Abstract
Background
Acute myeloid leukemia (AML) patients with mixed lineage leukemia (MLL) gene rearrangements always had a very poor prognosis. In this study, we report the incidence of MLL rearrangements in AML patients using gene analysis, as well as the clinical significance and prognostic features of these rearrangements.
Material/Methods
This retrospective study took place from April 2008 to November 2011 in the People’s Liberation Army General Hospital. A total 433 AML patients were screened by multiple nested reverse transcription polymerase chain reaction (RT-PCR) to determine the incidence of the 11 MLL gene rearrangements. There were 68 cases of MLL gene rearrangements, for a positive rate of 15.7%. A total of 24 patients underwent allogeneic hematopoietic stem cell transplantation (Allo-HSCT), and 34 patients received at least 4 cycles of chemotherapy. Ten patients were lost to follow-up.
Results
The median follow-up was 29 months. The complete remission (CR) rate was 85.4%. The overall survival (OS) was 57.4±5.9 months for the Allo-HSCT group and 21.0±2.1 months for the chemotherapy group. The Allo-HSCT group had superior survival compared with the chemotherapy group (5-year OS: 59±17% vs. 13±8%, P<0.01; 5-year disease-free survival [DFS]: 65±10% vs. 40±16%, P>0.05). Multivariate analysis showed that transplantation, platelets >50×109/L at onset, and CR are associated with a better OS in MLL rearranged AML patients. Patients with thrombocytopenia and extramedullary involvement were prone to relapse.
Conclusions
Our results suggest that Allo-HSCT is superior to chemotherapy alone for treating MLL rearranged AML patients. Patients treated with Allo-HSCT have a better prognosis and a longer survival. CR is an independent prognostic factor for OS, and extramedullary involvement is an independent prognostic factor for DFS. MLL rearranged AML patients with thrombocytopenia at onset <50×109 had very bad OS and DFS.
MeSH Keywords: Drug Therapy; Leukemia, Myeloid, Acute; Myeloid-Lymphoid Leukemia Protein; Thrombocytopenia; Transplantation, Homologous
Background
Acute myeloid leukemia (AML) is a malignant clonal disease of the hematopoietic stem cells. Main clinical manifestations of AML include infection, bleeding, anemia, and extramedullary tissue and organ infiltration. AML is usually a rapidly progressive disease and has a high mortality rate [1].
The mixed lineage leukemia (MLL) gene is located on chromosome 11q23, and contains 36 exons, totaling 92 kilobases [2]. MLL gene rearrangements in AML result in unique clinical and molecular genetic characteristics, and generally indicate a poorer prognosis [3]. There are 50–60 genes that are partners of MLL [4]. Therefore, study of the MLL gene in AML has important implications for diagnosis, prognosis, micro-residue monitoring, and choice of treatment options.
Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is one of the better treatment options for curing AML, especially in high-risk AML patients [5]. However, several recent studies showed that Allo-HSCT was not superior to chemotherapy alone for treating AML patients with MLL gene rearrangements. A study in MLL rearranged acute lymphoblastic leukemia (ALL) infants in first complete remission (CR1) undergoing Allo-HSCT showed that the 5-year event-free survival (EFS) was similar to that with chemotherapy alone (48.8% and 48.7%, respectively) [6]. Studies showed that Allo-HSCT did not significantly extend EFS in MLL rearranged ALL infants, or overall survival (OS) and disease-free survival (DFS) in MLL rearranged AML patients [7,8].
Previous reports indicated an uncertain effectiveness of Allo-HSCT treatment on OS in MLL rearranged AML patients. Our hypothesis was that Allo-HSCT was effective in MLL rearranged AML patients. Therefore, the current study aimed to investigate whether Allo-HSCT could improve OS and DFS in MLL rearranged AML patients.
Material and Methods
Patients
A total of 433 AML patients diagnosed between April 2008 and November 2011 were enrolled in this retrospective study performed at the Department of Hematology of the Chinese People’s Liberation Army General Hospital. MLL rearrangements were confirmed in 68 AML patients using the reverse transcription polymerase chain reaction (RT-PCR) method. The positive rate was 15.7% (68/433). Bone marrow mononuclear cells were purified by density centrifugation using a standard Ficoll-Hypaque method. Total RNA was isolated from bone marrow mononuclear cells using TriPure isolation reagent and reverse transcribed to cDNA using a kit from Promega. Each sample was confirmed by individual (split-out) PCR using specific primers for each transcript under identical cycling conditions and by DNA sequencing. Eleven MLL rearrangements were included (MLL-PTD, MLL-AF9, MLL-ELL, MLL-AF10, MLL-AF17, MLL-AF6, MLL-ENL, MLL-AF1Q, MLL-CBP, MLL-AF1P, MLL-AFX1). We divided the MLL rearranged AML patients into a chemotherapy alone group (more than 4 cycles of chemotherapy) and an Allo-HSCT group (Figure 1). Age ranged from 13 to 75 years (median, 47 years). There were 47 male and 21 female patients.
Figure 1.
