Abstract
Rationale
Despite the demonstrated advantageous systemic changes in response to regular exercise for individuals with cystic fibrosis (CF), exercise is still viewed as an elective rather than a vital component of therapy, and it is likely that these benefits extend to and are partially mediated by exercise-induced changes in ion regulation.
Objective
We sought to determine if exercise could provide comparable improvements in ion regulation in the CF lung as albuterol, measured using exhaled breath condensate (EBC) collection and nasal potential difference (NPD).
Methods
Fourteen CF (13-42yrs.) and sixteen healthy (18-42yrs.) subjects completed a randomized crossover study of albuterol and submaximal exercise. EBC was collected at baseline, 30- and 60-min post-albuterol administration, and at baseline and during three separate 15 min cycling exercise bouts at low, moderate, and vigorous intensity (25, 50 and 65% of the maximum workload, respectively). NPD was performed at 30- and 80-min post albuterol or following moderate and vigorous intensity exercise.
Results
CF subjects had lower EBC Cl−, but no difference in EBC Na+ at baseline when compared to healthy subjects. EBC Cl− increased four-fold with moderate exercise which was similar to that seen 60-min post albuterol administration for CF subjects. Neither exercise nor albuterol altered EBC Na+. The change in NPD voltage with amiloride (ΔAmil) was greater and there was minimal Cl− secretion (ΔTCC) seen at baseline in the CF compared to the healthy subjects. ΔAmil was greater with both albuterol and exercise when compared to baseline within both CF and healthy groups, but there was no significant difference in the ΔTCC response with either treatment.
Conclusion
Both exercise and albuterol can alter ion regulation increasing Cl− secretion to a significant and similar degree in individuals with CF.
Keywords: exhaled breath condensate (EBC), nasal potential difference (NPD), airway surface liquid, epithelial sodium channels (ENaC), beta-2 adrenergic receptor (ADBR2), purinergic receptor (P2Y2)
Introduction
The principle determinant of airway surface liquid (ASL) volume is the mass of salt on the airway surface 1. The pathologic ASL dehydration in CF is due to improper ion regulation with minimal Cl− secretion and an increase in Na+ absorption due to the loss or reduction in cystic fibrosis transmembrane conductance regulator (CFTR) function and its inhibitory regulation of the epithelial sodium channel (ENaC)2–4. Ameliorating this ion dysregulation is crucial in the treatment of CF. Although the majority of current therapies treat the ensuing symptoms, the ultimate goal for treatment of CF and the focus of current research is to treat the basic pathophysiology due to CFTR dysfunction.
The systemic therapeutic benefits of exercise in CF are well known, demonstrating the ability to reduce the characteristic 2–3% annual decline in pulmonary function, increase respiratory muscle endurance, improve exercise tolerance, aid in facilitating sputum clearance, increase aerobic capacity, and result in improved survival for individuals with CF who have higher aerobic fitness 5–10. More recently, the benefits of exercise have been extended to a more cellular level with demonstration of inhibition of ENaC activity with exercise, thereby potentially improving ion regulation. With the goals of treatment therapy in CF to 1) prevent or minimize decline in lung function and 2) ameliorate impairments in ion regulation, exercise has convincingly demonstrated its ability to facilitate the former and research is now suggesting it may also aid in the latter 11–13.
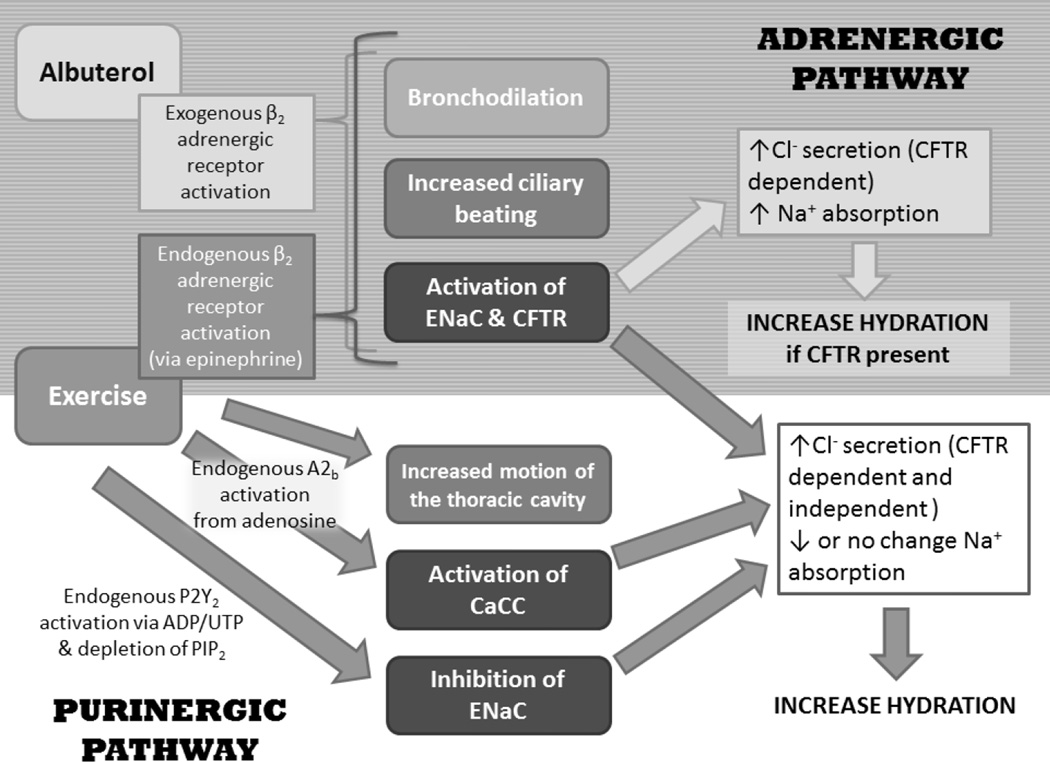
Albuterol, a common β2-agonist taken by individuals with CF, has the potential to be an exogenous stimulant of the adrenergic pathway to mediate stimulation of CFTR and ENaC 14–16. However, the resultant change in ENaC activity would be the net result of direct protein kinase A (PKA) activation of ENaC countered by PKA activation of CFTR which mediates inhibition of ENaC 17–19, with one side winning or the two cancelling each other out. Due to the lack of or reduction in the activity of CFTR-mediated ENaC inhibition in individuals with CF, ENaC activity in response to albuterol should increase which would only worsen the hyperabsorption of Na+. In contrast, exercise, in addition to activating the adrenergic pathway endogenously in response to the release of epinephrine, can also endogenously activate the purinergic pathway through the increase in nucleotide release from the airway epithelia that will follow the increase in ventilation 20. Albuterol may be a standard medication in the CF patient’s therapy regimen, but the impact on ion regulation is poorly understood in this patient population. Additionally, the potential for exercise to mediate activation of the same pathway as albuterol and also activate the purinergic pathway could be more beneficial for individuals with CF, as purinergic stimulation activates calcium-activated chloride channels (CaCC) offering a CFTR-independent means to both increase Cl− secretion and mediate inhibition of ENaC activity 21–23. For a summary of the ion transport pathways in the airway epithelia cells and the means of regulating the activity of CFTR, ENaC and the CFTR-independent means of Cl− secretion through CaCC, please refer to the supplement (Figure 7) and our hypothesis paper 24.
This study was designed to compare the effects of adrenergic and purinergic stimulation on ion regulation in the lungs by either endogenous activation through submaximal exercise, or by exogenous adrenergic activation via nebulization of the β2-agonist albuterol. The goal of this study was to determine if exercise could provide improvements to ion regulation that are comparable to the standard pharmacological therapy of albuterol for an individual with CF. To study ion regulation, two measurement techniques were used; exhaled breath condensate (EBC) collection, a novel measure of sodium and chloride content, and nasal potential difference (NPD) measurements, a standard measure of airway ion transport. We hypothesized that moderate intensity exercise would result in a decrease in EBC Na+ and sodium transport detected by NPD (ΔAmil) from baseline due to purinergic-mediated ENaC inhibition. With albuterol administration, we predicted an increase or no change in EBC Na+ and ΔAmil from baseline due to albuterol’s adrenergic activation of ENaC. For chloride transport, we predicted an even greater increase in EBC Cl− and chloride transport detected by NPD (ΔTCC and ΔATP) with exercise compared to albuterol due to activation of both CFTR and CaCC with exercise. A summary of the expected changes in ion channel regulation in response to albuterol and exercise is provided in Figure 1.
Figure 1. Summary of Expected Changes in Ion Channel Regulation in Response to Albuterol and Exercise.
Endogenous or exogenous activation of the purinergic (P2Y2) and adrenergic (β2) pathways are two mechanisms by which alterations in the ion composition of the ASL can be mediated. Such that water follows an increase in salt concentration in the ASL, increase the ASL depth and hydrate the lungs. Exercise endogenously activates both the adrenergic and purinergic pathways, whereas albuterol exogenously activates only the adrenergic pathway. CFTR: cystic fibrosis transmembrane conductance regulator; ENaC: epithelial Na+ channels; A2b: adenosine receptor (A2b); ADP/UDP: adenosine diphosphate/uridine diphosphate; PIP2: phosphatidylinositol 4,5-bisphosphate; CaCC: calcium-dependent chloride channels.
Methods
Subjects
Fourteen individuals with CF with mild to moderate lung disease (FEV1 >50% predicted), clinically diagnosed with a positive sweat test (>60mmol/L Cl−), and who were carriers of at least one ΔF508 allele were recruited for the present study. These subjects had not participated in another clinical trial within the past 30 days, or experienced a pulmonary exacerbation within two weeks. All participants with CF abstained from taking any long acting β-agonist (e.g. salmeterol) 12 hours prior to any study visit and did not take albuterol the morning of the visits. Individuals with CF continued any other medications (Table 1) or treatments (i.e. chest clearance) as normal. Additionally, sixteen healthy individuals of similar age, gender, height and weight were also recruited. Participants with CF were recruited through the University of Arizona Cystic Fibrosis Center. Control subjects were recruited through advertising posted around the University of Arizona and by word of mouth. The protocol was reviewed and approved by the University of Arizona Institutional Review Board. All participants provided written informed consent prior to study, and all aspects of the study were performed according to the Declaration of Helsinki.
