Abstract
Background
The present study investigated the effects of VEGF-A targeted by miR-126 on myocardial injury after acute myocardial infarction (AMI) in rats, along with the contributions of rosuvastatin to the synergic effect.
Material/Methods
SD rats were obtained to construct AMI models by ligating their left anterior descending coronary arteries (LAD). We conducted echocardiography to check the 6 involved indexes: left ventricular ejection fractions (LVEF), fractional shortening (FS), left ventricular end-systolic volume (LVV), left ventricular end-diastolic volume (LVVd), cardiac output (CO), and heart rate (HR). Moreover, antibody sandwich enzyme-linked immunosorbent assay was carried out to determine MI markers: creatine kinase (CK), CK Isoenzyme (CK-MB), and Troponin I (cTn I). Dual-Luciferase Reporter Assay was performed to confirm the targeting of miR-126 and VEGF-A. MTT assay provided insight into the proliferation of myocardial fibroblasts. Finally, RT-RCR and Western blot were used for the detection of miR-126 and VEGF-A expressions in vivo and in vitro.
Results
Luciferase activity assay showed that miR-126 transfection significantly decreased the relative luciferase activity in HEK293T cells when it was bound to normal 3′ UTR of VEGF-A (P<0.05). In comparison to the control group, rats in the AMI model group had significantly lower LVEF, FS, and CO, and substantially higher LVVs, LVVd, HR, CK/U, CK-MB/U, and cTn-1/U (all P<0.05). Down-regulated miR-126 and up-regulated VEGF-A were also observed in MI models (P<0.05).
Conclusions
miR-126 and rosuvastatin have protective effects on AMI risk, and VEGF-A antagonizes effects on AMI is imposed by.
MeSH Keywords: Myocardial Infarction, Rats, Vascular Endothelial Growth Factor A
Background
Myocardial infarction (MI) or acute myocardial infarction (AMI) is a major cause of death worldwide and is defined as massive cell damage (necrosis and apoptosis) due to the suspension of the blood flow in a specific part of the heart [1]. The prevalence of MI in China has witnessed a dramatic change as the Chinese economy developed over the last 2 decades and the mortality from ischemic heart disease has doubled and exceeds 1million deaths each year [2]. These figures appear to be increasing due to lifestyle-related stress and the growing aging population. In addition to coronary artery disease, particularly coronary atherosclerosis, other recognized risk factors include smoking, obesity, diabetes and genetic variants [3]. Due to limited the regeneration capability of cardio-myocytes, AMI therapies mainly focus on cardiovascular remodeling to prevent further impairment of the myocardium and thereby restore cardiac functions [4].
MicroRNAs (miRNAs) are non-coding small RNAs with an average length of 22 nucleotides. They induce post-transcriptional gene silencing by binding to the 30-untranslated region (UTR) of target mRNAs [5,6]. The epigenetic mechanism of miRNAs has an exploitable regulatory point contained in gene expressions, many of which are related to human disease. Therefore, circulating miRNAs have been verified as biological markers for early diagnosis of disease and they provide therapeutic targets for cardiovascular disease [7]. Several protective regulators in cardio-myocytes have been reported, including miR-24 and miR-214, while the miR-15 family is considered to be a negative regulator [8]. Among these miRNAs, miR-126 is highly expressed in vascular endothelial cells and is reported to play a role in post-infarct remodeling and other pathophysiological processes by targeting the VEGF-A gene [9,10]. Furthermore, it has been discovered that the down-regulation of miR-126 in gastric, oral, and metastatic colorectal cancer stimulates angiogenesis through the up-regulation of VEGF-A, suggesting a potential therapeutic strategy for preventing the neoplasm process via antagonizing the down-regulation of miR-126 in tumor tissues [11–13]. VEGF-A is an important angiogenic molecule and a 46kDa dimeric peptide that plays a key role in vascular growth and regulation. The down-regulation of miR-126 enhances cardiac repair in the infarcted heart and protects cardio-myocytes from ischemia injury [14]. In addition, serum miR-126 is believed to be a prospective biomarker and its down-modulation is usually observed in AMIs [15]. Studies have provided evidence for potential angiogenesis mediated by miR-126 in vitro and in vivo; therefore, we suspected that the interaction between miR-126 and VEGF-A is likely to have substantial influence on angiogenesis and may be an alternative therapeutic target for AMI.
On the other hand, rosuvastatin has the effect of inhibiting the cholesterol biosynthesis by its binding to HMG-CoA reductase, which further restores dyslipidemia and prevents arteriosclerosis (AS) and AMI. Rosuvastatin has shown angiogenic activity by changing cytokine expressions, including the expression of VEFG-A [16]. The inhibition of TIMP-1 and TIMP-2 are thought to be the potential mechanisms because TIMP-2 exhibits an antiangiogenic property that enhances the expression of VEGF-A and ER-α [17]. On the other hand, rosuvastatin is able to preserve and restore heart function through the regulation of VEGF-A when acute myocardial infarction occurs [18]. Compared to the conventional hypolipidemic effect of rosuvastatin, studies have provided little evidence of the above mechanisms and there is no study on the association among rosuvastatin, miR-126, and VEGF-A in AMI.
The present study explored this potential mechanism in the combined treatments of miR-126 inhibitors transfection and rosuvastatin to assess whether miR-126 inhibitors and rosuvastatin could protect the cardiac tissues once AMIs occur.
