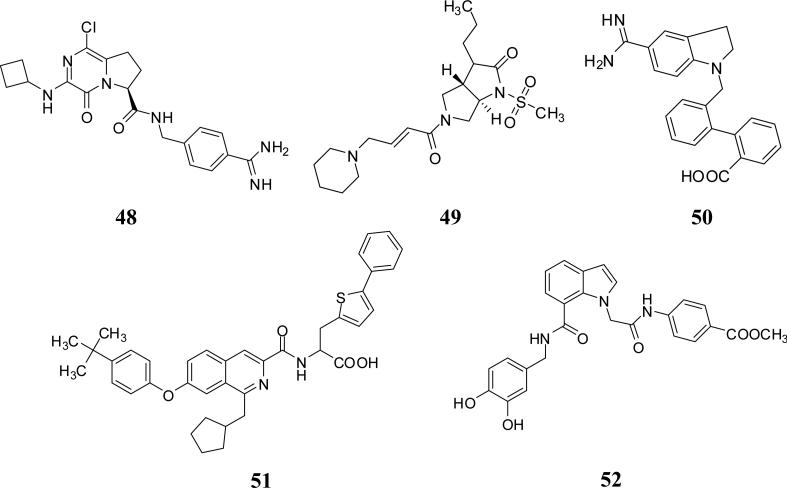
Earlier from 1999 to 2006, pyrrolopyrimidines such as inhibitor 48, pyrrolopyrrolidines such as inhibitor 49, indolines such as inhibitor 50, isoquinoline-3-carboxamides such as inhibitor 51, and indoles such as inhibitor 52 were patented as inhibitors of several serine proteases including FXIa (Figure 15). Unfortunately, these applications have not been followed up with reports on their development toward the clinic.
Figure 15.
Chemical structures of miscellaneous FXIa inhibitors with bicyclic saturated or unsaturated central domain.
Macrocyclic derivatives
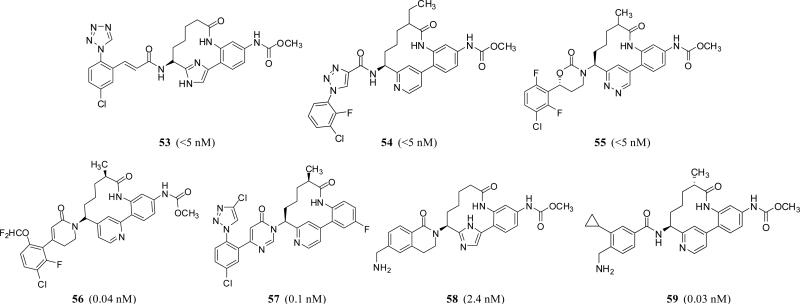
Biaryl macrocyclic peptidomimetics 53 – 59 (Figure 16) have also patented by Bristol Myers Squibb Co. as well as Merck Co. as selective FXIa inhibitors or dual inhibitors of FXIa and plasma kallikrein. Many of them are claimed to be useful in preventing and/or treating thrombosis, embolism, hypercoagulability, or fibrotic changes. Interestingly, some of these have claimed their utility in retinal vascular permeability associated with diabetic retinopathy and diabetic macular edema [74].
Figure 16.
Chemical structures of various macrocyclic FXIa inhibitors. Numbers in brackets represent the Ki values toward FXIa.
Molecules in this category from Bristol Myer Squibb are typically 12-membered lactams, which have variable P1 groups introduced at position-6 to putatively fit into S1 pocket of human FXIa. Part of this macro-lactam is a biaryl system in which aryl moieties involves 6-membered cycles such as benzene, pyridine, or pyridazine, and/or 5-membered heterocycles including imidazole or pyrazole. The P1 group could be substituted cinnamamide 53 (FXIa Ki <5 nM) [75, 76], substituted triazole-4-carboxamide 54 (FXIa Ki <5 nM) [77], or substituted 1,3-oxazinan-2-one 55 (FXIa Ki <5 nM) [77]. More recently, inhibitors with substituted dihydropyridinone or dihydropyrimidinone as P1 group have also been reported. Particularly, dihydropyridinone derivative 56 inhibited FXIa and plasma kallikrein with Ki values of 0.04 and 3 nM, respectively [78,79]. Dihydropyrimidinone derivative 57 inhibited FXIa with a Ki value of 0.1 nM, and inhibited plasma kallikrein with a Ki value of 7 nM [80].
Likewise, Merck macrocyclic inhibitors are structurally similar, yet they have constrained or unconstrained p-aminomethyl benzamide as P1 group as in inhibitors 58 and 59 which inhibited FXIa with Ki values of 2.4 and 0.03 nM, respectively [81]. Despite its metabolic instability, there generally seems a trend to exploit a methylcarbamate group so as to reach into the prime subsites of FXIa's active site, particularly S2’. Furthermore, it appears that this cyclization strategy was successfully exploited to enhance selectivity of these inhibitors against thrombin, FVIIa, FIXa, FXa, FXIIa, plasmin, activated protein C, trypsin, and chymotrypsin as well as to improve their chemical stability and pharmacokinetic profiles in general. The antithrombotic potential of these inhibitors was tested using different thrombosis models including the in vivo electrically induced carotid artery thrombosis model and arteriovenous shunt thrombosis model in rats and/or rabbits [74].
2.2 Sulfated glycosaminoglycan mimetics targeting the heparin allosteric site on FXIa
Inventors from the Virginia Commonwealth University (VCU) have reported sulfated nonsaccharide glycosaminoglycan mimetics (NSGMs) including sulfated galloyl glucopyranoses, galloyl inositols, and quinazolinone dimers as allosteric inhibitors of human FXIa by binding to or in the vicinity of the heparin-binding site in the catalytic domain [82]. The inventors argue that allosteric inhibitors offer significant advantages in comparison to the traditional active site inhibitors. An allosteric inhibitor is expected to be more selective than an orthosteric inhibitor because the active sites of serine proteases are rather similar (each prefers a P1 arginine) resulting in difficulties of obtaining selectivity, particularly against plasma kallikrein. Allosteric sites, on the other hand, are much less conserved and structurally significantly different allowing higher selectivity to be achieved [83, 84].
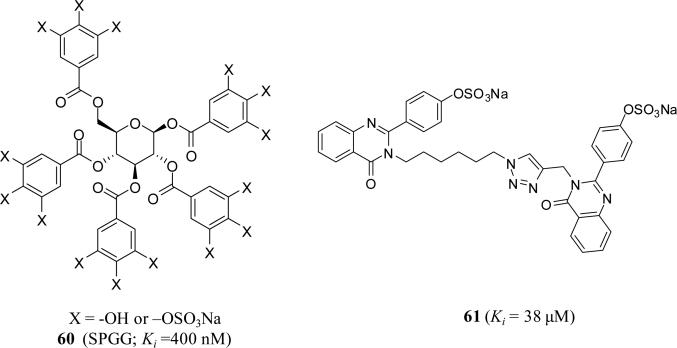
Particularly, sulfated pentagalloylglucoside (SPGG) 60 (Figure 17) displayed a Ki value of 400 nM against FXIa, which was at least 200-fold more selective than other relevant enzymes including thrombin, FXa, FIXa, FVIIa/TF, FXIIa, FXIIIa, plasma kallikrein, activated protein C, trypsin, and chymotrypsin. Importantly, SPGG prevented activation of FIX, the physiologic function of FXIa, and prolonged clotting time in human plasma only under APTT assay condition (at ~45 μM) but not under the conditions of PT assay (>350 μM). SPGG's effect on APTT of citrated human plasma was also not dependent on antithrombin or heparin cofactor II. SPGG also prolonged clotting time in human whole blood as determined by thromboelastography® [83, 84]. Toxicity studies using two cell lines showed no toxicity at SPGG levels as high as 200 mg/L (MTT assay) [85]. Biochemically, Michaelis-Menten kinetics, fluorescence emission scan and quenching experiments, competitive studies with UFH, and site-directed mutagenesis all revealed that SPGG binds in the heparin-binding site of FXIa catalytic domain. Interestingly, SPGG's anticoagulant potential was diminished by serum albumin as well as FXI, while it could be reversed by protamine or polybrene, which implies possible avenues for developing antidote strategy [86]. Structure-activity relationship studies indicated that replacing the pyranose moiety with inositol ones affords FXIa inhibitors with IC50 values of <200 nM.
Figure 17.
Chemical structures of two classes of SNGMs as allosteric inhibitors of human FXIa.