Table 1.
Subject Demographics
| Cystic Fibrosis | ||||
|---|---|---|---|---|
| Healthy | All | Heterozygous ΔF508 | Homozygous ΔF508 | |
| n | 16 | 14 | 5 | 9 |
| Gender male | 10 (63) | 12(86) | 4 (80) | 8 (89) |
| Age (years) | 24±6 | 22±8 | 26±9 | 20±7 |
| Height (cm) | 168±8 | 166±17 | 172±8 | 163±20 |
| Weight (kg) | 70±12 | 62±12 | 66±10 | 60±13 |
| BMI (kg/m2) | 24±3 | 22±2 | 22±3 | 22±2 |
| BSA (m2) | 1.8±0.2 | 1.7±0.3 | 1.8±0.2 | 1.6±0.3 |
| VO2PEAK (% predicted) | 106±23 | 78±18* | 73±14 | 80±20 |
| Exercise (hr/wk) | 5.5±4.5 | 6.8±4.5 | 7.2±5.3 | 6.6±4.4 |
| Medications n (%) | ||||
| Bronchodilators (albuterol, fluticasone/salmeterol) | 0 | 13 (93) | 5 (100) | 8 (89) |
| Pancreatic enzymes | 0 | 12 (86) | 5 (100) | 7 (78) |
| Dornase alfa | 0 | 11 (79) | 4 (80) | 7 (78) |
| Inhaled corticosteroids (fluticasone) | 0 | 1 (7) | 1 (20) | 0 |
| Hypertonic saline | 0 | 1 (7) | 0 | 1 (11) |
| Inhaled antibotics (tobramycin, aztre onam) | 0 | 9 (64) | 5 (100) | 4 (44) |
| Oral antibotics (sulfamethoxazole/trimethoprim azithromycin) | 0 | 5 (36) | 2 (40) | 1 (11) |
| Oral contraceptive | 3 (18) | 0 | 0 | 0 |
BMI = body mass index; BSA = body surface area; VO2peak = maximal oxygen consumption. Data are presented as mean±SD or No. (%)
p<0.025 vs. healthy. Statistics not performed on ΔF508 groups.
Protocol
Visit 1
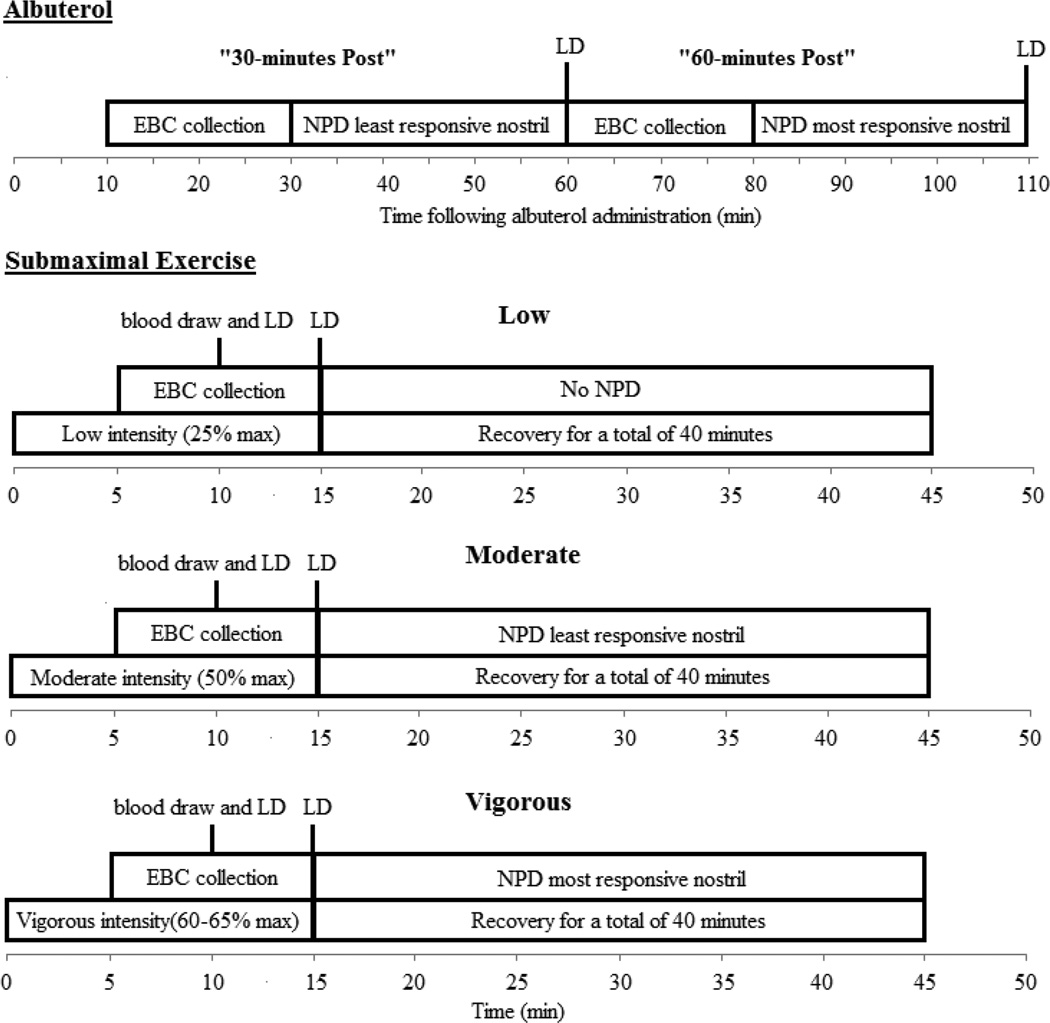
On this screening visit subjects performed basic spirometry, a NPD measurement on both nostrils and a maximal exercise test on a cycle ergometer. The specifics of the spirometry and exercise testing have been described in detail elsewhere 25. Subjects were randomized to one of the following four sequences, A: visit 2 albuterol and visit 3 submaximal exercise low-moderate-vigorous; B: visit 2 albuterol and visit 3 submaximal exercise vigorous-moderate-low; C: visit 2 submaximal exercise low-moderate-vigorous and visit 3 albuterol; D: visit 2 submaximal exercise vigorous-moderate-low and visit 3 albuterol. A summary of the measurement sequence and timing of measurements for the albuterol and submaximal exercise visits can be found in Figure 2.
Figure 2. Timeline of Measurements during Submaximal and Albuterol Visits.
EBC: exhaled breath condensate; NPD: nasal potential difference; LD: diffusion capacity of the lungs for nitric oxide. A baseline a 20 minute EBC collection with a midpoint blood draw was completed before these timelines started and a post EBC collection followed the final exercise bout. Submaximal exercise bouts were randomized: low-moderate-vigorous or vigorous-moderate-low.
Albuterol Visit
A baseline exhaled breath condensate (EBC) collection was performed for 20 minutes and a blood sample was taken at the midpoint of the EBC collection to assess serum ions (Na+, K− and Cl−). Subjects were then administered nebulized albuterol while wearing a nose piece (2.5 mg diluted in 3 mL normal saline) using a Power Neb2 nebulizer (Drive Medical, Port Washington, NY). Following nebulization two post albuterol EBC collections and NPD measurements were performed. Since EBC and NPD cannot be performed simultaneously, the NPD post measurements were performed at 30 and 80 minutes rather than 30 and 60. Also in order to perform NPD at both post albuterol time points, NPD measurements on each nostril were not done at the same time: the least responsive nostril (as noted from the baseline visit NPD) was used at 30-minutes post albuterol time point, and the most responsive nostril was used at the 80-minute post albuterol time point.
Submaximal Exercise Visit
Similar to the albuterol visit, a baseline EBC collection was performed for 20 minute and a baseline blood sample was taken at the midpoint of the EBC collection. The protocol for the submaximal cycling exercise bouts was the same for all intensities and has been described previously 25. Once the desired workload was reached, following a five minute build, tubing for EBC collection was connected to the breathing apparatus, and subjects continued to pedal at this workload for the remaining ten minutes of the exercise bout (15 minutes total). A blood sample was collected twelve minutes into exercise. Following the moderate and vigorous intensity workloads, the 40 minutes of recovery were utilized to perform a NPD measurement. Similar to the albuterol visit, the NPD tracing was split so that a measurement could be performed following both moderate and vigorous exercise, the least responsive nostril was used following the moderate intensity bout, and the most responsive nostril was used after the vigorous intensity bout. Finally, following all three exercise bouts a post-exercise EBC collection was completed either following the NPD measurement or following 30 minutes of recovery, depending on the intensity order randomization (vigorous last or low last respectively)
Experimental procedures
Collection of Exhaled Breath Condensate and Quantification of EBC Ions
Exhaled breath condensate samples were collected using the Jaeger EcoScreen as previously described 26. To collect EBC while subjects were cycling, plastic tubing was used to connect the inlet port of the EcoScreen to the exhalation port of a Y-valve connected to the backside of the switching valve with the pneumotach attached to on the front side (setup pictured in supplemental material Figure 8 A & B). Quantification of chloride was completed using ion chromatography and sodium and potassium were measured with inductively-coupled plasma mass spectrometry.
The calculation of net chloride efflux was used to account for the paracellular reabsorption of Cl− that will follow the reabsorption of Na+ to maintain electroneutral ion flux. Thus, the net chloride efflux calculation used was the gross chloride concentration plus the absolute value of the percent change in sodium from baseline multiplied by the gross chloride concentration for each time point:
Because the ratio of exhaled water to ASL droplets in the EBC sample is unpredictable and changing, we also applied a dilution factor to our EBC ion concentrations. The dilution factor for each sample was calculated using the total cation method 27:
Nasal Potential Difference Measurements
Since previous research attempting to describe ion transport during exercise and following administration of pharmacological therapies in CF has primarily used measurement of NPD 28–32, NPD was also measured in this study to compare with EBC measurements. NPD was conducted according to the Cystic Fibrosis Foundation Therapeutics Development Network Standard Operating Procedure 33. The values to represent the changes in potential difference (PD) discussed in this paper were calculated as follows:
Change with Amiloride (ΔAmil) = PDAMIL – PDRINGERS
Change with Cl− free + isoproterenol (ΔTotal Chloride Conductance (ΔTCC)) = PDISO – PDAMIL
Change with ATP (ΔATP) = PDATP – PDISO
Note: the PD used in the above calculations was the steady state value measured over the last ten seconds of the specified perfusion.