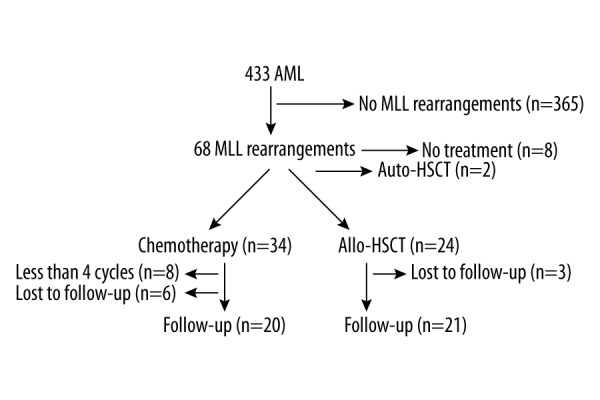
Patients’ flowchart.
Diagnostics and assessment of response to treatment
Bone marrow puncture, immunophenotyping, karyotype analysis, and multiplex nested RT-PCR were performed in all patients [9–12]. Complete remission (CR) was defined as post-chemotherapy bone marrow blasts <5%, absolute neutrophil count >1.0×109/L, and platelet (PLT) count >100×109/L. Relapse was defined as a recurrence of bone marrow blasts >5%, or the development of extramedullary disease infiltrates at any site.
Chemotherapy
Induction chemotherapy consisted of idarubicin (IA, 8–10 mg/m2), daunorubicin (DA, 45 mg/m2), or mitoxantrone (MA, 8 mg/m2 for 3 days), then cytarabine 100 mg/m2 for 7 days. Consolidation therapy consisted of intermediate-dose cytarabine 1–2 g/m2 every 12 h for 3 days. Maintenance treatment included IA, DA, MA, and homoharringtonine (HA, 2 mg/m2) for 7 days, then cytarabine 100 mg/m2 for 7 days and CAG (granulocyte-colony stimulating factor [G-CSF] 300 μg/d subcutaneous injection on days 1–14, cytarabine 10 mg subcutaneous injections every 12 h on days 1–14, and aclacinomycin 10 mg intravenous injection on days 1–10), FLAG (fludarabine 30 mg/m2/d on days 1–5, cytarabine 2 g/m2/d for 5 days, G-CSF 300 μg/d subcutaneous injection on day 1 to neutrophil recovery), and MAE (MA 8 mg/m2 for 3 days, cytarabine 0.25 g every 12 h for 5 days, and etoposide 100 mg/d intravenous injection on days 1–5). The chemotherapy alone group received at least 4 cycles of chemotherapy.
In the Allo-HSCT group after chemotherapy, 9 patients were given the conditioning regimen busulfan/cyclophosphamide (BU/CY) and antithymocyte globulin (ATG) [13]. G-CSF-mobilized peripheral blood stem cells were used as a graft resource. The conditioning regimen was BU/CY and ATG, consisting of cytosine arabinoside (4 g/m2/d intravenous injection) on days −10 and −9; busulfan (0.8 mg/kg intravenous injection) every 6 h on days −8, −7, and −6; semustine (250 mg/m2 intravenous injection) on day −5; cyclophosphamide (60 mg/kg intravenous injection) on days −4 and −3; and thymoglobulin (rabbit ATG [Sangstat-Genzyme] 2.5 mg/kg/d intravenous injection or porcine ATG [Bioproduct Inc.] 20 mg/kg/d) on days −5 to −2. Graft-versus-host disease (GVHD) prophylaxis included cyclosporine A (CsA), mycophenolate mofetil (MMF), and short-term methotrexate (MTX). The dosage of CsA was 2 mg/kg/d intravenous injection from day −7 until bowel function returned to normal, then patients were switched to oral CsA. Every 12 h, 0.5 g of MMF was administered orally from day −1 to day 28 after transplantation. MTX was also given (10 mg intravenous injection on days 1, 3, 6 and 11).
A total of 8 patients had a sibling matching donor. They were given the conditioning regimen BU/CY [14]. This regimen consisted of cytosine arabinoside (2 g/m2/d intravenous injection) on days −10 and −9; busulfan (0.8 mg/kg intravenous injection) every 6 h on days −8, −7, and -6; semustine (250 mg/m2 intravenous injection) on day −5; and cyclophosphamide (60 mg/kg intravenous injection) on days −4 and −3. GVHD prophylaxis included CSA, MMF, and MTX.
Two patients received the conditioning regimen of total body irradiation (TBI) + CY [15]. Two patients received the reduced-intensity conditioning regimen FB [16]. This regimen consisted of fludarabine 30 mg/m2/d on days −8, −7, −6, −5 and −4; cytarabine 2 g/m2/d on days −8 and −7; busulfan (0.8 mg/kg IV) every 6 h on days −6, −5, and −4; and semustine 250 mg/m2 on day −3. GVHD prophylaxis included CSA, MMF, and MTX.
OS and DFS
OS was defined as the time from diagnosis to death or last follow-up. DFS was defined as the time from diagnosis to the date of disease progression. The relapse rate was defined as the number of recurrences divided by the total numbers of treatments. The assessed variables included treatment choice (chemotherapy alone or Allo-HSCT), age (>60 years or not), white blood cell (WBC) count at diagnosis (>10×109/L or not), extramedullary infiltrates, and CR (if one cycle of chemotherapy produced CR or not).