Material and Methods
Cell extraction and cell culture
SD rats (Laboratory Animal Center of Southern Medical University) with an average age of 2–3 months and an average weight of 225–350 g were obtained to construct acute myocardial infarction (AMI) models, from which bone marrow mesenchymal stem cells (BMSCs) were isolated.
BMSC cells from rats were isolated using the density gradient centrifugation method [19]. HEK293T cells were purchased from the Institute of Biochemistry and Cell Biology (Shanghai). All cells were cultured in Dulbecco modified Eagle medium (Gibco, Carlsbad, CA) with 10% fetal bovine serum (Gibco, Carlsbad, CA) at 37°C in an incubator with 5% CO2. Proliferation of cells was inspected using 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT) assay and changes in cellular morphology were observed under a microscope.
MTT assay
MTT assay was used to analyze the proliferation of myocardial fibroblasts for each group. A total of 3×103 cells were cultured in 96-well plates and incubated for 24 h and then stained with 0.5 mg/ml MTT for 4 h. After that, supernatant was discarded and 200 μl of dimethylsulfoxide (DMSO) was added to dissolve precipitates. Samples were measured at 490 nm using an ELISA reader.
Luciferase activity assay
The 3′untranslated region (UTR) of VEGF-A, which contains miR-126 binding sites, was amplified using polymerase chain reaction (PCR) with prime sequence shown in Table 1 and was cloned into the downstream of the psiCHECK-2 luciferase vector (Promega, USA), named as wt 3′ UTR. The binding site was intentionally mutated using the GeneTailor Site-Directed Mutagenesis System (Invitrogen, USA) and the resultant mutant 3′ UTR was cloned into the same vector, named as mu 3′ UTR.
Table 1.
Primer sequences for luciferase reporter experiments.
| Gene | Sequence | |
|---|---|---|
| VEGF-A 3′ UTR | Sense | 5′-CCGCTCGAGGCCGGGCAGGAGGAAGGAG-3′ |
| Antisense | 5′-ATAAGAATGCGGCCGCTGAGATCAGAATTAAATTCTTTAATAC-3′ | |
| VEGF-A mutation 3′ UTR | Sense | 5′-AAGAGAAAGTGTTTTATATATCGATCTTATTTAATATCCCTTTTTA-3′ |
| Antisense | 5′-TAAAAAGGGATATTAAATAAGATCGATATATAAAACACTTTCTCT-3′ |
VEGF-A – vascular endothelial growth factor-A; UTR – untranslated region.
BMSC cells maintained in 48-well plates were co-transfected in 2 groups: one group was co-transfected with the combination of 200 ng pGL3-control luciferase reporter, 10 ng of pRL-TK vector and miR-126 vector, and in the other group miR-126 vector was replaced by the negative control vector. The transfected cells were analyzed by the Dual-Luciferase Reporter Assay System (Promega) 48 h after their transfection.
Lentivirus transduction and transfection
Two types of fragments containing separate miR-126 mimics and miR-126 inhibitors were cloned into the pCDH vector. Then, the pCDH vector, together with other packaging plasmids, was co-transfected into cells using the Lipofectamine LTX kit (Invitrogen, CA) and the viral particles therein were collected 48 h after transfection.
BMSC cells were infected with 3 types of recombinant lentivirus and 8 ug/ml of polybrene: the control BMSC (cells were transfected with the control vector), the mimics BMSC (cells were transfected with miR-126 mimics), and the inhibitors BMSC (cells were transfected with miR-126 inhibitors).
Animal model
We obtained 80 SD rats and they were randomly allocated into the control group (n=10) and model group (n=70). An AMI model was induced by ligating the left anterior descending coronary artery (LAD) of rats in the model group, while rats in the control group were treated with sham operation.
All rats in the experiment were further subdivided into 8 groups at 2 weeks after the operation: the control group (10 rats with sham operation, PBS was injected into the infarct region, 4-week intragastric administration of the solvent); the model group (10 rats had PBS injected into the infarct region, 4-week intragastric administration of the solvent); the model + rosuvastatin group (10 rats had PBS injected into the infarct region, 4-week intragastric administration of the rosuvastatin); the transfection control group (10 rats were injected with BMSC cells transfected with the control vector into the infarct region, 4-week intragastric administration of the solvent); the miR-126 mimics group (10 rats were injected with BMSC cells transfected with miR-126 mimics into the infarct region, 4-week intragastric administration of the solvent); the mimics + rosuvastatin group (10 rats were injected with BMSC cells transfected with miR-126 mimics into the infarct region, 4-week intragastric administration of the rosuvastatin); the miR-126 inhibitors group (10 rats were injected with BMSC cells transfected with miR-126 inhibitors into the infarct region, 4-week intragastric administration of the solvent); and the inhibitors + rosuvastatin group (10 rats were injected with BMSC cells transfected with miR-126 inhibitors into the infarct region, 4-week intragastric administration of the rosuvastatin). All rats were inspected at 6 weeks after the operation.
Echocardiographic measurement and tests of myocardial injury markers
Rats in each group were inspected with echocardiographic measurement (ATL-HDI5000, 15 MHz linear, and 12-MHz sectorial scanheads) 6 weeks after the operation. Six indexes were examined: including left ventricular ejection fractions (LVEF), fractional shortening (FS), left ventricular end-systolic volume (LVVs), left ventricular end-diastolic volume (LVVd), cardiac output (CO), and heart rate (HR).
Myocardial injury markers contained in blood samples – creatine kinase (CK), CK Isoenzyme (CK-MB), and Troponin I (cTn I) – were tested by the double antibody sandwich enzyme-linked immunosorbent assay (R&D, USA).