Along those lines, a dual element strategy was proposed to enhance the specificity and druggability of SNGMs while targeting the heparin–binding site(s) on FXIa. This strategy involves 1) initial attraction of an anionic sulfate group present on a SNGM to one or more arginines and lysines present in the heparin-binding site(s) of a heparin-binding protein followed by 2) recognition of an adjacent hydrophobic patch to form a complex. Enzymes devoid of either the heparin-binding site or the hydrophobic domain would not bind the sulfated NSGM and hence escape inhibition. Only enzymes possessing both binding domains will be targeted by the sulfated NSGM, yet the potency of inhibition will be strongly dependent on the complementarity of NSGM's hydrophobic scaffold with the hydrophobic domain on the enzyme. Exploiting this strategy, several quinazolinones reported to have moderate potency against human FXIa, while the efficacy for nearly all inhibitors was very high (>85%). Essentially no inhibition was observed at concentrations as high as 500 μM toward thrombin, FXa, trypsin and chymotrypsin [87]. Particularly, quinazolinone dimer 61 (Figure 17) inhibited human FXIa with a Ki value of 38 μM. It reduced the VMAX of substrate hydrolysis without influencing the KM demonstrating the noncompetitive inhibition phenomenon. Mutagenesis of residues of the heparin-binding site of FXIa introduced a nearly 5-fold loss in inhibition potency supporting recognition of an allosteric site. Fluorescence studies showed a sigmoidal binding profile indicating highly cooperative binding. Competition with a positively charged, heparin-binding polymer did not fully nullify inhibition suggesting importance of hydrophobic forces to binding. In human plasma studies, inhibitor 61 demonstrated more effect on clotting time as determined in APTT assay using human plasma [87].
2.3 Polypeptides
In 2015, a patent filed by the National University of Singapore claimed Fasxiator (BF01) and its recombinant form as an anticoagulant with minimal side effect of bleeding [88]. Fasxiator was isolated and sequenced from the venom of the banded krait snake, Bungarus fasciatus. Fasxiator is a Kunitz-type protease inhibitor that prolonged clotting time in APTT assay (APTTx2 = 3μM) without significant effect on clotting time in PT condition up to 100 μM. Recombinant Fasxiator (rFasxiator) was expressed, purified, and characterized to be a selective and slow inhibitor of FXIa with an IC50 of 1.5 μM and KD of 20.2 nM. rFasxiator was selective toward FXIa over thrombin, FXIIa, FXa, FIXa, FVIIa, plasmin, activated protein C, urokinase, and kallikrein at the highest concentration tested which was 120 μM, yet it inhibited chymotrypsin with an IC50 of 1 μM [89].
rFasxiator was found to inhibit the activation of FIX by FXIa with an IC50 value of 3 μM. rFasxiator has the reactive sequence of P315 – P2 – P1 – P1’– P2’ – P3’– P421’ as Arg–Cys–Asn –Ala–Leu– Ile–Pro. A series of mutants were subsequently generated to improve the potency and selectivity of recombinant rFasxiator. rFasxiator N17R,L19E potently inhibited FXIa with an IC50 value of ~ 1 nM and a selectivity index of > 100-fold over trypsin, plasmin, FVIIa, FXa, kallikrein, chymotrpsin, and activated protein C. rFasxiatorN17R,L19E was found to be a competitive slow-type inhibitor of FXIa with a Ki value of 0.86 nM. This protein variant possessed 10-fold more potent anticoagulant activity in human plasma than in murine plasma. rFasxiatorN17R,L19E prolonged APTT in a dose-dependent manner and doubled APTT at ~300 nM, but had no significant effect on PT up to 40 μM in human plasma. rFasxiatorN17R,L19E delayed the occlusion incidence of mice carotid artery in FeCl3-induced thrombosis model at a dose of 0.3 mg/animal [89].
Another polypeptide is Desmolaris. It is a naturally deleted form of tissue factor pathway inhibitor (TFPI) from the salivary gland of the vampire bat Desmodus rotundus that tightly binds FXIa. Screening assay showed that Desmolaris potently inhibits FXa, FXIa, and kallikrein but does not affect thrombin, FXIIa, FVIIa/TF, plasmin, tPA, uPA, chymase, matriptase, or proteinase 3. Desmolaris was also found to inhibit other enzymes such as trypsin, α-chymotrypsin, and neutrophil-derived elastase and cathepsin G. Desmolaris dose-dependently extended the PT and the APTT. At 300 nM, Desmolaris increased the APTT 9-fold and the PT only 2-fold. Mechanistically, recombinant Desmolaris (rDesmolaris) was found to be a slow, tight, and noncompetitive inhibitor of FXIa with a KD value of 0.63 nM by a mechanism modulated by heparin. Independent of protein S, Desmolaris also slowly, tightly, and noncompetitively inhibited FXa with a higher KD value of 16 nM. Furthermore, rDesmolaris bound to kallikrein (KD = 115 nM) and reduced bradykinin generation in kaolin-activated plasma. Truncated and mutated forms of Desmolaris determined that Arg32 in the Kunitz-1 domain is critical for protease inhibition whereas the C-terminal domain and Kunitz-2 domain mediate interaction of polypeptide with heparin and are required for optimal inhibition of FXIa and FXa. rDesmolaris (100 μg/kg) inhibited FeCl3-induced carotid artery thrombus in mice without affecting hemostasis. In mice models, rDesmolaris alleviated collagen- and epinephrine-mediated thromboembolism and the polyphosphate-induced increase in vascular permeability [90].
Researchers from Guangdong Medical College in China claimed a class of anticlotting polypeptides from the blood-feeding nematode Ancylostoma caninum to treat and prevent thromboembolic diseases [91]. Particularly, Ancylostoma caninum nematode anticoagulant peptide 10 (AcaNAP10) showed a potent anticoagulant activity and doubled the APTT and PT at estimated concentrations of 92.9 nM and 28.8 nM, respectively. AcaNAP10 demonstrated distinct mechanisms of action compared with known anticoagulants. It inhibited FXIa and FVIIa/TF with IC50 values of 26 nM and 124 nM, respectively. A double-reciprocal plot showed that recombinant AcaNAP10 (rAcaNAP10) was a mixed-type inhibitor of FXIa with a Kivalue of 18 nM. The subsequent patents were filed for modified variants of AcaNAP10 which were developed on the basis of AcaNAP10's activity toward FXIa. The patents described the structural sequences of AcaNAP10 and the advanced polypeptides as well as some aspects of their anticoagulant properties [92].
Along these lines researchers from Universite Libre de Bruxelles in Belgium filed to patent a polypeptide designated as Ir-CPI (recombinant Ixodes ricinus contact phase inhibitor), a Kunitz-type protein expressed by the salivary glands of the tick Ixodes ricinus [93]. Ir-CPI selectively inhibited activated human contact phase factors (FXIIa, FXIa, and kallikrein) and prolonged the APTT in vitro. It did neither inhibit the activities of FXa and/or FVIIa nor reduce their contents. The effects of Ir-CPI were also examined in vivo using both venous and arterial thrombosis models. IV adminstration of Ir-CPI in rats and mice dose-dependently reduced venous thrombus formation as well as the formation of arterial occlusive thrombi. Moreover, mice injected with Ir-CPI were found to be protected against epinephrine- and collagen-induced thromboembolism. The effective antithrombotic dose of Ir-CPI did not promote bleeding or impair blood coagulation parameters. Therefore, pharmaceutical preparations comprising Ir-CPI were claimed to treat and/or prevent thromboembolism, stroke, myocardial infarction, cerebrovascular diseases, cerebral ischemia, pulmonary embolism, renal vein thrombosis, and hepatic vein thrombosis. The cDNA sequence and encoded amino acid sequence of Ir-CPI are provided in the patent [94].
Other peptides that claimed to have an inhibitory activity against FXIa include Simukunin from Simulium vittatum (2012; University of Georgia Research Foundation, Inc., USA; U.S. Dept of Health and Human Services) [95, 96], human Kunitz-type protease inhibitors (hKTPi) (2009; Bayer Healthcare A.-G., Germany) [97], ecotin from Escherichia coli and its variants (2000; University of Claifornia, US and 1996; Corvas International, Inc., USA ) [98, 99], serine protease inhibitor peptides derived from human amyloid β-protein precursor (1999/6; Scios, Inc., US), Kunitz domain inhibitor proteins derived from Alzheimer's amyloid β-protein precursor inhibitor (KALI-DY) (1995; Genentech, Inc., US) [100], analogs (>5-80 amino acids) of the activated platelet binding site on FXI or FXIa (1995; Temple University and Thomas Jefferson University, US), serine protease derived-polypeptides (1993; Scripps Research Institute, US), and others. Yet, no reports have been published subsequently on the development of these peptides.