In order to perform an NPD measurement twice within a treatment visit, we could not perform NPD tracings on both nostrils at the same time and compare changes in the average of both nostrils as has been recommended in order to get the most sensitivity for detecting changes in Cl− activity 28. Instead we compared the changes between the baseline NPD measurement taken on visit 1 and treatment visits (right nostril at baseline compared to right nostril on albuterol and submaximal exercise and same with the left nostril). The calculation of the difference between resting and treatment responses was calculated by: Difference with treatment = Treatment values –Baseline (Visit 1) values for same nostril for ΔAmil, ΔTCC and ΔATP. As described by Rowe et al. the variability of NPD parameters from the placebo group in the ivacaftor and the lumacaftor trials are: ΔTCC: 0.21±4.15 and ΔAmil: 1.75±9.80. Further, for within subject analysis an n of 10 for CFTR and 5 for ENaC has been calculated to be a sufficient sample size to detect a 5mV change, the suggested effect size to provide a meaningful change in clinical status for an individual with CF, if it is present using the most-polarized nostril at screening carried forward method 28. As such our sample size of 14 CF and 16 healthy should have been a sufficient sample size to show a change in NPD.
Statistical Analysis
The SPSS statistical software package (v.19; SPSS, Inc., Chicago, IL) was used for all statistical analyses. After confirming equality of variance with a Levene’s Test, baseline and treatment differences (60-minutes post albuterol or moderate exercise) between healthy and CF groups were assessed using two-sided independent samples t-tests, and a Bonferroni correction of 0.025 to determine statistical significance. Within conditions, we assessed the effect of each treatment on EBC ions and NPD measurements using two-factor repeated measures ANOVA tests across all exercise intensities (baseline, low, moderate and vigorous) and across all albuterol time points (baseline, 30- and 60-minutes post). If a difference based on treatment was found, paired samples t-tests were performed between the moderate exercise and 60-minute post albuterol values for healthy and CF groups with a Bonferroni correction of 0.025 to determine statistical significance. Evaluation for differences in response between healthy and CF groups were also assessed at baseline and moderate intensity exercise, as well as baseline and 60-minutes post albuterol using two-sided independent samples t-tests and a Bonferroni correction of 0.025 to determine statistical significance. Then to analyze whether exercise can provide comparable changes to albuterol in ion regulation (EBC ions and NPD), we compared the percent change from baseline (EBC) or difference (NPD) between the moderate intensity exercise and 60-minutes post albuterol for both the CF and healthy groups, using a Bonferroni correction of 0.025 to determine statistical significance.