Statistical analysis
The Kaplan-Meier method was used to evaluate survival rate. Differences in survival rate were assessed using the log-rank test. In addition, a Cox regression model was used to identify prognosis variables. P values were two-tailed, and values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, Illinois, USA).
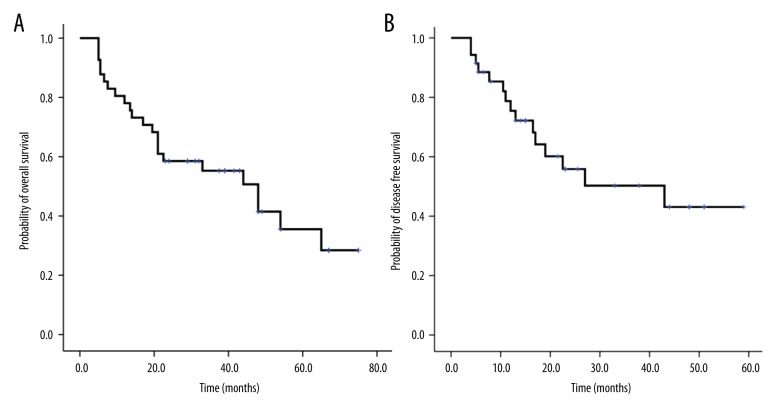
Results
The demographic and baseline characteristics of the two groups of MLL rearranged AML patients are presented in Table 1. Twenty-four patients were treated with Allo-HSCT (3 were lost to follow-up). Twenty-six patients were treated with chemotherapy for at least four cycles (six were lost to follow-up). Of the 41 followed-up patients, median follow-up duration was 29 months. Five-year OS and DFS rates were 35±9% and 54±10%, respectively (Figure 2A, 2B). OS was 57.4±5.9 months for the Allo-HSCT group and 21±2.1 months for the chemotherapy-alone group. Six and thirteen patients died in the Allo-HSCT and chemotherapy alone groups, respectively. The relapse rate in the Allo-HSCT group was significantly lower than that in the chemotherapy alone group (28.6% [6/21] vs. 40% [8/20]; P<0.05).
Table 1.
Demographic and clinical characteristics of MLL rearranged AML patients according to treatments received.
| Characteristic | Chemotherapy group, % | Allo-HCST group, % | P | ||
|---|---|---|---|---|---|
| Number of patients | 20 | 48.8 | 21 | 51.2 | NS |
| Median age (range) | 55 (15–75) | 37 (13–59) | <0.05 | ||
| Gender | |||||
| Female | 8 | 40 | 6 | 28.6 | NS |
| Male | 12 | 60 | 15 | 71.4 | NS |
| FAB type | |||||
| M1 | 0 | 0 | 1 | 4.8 | NS |
| M2 | 10 | 50 | 8 | 38.1 | NS |
| M4 | 4 | 20 | 4 | 14.3 | NS |
| M5 | 6 | 30 | 6 | 28.6 | NS |
| M6 | 0 | 0 | 2 | 9.5 | NS |
| No. of courses to CR | |||||
| 1 | 6 | 30 | 12 | 57.1 | NS |
| >1 | 10 | 50 | 8 | 38.1 | NS |
| No remission | 4 | 20 | 1 | 4.8 | NS |
Figure 2.
A) OS of 41 MLL rearranged AML patients. (B) DFS of 41 MLL rearranged AML patients.
In order to avoid losing important risk factors for OS and DFS, variables with a P value ≤0.10 in univariate analyses were included in the multivariate analysis (Table 2).
Table 2.
Univariate and multivariate analysis of OS and DFS (N=41).
| Parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| OS | ||||||
| Allo-HSCT vs. chemotherapy | 0.216 | 0.085–0.550 | 0.001 | 0.319 | 0.105–0.964 | 0.043 |
| CR or not | 0.358 | 0.131–0.981 | 0.046 | 0.133 | 0.031–0.576 | 0.007 |