2.4 Antisense Oligonucleotides (ASOs)
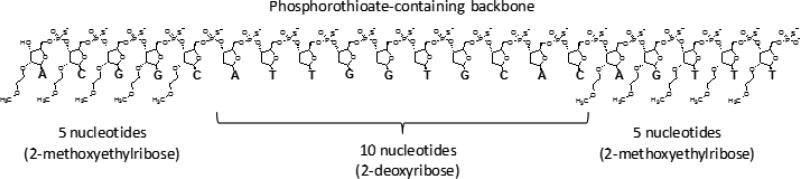
In general, ASOs are relatively short (8 – 50 nucleotides), single-stranded nucleic acid sequences, which could be either unmodified or chemically modified. They can be rationally designed to reduce a specific target protein level by different mechanisms. In case of FXI, ASOs are specifically designed to selectively hybridize with a mRNA target in liver through specific complementary base-pairing. They have ≤20 nucleotides linked by phosphorothioate instead of phosphodiester linkage) (Figure 18). The hybridization typically results in the selective and catalytic degradation of the targeted mRNA by a mechanism involving the nuclease RNAse H and leads to a corresponding reduction in FXI plasma levels [101]. Advantages of ASOs therapy include their dose-linear and predictable pharmacokinetics in humans, a relatively infrequent administration because of their prolonged t1/2, a relatively high degree of target selectivity, and their lack of common drug-drug interactions [102].
Figure 18.
The ASO “5–10–5” design of ISIS416858. It is a 20–base pair ASO designed to support RNaseH activity. The 2‘-methoxyethylribose is utilized only on the external 5 nucleotides on both ends while leaving the internal 10 nucleotides with the unmodified 2‘-deoxyribose. The phosphorothioate backbone is used along the length of the ASO to provide nuclease resistance. This provides increased target RNA binding affinity on the outer portions of the ASO, while still allowing RNaseH cleavage at the central domain of the ASO.
Initially, backbone-modified and base analog-containing ASOs directed against blood coagulation FXI mRNA were described by ISIS Pharmaceuticals for use in the treatment and prevention of thromboembolic complications in cardiovascular disease [103]. Disease conditions that are claimed to be ameliorated with these materials include deep vein thrombosis, pulmonary embolism, myocardial infarction, and stroke. These ASOs were also claimed to be beneficial in the prophylaxis against coagulation disorders in individuals at risk for thrombosis and embolism. A set of 170 antisense gapmers with 5'- and 3'-methoxy modifications were screened for their ability to inhibit FXI biosynthesis in HepG2 cells. This exercise identified 13 oligonucleotides that inhibited FXI synthesis in a dose-dependent manner with IC50 values in the range of 21–43 nM. Subsequently, promising ASOs were used to design more efficient inhibitors and to identify sites most sensitive to antisense inhibition. In FeCl3-induced deep vein thrombosis mice model, FXI synthesis inhibition of ≥95% was detected at 25 mg/kg for the most effective oligonucleotides which were also more effective than warfarin.
Subsequently, inflammatory diseases such as arthritis and colitis were also claimed by the same company to be ameliorated or prevented with the administration of ASOs targeted to FXI. Oligonucleotides effective in blocking inflammation in the collagen-induced arthritis model were identified. These oligonucleotides were also effective in the treatment of dextran sulfate-induced colitis [104]. Recently, ISIS Pharmaceuticals was granted a patent in which they claimed modified ASOs complementary to human FXI, for decreasing both FXI antigen and FXI activity. They claimed that the oligonucleotides may be used for treating or preventing thromboembolic and inflammatory disorders in patients, or as a prophylactic treatment in subjects with risk factors for these conditions [105]. Several studies have supported these claims and demonstrated the success of this strategy (Table 2).
Table 2.
Studies involving the use of ASOs targeting FXI.
| Subjects | Dose | Residual FXI Conc. and Activity | References |
|---|---|---|---|
| Mice | 50 mg/kg (ISIS404071) | <10% (conc.) and <10% (activity) | 106 |
| Mice | 50 mg/kg | 36% (activity) | 111 |
| Cynomolgus monkeys | 4–40 mg/kg (ISIS416858= ISIS-FXIRx) | 25% (activity) | 108 |
| Baboons | 25 mg/kg (modified ISIS-FXIRx) | 30% (conc.) and 30% (activity) | 109 |
| Rabbits | 15 mg/kg (ISIS564673) | 4% (conc.) and 1% (activity) | 110 |
| Human | 50–300 mg (ISIS416858= ISIS-FXIRx) | 0% (conc.) and 8% (activity) | 107 |
| Human | 200 & 300 mg (ISIS416858= ISIS-FXIRx) | at 200 mg 41% (activity) & at 300 mg 22% (activity) | 112 |
Systemic treatment of mice with FXI second generation ASO (ISIS404071) resulted in a potent, selective, and dose-dependent reduction of FXI mRNA levels in the liver and parallel reductions in plasma levels of FXI protein and activity. Comparable to warfarin and enoxaparin, FXI ASO treatment produced a potent, dose-dependent antithrombotic activity in FeCl3-induced inferior vena cava, mesenteric vein, and aortic thrombosis models as well as in stenosis-induced inferior vena cava thrombosis model. In contrast to warfarin and enoxaparin, ASO-mediated FXI inhibition did not cause bleeding in tail vein bleeding assay and hepatectomy surgical bleeding model. In addition, plasma-derived FXI concentrate was shown to effectively and rapidly reverse the anticoagulant effect of FXI antisense therapy. Co-administration of FXI ASO with enoxaparin or clopidogrel produced improved antithrombotic activity without increased bleeding [106].
The phase I study evaluating ISIS-FXIRx in healthy volunteers revealed that robust, sustained, and statistically significant reductions of up to 78% and 85% in FXI protein levels and of up to 71% and 78% in FXI activity upon the administration of 200 mg and 300 mg dose of ISIS-FXIRx, respectively. Reductions in activity were accompanied by APTT prolongation [107].
Furthermore, cynomolgus monkeys administered with a 2’-methoxyethoxy ASO (ISIS416858) (4, 8, 12, and 40 mg/kg/wk, sc) for up to 13 weeks produced a dose-dependent reduction in hepatic FXI level as well as in plasma FXI level and activity. Concomitantly, there was also an increase in APTT. The ASO (20 or 40 mg/kg/wk) reduced plasma FXI activity by 80% at 4 weeks of treatment leading to a 33% increase in APTT by 13 weeks with no effects on PT, platelets, or increased bleeding following partial tail amputation or gum and skin laceration. No toxicity was attributed to hepatic FXI reduction [108]. Likewise, in the baboon arteriovenous shunt thrombosis model, ASO-mediated reduction of FXI plasma levels by ≥ 50% resulted in a significant antithrombotic effect without an increased risk of bleeding in the standard template skin bleeding test [109]. Similarly, knockdown of FXI by FXI ASO (ISIS564673) in rabbits prolonged the APTT and decreased FXI activity level by more than 90%. Using a rabbit model of catheter thrombosis, catheters implanted in the jugular vein were assessed daily until they occluded, up to a maximum of 35 days. Compared with control, FXI ASO treatment prolonged the time to catheter occlusion by 2.3-fold [110]. Interestingly, on atherosclerotic plaque rupture in the carotid arteries of ApoE(−/−) mice, initial platelet adhesion and platelet plug formation were not impaired in mice treated with FXI ASO (50 mg/kg). Yet, the thrombus formation and fibrin deposition were significantly lower after 5 to 10 minutes in FXI ASO-treated animals without inducing a bleeding tendency. Furthermore, thrombi from these animals were less stable than thrombi from placebo-treated animals. Moreover, macrophage infiltration and collagen deposition were lower in the carotid arteries of FXI ASO-treated animals and no neutrophils were present. This suggests that FXI ASO safely prevent thrombus formation on acutely ruptured atherosclerotic plaques in mice with much less severe inflammatory response [111].
In an open-label, parallel-group Phase II study (NCT01713361), 300 patients who were undergoing elective primary unilateral total knee arthroplasty were assigned to receive one of two doses of FXI-ASO (200 mg or 300 mg) or 40 mg of enoxaparin once daily. The primary efficacy outcome was the incidence of venous thromboembolism and the principal safety outcome was major or clinically relevant nonmajor bleeding. The primary efficacy outcome occurred in 4% of patients who received the 300-mg dose of FXI-ASO and in 27% of patients who received the 200-mg dose of FXI-ASO, as compared with 30% of patients who received enoxaparin. The 300-mg regimen was superior, and the 200-mg regimen was noninferior, to enoxaparin. Bleeding occurred in 3% of the patients received FXI-ASO treatment and in 8% of the patients who received enoxaparin. Accordingly, this study demonstrated that reducing FXI levels in patients undergoing elective primary unilateral total knee arthroplasty was an effective method for prevention of postoperative venous thromboembolism and appeared to be safe with respect to the risk of bleeding [112].
2.5 Monoclonal Antibodies
Several companies have reported on the preparation and/or the use of monoclonal antibodies targeting FXI/FXIa system for treatment and/or prevention of thrombosis. These include Bayer of Germany in 2013, Prothix B.V. of Netherlands in 2009, Oregon Health & Science and Vanderbilt Universities of US in 2009, and GlaxoSmithKline of US in 2003. Antithrombotic efficacy and bleeding risk of these antibodies have been tested in several studies (Table 3).