Hematoxylin-eosin staining
Rats in each group were sacrificed using the injection of xylazine hydrochloride 6 weeks after the operation. Tissues extracted from infarct regions in the left atrial were harvested and treated with 4% paraformaldehyde. Five-μm-thick paraffin sections were embedded and then stained with HE assay. Samples were checked under the microscope and measured at 5 randomly selected sites.
RNA isolation and RT-PCR
Total RNA from tissues or cells were extracted using TRIzol reagent (Invitrogen). The ReverTra Ace qPCR RT Kit (Toyobo, Japan) was used to reversely transcribe total RNA into cDNA and real-time PCR (RT-PCR) was performed using THUNDERBIRD SYBR® qPCR Mix (Toyobo, Japan) and the CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The relevant primers are listed in Table 2. Target gene expression levels were normalized to those of the control gene (GADPH) and were calculated using the method of 2−ΔΔCT.
Table 2.
Primer sequences of VEGF-A, GADPH and miR-221 for implemention of RT-PCR.
| Gene | Sequence | |
|---|---|---|
| VEGF-A | Sense | 5′-TTCCATTATTAGACGTTCCCTG-3′ |
| Antisense | 5′-GTGTTTTCATCAAATTCTCGGT-3′ | |
| GADPH | Sense | 5′-TGGTATCGTGGAAGGACTCAT-3′ |
| Antisense | 5′-GTGGGTGTCGCTGTTGAAGTC-3′ | |
| miR-126 | Sense | 5′-CGACGCGTCGGGTTTACAGAACACCCATCA-3′ |
| Antisense | 5′-CCATGGGGCACAGCAACAAAACACT-3′ |
GADPH – phosphoglyceraldehyde dehydrogenase; RT-PCR – real time-polymerase chain reaction.
Western blot
Tissues and cells were harvested and lysed by radio immunoprecipitation assay (RIPA) buffer. Total protein was separated and evaluated by the Bradford method [20]. Then, the total protein was denatured in boiling water and transferred onto polyvinylidene fluoride (PVDF) membranes when sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was completed. The membranes were blocked in Tris-Buffered Saline Tween (TBST) with 5% skim milk for 1 h and then they were treated with primary antibodies against VEGF-A (1: 800 dilution, Zhongshan Biology Company, Beijing) at 4°C overnight. After membranes were washed, they were incubated with secondary antibodies (horseradish peroxidase-conjugated goat anti-goat, 1:2000 dilutions, Zhongshan Biology Company, Beijing). Samples and reduced glyceraldehydes-phosphate dehydrogenase (GAPDH) as the endogenous control were ultimately processed with enhanced chemiluminescence and quantified using Lab Works4.5 software (Mitov Software).
Statistical analysis
All statistical analyses were performed with SPSS 18.0 software (Chicago, IL). Data are presented in the form of mean ± standard deviation (SD). Two-tailed Student’s t-test or one-way analysis of variance (ANOVA) was used to assess significant different among different groups and P<0.05 provided evidence of statistical significance.
Results
MiR-126 suppressed VEGF-A expression by binding to the 3′ UTR
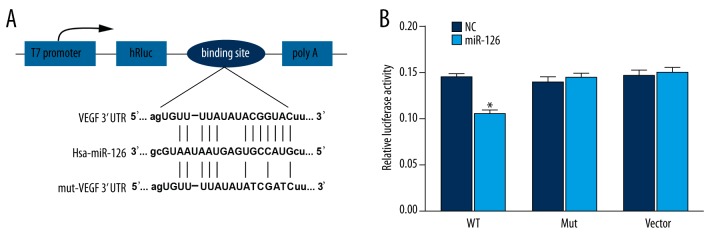
One highly conserved miR-126 binding site is likely to situate in 3′ UTR of VEGF-A, which is consistent with the results provided by miRanda software (Figure 1A). Luciferase activity assay confirmed that there is a direct interaction between miR-126 and VEGF-A. Apart from that, miR-126 transfection could significantly decrease the relative luciferase activity in HEK293T cells when miR-126 was bound to normal 3′ UTR of VEGF-A (P<0.05). Nonetheless, there was no significant difference in luciferase activities between NC and normal cells containing miR-126 for both groups of control and TIMP3 mutation 3′ UTR (Table 3, Figure 1B).
Figure 1.
Binding site prediction and results of luciferase report. (A) Putative targets predicted by TargetScan. (B) Results of luciferase report. * P<0.05 versus NC group.
Table 3.
Relative luciferase activity in wt VEGF-A, Mut VEGF-A and vector groups.
| Relative luciferase activity | Wt | Mut | Vector |
|---|---|---|---|
| NC | 0.144±0.005 | 0.139±0.007 | 0.146±0.006 |
| Transfection group | 0.105±0.004* | 0.144±0.005 | 0.149±0.007 |
Wt – wild type; Mut – mutation; NC – negative control.
P<0.05 versus NC group.
