Abstract
Objective In this study, we describe the chest computed tomography findings of influenza virus‐associated pneumonia in adult patients.
Methods Our retrospective study included 12 adult patients who had proven influenza virus ‐ associated pneumonia.
Results Out of 12 patients, six were diagnosed as having pure influenza virus pneumonia, five as having bronchopneumonia caused by bacteria associated with influenza A infection, and one as having a cryptogenic organizing pneumonia associated with influenza A infection.
Conclusion Radiographic findings of influenza virus pneumonia in adult patients consist of ground‐glass attenuation. Localized patchy consolidations were observed in cases of bronchopneumonia.
Keywords: Chest CT, influenza virus, pneumonia
Introduction
The influenza virus is a common cause of lower respiratory tract infections in adults. It can occur during winter outbreaks and particularly during pandemics. Clinical pneumonia attributable to the influenza virus is uncommon, but when it does occur, secondary bacterial infection as well as the influenza A virus itself need to be considered as the possible cause. 1 Influenza A virus pneumonia is usually mild, but it can be overwhelming in some patients and prove fatal within 24 hours after onset. 2 Older patients and patients with underlying heart disease, chronic bronchitis or cystic fibrosis have an increased risk of developing pneumonia. 3 Although isolated cases of influenza pneumonia in immunocompromised patients have been reported, 4 , 5 surprisingly little is known about the radiologic manifestations and clinical pictures of influenza virus‐associated pneumonia in adult patients 6 , 7 or avian influenza. 8 , 9
The aim of our study was to describe the radiographic and chest computed tomography (CT) findings of 12 adult patients with confirmed influenza virus‐associated pneumonia.
Materials and methods
Patients
Our retrospective study included 12 adult patients who had proven influenza virus‐associated pneumonia. The influenza virus infections were diagnosed using a rapid diagnostic kit between December 2003 and December 2006. All cases experienced an acute onset of high fever and symptoms compatible with an influenza virus infection. Influenza virus‐associated pneumonia was diagnosed as having a positive influenza antigen result using a commercially available diagnostic kit, detection of infiltration by chest X‐ray and chest CT, and the appropriate exclusion of other diagnoses.
The 12 patients in our study population were seven women and five men whose age ranged from 30 to 91 years (mean, 38 years; median, 32 years). Although some patients were very old, there was no pre‐existing lung disease in those patients. However, case 7 and case 9 had severe bronchial asthma (Table 1).
Table 1.
Patient characteristics
| Case | Age & sex | Background | Type | CRP/WBC | ICU | Sputum culture | Blood culture | Bacterial infection | Chest CT pattern | Therapy | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 M | Steroid | A | ND | – | ND | ND | – | Interstitial pneumonia | Oseltamivir | Alive |
| 2 | 86 M | Diabetes | A | 11·7/11 900 | – | ND | ND | – | Interstitial pneumonia | Amantadine | Died* |
| 3 | 68 M | Parkinson | A | 21/4100 | + | Pseudomonas aeruginosa, MRSA | ND | + | Interstitial pneumonia | Amantadine | Alive |
| 4 | 40 F | Asthma | A | 14·7/12 700 | + | ND | – | – | Interstitial pneumonia | Oseltamivir + antibiotics + steroid | Alive |
| 5 | 77 M | CHD | A | 9·5/8200 | + | – | – | – | Interstitial pneumonia + effusion | Oseltamivir + antibiotics | Alive |
| 6 | 79 M | CHD | A | 15·4/6600 | + | – | – | – | Interstitial pneumonia + effusion | Oseltamivir + antibiotics | Died* |
| 7 | 91 F | Diabetes, hypertension | A | 4·5/8900 | – | Streptococcus pneumoniae | – | + | Bronchopneumonia | Oseltamivir + antibiotics | Alive |
| 8 | 41 F | Diabetes | A | 11·8/26 200 | + | MSSA | MSSA | + | Bronchopneumonia | Oseltamivir + antibiotics | Alive |
| 9 | 60 F | Asthma | A | 18·4/16 300 | – | MRSA + MSSA | – | + | Bronchopneumonia | Oseltamivir + antibiotics | Alive |
| 10 | 30 F | – | A | 4·49/5000 | – | Streptococcus pneumoniae | ND | + | Bronchopneumonia | Oseltamivir + antibiotics | Alive |
| 11 | 82 F | Diabetes | A | 2·46/5300 | – | Gram stain (+), not identified | ND | + | Bronchopneumonia | Oseltamivir + antibiotics | Alive |
| 12 | 38 F | – | A | 8·85/14 300 | – | – | ND | – | Cryptogenic organizing pneumonia | Zanamivir | Alive |
CHD, coronary heart disease; ND, not done; MRSA, methicillin‐resistant Staphylococcus aureus; MSSA, methicillin‐sensitive Staphylococcus aureus.