Table 3.
Studies involving the use of monoclonal antibodies targeting FXI and/or FXIa.
| Monoclonal antibody | Binding site | Positive results in animal models | References |
|---|---|---|---|
|
076D-M007
076D-M028-H17 |
The former binds to the catalytic domain and the latter to the apple domain | 1) FeCl3-induced arterial occlusion (rabbits) 2) Ear bleeding assay (rabbits) 3) Collagen-initiated arteriovenous shunt thrombosis (baboons) 4) Template skin bleeding test (baboon) |
113 |
| XI-5108 | Binds the catalytic domain | 1) Balloon-induced injured neointima of the iliac artery (rabbits) 2) Endothelial denudation and vessel ligation-induced thrombosis in jugular vein (rabbits) |
114 & 115 |
| O1A6 (aXIMab) | Binds to A3 domain | 1) Collagen-initiated arteriovenous shunt thrombosis (baboons) 2) Template skin bleeding test (baboon) |
117 & 118 |
| 14E11 | Binds to A2 domain | 1) FeCl3-induced arterial occlusion (mice) 2) TF-induced pulmonary embolism (mice) 3) Acute ischemic stroke (mice) 4) Collagen-initiated arteriovenous shunt thrombosis (baboons) 5) Polymicrobial sepsis (mice) 6) Lethal listeriosis (mice) 7) Tail bleeding assay (mice) |
119-122 |
|
αFXI-175
αFXI-203 |
The former binds to A4 domain and the latter to A2 domain | 1) FeCl3-induced thrombosis in vena cava inferior (mice) 2) Tail bleeding assay (mice) |
123 |
In 2013, Bayer claimed an invention on antibodies capable of binding to FXI and/or FXIa and methods of use thereof, particularly methods of use as agents inhibiting platelet aggregation and thrombus formation without compromising hemostasis [113]. Two particular human monoclonal antibodies were discussed including 076D-M007-H04 and 076D-M028-H17. The patent presented the inhibition profiles of the former against human and rabbit FXIa. It also presented the inhibition by the latter for the conversion of human or rabbit FXI to the corresponding FXIa as mediated by thrombin and FXIIa. Only 067D-M007-H04 inhibited the proteolytic activity of FXIa suggesting binding to the catalytic domain as competitive inhibitor, while 076D-M007-H17 did not suggesting a potential binding site in the apple domain(s). Both antibodies reduced platelet expression and micro-aggregate formation. They also dose-dependently reduced thrombus weight in FeCl3-induced arterial thrombosis model in rabbits without affecting the ear bleeding times. APTT, PT, TT, and platelet deposition aspects of their antithrombotic potentials were also studied in baboons.
Along these lines, a mouse monoclonal antibody against FXI and FXIa known as XI-5108 was generated. The XI-5108 antibody was found to bind the catalytic domain of human and rabbit FXI/FXIa with KD values of 0.11 – 0.46 nM. The antibody inhibited FXIa-mediated generation of FXa and FXIa. In a model of balloon-induced injured neointima of the rabbit iliac artery, XI-5108 (3.0 mg/kg) significantly reduced thrombus growth and prolonged the APTT measurement, yet without affecting PT measurement, bleeding time, and collagen-induced platelet aggregation [114]. Likewise, subsequent studies showed that XI-5108 significantly reduced ex vivo plasma thrombin generation initiated by ellagic acid but not by TF, and in vivo thrombus formation in rabbit jugular vein under endothelial denudation and/or vessel ligation. The antibody significantly reduced surface areas covered by platelets and fibrin, as well as thrombin generation at a low shear rate in flow chambers [115].
A polyclonal antihuman FXI antibody (aFXI) (16–50 mg/kg, IV) was evaluated in a nonterminal baboon model in which a graft of varying materials was inserted in an arteriovenous shunt to induce a non-occlusive thrombus. At doses tested, aFXI produced a 3.2-fold APTT prolongation without affecting the PT. Heparin at a dose that increased the APTT by 3.8-fold was tested as a reference. Both aFXI and heparin elicited strong and comparable inhibition of platelet accumulation on different grafts coated with TF. The initial accumulation of platelets was affected to a lesser extent by these treatments. Template bleeding times were unaffected by aFXI, but were increased 1.4-fold by heparin [116]. Later same exercise was repeated with a mouse monoclonal anti-FXI antibody known as aXIMab. In 2009, Oregon Health & Science and Vanderbilt Universities claimed aXIMab antibodies capable of binding to an epitope on the apple domains of human FXI, particularly the A3 domain, for inhibiting thrombosis without compromising hemostasis. Specific aXIMab, known as O1A6, inhibited FXIa activity with an in vitro IC50 value of 2.5 nM. Pretreatment of baboons with O1A6 (2 mg/kg) inhibited plasma FXIa by at least 99% for 10 days, and suppressed thrombin-antithrombin complex and β-thromboglobulin formation within the vicinity of thrombi forming within collagen-coated vascular grafts. FXIa inhibition with O1A6 also inhibited platelet and fibrin deposition without an apparent increase in D-dimer release from thrombi, and prevented the occlusion of smaller-diameter grafts without affecting template bleeding times [117, 118].
A monoclonal antibody, 14E11, which targets A2 domain of FXI was found to selectively reduce the prothrombotic activation of FXI by FXIIa and not to affect FXIa or hemostatic activation of FXI by thrombin. Similar to FXI deficiency, 14E11 prevented FeCl3-induced arterial occlusion in mice. 14E11 also demonstrated some favorable effect in a TF-induced pulmonary embolism model. In baboons, 14E11 reduced platelet-rich thrombus growth in collagen-coated grafts inserted into an arteriovenous shunt [119]. In acute ischemic stroke/reperfusion injury murine model, 14E11-treated mice displayed reduced cerebral infarction and fibrin deposition, increased cortical reperfusion, and improved neurological behavior. The antibody-treated mice had no detectable hemostasis impairment as observed in tail bleeding assay [120]. Likewise, inhibition of FXI activation by 14E11 attenuated inflammation and coagulopathy while improving the survival of mouse model of polymicrobial sepsis [121]. Furthermore, FXI activation inhibition by 14E11 displayed reduced inflammation, coagulopathy, and bacterial growth in mouse model of lethal listeriosis [122].
Two mouse monoclonal anti-human FX/FXIa antibodies known as αFXI-175 and αFXI-203 were reported recently [123]. Both antibodies were able to dose-dependently prolong coagulation in normal plasma with a maximum inhibition of ~85%. Complete inhibition of FXI (<1% FXI activity) was obtained by adding the combination of αFXI-175 and αFXI-203 to normal human plasma. The antibodies reacted with both FXI and FXIa with KD values of 3-5 nM. αFXI-175 predominantly binds to A4 domain and αFXI-203 interacts with A2 domain. Accordingly, it appears that αFXI-175 interferes with factor IX activation by FXIa whereas αFXI-203 prevents the binding of FXI(a) to HK. Both antibodies inhibited thrombin generation initiated via the intrinsic pathway. In contrast, upon TF-initiated thrombin generation, αFXI-203 did not inhibit thrombin generation, while αFXI-175 inhibited thrombin generation only at low concentrations of TF. In the FeCl3-induced murine thrombosis model, the vena cava inferior remained patent for 25 min in mice treated with αFXI-175 and for 12.5 min in αFXI-203 treated animals, which was significantly longer than in placebo-treated animals. Neither antibody caused severe blood loss in tail bleeding assays [123].
3. Expert opinion
The definition of an “ideal” anticoagulant is extensive because it should satisfy a large number of disease and practical conditions. In addition to excellent pharmacodynamic profile, it should 1) have predictable pharmacokinetics; 2) lack significant toxicity arising from effects on the liver (hepatotoxicity), bones (osteoporosis), and platelets (thrombocytopenia); 3) be rapidly reversible through the use an effective and inexpensive antidote; 4) not require continuous monitoring, assessment, and/or dose adjustment; 5) be safe in compromised patient populations such as pregnant women and cancer patients; 6) be available in relatively inexpensive oral and/or parenteral forms; and 7) result in any elevation of internal bleeding risk. Despite recent advances in anticoagulant therapy, there are no “ideal” anticoagulants available in clinic to prevent and/or treat thromboembolic diseases. At best most agents fulfill some of these criteria. Therefore, the search for an ideal anticoagulant continues.