In vitro cell culture
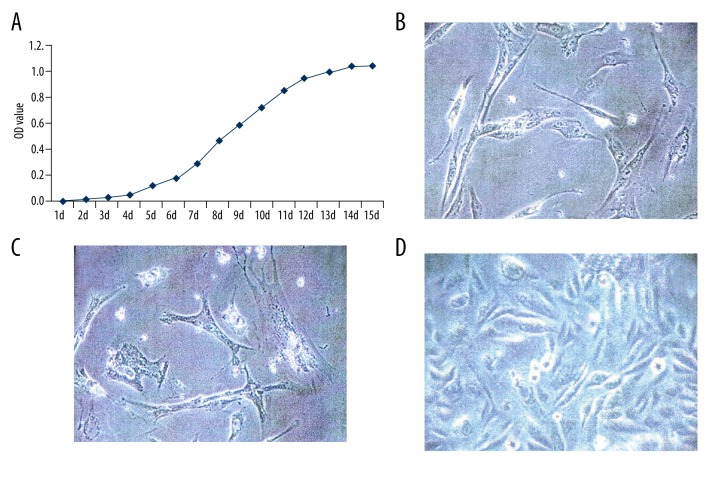
As suggested by MTT assay (Figure 2A), the growth curve of BMSC cells depicted an “S” shape. Briefly, the growth of cells stayed in a logarithmic phase from day 6 to day 11 and peaked on day 12. Furthermore, cells at various growth stages had different cell shapes, as observed by the optical microscope (×400). For instance, cells at early stages were usually large and round (Figure 2B), while those at late stage appeared to have shuttle or polygon shapes (Figure 2C). The final selected cells were purified BMSC cells that had undergone more than 3 generation passages (Figure 2D).
Figure 2.
Proliferation test of BMSC cells. (A) The growth curve of BMSC cells checked by MTT assay. (B) Morphology of primary BMSC cells 24 h after culture (×400). (C) Morphology of primary BMSC cells 72 h after culture (×400). (D) BMSC cells having undergone 3 generations of passage at the fusion state.
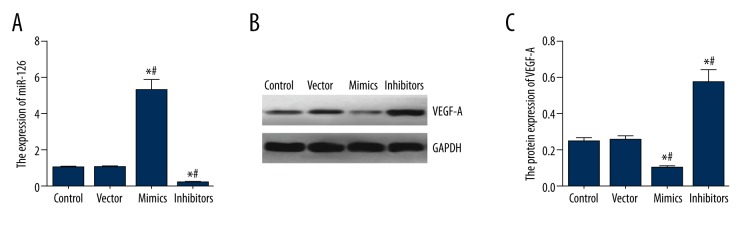
Expressions of miR-126 and VEGF-A in transfected cells
As shown in Table 4 and Figure 3, expressions of miR-126 and VEGF-A in the control group were not significantly different from those in the vector group (all P>0.05). Compared with control and vector groups, miR-126 mimics group had significantly higher miR-126 expressions and lower VEGF-A protein expressions, whereas the inhibitors group had significantly lower miR-126 expressions and higher VEGF-A protein expressions (all P<0.05).
Table 4.
Expression miR-126 and VEGF-A in transfection cells.
| Group | Control | Vector | Mimics | Inhibitors |
|---|---|---|---|---|
| miR-126 | 0.994±0.032 | 1.029±0.057 | 5.301±0.561*,# | 0.224±0.021*,# |
| VEGF-A protein | 0.245±0.018 | 0.252±0.021 | 0.096±0.010*,# | 0.574±0.066*,# |
P<0.05 versus control group;
P<0.05 versus vector group.
Figure 3.
Expressions of miR-126 and VEGF-A in transfected BMSC cells. (A) Quantitation of miR-126 in cells within groups of control, vector, mimics and inhibitors. (B) Western blot analysis of VEGF-A in BMSC cells normalized to GAPDH. (C) Quantitation of VEGF-A in cells within groups of control, vector, mimics and inhibitors. Data are presented as mean ±SD for 3 independent experiments. * P<0.05 versus control group; # P<0.05 versus vector group.
Results of echocardiographic measurement and myocardial injury markers
Compared with the control group, rats in the model group had significantly lower LVEF, FS and CO as along with significantly higher LVVs, LVVd, and HR (all P<0.05, Table 5, Figure 4A–4F). Moreover, the above physiological indexes among 5 groups of rats (model + rosuvastatin, transfection control, mimics + rosuvastatin, miR-126 inhibitors, and inhibitors + rosuvastatin) were extremely close. However, the cardiac capability of rats in these 5 groups appeared to be more favorable than in the mimics group. Of note, 5 inter-group comparisons were observed to be statistically different with respect to LVEF and other indexes: comparisons between groups of model + rosuvastatin and model, groups of miR-126 mimics and model + rosuvastatin, groups of mimics + rosuvastatin and miR-126 mimics, groups of miR-126 inhibitors and miR-126 mimics, groups of inhibitors + rosuvastatin, and control (all P<0.05, Table 5, Figure 4A–4F).
Table 5.
Results of echocardiographic measurement and myocardial injury markers tests.