Results
There were no differences in gender, age, height, weight, body mass index, or body surface area between the 14 individuals with CF and 16 healthy individuals (Table 1). Baseline pulmonary function (FEV1, FEV1/FVC and FEF25–75) was significantly lower for CF patients (Table 2).
Table 2.
Baseline Pulmonary Function Parameters
| Cystic Fibrosis | ||||
|---|---|---|---|---|
| Healthy | All | Heterozygous ΔF508 | Homozygous ΔF508 | |
| FVC (L) | 4.6±0.9 | 4.0±1.2 | 3.6±1.4 | 4.2±1.0 |
| FVC (% predicted) | 100±9 | 96±30 | 77±21 | 106±30 |
| FEV1 (L) | 3.8±0.9 | 2.9±1.1* | 2.7±1.3 | 3.1±1.0 |
| FEV1 (% predicted) | 99±12 | 83±28 | 68±26 | 91±26 |
| FEV1/FVC (%) | 84±4 | 73±10 * | 72±10 | 74±10 |
| FEF25–75 (L/s) | 4.0±1.2 | 2.4±1.4 * | 2.1±1.3 | 2.5±1.5 |
| FEF25–75 (% predicted) | 96±26 | 60±30 * | 51±28 | 65±32 |
FVC=forced vital capacity; FEV1=forced expiratory volume after one second of FVC; FEF25–75= forced expiratory flow at 25-75% of FVC. Predicted values for all pulmonary function measures were based on predicted equations from NHANES III. Data presented are mean±SD
p<0.025 vs. healthy. Statistics not performed on ΔF508 groups.
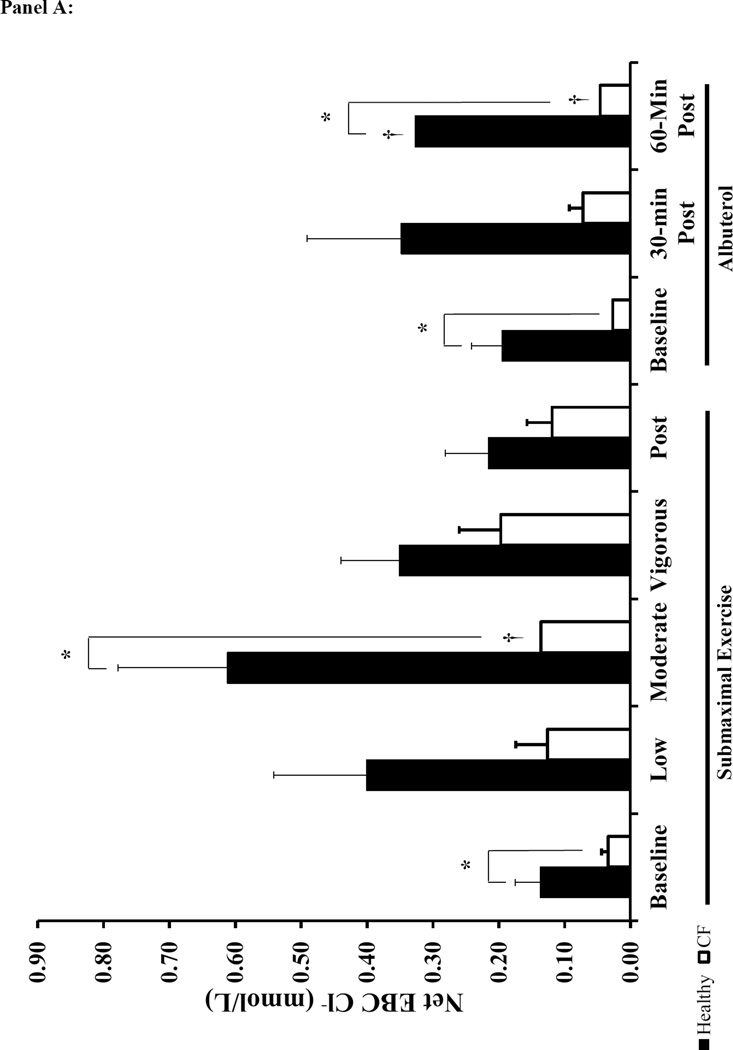
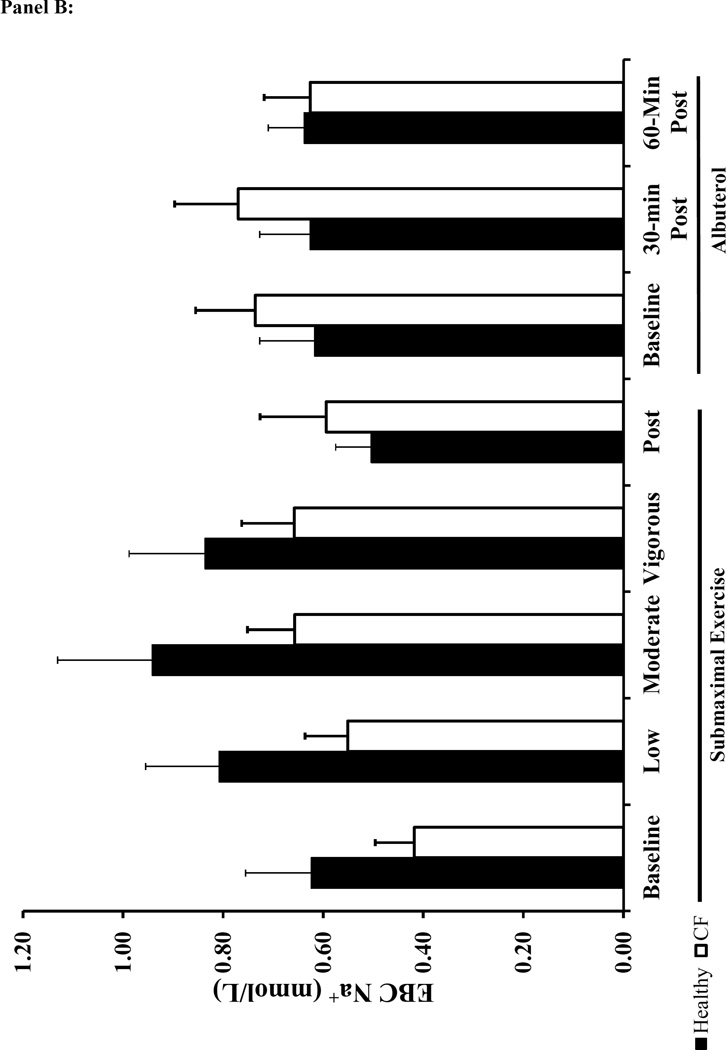
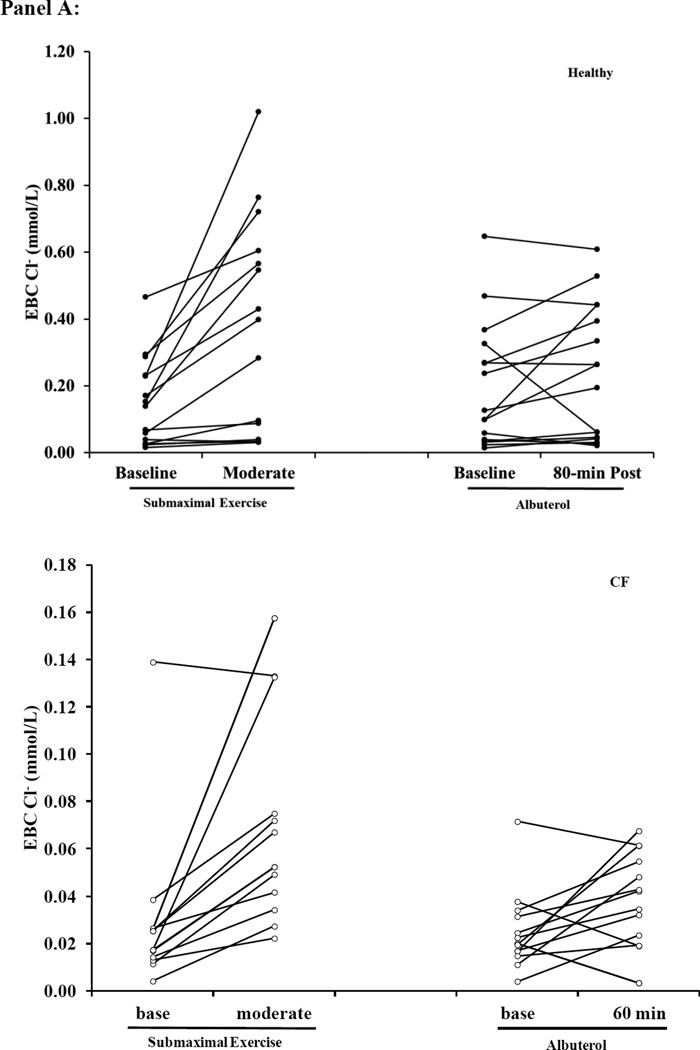
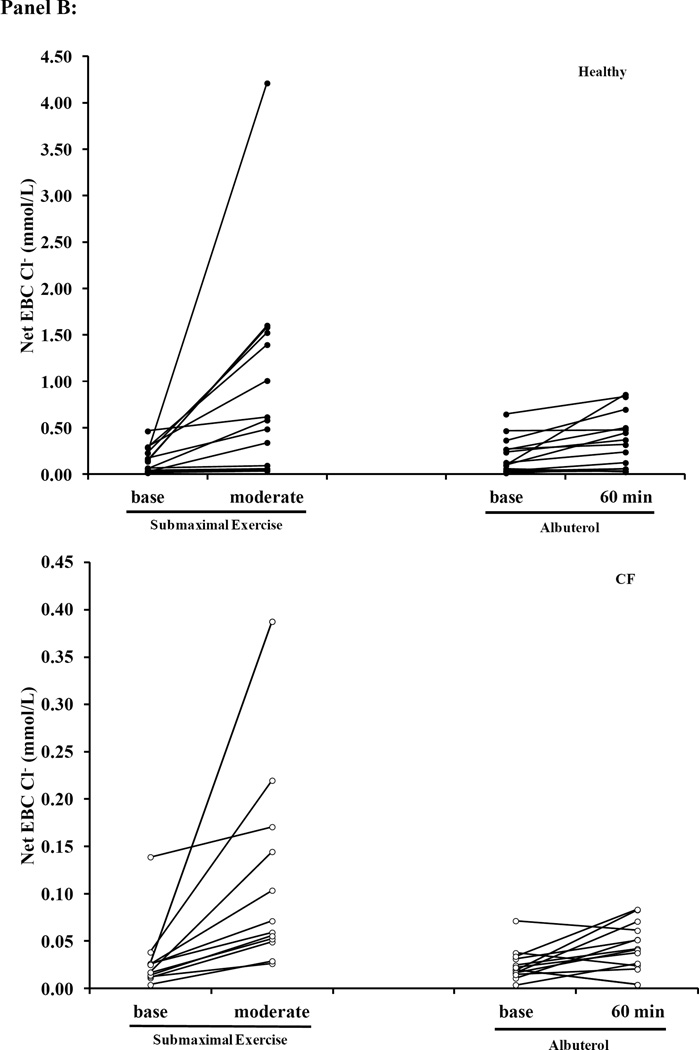
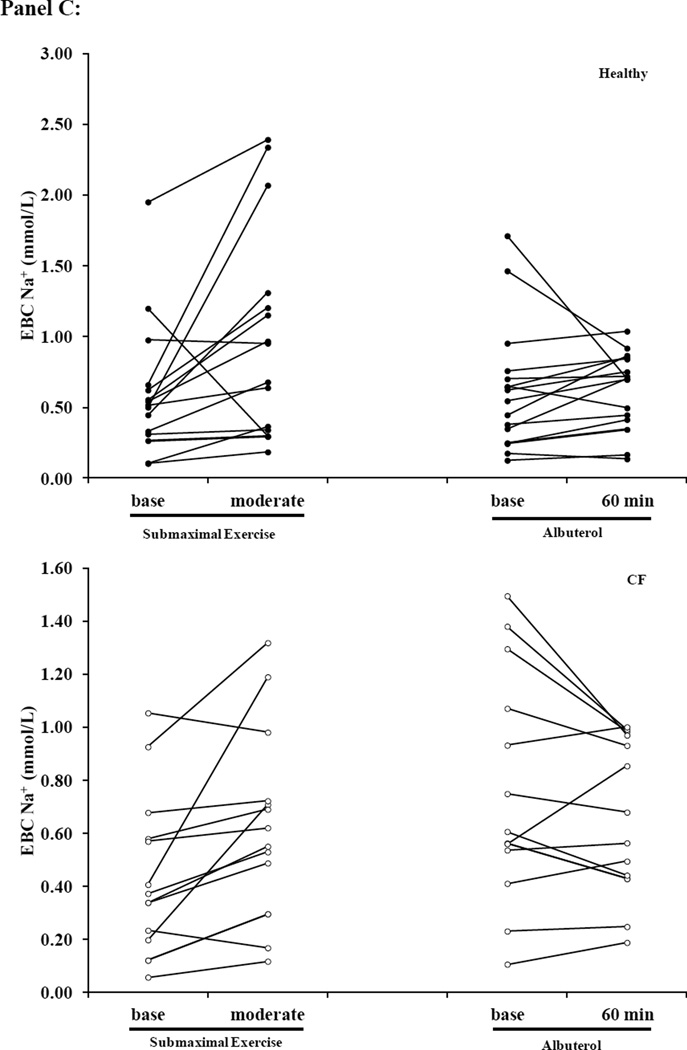
Individuals with CF presented lower EBC Cl− concentrations, but there was no difference in EBC Na+ at baseline when compared to healthy subjects (Figure 3). Albuterol administration increased Net EBC Cl− at 60-minutes post administration for both healthy and CF subjects (Figure 3A). Moderate intensity exercise increased both raw EBC Cl− and Net EBC Cl− in CF subjects and Net EBC Cl− in healthy subjects. In contrast, neither submaximal exercise nor albuterol altered EBC Na+ (Figure 3B). When comparing the Net EBC Cl− between albuterol administration by 60-minutes post and moderate intensity exercise, we found that the percent change from baseline in Net EBC Cl− was greater with moderate intensity exercise in healthy subjects, but the increase in Net EBC Cl− was similar between moderate intensity exercise and 60 minutes post albuterol administration for CF subjects (Healthy: 162±139% vs. 65±103%; CF: 220±220% vs. 105±171, moderate exercise vs. 60-minutes post albuterol respectively, p<0.025 for healthy).
Figure 3. Effects of Submaximal Exercise and Albuterol on EBC Na+ and Net Cl−.