All anticoagulants approved to date are directed toward thrombin and FXa, serine proteases in the common coagulation pathway. Each of these suffers from some form of bleeding risk. It is expected that factors of intrinsic or extrinsic pathways may serve as better targets for developing anticoagulants. In fact, over the last two decades, our understanding for roles of these factors in hemostasis and consequences of their genetic deficiencies in humans has advanced considerably, which has provided a compelling rationale for inhibiting FXIa to treat and/or prevent thromboembolism without associated hemorrhagic conditions. This coupled with the availability of advanced structural and molecular biology tools led to a variety of strategies targeting FXI/FXIa with the goal of developing antithrombotic therapies that are largely, or completely, devoid of effects on hemostasis. These strategies have resulted in the large number of small and large molecules as FXI/FXIa inhibitors. It is important to mention here that several other coagulation factors including FXIIa, FIXa, and FVIIa have been targeted for inhibition by many research groups. For example, a number of pharmacological and clinical studies have shown conclusively that inhibition of FVIIa affords excellent antithrombotic efficacy along with low bleeding liability; similar to the potential of FXIa inhibition. Yet, a Scifinder®-facilitated survey indicates that the number of patents filed for FVIIa inhibitors over the last five years is about 6 times less than those filed for FXIa inhibitors suggesting a major trend.
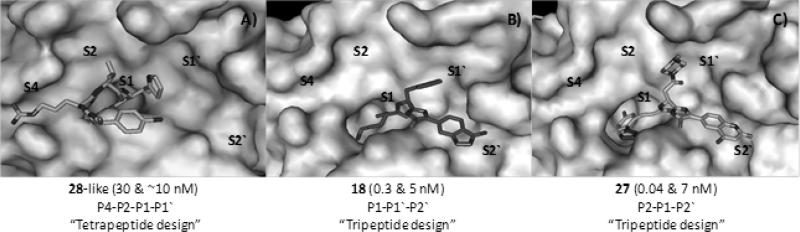
Despite many advances, further development efforts are necessary for FXI/FXIa inhibitors to enter the clinic. For example, the routes of administration and delivery as well as the pharmacokinetic profiles are of concern for all of the new category of inhibitors. Furthermore, the non-selectivity of the active site peptidomimetic inhibitors, particularly toward plasma kallikrein seems to be persistent issue, although different design strategies have been exploited (Figure 19). In addition, a reversal agent to treat cases of excessive anticoagulation might be difficult to develop. In case of polypeptides, their non-selectivity, the lack of reversal strategy, the parenteral administration, and their potential immunogenicity may represent the main issues.
Figure 19.
Co-crystal structures for complexes of FXIa with 3 active site inhibitors showing different design approaches used. Numbers given represent Ki toward FXIa and plasma kallikrein, respectively. Crystal structures depicted are A) 1ZOM for inhibitor 28, B) 4TY7 for inhibitor 18, and C) 4Y8Z for inhibitor 27. The figure was generated in PyMOL (http://www.pymol.org/).
In contrast, results from Phase I and II studies involving ASOs seem to be very encouraging. ASOs could possess excellent selectivity for FXI and, in turn, it is possible that FXI protein could also be used as a reversal strategy in some cases. Yet, ASOs have also some potential limitations. ASOs have a delayed onset of action and should be parenterally administered. They also have a very long elimination half-life and may suppress FXI activity by >50% for more than 1 month after the last dose. This may increase and complicate the risk of bleeding, particularly with the current lack of well-established reversal strategy. Despite initial success in human, their clinical effectiveness should also be compared against the new oral anticoagulants. Given the high cost of ASOs, cost-effective analyses is necessary to identify their economical advantages. Likewise, monoclonal antibodies could also provide an alternative strategy to achieve selectivity over other coagulation factors as well as achieve a fast onset of action. Moreover, dosing additional FXI protein could serve as a reversal strategy. However, their expected high cost and the need for parenteral administration are likely to be limitations in developing a widely used strategy, especially with much cheaper heparins used in the clinic.
An interesting strategy that appears gaining momentum in this field is the allosteric inhibition of FXIa by targeting the heparin binding site in the catalytic domain. Allosteric modulation is fundamentally exploited by nature to confer specificity of recognition, and therefore it has been used to engineer selectivity in inhibitors targeting FXIa, particularly because FXIa's heparin allosteric sites are dissimilar to these belong to other serine proteases in coagulation and other systems. This concept has been uniquely demonstrated by sulfated NSGMs, e.g., SPGG, which represents a first generation non-peptide mimetic agent to selectively influence FXIa activity. Yet, its efficacy to reduce thrombosis in both venous and arterial thrombosis models and its impact on bleeding remain to be rigorously established.
Finally, the types of thromboembolic disorders that can be prevented or treated using FXIa inhibitors remain unknown. However, the results of animal models of mice, rats, rabbits, and baboons as well as humans in addition to the statistical data from human populations suggest that this therapy might be exploited to prevent both arterial and venous thrombosis.
Article Highlight Box.
FXIa, a serine protease in the intrinsic coagulation pathway, is a promising drug target for safer anticoagulation with respect to bleeding consequences.
The past 3 years have realized a large number of industrial and academic patent applications describing new advances in the discovery of novel FXI/FXIa inhibitors.
New agents include small peptidomimetics, sulfated glycosaminoglycan mimetics, polypeptides, antisense oligonucleotides, and monoclonal antibodies.
Antithrombotic activity and safety of several FXI/FXIa inhibitors have been confirmed in arterial and venous animal models.
Pharmacokinetics appears to be the major concern while developing these inhibitors for the clinical use.
Acknowledgments
This work was supported by funding from the NIH through grants HL107152, HL090586 and HL128639 to UR Desai.
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (**) or of considerable interest (***) to readers.
- 1.Raskob GE, Angchaisuksiri P, Blanco AN. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34:2363–71. doi: 10.1161/ATVBAHA.114.304488. [DOI] [PubMed] [Google Scholar]
- 2.Mahan CE, Holdsworth MT, Welch SM, et al. Deep-vein thrombosis: a United States cost model for a preventable and costly adverse event. Thromb Haemost. 2011;106:405–15. doi: 10.1160/TH11-02-0132. [DOI] [PubMed] [Google Scholar]
- 3.Henry BL, Desai UR. Anticoagulants: Drug discovery and development. In: Rotella D, Abraham DJ, editors. Burger's Medicinal Chemistry. 7th ed. John Wiley and Sons; New York: 2010. pp. 365–408. [Google Scholar]
- 4.Garcia DA1, Baglin TP, Weitz JI, et al. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e24S–43S. doi: 10.1378/chest.11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ageno W1, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e44S–88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer KA. Pros and cons of new oral anticoagulants. Hematology Am Soc Hematol Educ Program. 2013;2013:464–70. doi: 10.1182/asheducation-2013.1.464. [DOI] [PubMed] [Google Scholar]
- 7.Lee AYY. Anticoagulation in the treatment of established venous thromboembolism in patients with cancer. J Clin Oncol. 2009;27:4895–901. doi: 10.1200/JCO.2009.22.3958. [DOI] [PubMed] [Google Scholar]
- 8.Bates SM, Greer IA, Pabinger I, et al. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy, 8th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2008;133:844S–86S. doi: 10.1378/chest.08-0761. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Seiffert D, Hawes B. Inhibition of Factor XI activity as a promising antithrombotic strategy. Drug Discov Today. 2014;19:1435–9. doi: 10.1016/j.drudis.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Bane CE, Jr, Gailani D. Factor XI as a target for antithrombotic therapy. Drug Discov Today. 2014;19:1454–8. doi: 10.1016/j.drudis.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schumacher WA, Luettgen JM, Quan ML, et al. Inhibition of factor XIa as a new approach to anticoagulation. Arterioscler Thromb Vasc Biol. 2010;30:388–92. doi: 10.1161/ATVBAHA.109.197178. [DOI] [PubMed] [Google Scholar]
- 12.Löwenberg EC, Meijers JC, Monia BP, et al. Coagulation factor XI as a novel target for antithrombotic treatment. J Thromb Haemost. 2010;8:2349–57. doi: 10.1111/j.1538-7836.2010.04031.x. [DOI] [PubMed] [Google Scholar]