*Died of respiratory failure.
Chest radiographs and chest CT scans of the chest were obtained for all patients. As all patients had a serious illness, chest CT scans were immediately performed for each patient. Influenza virus‐associated pneumonia was defined as the presence of infiltrates in chest radiographs and chest CT scans in patients with clinically proven influenza‐virus infection confirmed by the rapid diagnosis kit.
Assessment of chest CT findings
Two observers reviewed the CT scans and reached a consensus decision about the pattern and distribution of the findings. When evaluating chest CTs, all clinical information was blinded before evaluating patterns of infiltration. Chest CT scans were assessed for the presence of ground‐glass opacifications, consolidation, as well as large and small nodules. The presence of zonal (upper, middle, or lower and central or peripheral) and lateral (unilateral or bilateral) predominance was also assessed. Consolidation was defined as an area of opacification that obscured the underlying vessels; in contrast, ground‐glass opacity was defined as a hazy increase in lung attenuation with no obscuration of underlying vessels.
With regard to evaluating patterns in the radiologic findings, it was very difficult to classify patterns in the chest X‐rays, especially for patients with pleural effusion. Therefore, we used only chest CTs to assess patterns in the radiologic findings. Based on the two observers’ opinions, the patterns of chest CT scans were categorized as follows: interstitial pneumonia (mainly ground‐glass opacity), bronchopneumonia (mainly patchy opacification) and a type compatible with cryptogenic organizing pneumonia (COP; only in this category did observers considered histological findings and steroid‐responsiveness). If a lobular consolidation was observed, we considered that type of pneumonia to be bronchopneumonia. Lobular consolidation was considered present when the consolidation involved the entire secondary lobule but spared the adjacent lobules. If a ground‐glass attenuation was mainly observed, we considered that type of pneumonia to be interstitial pneumonia.
Results
Seven of the twelve patients had other organisms identified in sputum or blood cultures (Table 1). In patients with superimposed bacterial pneumonia, purulent sputum was observed.
Based on chest CT findings, six of the twelve patients were categorized as having pure influenza pneumonia (principally the interstitial pattern). Of these six patients, four had no pleural effusion (Figure 1), and two patients had pleural effusion (Figure 2). In these six patients, ground‐glass opacities were the predominant finding; in five of the six, the opacities were bilateral and the opacities were unilateral in one patient. The ground‐glass opacities showed upper lobe predominance in all six patients. Pseudomonas aeruginosa and methicillin‐resistant Staphylococcus aureus (MRSA) were cultured in one patient with interstitial pneumonia. In this patient, the detection of P. aeruginosa and MRSA reflects colonization by these bacteria. As phagocytized bacteria were not detected by Gram stain of sputum from the patient we judged that these bacteria were not significantly important as causative pathogens. No bacteria were cultured in the remaining five patients who were categorized as having interstitial pneumonia.
Figure 1.
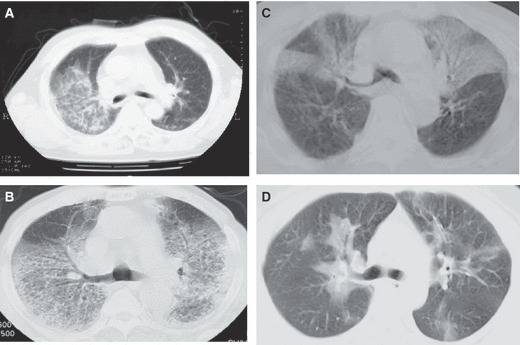
Examples of chest computed tomography (CT) findings classified as pure influenza virus pneumonia. (A) Case 1, (B) case 2, (C) case 3, (D) case 4. These chest CT scans demonstrate the ground‐glass attenuation. Some of them are diffusely distributed (A, C) and some of them are distributed as peribronchial infiltration (C, D).
Figure 2.
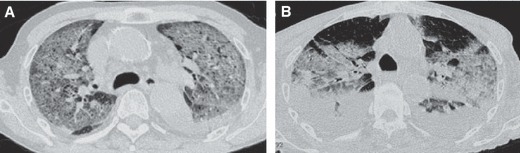
Examples of chest computed tomography (CT) findings classified as pure influenza virus pneumonia and pleural effusion. (A) Case 5, (B) case 6. These chest CT scans demonstrate the ground‐glass attenuation as well as pleural effusion.