Panel A: Net EBC Cl− (see methods for equation). Panel B: EBC Na+. The black bars represent healthy subjects and the white bars represent CF subjects. The error bars represent the standard error of the mean. * = p<0.025 healthy vs. CF and † = p<0.025 vs. baseline
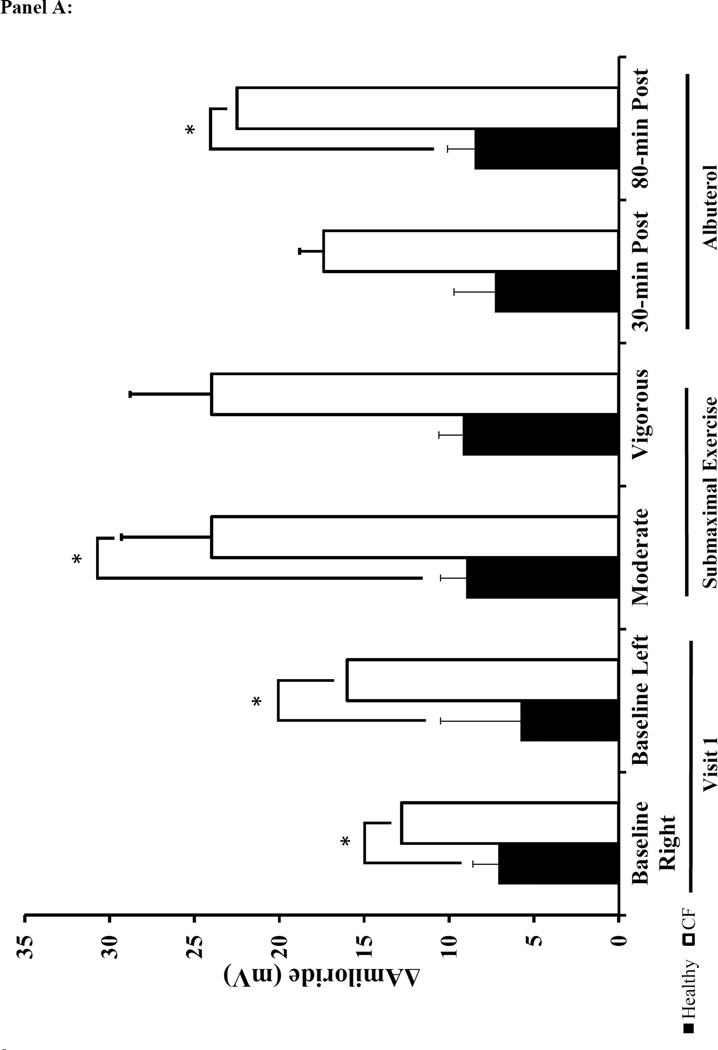
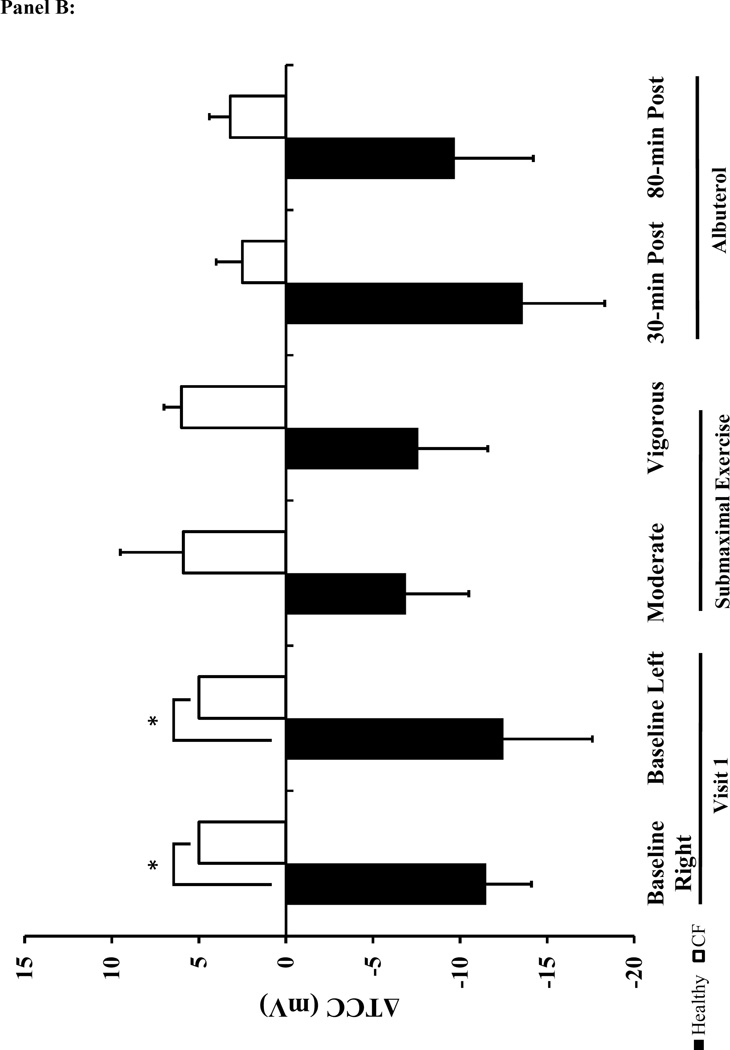
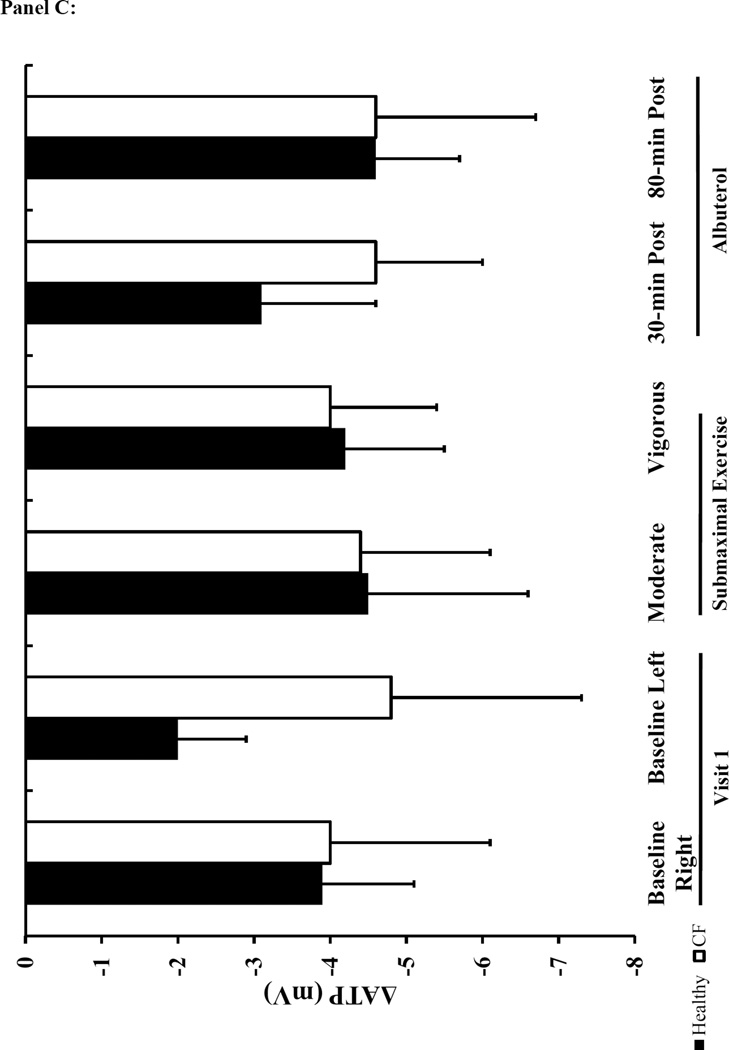
The baseline NPD responses were consistent with the diagnosis of CF and prior literature 28,29,34–36. Specifically, the baseline Ringer’s PD, change in PD with amiloride (ΔAmil) was greater in the CF subjects compared to the healthy participants. Similarly, there was a pronounced decrement in total Cl− conductance (Δ TCC) in CF patients (Figure 4 and supplement Table 3). The CaCC-mediated Cl− flux (ΔATP) was similar between CF and healthy subjects at baseline. There was a significant effect of treatment and the interaction between treatment and condition for ΔAmil in the two-factor repeated measures ANOVA analysis (p<0.01). There was a similar increase in Na+ flux (ΔAmil) above baseline levels with albuterol and exercise within both CF and healthy groups (Figure 4A). When comparing the response between healthy controls and CF subjects, the increase in ΔAmil with albuterol was greater in CF subjects at 80-minutes post albuterol, but there was no difference between groups following moderate exercise. In contrast to our expectations, exercise did not result in any significant effect on Cl− transport measured with NPD (ΔTCC) (Figure 4B). However, ΔTCC tended to be lower with exercise in healthy individuals compared to the ΔTCC at measured at baseline.
Figure 4. NPD Measured Changes in Na+ and Cl− in Response to Submaximal Exercise and Albuterol.
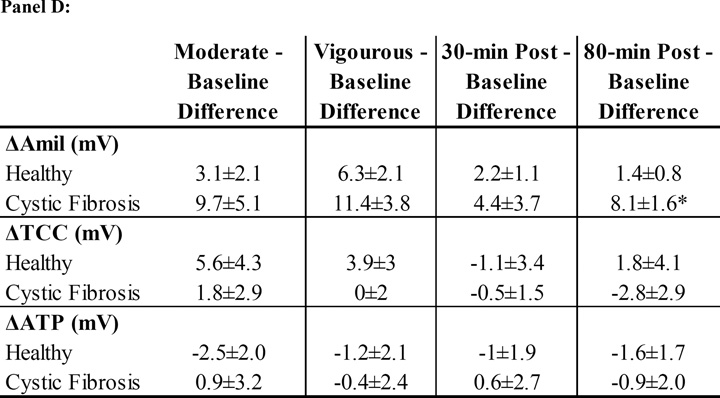
Panel A: ΔAmil=change with amiloride (measure of changes in Na+ flux). Panel B: ΔTCC=total Cl− conductance. Panel C: ΔATP=change with ATP. The black bars represent healthy subjects and the white bars represent cystic fibrosis subjects. Panel D: The table lists the mean difference in NPD response between the baseline tracing and the tracings performed on the two treatment visits (treatment-baseline). The most responsive nostril served to compare the difference in NPD response between baseline to vigorous and baseline to 80-min post albuterol. The other nostril served to compare the difference between baseline to moderate and baseline to 30-minutes post albuterol. Data presented at mean±SEM. *p<0.025 vs. healthy † p<0.025 vs. moderate intensity
We also evaluated the individual change of ions concentrations in EBC using scatter plots (Figure 5A–C). We found that the variability of EBC Na+ was similar between healthy and CF subjects, but the variability of EBC Cl− was greater in healthy subjects. These scatter plots also identified outliers which we confirmed with box plot analysis. We re-evaluated EBC ions with these outliers removed and found that the changes in response to albuterol or exercise remained significant. We also applied the cation dilution factor correction. With this correction the difference in EBC Cl− at baseline between healthy and CF subjects remained, but the increase in EBC Cl− following albuterol and submaximal exercise was no longer significant, but still followed the same trend (Supplement Table 4).
Figure 5. Scatter plots of EBC Na+, Cl− and Net Cl− during Submaximal and Albuterol Visits.
Panel A: EBC Cl− (Top: Healthy Bottom: Cystic Fibrosis (CF)) Panel B: Net EBC Cl− (Top: Healthy; Bottom: CF) Panel C: EBC Na+ (Top: Healthy; Bottom: CF). Healthy subjects are represented by the black circles and the open circles represent CF subjects. The y-axis scaling is different between the two groups, so that the individual changes for subjects within each condition can be appreciated, and due to the difference in Cl− secretion levels between healthy and CF subjects we have kept the scale specific to each condition.