- 13.He R, Chen D, He S. Factor XI: hemostasis, thrombosis, and antithrombosis. Thromb Res. 2012;129:541–50. doi: 10.1016/j.thromres.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 14.Emsley J, McEwan PA, Gailani D. Structure and function of factor XI. Blood. 2010;115:2569–77. doi: 10.1182/blood-2009-09-199182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gailani D, Smith SB. Structural and functional features of factor XI. J Thromb Haemost. 2009;7:75–8. doi: 10.1111/j.1538-7836.2009.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Sun MF, Gailani D, et al. Characterization of a heparin-binding site on the catalytic domain of factor XIa: mechanism of heparin acceleration of factor XIa inhibition by the serpins antithrombin and C1-inhibitor. Biochemistry. 2009;48:1517–24. doi: 10.1021/bi802298r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badellino KO, Walsh PN. Localization of a heparin binding site in the catalytic domain of FXIa. Biochemistry. 2001;40:7569–80. doi: 10.1021/bi0027433. [DOI] [PubMed] [Google Scholar]
- 18.Mackman N, Tilley RE, Nigel S, et al. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–93. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 19.Gailani D, Renne' T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2507–13. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- 20.Gailani D, Renne' T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease? J Thromb Haemost. 2007;5:1106–12. doi: 10.1111/j.1538-7836.2007.02446.x. [DOI] [PubMed] [Google Scholar]
- 21***.Gailani D, Lasky NM, Broze GJ., Jr A murine model of factor XI deficiency. Blood Coagul Fibrinolysis. 1997;8:134–44. doi: 10.1097/00001721-199703000-00008. [First description and characterization of FXI knockout mice model.] [DOI] [PubMed] [Google Scholar]
- 22**.Wang X, Cheng Q, Xu L, et al. Effects of factor IX or factor XI deficiency on ferric chloride induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [Protection against arterial thrombosis in FXI deficient mice.] [DOI] [PubMed] [Google Scholar]
- 23**.Wang X, Smith PL, Hsu MY, et al. Effects of factor XI deficiency on ferric chloride-induced vena cava thrombosis in mice. J Thromb Haemost. 2006;4:1982–8. doi: 10.1111/j.1538-7836.2006.02093.x. [Protection against venous thrombosis in FXI deficient mice.] [DOI] [PubMed] [Google Scholar]
- 24.Rosen ED, Gailani D, Castellino FJ. FXI is essential for thrombus formation following FeCl3- induced injury of the carotid artery in the mouse. Thromb Haemost. 2002;87:774–6. [PubMed] [Google Scholar]
- 25.Renné T, Pozgajová M, Grüner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–81. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinschnitz C, Stoll G, Bendszus M, et al. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203:513–8. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–56. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28**.Jin L, Pandey P, Babine RE, et al. Mutation of surface residues to promote crystallization of activated factor XI as a complex with benzamidine: an essential step for the iterative structure-based design of factor XI inhibitors. Acta Crystallogr D Biol Crystallogr. 2005;61:1418–25. doi: 10.1107/S0907444905024340. [First X-ray crystal structure of FXIa catalytic domain in complex with small molecule.] [DOI] [PubMed] [Google Scholar]
- 29.Fradera X, Kazemier B, Carswell E, et al. High-resolution crystal structures of factor XIa coagulation factor in complex with nonbasic high-affinity synthetic inhibitors. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012;68:404–8. doi: 10.1107/S1744309112009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanessian S, Larsson A, Fex T, et al. Design and synthesis of macrocyclic indoles targeting blood coagulation cascade Factor XIa. Bioorg Med Chem Lett. 2010;20:6925–8. doi: 10.1016/j.bmcl.2010.09.141. [DOI] [PubMed] [Google Scholar]
- 31**.Lin J, Deng H, Jin L, et al. Design, synthesis, and biological evaluation of peptidomimetic inhibitors of factor XIa as novel anticoagulants. J Med Chem. 2006;49:7781–91. doi: 10.1021/jm060978s. [In vitro and in vivo studies of a peptidomimetic inhibitor which binds into S4-S2-S1-S1' domain of FXIa active site.] [DOI] [PubMed] [Google Scholar]
- 32.Lazarova TI, Jin L, Rynkiewicz M, et al. Synthesis and in vitro biological evaluation of aryl boronic acids as potential inhibitors of factor XIa. Bioorg Med Chem Lett. 2006;16:5022–7. doi: 10.1016/j.bmcl.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 33**.Schumacher WA, Seiler SE, Steinbacher TE, et al. Antithrombotic and hemostatic effects of a small molecule factor XIa inhibitor in rats. Eur J Pharmacol. 2007;570:167–74. doi: 10.1016/j.ejphar.2007.05.043. [First demonstration of FXIa inhibitors efficacy in thrombosis model in rats.] [DOI] [PubMed] [Google Scholar]
- 34**.Wong PC, Crain EJ, Watson CA, et al. A small-molecule factor XIa inhibitor produces antithrombotic efficacy with minimal bleeding time prolongation in rabbits. J Thromb Thrombolysis. 2011;32:129–37. doi: 10.1007/s11239-011-0599-0. [First demonstration of FXIa inhibitors efficacy in thrombosis model in rabbits.] [DOI] [PubMed] [Google Scholar]
- 35.Buchanan MS, Carroll AR, Wessling D, et al. Clavatadine A, a natural product with selective recognition and irreversible inhibition of factor XIa. J Med Chem. 2008;51:3583–7. doi: 10.1021/jm800314b. [DOI] [PubMed] [Google Scholar]
- 36.Preparation of substituted phenylalanine derivatives as factor XI modulators for treating thrombotic and thromboembolic diseases. Bayer Pharma; Germany: 2015. WO2015044174A1, WO2015044165A1, WO2015044170A1, WO2015044173A1, WO2015044169A1, WO2015044167A1, WO2015044172A1, WO 2015044163A1. [Google Scholar]
- 37.FXIa inhibitors. Merck Sharp & Dohme; USA: 2015. WO2015164308A1. [Google Scholar]
- 38.Preparation of pyridazine derivatives as factor XIa inhibitors. Bristol Myers Squibb Co.; USA: 2009. WO2009114677A1. [Google Scholar]
- 39.Preparation of 5-membered heterocycles as serine protease inhibitors for treatment of thromboembolic disorders. Bristol Myers Squibb Co.; USA: 2005. US20050282805A1. [Google Scholar]
- 40.Pyridone or pyrimidone derivatives as coagulation factor XIa inhibitors and their preparation, pharmaceutical compositions and use in the treatment of diseases. Sichuan Haisco Pharmaceutical Co.; China: 2015. WO2015120777A1. [Google Scholar]
- 41.Preparation of 2-oxopyridine compounds as therapeutic Factor XIa inhibitors. Merck Sharp & Dohme; USA: 2014. WO2014160592A2. [Google Scholar]
- 42.Factor XIa inhibitors for treating or preventing thromboses, embolisms, hypercoagulability or fibrotic changes. Merck Sharp & Dohme; USA: 2015. WO2015123091A1. [Google Scholar]
- 43.Preparation of substituted oxopyridine derivatives as factor XIa and plasma kallikrein inhibitors. Bayer Pharma; Germany: 2015. WO2015063093A1. [Google Scholar]
- 44.Preparation of substituted oxopyridine derivatives and use thereof in the treatment of cardiovascular disorders. Bayer Pharma; Germany: 2014. WO2014154794A1. [Google Scholar]
- 45.Substituted oxopyridine derivatives and use thereof as factor XIa /plasma. Bayer Pharma; Germany: 2015. WO2015011087A1. [Google Scholar]
- 46.Dipeptide derivatives having factor XIa-inhibiting activities. LegoChem Bioscience Co.; S. Korea: 2013. KR2013131775A. [Google Scholar]
- 47**.Hu Z, Wong PC, Gilligan PJ, et al. Discovery of a Potent Parenterally Administered Factor XIa Inhibitor with Hydroxyquinolin-2(1H)-one as the P2′ Moiety. ACS Med Chem Lett. 2015;6:590–5. doi: 10.1021/acsmedchemlett.5b00066. [In vitro and in vivo studies of a small molecule peptidomimetic inhibitor which binds into S1-S2-S2′ domain of FXIa active site.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Hangeland JJ, Friends TJ, Rossi KA, et al. Phenylimidazoles as potent and selective inhibitors of coagulation factor XIa with in vivo antithrombotic activity. J Med Chem. 2014;57:9915–32. doi: 10.1021/jm5010607. [In vitro and in vivo studies of small molecule, active site peptidomimetic FXIa inhibitor with P1-P1'-P2' design.] [DOI] [PubMed] [Google Scholar]
- 49.Fjellström O, Akkaya S, Beisel HG, et al. Creating novel activated factor XI inhibitors through fragment based lead generation and structure aided drug design. PLoS One. 2015;10:e0113705. doi: 10.1371/journal.pone.0113705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crystal structure of coagulation factor XIa-inhibitor complexes yield a pharmacophore structure useful for the design of compounds for treatment of thrombosis. Suntory Pharmaceutical Research Laboratories; USA: 2004. WO2004103270A2. [Google Scholar]