Five of the twelve patients were categorized as having bronchopneumonia. Of the five patients categorized as having bronchopneumonia, bacteria were cultured from the sputum specimens of four patients: two showing S. aureus and two showing Streptococcus pneumoniae. In the remaining patient who was categorized as having bronchopneumonia, no bacteria were cultured even though Gram‐positive rods as well as Gram‐negative bacilli were observed in the sputum.
All patients were treated by oral oseltamivir. In addition, patients with superimposed bacterial infection and a patient with COP were treated by broad‐spectrum antibiotics, which was then followed by deescalated antibiotic therapy based on data from the drug sensitivity test.
The chest CT findings for those categorized as having bronchopneumonia are shown in Figure 3. In all five patients, patchy consolidations are observable, and pneumothorax is complicated in one patient who had bronchopneumonia caused by S. aureus (Figure 3c).
Figure 3.
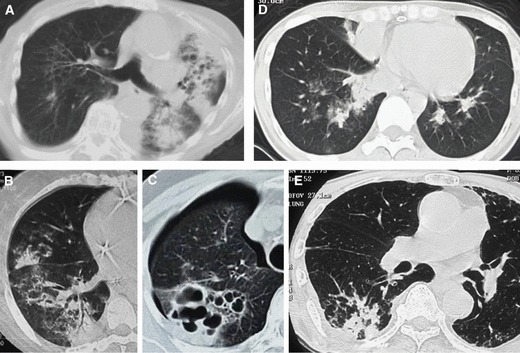
Examples of chest computed tomography (CT) findings classified as bronchopneumonia caused by bacteria. (A) Case 7, (B) case 8, (C) case 9, (D) case 10, (E) case 11. These chest CT scans demonstrate bronchopneumonia. Lobular consolidation was observed in four cases (a, b, d, e). In one case (c), cavity formation was observed from lobular consolidations.
One patient was categorized as having a pattern of COP, and had a biopsy that was histologically compatible with COP (Figure 4). In this case, our initial diagnosis was bacterial pneumonia. However, purulent sputum was not observed. In addition, although broad‐spectrum antibiotics were administered, there was no improvement in the radiologic findings. Furthermore, as there have been a few case reports describing COP associated with influenza viral infection, we decided to perform a lung biopsy. Steroid pulse therapy was very effective with this patient. None of the patients had mediastinal lymphadenopathy.
Figure 4.
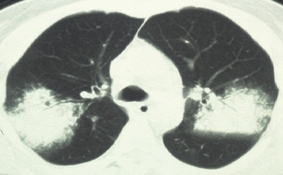
Chest computed tomography findings classified as cryptogenic organizing pneumonia pattern (case 12). Bilateral air‐space consolidations with airbronchogram are observed.
Discussion
Influenza is classified into three types (A, B or C) according to the nucleoprotein antigens and matrix protein components. Consistent with the present study, pure influenza pneumonia is usually reported in association with type A influenza viruses. Although fatal pneumonia can result from the primary viral infection, it has been reported that most deaths are related to bacterial superinfections. 10
The radiographic findings of influenza virus pneumonia in immunocompetent patients have rarely been reported. Kim et al. 6 evaluated the high‐resolution CT findings of influenza virus pneumonia in two immunocompetent patients and reported that both lungs had areas of multi‐focal peribronchovascular or subpleural consolidation. For one patient, the chest CT scan shows multifocal peribronchovascular or subpleural consolidation and ground‐glass attenuation in both lungs. Some lesions have a lobular distribution. The other patient showed diffuse ground‐glass opacities with irregular linear areas of increased attenuation as shown in our patients diagnosed with pure influenza pneumonia. In addition, Tanaka et al. 7 reported the high‐resolution CT findings of influenza virus pneumonia in a immunocompetent patient as consisting of bilateral areas of ground‐glass attenuation with a lobular distribution.