| Extramedullary infiltrates or not | 0.820 | 0.234–2.769 | 0.749 | 1.230 | 0.323–4.680 | 0.762 |
| PLT count >50 vs. <50×109/L | 0.221 | 0.073–0.674 | 0.008 | 0.238 | 0.073–0.774 | 0.017 |
| WBC count >10 vs. <10×109/L | 0.883 | 0.372–2.091 | 0.776 | 0.999 | 0.345–2.816 | 0.998 |
| DFS | ||||||
| Allo-HSCT vs. chemotherapy | 0.276 | 0.05–1.525 | 0.14 | 0.478 | 0.134–1.714 | 0.258 |
| CR or not | 1.534 | 0.199–11.84 | 0.618 | 18653.8 | 0.000 | 0.985 |
| Extramedullary infiltrates or not | 4.447 | 1.491–13.26 | 0.007 | 11.353 | 2.302–55.98 | 0.003 |
| PLT count >50 vs. <50×109/L | 0.154 | 0.033–0.714 | 0.017 | 0.094 | 0.016–0.541 | 0.008 |
| WBC count >10 vs. <10×109/L | 0.702 | 0.238–2.066 | 0.520 | 1.431 | 0.337–6.081 | 0.627 |
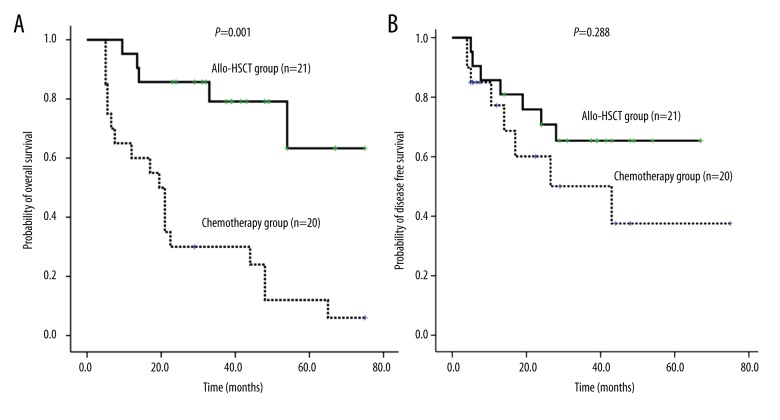
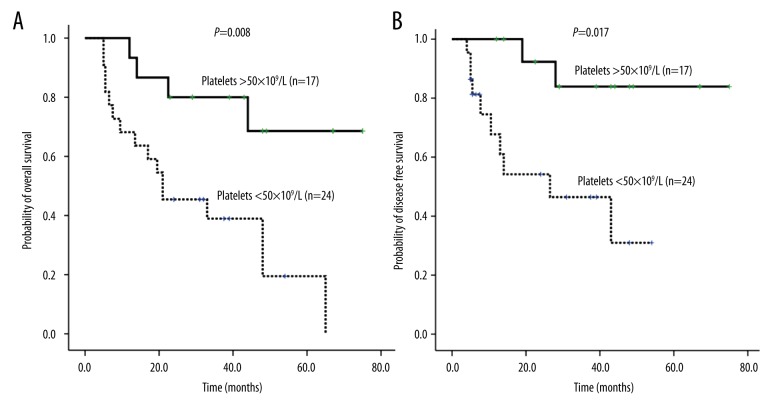
Using univariate analysis, OS for the Allo-HSCT group was significantly higher than that for the chemotherapy alone group (5 years; 59±17% vs. 13±08%, RR=0.216, 95% CI 0.085–0.550, P=0.001; Figure 3A, Table 2). Platelet count at onset (>50×109/L or ≤50×109/L) (RR=0.221, 95% CI: 0.073–0.674; P=0.008, Figure 4A, Table 2) and CR or not (RR=0.358, 95% CI: 0.131–0.981; P=0.046, Figure 5, Table 2) were independent prognostic factors for the OS. Transplantation, platelets >50×109/L, and CR were associated with a better prognosis in MLL rearranged AML patients. Extramedullary involvement and WBC >10×109/L did not affect the OS.
Figure 3.
A) Comparison of 5-year OS between Allo-HSCT and chemotherapy alone groups in MLL rearranged AML patients.
(B) Comparison of 5-year DFS between Allo-HSCT and chemotherapy alone groups in MLL rearranged AML patients.
Figure 4.
A) The number of platelets at onset affected the OS of 41 MLL rearranged AML patients. (B) The number of platelets at onset affected the DFS of 41 MLL rearranged AML patients.
Figure 5.
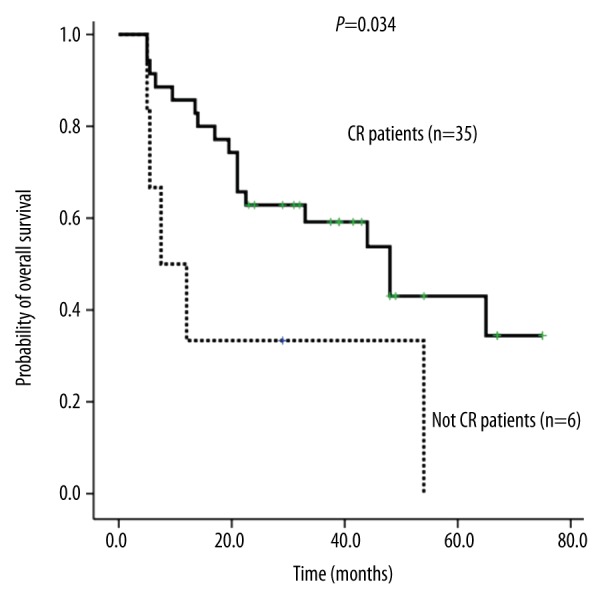
The status of CR affected the OS of 41 MLL rearranged AML patients.
Platelet count at onset (>50×109/L or ≤50×109/L) (RR=0.154, 95% CI: 0.033–0.714; P=0.017, Figure 4B, Table 2) and whether there was extramedullary involvement (RR=4.447, 0.95% CI: 1.491–13.263; P=0.007, Figure 6, Table 2) were independent prognostic factors for DFS. MLL rearranged AML patients with thrombocytopenia and extramedullary involvement were prone to relapse. CR transplantation and WBC >10×109/L did not affect the DFS.
Figure 6.