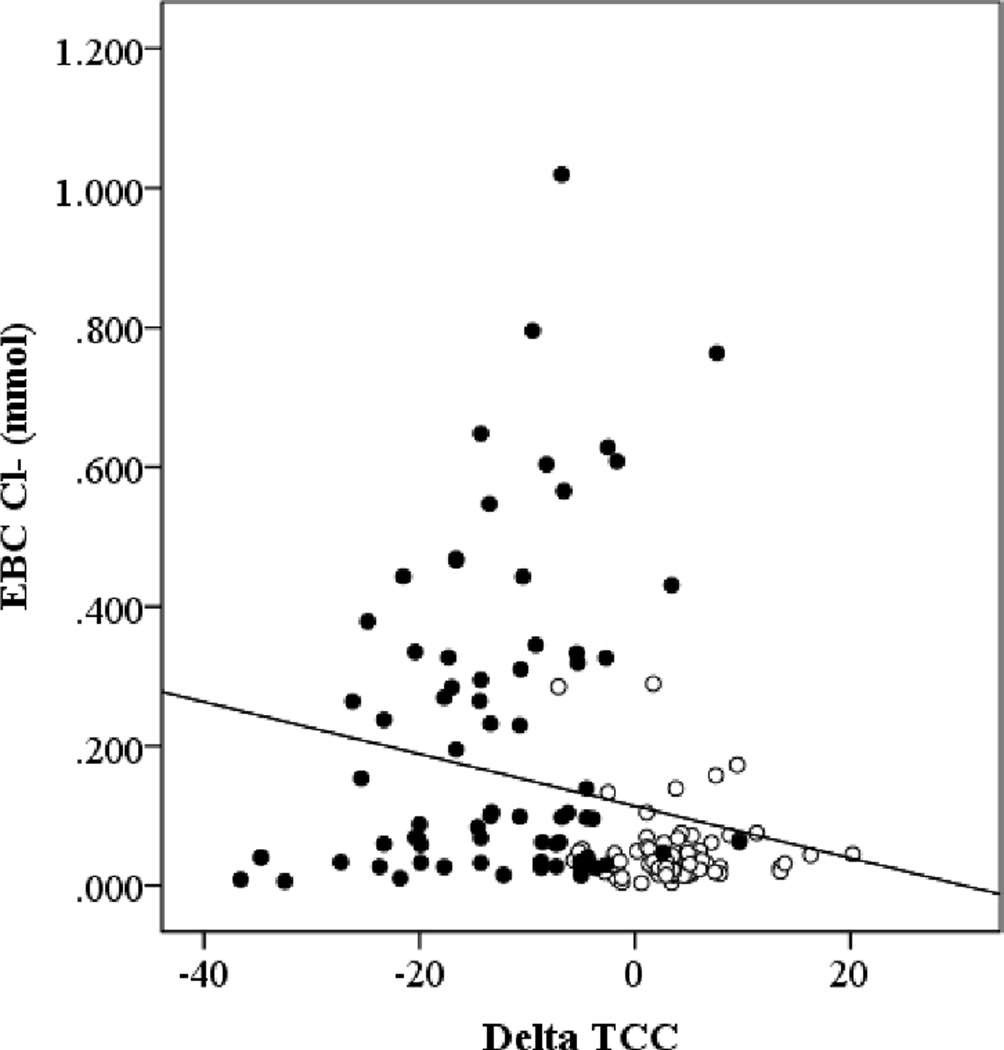
Due to the difference in anatomic location of measurement of ion regulation between EBC and NPD, we determined if there was a relationship between EBC Na+ and NPD ΔAmil or EBC Cl− and NPD ΔTCC. There was no relationship between EBC Na+ and ΔAmil, but there was a negative relationship between EBC Cl− and ΔTCC using all of the time points when healthy and CF subjects were considered together (Figure 6).
Figure 6. Relationship between EBC Cl− and NPD measured Cl− Flux (ΔTCC).
The black circles represent healthy subjects and the open circles represent CF subjects. All of the time points for both the albuterol and submaximal exercise visits have been included in the plot. ρ=−0.327, p=0.000.
Discussion
In this study we aimed to demonstrate the benefits of exercise at a more cellular level regarding its influence on ion regulation of the ASL, thereby expanding the concept of why exercise acts therapeutically for individuals with CF, beyond just augmenting mucus clearance 7,8. We tested whether albuterol and exercise could improve ion regulation in CF, using measurements of EBC ions and NPD tracings to monitor changes in ion flux. In this study, we found that albuterol administration increased Net EBC Cl− from baseline by 60-minutes post, replicating our previous findings, and additionally showed that moderate exercise, which we have previously identified as ideal for facilitating bronchodilation and improving the diffusing capacity of the lung25, also increased Net EBC Cl−. Further, we demonstrated that in CF patients the increase in Cl− flux measured by EBC Cl− was not statistically different between 60-minutes post albuterol and moderate intensity exercise. Collectively suggesting, at least in the short term, moderate exercise can provide similar benefits for mediating Cl− efflux as their standard medication albuterol. This data showing an increase in Cl− efflux further supports the mechanism we proposed25 that the increase in alveolar-capillary membrane conductance achieved with moderate exercise is due to improved mucus clearance being mediated in part by increased hydration of the ASL.
The observation of an increase in net EBC Cl− with albuterol is logical given our understanding of CFTR activity in response to adrenergic stimulation in healthy airway epithelium; however, this requires further explanation when observed in individuals with CF, where CFTR activity is reduced. First, it is important to highlight that although CFTR activity is known to be reduced, it is not completely absent even in individuals homozygous for the ΔF508 mutation. Even in these individuals there is ΔF508 CFTR protein expression in the respiratory epithelium in a smaller proportion of cells (22% vs. 56%; CF vs. healthy) 37, and some of these channels are functional, but with altered kinetics38,39. Also, administration of a long acting β-agonist, salmeterol, in airway submucosal gland serous cells has demonstrated restoration of CFTR function, by an 83% increase in ΔF508 CFTR mediated Cl− efflux 40 and through downstream PKA actions there is dimerization of CFTR causing inhibition of endocytic retrieval and increased membrane expression 41,42. Although we believe the increase in net exhaled Cl− with albuterol is partially CFTR-mediated, the use of saline as a vehicle is likely also responsible for the increased Cl− concentration, especially for individuals with CF. The addition of saline to the ASL may also help explain the variability in exhaled Na+ rather than the decrease one would expect in response to activation from β2 adrenergic receptor–mediated activation. With saline as the vehicle for the nebulized albuterol, this would increase the concentration of salt in the ASL, but also increase the concentration gradient for Na+ reabsorption through ENaC. As such, the resultant EBC Na+ measured is the end product of adding more Na+ to the airway surface, but also increasing ENaC activity and increasing the concentration gradient to mediate its reabsorption.
Since exercise activates both CFTR-dependent and CFTR-independent, purinergic activation of CaCC, means of Cl− secretion, it makes sense mechanistically that the increase in net exhaled Cl− tends to be greater with exercise than with albuterol for individuals with CF (Figure 3A & Figure 5B). With exercise there is also a variable response in exhaled Na+ for both healthy controls and individuals with CF, but we believe this is due to activation of two different pathways with opposing effects on ENaC activity. With exercise there are more CF patients who demonstrate an increase in EBC Na+ (Figure 5C), whereas with albuterol there is minimal change or a decrease, again highlighting a potential benefit of exercise over albuterol due to its ability to activate the purinergic pathway and thereby activate a means of inhibiting ENaC rather than activating the solely the adrenergic pathway which can only activate ENaC, and would theoretically only further aggravate the hyperabsorption of Na+ that is already occurring in individuals with CF.
Previous work has demonstrated that exercise inhibits ENaC as observed by a reduction in the ΔAmil response from baseline with exercise 12,43. Our study did not demonstrate this same finding, but instead found ΔAmil was larger with exercise for both CF and healthy subjects. One possible explanation for this difference is that our study measured NPD after completion of exercise rather than during exercise as was done in these previous studies. Since the purinergic pathway is activated by increases in shear stress via increases in tidal breathing during exercise and it is the activation of this pathway that is an important mediator of this exercise-induced inhibition of ENaC 20,44, performing the NPD recordings after the completion of exercise when tidal breathing has returned to normal and halted further purinergic stimulation may have limited the exercise-induced ENaC inhibition and reduction in the ΔAmil response detectable by NPD. Also subjects were wearing a nose piece during exercise to allow us to collect all their exhaled air for an EBC sample, but doing this could limit shear stress on the nasal epithelia and considerably limit purinergic pathway activation at this tissue. ΔAmil was also larger with albuterol when compared to baseline, which is solely adrenergic stimulation and has the potential to activate ENaC. As such, the similar change in ΔAmil with exercise and albuterol suggests that adrenergic pathway stimulation, but not purinergic pathway activation, was occurring during the post exercise NPD recordings. Because we used NPD as a second method to measure changes in ion regulation in response to albuterol or submaximal exercise, the results may have been altered from what would have been observed if NPD was measured alone: there would have been no nose clip worn during exercise to potentially effect purinergic stimulation of the nasal epithelium. As such future work needs to evaluate the changes in NPD following both treatments independently from EBC collections: performing only one exercise bout at the moderate intensity that has been identified as ideal through this initial work, not performing measurement of diffusion capacity which will allow for the subjects to breathe on a mask rather than a mouthpiece with nose clip, and performing NPD at an initial baseline visit and then performing NPD again post exercise using either the average of both nostrils or the most polarized nostril from each visit and comparing the NPD changes between baseline and post exercise.
In contrast to our expectations and EBC findings, we did not see a significant change in Cl− transport measured with NPD with either submaximal exercise or albuterol. However, the trend for a reduced response following submaximal exercise in healthy, but not CF subjects was in agreement with our hypothesis. First, submaximal exercise reduced the total Cl− flux (ΔTCC) when compared to baseline levels in healthy subjects, suggesting that exercise was already mediating Cl− efflux such that the response to the NPD Cl− flux stimulus was diminished. Second, there was no difference between the baseline and submaximal exercise ΔTCC values in CF subjects, which would be expected due to reduced functional CFTR available to respond to any stimulus. We were also surprised that there was no difference in the change in Cl− efflux with ATP perfusion between baseline and post exercise. We had hypothesized that the activation of the purinergic pathway would have increased CaCC-mediated Cl− secretion, such that the response to NPD stimulus would have been reduced. Again this may be due to the limited purinergic activation at the nasal epithelia during the NPD measurements due to the timing of the NPD and having our subjects use a nose piece during exercise. The negative relationship between ΔTCC and EBC Cl− overall, suggests that the two measurements are related as a more negative ΔTCC reflects more Cl− transport and a higher EBC Cl− suggests more Cl− in the ASL.