| Group | Control | Model | Model + rosuvastatin | Transfection control | MiR-126 mimics | Mimics + rosuvastatin | MiR-126 inhibitors | Inhibitors + rosuvastatin |
|---|---|---|---|---|---|---|---|---|
| LVEF/% | 67.7±6.1 | 42.8±7.9a | 50.2±3.8ab | 44.2±4.4a | 39.2±3.7ac | 46.8±4.8ae | 48.2±5.2ae | 52.9±4.5abde |
| FS (%) | 36.3±2.4 | 22.4±4.1a | 27.7±5.4a | 23.7±4.9a | 20.3±4.3ac | 25.3±5.1a | 26.5±5.5a | 28.4±3.9ae |
| LVVs (ml) | 3.0±0.3 | 3.7±0.6a | 3.2±0.4 | 3.6±0.5 | 3.8±0.6a | 3.3±0.4 | 3.2±0.4 | 3.1±0.2e |
| LVVd (ml) | 5.4±0.7 | 6.2±0.4a | 5.8±0.4 | 6.1±0.4a | 6.4±0.5a | 5.9±0.5 | 5.8±0.5 | 5.7±0.4e |
| CO (ml) | 2.3±0.4 | 1.5±0.3a | 1.7±0.3a | 1.5±0.3a | 1.5±0.4a | 1.6±0.3a | 1.6±0.3a | 1.8±0.4a |
| HR (·min−1) | 444±50 | 521±58a | 482±48 | 514±54 | 532±60a | 504±49 | 495±42 | 478±52 |
| CK (U/ml) | 92.7±19.3 | 303.3±60.3a | 192.7±32.8ab | 275.3±52.0ac | 332.1±62.5ac | 212.7±41.6abde | 189.5±27.2abde | 142.2±25.3bdef |
| CK-MB (U/ml) | 103.5±21.1 | 236.9±36.5a | 145.2±27.2b | 227.5±35.3ac | 251.5±38.4ac | 184.8±31.2abde | 162.4±25.9abde | 117.6±24.0bdefg |
| cTn I (pg/ml) | 59.2±11.7 | 167.2±29.9a | 85.8±14.0b | 149.5±27.6ac | 183.1±33.4acd | 125.9±21.6abce | 113.1±17.5abde | 65.4±12.4bdefg |
LVEF – left ventricular ejection fractions; FS – fractional shortening; LVVs – left ventricular end-systolic volume; LVVd – left ventricular end-diastolic volume; CO – cardiac output; HR – heart rate; CK – creatine kinase; CK-MB – CK isoenzyme; cTn I – Troponin I.
P<0.05 versus control group;
P<0.05 versus model group;
P<0.05 versus model + rosuvastatin group;
P<0.05 versus transfection control group;
P<0.05 versus mimics group;
P<0.05 versus mimics + rosuvastatin group;
P<0.05 versus inhibitors group.
Figure 4.
Results of echocardiographic measurement and myocardial injury markers tests. (A–F) Results of echocardiographic measurement. (G–I) Results of myocardial injury markers tests. Data are presented as mean ±SD for 3 independent experiments. LVEF – left ventricular ejection fractions; FS – fractional shortening; LVVs – left ventricular end-systolic volume; LVVd – left ventricular end-diastolic volume; CO – cardiac output; HR – heart rate; CK – creatine kinase; CK-MB – CK Isoenzyme; cTn I: Troponin I. a P<0.05 versus control group; b P<0.05 versus model group; c P<0.05 versus model + rosuvastatin group; d P<0.05 versus transfection control group; e P<0.05 versus mimics group; f P<0.05 versus mimics + rosuvastatin group; g P<0.05 versus inhibitors group.
The expression of myocardial injury markers – CK/U, CK-MB/U, and cTn-1/U among the model, transfection control, and miR-126 mimics group – appeared to be similar.
For myocardial injury markers – CK/U, CK-MB/U, and cTn-1/U – the expression within groups of model, transfection control, and miR-126 mimics were fairly similar and were higher than those within the control group. Nonetheless, the other 4 groups – model + rosuvastatin, mimics + rosuvastatin, miR-126 inhibitors, and inhibitors + rosuvastatin – showed significantly lower expression levels of three markers than the above 3 groups (all P<0.05, Table 5, Figure 4G–4I).
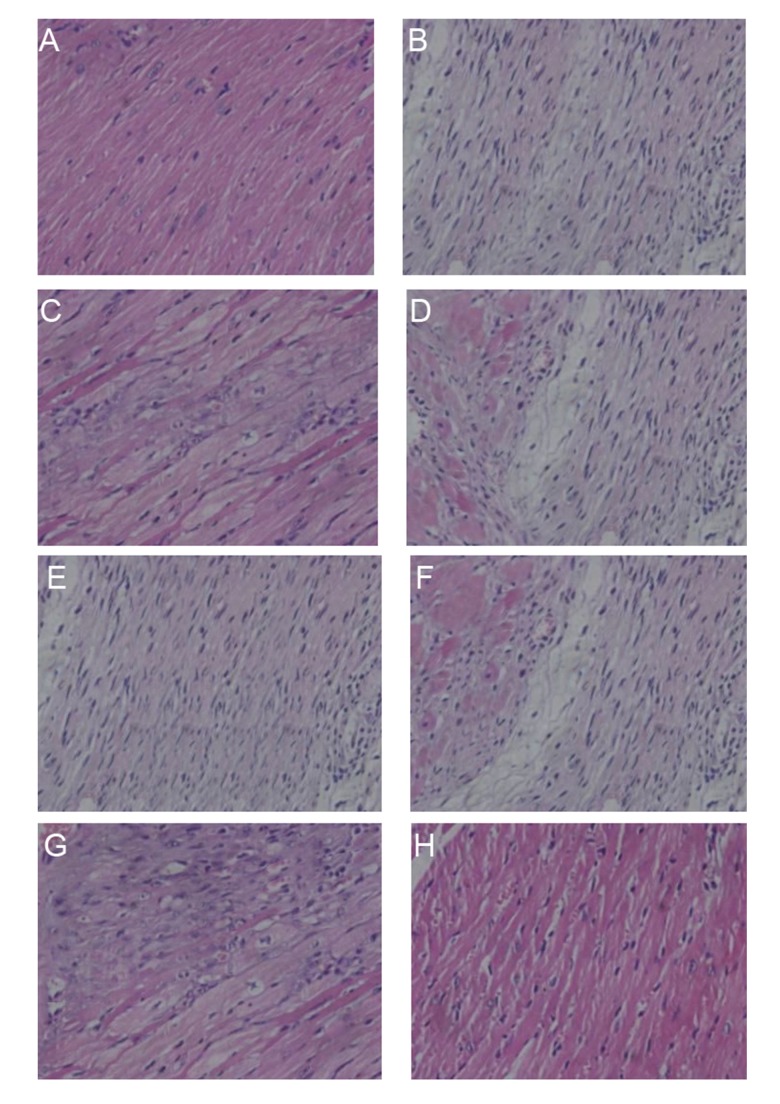
Result of HE staining
As shown in Figure 5, various symptoms, such as disordered fibers, broken nuclear, condensation nuclear, and fragmented myocardial, were discovered in the MI area of rats in both the model and mimics group (Figure 5B, 5E). In contrast, cardiac tissues after AMI were protected after treatments of BMSC, miR-126 inhibitors transfection, and injection of rosuvastatin (Figure 5C, 5D, 5F, 5G, respectively).