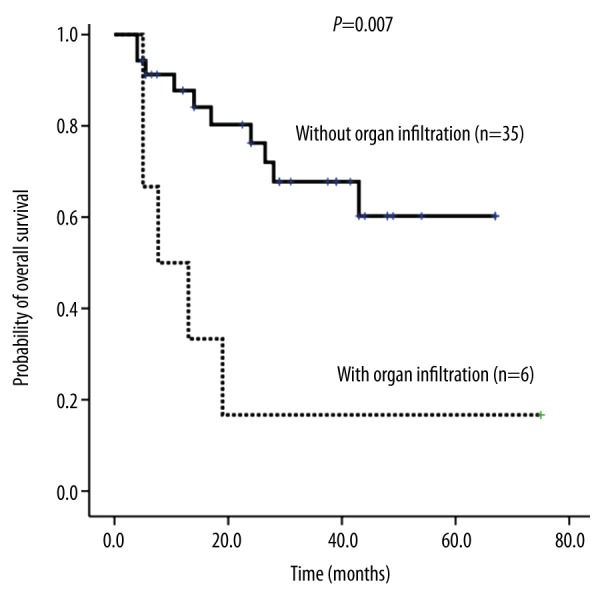
Organ infiltration affected the DFS of 41 MLL rearranged AML patients.
In univariate analysis, patients with MLL subtypes had no significant differences in overall and disease free survival.
Despite the transplantation group demonstrating better OS results in univariate analysis, there was no significant difference in DFS between the Allo-HSCT group and the chemotherapy alone group (5-year DFS: 65±10% vs. 40±16%, respectively, P>0.05; RR=0.276; 95% CI: 0.05–1.525; Figure 3B, Table 2).
Discussion
In this present study, the 5-year OS and DFS for the chemotherapy alone group were similar to those reported in the literature [17]. The 5-year OS and DFS in the Allo-HSCT group were also similar to the rates previously reported [6,18]. However, the CR rate was 85.4%, which was superior to previously reported rates [7,19].
A previous study [7] on 51 11q23 AML patients in CR1 (including 12 who underwent Allo-HSCT, and 39 who underwent two courses of cytarabine consolidation chemotherapy) reported that 1-year DFS and 2-year OS rates were 41.5% and 62.5%, respectively, in the Allo-HSCT group, while these rates were only 20% for the chemotherapy alone group. There was no statistically significant difference between the groups, but the transplantation group had a better prognosis.
Prognostic factors were analyzed in 180 patients with AML who underwent allogeneic stem cell transplantation (Allo-SCT) [8]. The CR rate was 71%, the recurrence rate was 37.8% (68/180), the mortality rate was 9%, and the 4-year OS rate was 29%. Multivariate analysis did not show a clear effect of Allo-SCT on OS. However, in Allo-HSCT patients, the relapse-free survival (RFS) rate was significantly better than that in patients treated with chemotherapy or autologous transplantation.
In our study, Allo-HSCT had survival advantages in terms of OS compared with chemotherapy alone (5-year OS: 59±17% vs. 13±08%, P<0.05). However, multivariate analysis did not show a clear effect of Allo-HSCT on DFS compared with chemotherapy alone. There was no statistically significant difference in DFS, but the Allo-HSCT group had a better prognosis. There may be a relationship with the small number of cases. To verify the result, we need to expand the number of patients.
Thirty-six (7.2%) patients with MLL rearrangements from the 494 patients with acute leukemia were reported in the Daopei Hospital, China. Of the 36 patients, 29 had chromosomal aberrations, and 22 of them had chromosome 11 aberrations. All patients received chemotherapy; the total response rate was 47.2% and the recurrence rate was 40%. The median survival time of the 36 patients was 16 months (range 2–46 months), and the 2-year OS rate was 41.4%. However, the 2-year OS rate of the 9 patients who received HSCT was 87.5%. It was concluded that MLL rearranged acute leukemia patients had a poor response to chemotherapy, a high recurrence rate, and a poor prognosis. Hematopoietic stem cells transplantation may be a reasonable treatment option for improving survival in these patients [19]. In the current study, 68 of 433 (15.7%) AML patients were found to have MLL rearrangements. The CR rate of MLL rearranged AML patients was (35/41) 85.4%. OS was 57.4±5.9 months for the Allo-HSCT group and 21.0±2.1 for the chemotherapy alone group. The positivity of MLL rearrangements, CR rate, and 5-year OS rate were higher than those in previous studies [19].
Hematopoietic stem cell transplantation seemed to be a valuable treatment option for a subgroup of infants with MLL-rearranged acute lymphoblastic leukemia who had additional poor prognostic factors [20]. Results from the Interfant-99 Study showed improved outcomes with HSCT.
Cytogenetic analysis has an important role in the diagnosis and prognosis of leukemia, but conventional methods not only are time-consuming, but also have a low detection rate. Compared with cytogenetic analysis, RT-PCR is sensitive and rapid. In China, Huang et al. used multiplex nested RT-PCR and karyotype analysis techniques in combination to improve the detection rate of clonal chromosomal AML patients [21].
The MLL rearrangements with 11q23 chromosomal abnormalities showed poor leukemia prognosis [22–24]. In our study, a total of 40 patients underwent combined karyotype analysis. Karyotype abnormalities of chromosome structures were detected in 28 patients (70%), and we only detected as few as 5 cases of chromosome 11q23 abnormalities.