- 51.Blood coagulation factor XI inhibitors and methods for treatment of thrombosis. Suntory Pharmaceutical Research Laboratories; USA: 2004. p. WO 2004089297A2. [Google Scholar]
- 52.Lin J, Deng H, Jin L, Pandey P, et al. Design, synthesis, and biological evaluation of peptidomimetic inhibitors of factor XIa as novel anticoagulants. J Med Chem. 2006;49:7781–91. doi: 10.1021/jm060978s. [DOI] [PubMed] [Google Scholar]
- 53.Methods of treating thrombosis with reduced risk of increased bleeding times by administration of a small mol. inhibitor of Factor XIa. Bristol Myers Squibb Co.; USA: 2004. US20040180855A1. [Google Scholar]
- 54.Preparation of β-lactam derivatives as inhibitors of factor XIa or kallikrein. Exithera Pharmaceuticals; USA: 2015. WO2015120062A2. [Google Scholar]
- 55.Preparation of substituted azetidinones as inhibitors of tryptase, thrombin, trypsin and Factors Xa, VIIa and XIa. Daiamed; USA: 2006. WO2006108039A2. [Google Scholar]
- 56.Preparation of condensed 5-oxazolidinone derivatives as factor XIa inhibitors useful for oral prodrugs. Dainippon Sumitomo Pharma Co.; Japan: 2015. WO2015107724A1. [Google Scholar]
- 57.Preparation of 3-substituted proline derivatives as FXIa inhibitors. Dainippon Sumitomo Pharma Co.; Japan: 2015. JP2015083542A. [Google Scholar]
- 58.Preparation of 1-(heteroarylcarbonyl)proline derivatives as FXIa inhibitors. Dainippon Sumitomo Pharma Co.; Japan: 2015. JP2015013821A. [Google Scholar]
- 59.Preparation of 1-(cycloalkylcarbonyl)proline derivatives having excellent FXIa inhibitory activity. Dainippon Sumitomo Pharma Co.; Japan: 2014. WO2014014050A1. [Google Scholar]
- 60.Preparation of 3-substituted proline derivatives as FXIa inhibitors. Dainippon Sumitomo Pharma Co.; Japan: 2013. WO2013118805A1. [Google Scholar]
- 61.Pharmaceutical composition comprising 1,2,4-substituted pyrrolidine derivative as factor XIa inhibitor. Ono Pharmaceutical Co.; Japan: 2015. JP2015120685A. [Google Scholar]
- 62.Preparation of 1,2,4-substituted pyrrolidines as factor XIa inhibitors useful in the treatment thromboembolic diseases. Ono Pharmaceutical Co.; Japan: 2013. WO2013174937A1. [Google Scholar]
- 63.Factor XIa inhibitors for treatment of thrombosis, embolisms, hypercoagulability or fibrotic changes. Merck Sharp & Dohme; USA: 2015. WO2015054087A1. [Google Scholar]
- 64.Factor XIa inhibitors for treatment or prevention of thromboses, embolisms, hypercoagulability or fibrotic changes. Merck Sharp & Dohme; USA: 2015. WO2015047973A1. [Google Scholar]
- 65.Preparation of azolyl arylpropionamides, arylacrylamides, arylpropynamides, or arylmethylurea analogs as factor XIa inhibitors. Bristol-Myers Squibb Co.; USA: 2008. WO2008076805A2. [Google Scholar]
- 66.Pyridinone and pyrimidinone derivatives as factor XIa inhibitors and their preparation and use in the treatment of thromboembolic diseases. Ono Pharmaceutical Co.; Japan: 2013. WO2013093484A1. [Google Scholar]
- 67.Preparation of tetrahydroisoquinoline derivatives for use as coagulation factor XIa inhibitors. Bristol-Myers Squibb Co.; USA: 2014. WO2014160668A1. [Google Scholar]
- 68.Preparation of guanidine and amine substituted tetrahydroisoquinoline compounds as factor XIa inhibitors. Bristol-Myers Squibb Co.; USA: 2014. WO2014059214A1. [Google Scholar]
- 69.Preparation of guanidine substituted tetrahydroisoquinoline compounds as factor XIa inhibitors. Bristol-Myers Squibb Co.; USA: 2014. WO2014059202A1. [Google Scholar]
- 70.Substituted tetrahydroisoquinoline compounds as factor XIa inhibitors. Bristol-Myers Squibb Co.; USA: 2013. WO2013056060A1. [Google Scholar]
- 71.Preparation of substituted tetrahydroisoquinoline compounds as factor XIa inhibitors. Bristol-Myers Squibb Co.; USA: 2013. WO2013055984A1, WO 2013056034A1. [Google Scholar]
- 72.Quan ML, Wong PC, Wang C, et al. Tetrahydroquinoline derivatives as potent and selective factor XIa inhibitors. J Med Chem. 2014;57:955–969. doi: 10.1021/jm401670x. [DOI] [PubMed] [Google Scholar]
- 73.Preparation of tetrahydroquinoline derivatives as inhibitors of serine protease enzymes of the coagulation cascade and/or contact activation system. Bristol-Myers Squibb Co.; USA: 2004. WO2004080971A1. [Google Scholar]
- 74.Abdel-Magid Inhibitors of factor XIa and plasma kallikrein may treat thromboembolic disorders and many diabetes complications. ACS Med Chem Lett. 2014;5:286–287. doi: 10.1021/ml500084u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Macrocycle derivatives as factor XIa inhibitors and their preparation and use for the treatment of thromboembolic and inflammatory diseases. Bristol-Myers Squibb Co.; USA: 2011. WO2011100402A1. [Google Scholar]
- 76.Preparation of tetrazolylphenyldiazatricyclooctadecapen taenylacrylamide derivatives and analogs for use as factor XIa inhibitors. Bristol-Myers Squibb Co.; USA: 2011. WO 2011100401A1. [Google Scholar]
- 77.Novel macrocycles as factor XIa inhibitors and their preparation. Bristol-Myers Squibb Co.; USA: 2013. WO 2013022818A1, WO2013022814A1. [Google Scholar]
- 78.Preparation of macrocycles condensed with heterocycles as factor IXa inhibitors. Bristol-Myers Squibb Co.; USA: 2015. WO2015116882A1. [Google Scholar]
- 79.Dihydropyridones as factor XIa inhibitors and their preparation. Bristol-Myers Squibb Co.; USA: 2014. WO2014022766A1, WO2014022767A1. [Google Scholar]
- 80.Preparation of macrocycles with heterocyclic p2' groups as factor XIa inhibitors. Bristol-Myers Squibb Co.; USA: 2015. WO2015116885 A1, WO2015116886A1. [Google Scholar]
- 81.Factor XIa inhibitors for treating or preventing thromboses, embolisms, hypercoagulability or fibrotic changes. Merck Sharp & Dohme Corp.; USA: 2015. WO2015123090A1, WO2015123093A1. [Google Scholar]
- 82.Preparation of allosteric modulators of factor XIa by targeting hydrophobic domains adjacent to its heparin-binding site. Virginia Commonwealth University; USA: 2014. WO2014075045 A1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Horani RA, Ponnusamy P, Mehta AY, et al. Sulfated pentagalloylglucoside is a potent, allosteric, and selective inhibitor of factor XIa. J Med Chem. 2013;56:867–78. doi: 10.1021/jm301338q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84***.Al-Horani RA, Desai UR. Designing allosteric inhibitors of factor XIa. Lessons from the interactions of sulfated pentagalloylglucopyranosides. J Med Chem. 2014;57:4805–18. doi: 10.1021/jm500311e. [Discovery of first sulfated non-heparin mimetic as FXIa allosteric inhibitor.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patel NJ, Karuturi R, Al-Horani RA, et al. Synthetic, non-saccharide, glycosaminoglycan mimetics selectively target colon cancer stem cells. ACS Chem Biol. 2014;9:1826–33. doi: 10.1021/cb500402f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al-Horani RA, Gailani D, Desai UR. Allosteric inhibition of factor XIa. Sulfated non-saccharide glycosaminoglycan mimetics as promising anticoagulants. Thromb Res. 2015;136:379–87. doi: 10.1016/j.thromres.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karuturi R, Al-Horani RA, Mehta SC, et al. Discovery of allosteric modulators of factor XIa by targeting hydrophobic domains adjacent to its heparin-binding site. J Med Chem. 2013;56:2415–28. doi: 10.1021/jm301757v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Use of BF01 peptide inhibiting Factor XIa for use as anticoagulant with minimal side effects of bleeding. National University of Singapore; Singapore: 2015. WO2015002611A1. [Google Scholar]
- 89***.Chen W, Carvalho LP, Chan MY, et al. Fasxiator, a novel factor XIa inhibitor from snake venom, and its site-specific mutagenesis to improve potency and selectivity. J Thromb Haemost. 2015;13:248–61. doi: 10.1111/jth.12797. [In vitro and in vivo studies of rFasxiator as an engineered polypeptide inhibitor of FXIa.] [DOI] [PubMed] [Google Scholar]
- 90.Ma D, Mizurini DM, Assumpção TC, et al. Desmolaris, a novel factor XIa anticoagulant from the salivary gland of the vampire bat (Desmodus rotundus) inhibits inflammation and thrombosis in vivo. Blood. 2013;122:4094–106. doi: 10.1182/blood-2013-08-517474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li D, He Q, Kang T, et al. Identification of an anticoagulant peptide that inhibits both fXIa and fVIIa/tissue factor from the blood-feeding nematode Ancylostoma caninum. Biochem Biophys Res Commun. 2010;392:155–9. doi: 10.1016/j.bbrc.2009.12.177. [DOI] [PubMed] [Google Scholar]