With the ultimate goal being to treat the cause rather than the symptoms of CF, outcome measures which can demonstrate therapeutic alteration of ion transport are crucial. Currently, NPD and sweat chloride are the primary endpoints used as a measure of changes in CFTR activity. Although informative, both NPD and sweat chloride are not perfect measurements. NPD measures CFTR activity in the back of the nose where respiratory epithelium initiates, but this is still upper airway epithelium not lower airway. Since the lung pathophysiology of CF initiates in the lower airways a measure of CFTR activity in this region is desirable 45,46. Also, although the standardization of the NPD procedure has made it more reliable and less challenging to perform it is still not perfect and not the most comfortable or pleasant for the subject. Sweat chloride is simple to perform and still the gold standard for diagnosing CF, but recent clinical trial findings have prompted questioning of its reliability as an endpoint to establish efficacy 47–49. These limitations make EBC a potentially attractive outcome measure for determining changes in Na+ and Cl− regulation, as EBC droplets are most likely being generated from both larger and small airways are likely contributors. In addition, EBC collection is comfortable and easy to complete for the subject. In this study we have demonstrated that EBC could be used to measure and compare changes between treatments within a subject. As such with both techniques we cannot definitely conclude where in the lung the changes are occurring at this time. However it should be noted that this is the first study to attempt to quantify Cl− changes that are occurring from within the lung during exercise using EBC in patients with CF. NPD is a validated measure of ion channel activity and the fact that there was a relationship between EBC and NPD helps to validate EBC as a measure of changes in ASL ion concentration and as such an indirect measure of ion channel activity. Also we have previously found that 1)there is a relationship between EBC Na+ and epithelial sodium channel genotypes, where the less active ENaC genotype demonstrated lower EBC Na+ values 50; 2) CF subjects have lower EBC Cl− at baseline than healthy subjects as would be expected and ENaC genotype influences lung diffusion capacity 51. Collectively, these findings strengthen the claim that EBC can be utilized as a measure of ion channel activity.
Previous work has demonstrated that a 5mV change indicates a meaningful difference in clinical phenotype for an individual with CF 34, but this information is unknown for EBC. Before EBC will be added as an endpoint in clinical trials, as described by Horvath et al., a few areas need to be address: mechanism and site of EBC droplet formation, standardization of dilution markers, improvement of reproducibility, and quantification of what the minimal clinically important difference for EBC Cl− is for an individual with CF52. Comparison of bronchoalveolar lavage fluid collected with deionized water from various locations in the bronchial tree to that of the EBC could help to identify location of droplet formation. Previous work demonstrated that it is important to measure dilution of the EBC contents53; we did this using the dilution correction factor of the ratio of total cations in serum to EBC. Although this has been done previously, it appeared to make our variable data even more variable. A dilution correction can also be calculated using urea, because its concentration in the ASL should be isotonic with the blood, and thereby provide a better measure of dilution, but we were unable to do so due to resource availability. Our initial research looking at the variability of EBC ions demonstrated that within subjects across three measurements on the same day the coefficient of variation is 30% for EBC Na+ 54. Review of the variability in response to the variability of EBC Cl− in response to saline where the coefficient of variation within subjects across baseline, 30, 60 and 90-minutes post nebulized saline was 46%26. Our results demonstrate changes from baseline exceed this inherent within subject variability suggesting that our results are not by chance or measurement error, but what is still unclear is if the level of change we observed is clinically significant. We did not demonstrate a difference both statistically or clinically for NPD, but as described above the timing and use of a nose piece may have limited the purinergic pathway activation. Additionally, having to use the baseline results from the prior screening visit to compare changes we felt carrying forward the most polarized nostril was best for our situation, but this likely reduced our sensitivity. As this analysis method tended to show the smallest magnitude of change compared to using the average of both nostrils, or the most polarized nostril at each visit in the optimization data analysis of the Ivacaftor trial 28. For both these reasons, our NPD results may be underrepresenting the true change in Cl− and Na+ transport, and the fact that we did not find a significant effect of exercise or albuterol on NPD measures does not mean it wasn’t present. What is known is that individuals with CF who are more active have a better survival, and participation in exercise has been shown to improve measures of clinical status such as FEV1. So on its own or following a single bout, the increases observed in EBC Cl−, used as an indirect measure of Cl− flux, may not be enough to alter the clinical phenotype, but a habit of exercise and its potential to help ameliorate some of the impairments in ion regulation are likely clinically significant.
This study began to look at exercise within the framework of being a medicine, we determined (1) the optimal “dose” was moderate intensity exercise 25, and (2) that exercise provided beneficial effects comparable to one of the current standard pharmacological therapies, albuterol. To build upon this research, it will be important for future studies to determine the minimal clinically significant difference for EBC Cl−, investigate the duration of the effects on ion regulation of a single bout of moderate intensity exercise, to assess the effects of an active lifestyle on ion regulation in the lungs on a longer time scale, and to determine if the benefits are comparable between different types of exercise.
Conclusion
Our results suggest that moderate intensity exercise can provide significant changes in Net EBC Cl− similar to its pharmacological counterpart albuterol, when compared over a short duration. We found ΔAmil was larger with exercise conflicting with the exercise-induced inhibition of ENaC as has been previously demonstrated. We hypothesize this is due to study design: NPD was measured after completion of exercise and subjects wore a nosepiece during exercise to facilitate EBC collection. Further research is required to clarify these findings. Collectively, our work suggests that exercise is medicine at the level of ion regulation as both exercise and albuterol can improve Cl− secretion to a similar degree. Second, EBC holds the potential to be a viable method to assess ion regulation in the lungs and has the potential for application clinically to determine if a pharmacological therapy is ameliorating or improving ion regulation in CF.
Supplementary Material
Acknowledgments
We extend our gratitude to our subjects who willingly gave up their time and at times comfort to participate in this study and support research in cystic fibrosis. This work was performed at the University of Arizona and supported by the PEO Scholar Award, American College of Sports Medicine Doctoral Student Research Grant, Caldwell Health Sciences Research Fellowship and National Institute of Health (HL108962-01).
Acronym List
- ASL
airway surface liquid
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- Cl−
chloride
- CaCCs
calcium-activated chloride channels
- ENaC
epithelial sodium channel
- EBC
exhaled breath condensate
- FEV1
forced expiratory volume in one second
- FEF25–75
forced expiratory flow at 25–75% of FVC
- FVC
force vital capacity
- Na+
sodium
- NPD
nasal potential difference
- P2Y2
purinergic receptor
- PKA
protein kinase A
- ΔAmil
change in NPD with amiloride
- ΔTCC
total chloride conductance: change with Cl− free + isoproterenol in NPD
- ΔATP
change in NPD with ATP
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
CMW, WJM, and EMS were responsible for the study concept and design, CMW was responsible for data analysis and interpretation, MAM was responsible for subject recruitment, BL and SMR provide insight, expertise and assistance in analysis of NPD data, and all other authors assisted CMW with data collection. All authors were significant manuscript revisers and reviewers.
Contributor Information
Courtney M. Wheatley, Email: wheatley.courtney@mayo.edu.
Sarah E. Baker, Email: baker.sarah@mayo.edu.
Mary A. Morgan, Email: uagrad06@gmail.com.
Marina G. Martinez, Email: mgm2@email.arizona.edu.
Bo Liu, Email: bliu@peds.uab.edu.
Steven M. Rowe, Email: smrowe@uab.edu.
Wayne J. Morgan, Email: wjmorgan@arc.arizona.edu.
Eric C. Wong, Email: ecwong47@gmail.com.
Stephen R. Karpen, Email: karpen@pharmacy.arizona.edu.
Eric M. Snyder, Email: esnyder@umn.edu.