MLL rearrangements in the M5 subtype have been reported to have a higher incidence than other types in China [25]. In addition, it has been reported that MLL rearrangements are likely to occur in treatment-related leukemia, such as after use of agents such as etoposide, epirubicin, and cyclophosphamide in subtype M4 or M5 leukemia [26,27]. In our study, MLL gene rearrangements of the M5 subtype occurred in the largest number of cases (30.9%, 21/68). Treatment-related leukemia prognosis was poor when considering MLL rearrangements.
Of 988 AML patients, 114 (11.54%) were found to be positive for MLL rearrangements using the RT-PCR method [28]. Another study showed that the incidence of MLL rearrangements in AML patients was 14.3% (31/217) [29], while the incidence of MLL rearrangements was 15.7% (68/433) in our study.
Extramedullary infiltrates appeared in 25.1% of children with AML [30]. The most common extramedullary infiltrated organs were the skin, soft tissues or bones, gums, and central nervous system. Extramedullary leukemia (EML) patients had a high initial WBC count and a high proportion of M4/M5 morphological variants. Extramedullary infiltrates at diagnosis had no prognostic significance in children with AML. Extramedullary infiltrates were reported in 30.5% of AML adult patients [31]. Patients with EML had a high initial WBC count and a high proportion of M4/M5 morphological variants. 11q23 abnormalities were associated with EML (P=0.002), especially with lymphadenopathy (P=0.011) and gingival hyperplasia (P=0.0016). EML at diagnosis was associated with CD56 expression by leukemic blasts, 11q23 karyotypic abnormalities, low CR rate, and poor OS. The incidence of granulocytic sarcoma was significantly different between children (3.8%) and adults (0.8%) in 11q23-positive AML patients [32].
In the current study there were six EML patients: three had the M4 subtype, two had the M2 subtype, and one had the M5 subtype. Two patients had MLL-PTD, two had MLL-AF10, one had MLL-AF9, and one had MLL-AF17. In multivariate analysis, the presence of extramedullary infiltrates was a highly hazardous factor for DFS. In our study, six AML patients had extramedullary infiltrates: two had skin infiltration, two had lymph node infiltration, one had orbital and breast infiltration, and one had multiple bone and liver infiltration. The rate of extramedullary infiltrates in AML patients with MLL gene rearrangements was 8.8% (6/68).
The latest reports from Peking University People’s Hospital on the role of allo-HSCT in the treatment of AML patients were published in 2014 [33]. From October 2007 to 2012, a retrospective cohort study was conducted on whether transplantation improved DFS and OS in MLL rearranged AML patients. A total of 56 patients with MLL rearranged AML patients underwent Allo-HSCT. The 3-year recurrence rate was 25.3%. The CR1 patients had a lower recurrence rate (17.9% vs. 48.1%, P=0.03). Allo-HSCT is an effective treatment for MLL rearranged AML patients. In our study, the recurrence rate in the Allo-HSCT group (6/21) was 28.6%. In the Allo-HSCT group, 5-year OS and DFS were 59±17% and 40±16%, respectively.
Ferra et al. reported that not reaching CR is a risk factor for OS [34]. It is also a risk factor for recurrence of disease. In our study, multiple- and single-factor analysis showed that CR is an independent prognostic factor for OS (P<0.05).
Bhatnagar et al. [35] applied TBI (1200 cGy) and MEL (100–110 mg/m2) in peripheral blood hematopoietic stem cell transplantation (PBSCT) for treatment of relapsed or refractory AML (n=14), ALL (n=10), non-Hodgkin lymphoma (NHL) (n=18), or other malignant disease (n=6). The median age was 48 years (range: 22–68 years). All patients received tacrolimus and methotrexate (MTX) for prevention of GVHD. The median absolute neutrophil count (ANC) recovery time was 12 days. A total of 44 patients could be evaluated: 28 (64%) reached CR and 7 (15%) reached PR. The median follow-up was 30 months (4~124 months) on the survival of patients, the 1- and 5-year recurrence rates were 45% and 22.5%, respectively. Multivariate analysis showed that a pre-transplant platelet count <80×109/L and LDH >500 IU/L were the risk factors of RFS; age <53 years old and CR were independent prognostic factors of OS. The conclusion was that MLL rearranged AML patients with thrombocytopenia at onset <50×109/L had a very bad OS and DFS. Reaching CR resulted in a better prognosis in MLL rearranged AML patients.
We also observed that Allo-HSCT provided a survival advantage to OS compared with chemotherapy alone. The median age of patients in the Allo-HSCT group was younger than that in the chemotherapy alone group, but multivariate analysis showed that the age has no effect on outcomes. A previous study suggested that outcomes of transplantation were not associated with patient age [36]. Others investigators suggested that age was associated with poor transplantation outcomes [37,38]
The main limitation of the present study is the small number of patients. Our results will need to be confirmed in a larger sample.