Figure 5.
HE staining of fibrous tissue in atria of rats in groups of control (A), model (B), model + rosuvastatin (C), transfection control (D), mimics (E), mimics + rosuvastatin (F), inhibitors (G), and inhibitors + rosuvastatin (H) (×400).
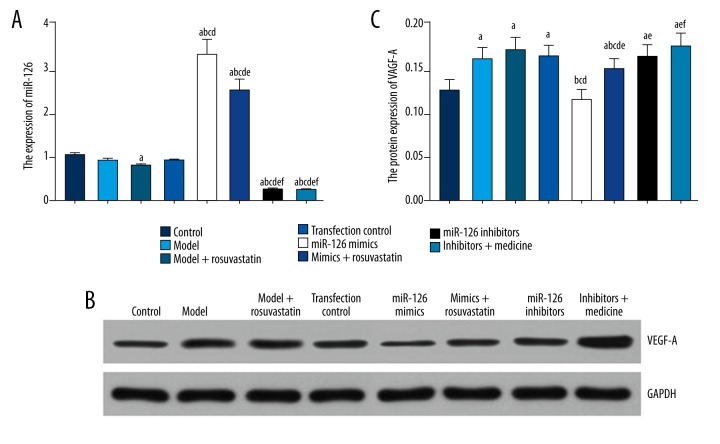
Expressions of miR-126 and VEGF-A in cardiac tissues
As shown in Table 6 and Figure 6, down-regulated miR-126 and up-regulated VEGF-A expressions were both observed in MI models. Furthermore, this trend can be differentiated using triple treatments (i.e., injection of BMSC cells, miR-126 inhibitors transfection, and rosuvastatin). Additionally, 5 inter-group comparisons displayed significant differences in the expressions of miR-126 and VEGF-A, including comparisons between groups of model and control, groups of miR-126 mimics and transfection control, groups of miR-126 inhibitors and transfection control, groups of miR-126 mimics and mimics + rosuvastatin, and groups of miR-126 inhibitors and inhibitors + rosuvastatin (all P<0.05, Table 6, Figure 6).
Table 6.
Expression level of miR-126 and VEGF-A protein in cardiac tissue.
| Group | Control | Model | Model + rosuvastatin | Transfection control | MiR-126 mimics | Mimics + rosuvastatin | MiR-126 inhibitors | Inhibitors + rosuvastatin |
|---|---|---|---|---|---|---|---|---|
| miR-126 | 1.014± 0.057 | 0.892± 0.046 | 0.775± 0.044a | 0.887± 0.044 | 3.278± 0.342abcd | 2.478± 0.247abcde | 0.245± 0.056abcdef | 0.224± 0.041abcdef |
| VEGF-A protein | 0.124± 0.012 | 0.159± 0.013a | 0.169± 0.015a | 0.162± 0.013a | 0.113± 0.012bcd | 0.148± 0.013ace | 0.162± 0.014ae | 0.173± 0.015aef |
P<0.05 versus control group;
P<0.05 versus model group;
P<0.05 versus model + rosuvastatin group;
P<0.05 versus transfection control group;
P<0.05 versus mimics group;
P<0.05 versus mimics + rosuvastatin group;
P<0.05 versus inhibitors group.
Figure 6.
Expressions of miR-126 and VEGF-A in cardiac tissues of model rats. (A) Quantitative analyses of miR-126 in rats within groups of control, model, model + rosuvastatin, transfection control, mimics, mimics + rosuvastatin, inhibitors, and inhibitors + rosuvastatin. (B) Western blot analysis of VEGF-A in cardiac tissue normalized to GAPDH. (C) Quantitative analyses of protein levels of VEGF-A in rats within groups of control, model, model + rosuvastatin, transfection control, mimics, mimics + rosuvastatin, inhibitors, and inhibitors + rosuvastatin. Data are presented as mean ±SD for 3 independent experiments. a P<0.05 versus control group; b P<0.05 versus model group; c P<0.05 versus model + rosuvastatin group; d P<0.05 versus transfection control group; e P<0.05 versus mimics group; f P<0.05 versus mimics + rosuvastatin group; g P<0.05 versus inhibitors group.