- 92.Anticoagulant polypeptide and its application as selective inhibitor of coagulation factor XIa. GuangDong Medical College; China: 2011. p. CN102241734A. WO2012116663A1 2012. [Google Scholar]
- 93.Decrem Y, Rath G, Blasioli V, Cauchie P, et al. Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J Exp Med. 2009;206:2381–95. doi: 10.1084/jem.20091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anticoagulant polypeptide from salivary glands of Ixodes ricinus. Universite Libre de Bruxelles; Belgium: 2009. WO2009141382A1, EP2123670A1. [Google Scholar]
- 95.An anticoagulant proteinase inhibitor from the salivary glands of Simulium vittatum and its therapeutic uses. University of Georgia Research Foundation, Inc.; U.S. Dept of Health and Human Services; USA: 2012. WO2012162611A1. [Google Scholar]
- 96.Tsujimoto H, Kotsyfakis M, Francischetti IM, et al. Simukunin from the salivary glands of the black fly Simulium vittatum inhibits enzymes that regulate clotting and inflammatory responses. PLoS One. 2012;7:e29964. doi: 10.1371/journal.pone.0029964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Inhibitory domains of human Kunitz-type protease inhibitors and their manufacture for therapeutic use. Bayer Healthcare A.-G.; Germany: 2009. WO2009030464A2. [Google Scholar]
- 98.Ecotin variants as inhibitors or activators of serine proteinases. University of California; USA: 2000. WO2000061782A1, WO2000061634A2. [Google Scholar]
- 99.Ecotin as a factor Xa, XIa, and XIIa inhibitor. Corvas International; USA: 1996. US5585259A. [Google Scholar]
- 100.Kunitz domain inhibitor proteins derived from Alzheimer's amyloid β-protein precursor inhibitor. Genentech; USA: 1995. WO9523860A2. [Google Scholar]
- 101.Lippi G, Harenberg J, Mattiuzzi C, et al. Next generation antithrombotic therapy: focus on antisense therapy against coagulation factor XI. Semin Thromb Hemost. 2015;41:255–62. doi: 10.1055/s-0035-1546466. [DOI] [PubMed] [Google Scholar]
- 102.DeVos SL, Miller TM. Antisense oligonucleotides: treating neurodegeneration at the level of RNA. Neurotherapeutics. 2013;10:486–97. doi: 10.1007/s13311-013-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Antisense nucleic acids inhibiting clotting factor XI biosynthesis in the treatment of cardiovascular disorders. ISIS Pharmaceuticals; USA: 2010. WO2010045509A2. [Google Scholar]
- 104.Antisense inhibitors of blood coagulation factor XI gene expression as modulators of inflammation. ISIS Pharmaceuticals; USA: 2010. WO 2010121074A1. [Google Scholar]
- 105.Administration of human Factor XI modified antisense oligonucleotides that decrease Factor XI activity to treat or prevent thromboembolic or inflammatory disorders. ISIS Pharmaceuticals; USA: 2013. WO2013070771A1. [Google Scholar]
- 106.Zhang H, Löwenberg EC, Crosby JR, et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116:4684–92. doi: 10.1182/blood-2010-04-277798. [DOI] [PubMed] [Google Scholar]
- 107***.Que Liu ED, Claudette B, Shuting X, et al. Isis-fxirx, a novel and specific antisense inhibitor of factor xi, significantly reduces fxi antigen and activity and increases APTT without causing bleeding in healthy volunteers. Blood. 2011;118:209. [Phase I clinical trial for ISIS-FXIRx in healthy volunteers.] [Google Scholar]
- 108.Younis HS, Crosby J, Huh JI, et al. Antisense inhibition of coagulation factor XI prolongs APTT without increased bleeding risk in cynomolgus monkeys. Blood. 2012;119:2401–8. doi: 10.1182/blood-2011-10-387134. [DOI] [PubMed] [Google Scholar]
- 109.Crosby JR1, Marzec U, Revenko AS, et al. Antithrombotic effect of antisense factor XI oligonucleotide treatment in primates. Arterioscler Thromb Vasc Biol. 2013;33:1670–8. doi: 10.1161/ATVBAHA.113.301282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yau JW, Liao P, Fredenburgh JC, et al. Selective depletion of factor XI or factor XII with antisense oligonucleotides attenuates catheter thrombosis in rabbits. Blood. 2014;123:2102–7. doi: 10.1182/blood-2013-12-540872. [DOI] [PubMed] [Google Scholar]
- 111.van Montfoort ML, Kuijpers MJ, Knaup VL, et al. Factor XI regulates pathological thrombus formation on acutely ruptured atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2014;34:1668–73. doi: 10.1161/ATVBAHA.114.303209. [DOI] [PubMed] [Google Scholar]
- 112***.Büller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232–40. doi: 10.1056/NEJMoa1405760. [Phase II clinical trial for ISIS-FXIRx in patients undergoing elective primary unilateral total knee arthroplasty.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Preparation of human antibodies capable of binding to the coagulation factor XI and/or its activated form factor XIa for treatment of thrombosis. Bayer Pharma; Germany: 2013. WO2013167669A1. [Google Scholar]
- 114.Yamashita A, Nishihira K, Kitazawa T, et al. Factor XI contributes to thrombus propagation on injured neointima of the rabbit iliac artery. J Thromb Haemost. 2006;4:1496–501. doi: 10.1111/j.1538-7836.2006.01973.x. [DOI] [PubMed] [Google Scholar]
- 115.Takahashi M, Yamashita A, Moriguchi-Goto S, et al. Inhibition of factor XI reduces thrombus formation in rabbit jugular vein under endothelial denudation and/or blood stasis. Thromb Res. 2010;125:464–70. doi: 10.1016/j.thromres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 116.Gruber A, Hanson SR. Factor XI-dependence of surface- and tissue factor initiated thrombus propagation in primates. Blood. 2003;102:953–955. doi: 10.1182/blood-2003-01-0324. [DOI] [PubMed] [Google Scholar]
- 117.Anti-human factor XI monoclonal antibodies for treating thrombosis, metastatic cancer and acute inflammatory reaction. Oregon Health & Science University and Vanderbilt University; USA: 2009. WO2009067660A2. [Google Scholar]
- 118***.Tucker EI, Marzec UM, White TC, et al. Prevention of vascular graft occlusion and thrombus- associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–44. doi: 10.1182/blood-2008-06-163675. [Antithrombotic efficacy of O1A6 (aXIMab) monoclonal antibody in collagen-initiated thrombosis in baboon model.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheng Q, Tucker EI, Pine MS, et al. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–9. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120***.Leung PY, Hurst S, Berny-Lang MA, et al. Inhibition of factor XII-mediated activation of factor XI provides protection against experimental acute ischemic stroke in mice. Transl Stroke Res. 2012;3:381–9. doi: 10.1007/s12975-012-0186-5. [Antithrombotic efficacy of 14E11 monoclonal antibody in acute ischemic stroke model in mice.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tucker EI, Verbout NG, Leung PY, et al. Inhibition of factor XI activation attenuates inflammation and coagulopathy while improving the survival of mouse polymicrobial sepsis. Blood. 2012;119:4762–8. doi: 10.1182/blood-2011-10-386185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luo D, Szaba FM, Kummer LW, et al. Factor XI-deficient mice display reduced inflammation, coagulopathy, and bacterial growth during listeriosis. Infect Immun. 2012;80:91–9. doi: 10.1128/IAI.05568-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van Montfoort ML, Knaup VL, Marquart JA, et al. Two novel inhibitory anti-human factor XI antibodies prevent cessation of blood flow in a murine venous thrombosis model. Thromb Haemost. 2013;110:1065–73. doi: 10.1160/TH13-05-0429. [DOI] [PubMed] [Google Scholar]
- 124.Suleymanov OD, Szalony JA, Salyers AK, Lachance RM, Parlow JJ, South MS, Wood RS, Nicholson NS. Pharmacological interruption of acute thrombus formation with minimal hemorrhagic complications by a small molecule tissue factor/factor VIIa inhibitor: Comparison to factor Xa and thrombin inhibition in a nonhuman primate thrombosis model. J Pharmacol Exp Ther. 2003;306:1115–21. doi: 10.1124/jpet.103.052779. [DOI] [PubMed] [Google Scholar]
- 125.Szalony JA, Suleymanov OD, Salyers AK, Panzer-Knodle SG, Blom JD, LaChance RM, Case BL, Parlow JJ, South MS, Wood RS, Nicholson NS. Administration of a small molecule tissue factor/factor VIIa inhibitor in a non-human primate thrombosis model of venous thrombosis: effects on thrombus formation and bleeding time. Thromb Res. 2003;112:167–74. doi: 10.1016/j.thromres.2003.10.017. [DOI] [PubMed] [Google Scholar]