Conclusions
Our study suggested that Allo-HSCT provided a significant long-term survival advantage for MLL rearranged AML patients, especially in OS. In addition, whether MLL rearranged AML patients reach CR or not is an independent prognostic factor. Not reaching CR indicates a very poor prognosis. We first reported that platelet count is an independent prognostic factor for OS and DFS. MLL rearranged AML patients with a platelet count <50×109/L before treatment had a very short OS, and very high mortality and recurrence rates. We observed that MLL rearranged AML patients with extramedullary infiltrates can easily relapse and that Allo-HSCT is superior to chemotherapy alone for treating MLL rearranged AML patients to get a better prognosis and a longer survival. The findings of this study should be valuable for providing guidance for the treatment and prognostic analysis of MLL rearranged AML patients. It has very important clinical value.
Acknowledgments
The authors thank all of the physicians, nurses, and laboratory colleagues for their work for this study.
Footnotes
Source of support: Capital Medical Development Scientific Research Fund (SF2001-5001-07), grants from the national Nature Science Foundation of China (No. 81400135), and China Postdoctoral Foundation (Grant No. 2015M57275)
References
- 1.Huang XJ. Haematology. Beijing: People’s Medical Publishing House; 2009. pp. 84–85. [Google Scholar]
- 2.Tamai H, Inokuchi K. 11q23/MLL acute leukemia: update of clinical aspects. J Clin Exp Hematop. 2010;50:91–98. doi: 10.3960/jslrt.50.91. [DOI] [PubMed] [Google Scholar]
- 3.Meyer C, Kowarz E, Hofmann J, et al. New insights to the MLL recombinome of acute leukemias. Leukemia. 2009;23:1490–99. doi: 10.1038/leu.2009.33. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Benito M, Loayza-Puch F, Oude Vrielink JA, et al. 3′UTR-mediated gene silencing of the Mixed Lineage Leukemia (MLL) gene. PLoS One. 2011;6:e25449. doi: 10.1371/journal.pone.0025449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang XJ, Zhu HH, Chang YJ, et al. The superiority of haploidentical related stem cell transplantation over chemotherapy alone as postremission treatment for patients with intermediate- or high-risk acute myeloid leukemia in first complete remission. Blood. 2012;119:5584–90. doi: 10.1182/blood-2011-11-389809. [DOI] [PubMed] [Google Scholar]
- 6.Dreyer ZE, Dinndorf PA, Camitta B, et al. Analysis of the role of hematopoietic stem-cell transplantation in infants with acute lymphoblastic leukemia in first remission and MLL gene rearrangements: a report from the Children’s Oncology Group. J Clin Oncol. 2011;29:214–22. doi: 10.1200/JCO.2009.26.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamai H, Yamaguchi H, Hamaguchi H, et al. Clinical features of adult acute leukemia with 11q23 abnormalities in Japan: A co-operative multicenter study. Int J Hematol. 2008;87:195–202. doi: 10.1007/s12185-008-0034-2. [DOI] [PubMed] [Google Scholar]
- 8.Krauter J, Wagner K, Schafer I, et al. Prognostic factors in adult patients up to 60 years old with acute myeloid leukemia and translocations of chromosome band 11q23: Individual patient data-based meta-analysis of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. 2009;27:3000–6. doi: 10.1200/JCO.2008.16.7981. [DOI] [PubMed] [Google Scholar]
- 9.Bacher U, Haferlach C, Schnittger S, et al. Diagnostics of acute leukemias: Interaction of phenotypic and genetic methods. Pathologe. 2012;33:528–38. doi: 10.1007/s00292-012-1653-1. [DOI] [PubMed] [Google Scholar]
- 10.Pallisgaard N, Hokland P, Riishoj DC, et al. Multiplex reverse transcription-polymerase chain reaction for simultaneous screening of 29 translocations and chromosomal aberrations in acute leukemia. Blood. 1998;92:574–88. [PubMed] [Google Scholar]
- 11.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–83. [PubMed] [Google Scholar]
- 12.Cox MC, Panetta P, Venditti A, et al. Fluorescence in situ hybridization and conventional cytogenetics for the diagnosis of 11q23+/MLL+ translocation in leukaemia. Br J Haematol. 2003;121:953–55. doi: 10.1046/j.1365-2141.2003.04382.x. [DOI] [PubMed] [Google Scholar]
- 13.Huang XJ, Liu DH, Liu KY, et al. Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15:257–65. doi: 10.1016/j.bbmt.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347–53. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 15.Thomas ED, Buckner CD, Banaji M, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49:511–33. [PubMed] [Google Scholar]
- 16.Platzbecker U, Thiede C, Fussel M, et al. Reduced intensity conditioning allows for up-front allogeneic hematopoietic stem cell transplantation after cytoreductive induction therapy in newly-diagnosed high-risk acute myeloid leukemia. Leukemia. 2006;20:707–14. doi: 10.1038/sj.leu.2404143. [DOI] [PubMed] [Google Scholar]
- 17.Dohner K, Tobis K, Ulrich R, et al. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: A study of the Acute Myeloid Leukemia Study Group Ulm. J Clin Oncol. 2002;20:3254–61. doi: 10.1200/JCO.2002.09.088. [DOI] [PubMed] [Google Scholar]