Discussion
This study investigated the role of VEGF in the effects of miR-126 and rosuvastatin on myocardial infarction. VEGF is an important neovascularization regulator that can effectively enhance the establishment of collateral circulation in ischemic myocardium [21]. The main mechanism of VEGF related to angiogenesis is its specific effects on vascular endothelial cells, inducing endothelial cell proliferation, sprouting, migration, and luminal formation [22]. The VEGF gene promoter region contains the hypoxia-inducible factor-1 (HIF-1) reactive element, which directly induces the expression of VEGF under hypoxic conditions [23]. Animal and clinical trials have demonstrated that the expression of VEGF was increased in myocardial infarction [24]. We examined the expression of VEGF-A in myocardial infarction using a rat model and discovered that VEGF expression appeared to be increased.
MiR126 is specifically expressed in vascular endothelial cells and vascular smooth muscle cells and has an important role in the process of angiogenesis through regulation of cell proliferation, differentiation, and apoptosis [25]. Moreover, MiR126 can directly target Spred1 and PI3KR2 and enhance the VEGF signaling pathway. When miR-l26 is down-regulated, the over-expression of Spred1 and PI3KR2 inhibits the MAPK and PI3K signaling pathway, which affects angiogenic factor signals and results in the disruption of angiogenesis [26,27]. This study discovered that mice with knockout miRl26 could not form complete vessels and some mice died in the embryonic period. Apart from that, mice survived during the embryonic period, ultimately dying from cardiac rupture or myocardial infarction due to vascular abnormalities [26]. Yang et al. also demonstrated that miR-126 could inhibit the expression of VEGF and disrupt its functions in oral squamous cell carcinoma [28].
We transfected miR-126 into BMSC cells to assess the relationship between miR-126 and VEGF-A in vitro and in vivo. The injected miR126 inhibitors contributed to an increased expression of VEGF-A and further experiments discovered that miR-126 could target the 3′untranslated region of VEGF-A and inhibit its expressions. Then, we constructed a myocardial infarction model to further demonstrate the above results. Lower expression of VEGF-A in the group of BMSC cells transfected with miR-126 mimics was observed compared with the miR-126 inhibitors group. The myocardial infarction model suggested that myocardial injury was moderated for those groups with increased VEGF-A expressions, suggesting a potentially protective effect of VEGF-A on cardiac functions.
Statins are HMG-CoA reductase inhibitors that can reduce the synthesis and secretion of intracellular cholesterol, facilitate serum cholesterol removal, and inhibit the synthesis of apolipoprotein [29]. The effect of statins has been recognized in the prevention of coronary heart disease [30]. In addition, recent studies have found that statins have other pharmacological effects, including narrowing of atherosclerotic plaques, anti-inflammatory or anti-oxidative stress, and improving endothelial cell function [30]. Furthermore, statins can significantly reduce the incidence of long-term cardiovascular events [31,32]. Our results also show that myocardial injury was significantly reduced for rats given rosuvastatin compared with the control group. The myocardial infarction model suggested a further decreased expression of miR-126 and a highly increased expression of VEGF-A in rosuvastatin groups compared with control groups. Therefore, we suspected that rosuvastatin might have a protective effect on ischemic myocardium through reducing the level of miR-126 and increasing the expression of VEGF-A.
The limitation in use of lipid-lowering drugs (statins) is the intolerance of certain patients to them, and nutraceuticals may be safer. It has been documented that combination therapies of statins and nutraceuticals (e.g., fish oil supplementation and berberlin) can facilitate more reductions of total cholesterol and triacylglycerol than can statins mono-therapy [33,34]. Dosages of statins in the combination therapy would be dramatically lower than in mono-therapies, and thus the adverse effects induced by statins could be avoided to a greater degree. Further in vivo investigations are needed to exploring the effects of nutraceuticals on AMI.
Conclusions
In this study, rats with myocardial infarction were used to explore the relationship between rosuvastatin, miR-126, and VEGF-A. As suggested by the myocardial infarction model, there was a decrease in the expression of miR-126, but VEGE-A expression levels increased. Rosuvastatin also inhibited the angiogenesis of infarcted myocardial tissues in rats through the down-regulation of VEGF-A, further suggesting a protective effect on myocardial injury. Nevertheless, the intrinsic role of VEGF-A in molecular regulation and myocardial injury remains to be clarified.