Information about the radiographic findings of influenza pneumonia in immunocompromised patients is also limited. Leung et al. 11 in their study of 59 cases of pulmonary infection in bone marrow transplant recipients included one case of influenza virus B pneumonia, a patient whose chest radiograph showed 20–30 nodules with diameters of 6–10 mm in the middle and lower zones of both lungs with no other associated findings. In a retrospective study of the radiographic findings of viral infections in 21 lung transplant recipients, the authors reported that one patient with influenza virus pneumonia showed bilateral homogeneous opacities that progressed to show patterns associated with acute respiratory distress syndrome, whereas the other patients had only minimal abnormalities. 12 In addition, Oikonomou et al. reported that the radiographic findings consisted mainly of unilateral or bilateral patchy areas of consolidation with or without associated poorly defined nodular opacities. 13
As influenza infection complicated by pneumonia is not very frequent, there may be some selection bias in our study. However, we checked every patient with an influenza virus infection which was diagnosed both clinically and by the detection of influenza antigen, and we selected those patients with chest X‐ray abnormalities. In patients with superimposed bacterial pneumonia, purulent sputum was observed. In addition, information obtained from Gram stain was also helpful in evaluating bacterial superinfection. In the present study, the chest CT patterns of 12 patients with influenza virus‐associated pneumonia were demonstrated. Importantly, chest CT patterns, especially lobular consolidations, were very helpful in diagnosing bacterial‐superimposed bacterial infections. In addition, diffuse ground‐glass attenuation was frequently observed in pure influenza‐virus pneumonia. As the treatment strategy for influenza virus‐associated pneumonia is different in the presence and absence of a superimposed bacterial infection, evaluation of radiologic patterns by chest CT scans seemed to be clinically important. Furthermore, the clinician should be aware of the existence of COP associated with influenza virus infections in order to choose the appropriate treatment by corticosteroids.
Our study has two major limitations. First, the small number of patients does not allow us to make generalized statements about the range of potential abnormalities. Second, no correlation with pathological findings was possible because it was a retrospective study. However, we believe that physicians will find these chest CT findings very useful.
In summary, several patterns of chest CT findings of influenza A virus‐associated pneumonia were demonstrated.
References
- 1. Greenberg SB.. Viral pneumonia. Infect Dis Clin North Am 1991;5:603–621. [PubMed] [Google Scholar]
- 2. Oliveira EC, Marik PE, Colice G. Influenza pneumonia: a descriptive study. Chest 2001;119:1717–1723. [DOI] [PubMed] [Google Scholar]
- 3. Bradley SF. Influenza in the elderly: prevention is the best strategy in high‐risk populations. Postgrad Med 1996;99:138–139. [PubMed] [Google Scholar]
- 4. Ljungman P, Andersson J, Aschan J et al. Influenza A in immunocompromised patients. Clin Infect Dis 1993;17:244–247. [DOI] [PubMed] [Google Scholar]
- 5. Yousuf HM, Englund J, Couch R et al. Influenza among hospitalized adults with leukemia. Clin Infect Dis 1997;24:1095–1099. [DOI] [PubMed] [Google Scholar]
- 6. Kim EA, Lee KS, Primack SL et al. Viral pneumonias in adults: radiologic and pathologic findings. RadioGraphics 2002;22(Suppl.):S137–S149. [DOI] [PubMed] [Google Scholar]
- 7. Tanaka N, Matsumoto T, Kuramitsu T et al. High‐resolution CT findings in community‐acquired pneumonia. J Comput Assist Tomogr 1996;20:600–608. [DOI] [PubMed] [Google Scholar]
- 8. Qureshi NR, Hien TT, Farrar J, Gleeson FV. The radiologic manifestations of H5N1 avian influenza. J Thorac Imaging 2006;21:259–264. [DOI] [PubMed] [Google Scholar]
- 9. Bay A, Etlik O, Oner AF et al. Radiological and clinical course of pneumonia in patients with avian influenza H5N1. Eur J Radiol 2007;61:245–250. [DOI] [PubMed] [Google Scholar]
- 10. Duchini A, Hendry RM, Redfield DC, Pockros PJ. Influenza infection in patients before and after liver transplantation. Liver Transpl 2000;6:531–542. [DOI] [PubMed] [Google Scholar]
- 11. Leung AN, Gosselin MV, Napper CH et al. Pulmonary infections after bone marrow transplantation: clinical and radiographic findings. Radiology 1999;210:699–710. [DOI] [PubMed] [Google Scholar]
- 12. Matar LD, McAdams HP, Palmer SM et al. Respiratory viral infections in lung transplant recipients: radiologic findings with clinical correlation. Radiology 1999;213:735–742. [DOI] [PubMed] [Google Scholar]
- 13. Oikonomou A, Muller NL, Nantel S. Radiographic and high‐resolution CT findings of influenza virus pneumonia in patients with hematologic malignancies. AJR Am J Roentgenol 2003;181:507–511. [DOI] [PubMed] [Google Scholar]