References
- 1.Boucher RC. Molecular insights into the physiology of the 'thin film' of airway surface liquid. J Physiol. 1999;516(Pt 3):631–638. doi: 10.1111/j.1469-7793.1999.0631u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsui H, Grubb BR, Tarran R, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95(7):1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 3.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10(5):487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 4.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 5.Schneiderman-Walker J, Pollock SL, Corey M, et al. A randomized controlled trial of a 3-year home exercise program in cystic fibrosis. J Pediatrics. 2000;136(3):304–310. doi: 10.1067/mpd.2000.103408. [DOI] [PubMed] [Google Scholar]
- 6.Orenstein DM, Franklin BA, Doershuk CF, et al. Exercise conditioning cardiopulmonary fitness in cystic fibrosis. The effects of a three-month supervised running program. Chest. 1981;80(4):392–398. doi: 10.1378/chest.80.4.392. [DOI] [PubMed] [Google Scholar]
- 7.Dwyer TJ, Alison JA, McKeough ZJ, Daviskas E, Bye PT. Effects of exercise on respiratory flow and sputum properties in patients with cystic fibrosis. Chest. 2011;139(4):870–877. doi: 10.1378/chest.10-1158. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin DR, Hill AL, Peckham DG, Knox AJ. Effect of addition of exercise to chest physiotherapy on sputum expectoration and lung function in adults with cystic fibrosis. Respiratory Medicine. 1994;88(1):49–53. doi: 10.1016/0954-6111(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 9.Nixon PA, Orenstein DM, Kelsey SF, Doershuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. N Engl J Med. 1992;327(25):1785–1788. doi: 10.1056/NEJM199212173272504. [DOI] [PubMed] [Google Scholar]
- 10.Pianosi P, Leblanc J, Almudevar A. Peak oxygen uptake and mortality in children with cystic fibrosis. Thorax. 2005;60(1):50–54. doi: 10.1136/thx.2003.008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsuwaidan S, Li Wan Po A, Morrison G, et al. Effect of exercise on the nasal transmucosal potential difference in patients with cystic fibrosis and normal subjects. Thorax. 1994;49(12):1249–1250. doi: 10.1136/thx.49.12.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebestreit A, Kersting U, Basler B, Jeschke R, Hebestreit H. Exercise inhibits epithelial sodium channels in patients with cystic fibrosis. Am J Respir Crit Care Med. 2001;164(3):443–446. doi: 10.1164/ajrccm.164.3.2007168. [DOI] [PubMed] [Google Scholar]
- 13.Schmitt L, Wiebel M, Frese F, et al. Exercise reduces airway Na-reabsorption in cystic fibrosis but not in exercise asthma. Eur Respir J. doi: 10.1183/09031936.00197309. [DOI] [PubMed] [Google Scholar]
- 14.Haws C, Krouse ME, Xia Y, Gruenert DC, Wine JJ. CFTR channels in immortalized human airway cells. The American journal of physiology. 1992;263(6 Pt 1):L692–L707. doi: 10.1152/ajplung.1992.263.6.L692. [DOI] [PubMed] [Google Scholar]
- 15.Hwang TC, Nagel G, Nairn AC, Gadsby DC. Regulation of the gating of cystic fibrosis transmembrane conductance regulator C1 channels by phosphorylation and ATP hydrolysis. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(11):4698–4702. doi: 10.1073/pnas.91.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen XJ, Eaton DC, Jain L. Beta-adrenergic regulation of amiloride-sensitive lung sodium channels. American journal of physiology. Lung cellular and molecular physiology. 2002;282(4):L609–L620. doi: 10.1152/ajplung.00356.2001. [DOI] [PubMed] [Google Scholar]
- 17.Stutts MJ, Canessa CM, Olsen JC, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269(5225):847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 18.Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol Chem. 1997;272(22):14037–14040. doi: 10.1074/jbc.272.22.14037. [DOI] [PubMed] [Google Scholar]
- 19.Kunzelmann K, Schreiber R, Nitschke R, Mall M. Control of epithelial Na+ conductance by the cystic fibrosis transmembrane conductance regulator. Pflugers Archiv : European journal of physiology. 2000;440(2):193–201. doi: 10.1007/s004240000255. [DOI] [PubMed] [Google Scholar]
- 20.Chambers LA, Rollins BM, Tarran R. Liquid movement across the surface epithelium of large airways. Respir Physiol Neurobiol. 2007;159(3):256–270. doi: 10.1016/j.resp.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mall M, Gonska T, Thomas J, et al. Modulation of Ca2+-activated Cl- secretion by basolateral K+ channels in human normal and cystic fibrosis airway epithelia. Pediatr Res. 2003;53(4):608–618. doi: 10.1203/01.PDR.0000057204.51420.DC. [DOI] [PubMed] [Google Scholar]
- 22.Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177(2):119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- 23.Com G, Clancy JP. Adenosine receptors cystic fibrosis airway hydration. Handb Exp Pharmacol. 2009;(193):363–381. doi: 10.1007/978-3-540-89615-9_12. [DOI] [PubMed] [Google Scholar]
- 24.Wheatley CM, Wilkins BW, Snyder EM. Exercise is medicine in cystic fibrosis. Exerc Sport Sci Rev. 2011;39(3):155–160. doi: 10.1097/JES.0b013e3182172a5a. [DOI] [PubMed] [Google Scholar]
- 25.Wheatley CM, Baker SE, Morgan MA, Martinez MG, Morgan WJ, Wong EC, Karpen SR, Snyder EM. Effects of Exercise Intensity Compared to Albuterol in Individuals with Cystic Fibrosis. Respiratory Medicine. 2014 doi: 10.1016/j.rmed.2014.12.002. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheatley CM, Morgan WJ, Cassuto NA, et al. Exhaled Breath Condensate Detects Baseline Reductions in Chloride and Increases in Response to Albuterol in Cystic Fibrosis Patients. Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine. 2013;7:79–90. doi: 10.4137/CCRPM.S12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Effros RM, Biller J, Foss B, et al. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am J Respir Crit Care Med. 2003;168(12):1500–1505. doi: 10.1164/rccm.200307-920OC. [DOI] [PubMed] [Google Scholar]
- 28.Rowe SM, Liu B, Hill A, et al. Optimizing Nasal Potential Difference Analysis for CFTR Modulator Development: Assessment of Ivacaftor in CF Subjects with the G551D–CFTR Mutation. PLoS One. 2013;8(7):e66955. doi: 10.1371/journal.pone.0066955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clancy JP, Bebök Z, Ruiz F, et al. Evidence that Systemic Gentamicin Suppresses Premature Stop Mutations in Patients with Cystic Fibrosis. American Journal of Respiratory and Critical Care Medicine. 2001;163(7):1683–1692. doi: 10.1164/ajrccm.163.7.2004001. [DOI] [PubMed] [Google Scholar]
- 30.Rowe SM, Accurso F, Clancy JP. Detection of cystic fibrosis transmembrane conductance regulator activity in early-phase clinical trials. Proceedings of the American Thoracic Society. 2007;4(4):387–398. doi: 10.1513/pats.200703-043BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D–CFTR mutation. The New England journal of Medicine. 2010;363(21):1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilschanski M, Yahav Y, Yaacov Y, et al. Gentamicin-induced correction of CFTR function in patients with cystic fibrosis and CFTR stop mutations. The New England journal of Medicine. 2003;349(15):1433–1441. doi: 10.1056/NEJMoa022170. [DOI] [PubMed] [Google Scholar]
- 33.Solomon GM, Konstan MW, Wilschanski M, et al. An international randomized multicenter comparison of nasal potential difference techniques. Chest. 2010;138(4):919–928. doi: 10.1378/chest.10-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sermet-Gaudelus I, Girodon E, Sands D, et al. Clinical phenotype and genotype of children with borderline sweat test and abnormal nasal epithelial chloride transport. American Journal of Respiratory and Critical Care Medicine. 2010;182(7):929–936. doi: 10.1164/rccm.201003-0382OC. [DOI] [PubMed] [Google Scholar]
- 35.Rowe SM, Accurso F, Clancy JP. Detection of cystic fibrosis transmembrane conductance regulator activity in early-phase clinical trials. Proc Am Thorac Soc. 2007;4(4):387–398. doi: 10.1513/pats.200703-043BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305(25):1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- 37.Penque D, Mendes F, Beck S, et al. Cystic fibrosis F508del patients have apically localized CFTR in a reduced number of airway cells. Laboratory investigation; a journal of technical methods and pathology. 2000;80(6):857–868. doi: 10.1038/labinvest.3780090. [DOI] [PubMed] [Google Scholar]
- 38.Drumm ML, Wilkinson DJ, Smit LS, et al. Chloride conductance expressed by delta F508 and other mutant CFTRs in Xenopus oocytes. Science. 1991;254(5039):1797–1799. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]
- 39.Dalemans W, Barbry P, Champigny G, et al. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991;354(6354):526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- 40.Delavoie F, Molinari M, Milliot M, et al. Salmeterol restores secretory functions in cystic fibrosis airway submucosal gland serous cells. American journal of respiratory cell and molecular biology. 2009;40(4):388–397. doi: 10.1165/rcmb.2008-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riordan JR. Assembly of functional CFTR chloride channels. Annual review of physiology. 2005;67:701–718. doi: 10.1146/annurev.physiol.67.032003.154107. [DOI] [PubMed] [Google Scholar]
- 42.Lukacs GL, Segal G, Kartner N, Grinstein S, Zhang F. Constitutive internalization of cystic fibrosis transmembrane conductance regulator occurs via clathrin-dependent endocytosis and is regulated by protein phosphorylation. The Biochemical journal. 1997;328(Pt 2):353–361. doi: 10.1042/bj3280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitt L, Wiebel M, Frese F, et al. Exercise reduces airway sodium ion reabsorption in cystic fibrosis but not in exercise asthma. Eur Respir J. 2011;37(2):342–348. doi: 10.1183/09031936.00197309. [DOI] [PubMed] [Google Scholar]
- 44.Button B, Boucher RC. Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Respir Physiol Neurobiol. 2008;163(1–3):189–201. doi: 10.1016/j.resp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenfeld M, Gibson RL, McNamara S, et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatric Pulmonology. 2001;32(5):356–366. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 46.de Jong PA, Lindblad A, Rubin L, et al. Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax. 2006;61(1):80–85. doi: 10.1136/thx.2005.045146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durmowicz AG, Witzmann KA, Rosebraugh CJ, Chowdhury BA. Response. Chest. 2013;144(4):1418–1419. doi: 10.1378/chest.13-1517. [DOI] [PubMed] [Google Scholar]
- 48.Durmowicz AG, Witzmann KA, Rosebraugh CJ, Chowdhury BA. Change in sweat chloride as a clinical end point in cystic fibrosis clinical trials: the ivacaftor experience. Chest. 2013;143(1):14–18. doi: 10.1378/chest.12-1430. [DOI] [PubMed] [Google Scholar]
- 49.Heltshe SL, Mayer-Hamblett N, Rowe SM. Understanding the relationship between sweat chloride and lung function in cystic fibrosis. Chest. 2013;144(4):1418. doi: 10.1378/chest.13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker SE, Wheatley CM, Cassuto NA, Foxx-Lupo WT, Sprissler R, Snyder EM. Genetic variation of alphaENaC influences lung diffusion during exercise in humans. Respir Physiol Neurobiol. 2011;179(2–3):212–218. doi: 10.1016/j.resp.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baker SE, Wong EC, Wheatley CM, et al. Genetic Variation of SCNN1A Influences Lung Diffusing Capacity in Cystic Fibrosis. Med Sci Sports Exerc. 2012 doi: 10.1249/MSS.0b013e318266ebc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horvath I, Hunt J, Barnes PJ, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. The European respiratory journal. 2005;26(3):523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 53.Effros RM, Hoagland KW, Bosbous M, et al. Dilution of respiratory solutes in exhaled condensates. Am J Respir Crit Care Med. 2002;165(5):663–669. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- 54.Wheatley CM, Cassuto NA, Foxx-Lupo WT, Snyder EM. Variability in measures of exhaled breath na, influence of pulmonary blood flow and salivary na. Clin Med Insights Circ Respir Pulm Med. 2010;4:25–34. doi: 10.4137/ccrpm.s4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.