Footnotes
Source of support: Departmental sources
References
- 1.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–84. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 2.Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet. 2013;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu ML, De Venecia T, Patnaik S, Figueredo VM. Atrial myocardial infarction: A tale of the forgotten chamber. Int J Cardiol. 2016;202:904–9. doi: 10.1016/j.ijcard.2015.10.070. [DOI] [PubMed] [Google Scholar]
- 4.Zangi L, Lui KO, von Gise A, et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS. Post-transcriptional gene silencing by siRNAs and miRNAs. Curr Opin Struct Biol. 2005;15:331–41. doi: 10.1016/j.sbi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Jing Q, Huang S, Guth S, et al. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120:623–34. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Chen C, Wang D. Circulating microRNAs in cardiovascular diseases: from biomarkers to therapeutic targets. Front Med. 2014;8:404–18. doi: 10.1007/s11684-014-0379-2. [DOI] [PubMed] [Google Scholar]
- 8.Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol. 2015;12:135–42. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- 9.Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–84. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnem T, Lonvik K, Eklo K, et al. Independent and tissue-specific prognostic impact of miR-126 in nonsmall cell lung cancer: Coexpression with vascular endothelial growth factor-A predicts poor survival. Cancer. 2011;117:3193–200. doi: 10.1002/cncr.25907. [DOI] [PubMed] [Google Scholar]
- 11.Cheng D, Shi H, Zhang K, et al. RAD51 Gene 135G/C polymorphism and the risk of four types of common cancers: A meta-analysis. Diagn Pathol. 2014;9:18. doi: 10.1186/1746-1596-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen TF, Christensen R, Andersen RF, et al. MicroRNA-126 and epidermal growth factor-like domain 7-an angiogenic couple of importance in metastatic colorectal cancer. Results from the Nordic ACT trial. Br J Cancer. 2013;109:1243–51. doi: 10.1038/bjc.2013.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasahira T, Kurihara M, Bhawal UK, et al. Downregulation of miR-126 induces angiogenesis and lymphangiogenesis by activation of VEGF-A in oral cancer. Br J Cancer. 2012;107:700–6. doi: 10.1038/bjc.2012.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang JM, Luo B, Xiao JH, et al. VEGF-A promotes cardiac stem cell engraftment and myocardial repair in the infarcted heart. Int J Cardiol. 2015;183:221–31. doi: 10.1016/j.ijcard.2015.01.050. [DOI] [PubMed] [Google Scholar]
- 15.Hsu A, Chen SJ, Chang YS, et al. Systemic approach to identify serum microRNAs as potential biomarkers for acute myocardial infarction. Biomed Res Int. 2014;2014:418628. doi: 10.1155/2014/418628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaitone SA, Abo-Gresha NM. Rosuvastatin promotes angiogenesis and reverses isoproterenol-induced acute myocardial infarction in rats: role of iNOS and VEGF. Eur J Pharmacol. 2012;691:134–42. doi: 10.1016/j.ejphar.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui AJ, Gustafsson T, Sylven C, Crisby M. Rosuvastatin inhibits TIMP-2 and promotes myocardial angiogenesis. Pharmacology. 2014;93:178–84. doi: 10.1159/000360860. [DOI] [PubMed] [Google Scholar]
- 18.Semenova AE, Sergienko IV, Masenko VP, et al. [Effect of rosuvastatin therapy and myocardial revascularization on angiogenesis in coronary artery disease patients]. Kardiologiia. 2007;47:4–8. [in Russian] [PubMed] [Google Scholar]
- 19.Li X, Zhang Y, Qi G. Evaluation of isolation methods and culture conditions for rat bone marrow mesenchymal stem cells. Cytotechnology. 2013;65:323–34. doi: 10.1007/s10616-012-9497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian X, Dong H, Hu X, et al. Analysis of the interferences in quantitation of a site-specifically PEGylated exendin-4 analog by the Bradford method. Anal Biochem. 2014;465:50–52. doi: 10.1016/j.ab.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Moon HH, Kim HA, et al. Hypoxia-inducible vascular endothelial growth factor-engineered mesenchymal stem cells prevent myocardial ischemic injury. Mol Ther. 2011;19:741–50. doi: 10.1038/mt.2010.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konopka A, Janas J, Piotrowski W, Stepinska J. Concentration of vascular endothelial growth factor in patients with acute coronary syndrome. Cytokine. 2013;61:664–99. doi: 10.1016/j.cyto.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Nowak D, Kozlowska H, Gielecki JS, et al. Cardiomyopathy in the mouse model of Duchenne muscular dystrophy caused by disordered secretion of vascular endothelial growth factor. Med Sci Monit. 2011;17(11):BR332–38. doi: 10.12659/MSM.882043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kranz A, Rau C, Kochs M, Waltenberger J. Elevation of vascular endothelial growth factor-A serum levels following acute myocardial infarction. Evidence for its origin and functional significance. J Mol Cell Cardiol. 2000;32:65–72. doi: 10.1006/jmcc.1999.1062. [DOI] [PubMed] [Google Scholar]
- 25.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–71. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo C, Sah JF, Beard L, et al. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer. 2008;47:939–46. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Wu H, Ling T. Suppressive effect of microRNA-126 on oral squamous cell carcinoma in vitro. Mol Med Rep. 2014;10:125–30. doi: 10.3892/mmr.2014.2171. [DOI] [PubMed] [Google Scholar]
- 29.Semenova AE, Sergienko IV, Masenko VP, et al. The influence of rosuvastatin therapy and percutaneous coronary intervention on angiogenic growth factors in coronary artery disease patients. Acta Cardiol. 2009;64:405–9. doi: 10.2143/AC.64.3.2038029. [DOI] [PubMed] [Google Scholar]
- 30.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: A randomized controlled trial. JAMA. 2004;291:1071–80. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg EO, Scherrer-Crosbie M, Picard MH, et al. Rosuvastatin reduces experimental left ventricular infarct size after ischemia-reperfusion injury but not total coronary occlusion. Am J Physiol Heart Circ Physiol. 2005;288:H1802–9. doi: 10.1152/ajpheart.00962.2004. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Dai H, Xing M, et al. Effect of a single high loading dose of rosuvastatin on percutaneous coronary intervention for acute coronary syndromes. J Cardiovasc Pharmacol Ther. 2013;18:327–33. doi: 10.1177/1074248412474346. [DOI] [PubMed] [Google Scholar]
- 33.Oelrich B, Dewell A, Gardner CD. Effect of fish oil supplementation on serum triglycerides, LDL cholesterol and LDL subfractions in hypertriglyceridemic adults. Nutr Metab Cardiovasc Dis. 2013;23:350–57. doi: 10.1016/j.numecd.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Eussen S, Klungel O, Garssen J, et al. Support of drug therapy using functional foods and dietary supplements: Focus on statin therapy. Br J Nutr. 2010;103:1260–77. doi: 10.1017/S0007114509993230. [DOI] [PubMed] [Google Scholar]