- 18.Garrido SM, Bryant E, Appelbaum FR. Allogeneic stem cell transplantation for relapsed and refractory acute myeloid leukemia patients with 11q23 abnormalities. Leuk Res. 2000;24:481–86. doi: 10.1016/s0145-2126(00)00011-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Yin YM, Zhao YL, et al. [Clinical and molecular biologic characteristics of 36 cases of leukemia with 11q23/mll]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18:1381–85. [in Chinese] [PubMed] [Google Scholar]
- 20.Mann G, Attarbaschi A, Schrappe M, et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukemia: Results from the Interfant-99 Study. Blood. 2010;116:2644–50. doi: 10.1182/blood-2010-03-273532. [DOI] [PubMed] [Google Scholar]
- 21.Huang L, Li CR, Zhang H, et al. Combined application of multiplex reverse transcription-polymerase chain reaction and karyotype analysis to detection of clonal chromosomal aberrations in acute myeloid leukemia. Ai Zheng. 2007;26:1029–33. [PubMed] [Google Scholar]
- 22.Shen Y, Zhu YM, Fan X, et al. Gene mutation patterns and their prognostic impact in a cohort of 1185 patients with acute myeloid leukemia. Blood. 2011;118:5593–603. doi: 10.1182/blood-2011-03-343988. [DOI] [PubMed] [Google Scholar]
- 23.Ilencikova D, Sykora J, Mikulasova Z, et al. Identification of molecular markers in children with acute myeloid leukemia (AML) Klin Onkol. 2012;25:26–35. [PubMed] [Google Scholar]
- 24.Xu LL, Liu XL, Du QF, et al. [Multiprobe fluorescence in situ hybridization panel in detection of the common cytogenetic abnormalities of acute myeloid leukemia]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2011;27:324–26. [in Chinese] [PubMed] [Google Scholar]
- 25.Zhao JF WL, Zhang XL. [The relative research of acute monocytic leukemia and MLL rearrangements]. Shan Dong Yi Yao. 2011;51:85–86. [in Chinese] [Google Scholar]
- 26.Bielorai B, Meyer C, Trakhtenbrot L, et al. Therapy-related acute myeloid leukemia with t(2;11)(q37;q23) after treatment for osteosarcoma. Cancer Genet Cytogenet. 2010;203:288–91. doi: 10.1016/j.cancergencyto.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Chin LK, Cheah CY, Michael PM, et al. 11q23 rearrangement and duplication of MLLT1-MLL gene fusion in therapy-related acute myeloid leukemia. Leuk Lymphoma. 2012;53:2066–68. doi: 10.3109/10428194.2012.666663. [DOI] [PubMed] [Google Scholar]
- 28.Shih LY, Liang DC, Fu JF, et al. Characterization of fusion partner genes in 114 patients with de novo acute myeloid leukemia and MLL rearrangement. Leukemia. 2006;20:218–23. doi: 10.1038/sj.leu.2404024. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim S, Estey EH, Pierce S, et al. 11q23 abnormalities in patients with acute myelogenous leukemia and myelodysplastic syndrome as detected by molecular and cytogenetic analyses. Am J Clin Pathol. 2000;114:793–97. doi: 10.1309/XY44-L8TE-PWU5-62MP. [DOI] [PubMed] [Google Scholar]
- 30.Bisschop MM, Revesz T, Bierings M, et al. Extramedullary infiltrates at diagnosis have no prognostic significance in children with acute myeloid leukaemia. Leukemia. 2001;15:46–49. doi: 10.1038/sj.leu.2401971. [DOI] [PubMed] [Google Scholar]
- 31.Chang H, Brandwein J, Yi QL, et al. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk Res. 2004;28:1007–11. doi: 10.1016/j.leukres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Johansson B, Fioretos T, Kullendorff CM, et al. Granulocytic sarcomas in body cavities in childhood acute myeloid leukemias with 11q23/MLL rearrangements. Genes Chromosomes Cancer. 2000;27:136–42. [PubMed] [Google Scholar]
- 33.Wang Y, Liu QF, Qin YZ, et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of patients with mixed-lineage-leukemia-rearranged acute leukemia: Results from a prospective, multi-center study. Am J Hematol. 2014;89:130–36. doi: 10.1002/ajh.23595. [DOI] [PubMed] [Google Scholar]
- 34.Ferra C, Sanz J, de la Camara R, et al. Unrelated transplantation for poor-prognosis adult acute lymphoblastic leukemia: Long-term outcome analysis and study of the impact of hematopoietic graft source. Biol Blood Marrow Transplant. 2010;16:957–66. doi: 10.1016/j.bbmt.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Bhatnagar B, Rapoport AP, Fang HB, et al. Single center experience with total body irradiation and melphalan (TBI-MEL) myeloablative conditioning regimen for allogeneic stem cell transplantation (SCT) in patients with refractory hematologic malignancies. Ann Hematol. 2014;93:653–60. doi: 10.1007/s00277-013-1908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong L, Wu T, Gao ZY, et al. The outcomes of family haploidentical hematopoietic stem cell transplantation in hematologic malignancies are not associated with patient age. Biol Blood Marrow Transplant. 2011;17:1205–13. doi: 10.1016/j.bbmt.2010.12.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallen H, Gooley TA, Deeg HJ, et al. Ablative allogeneic hematopoietic cell transplantation in adults 60 years of age and older. J Clin Oncol. 2005;23:3439–46. doi: 10.1200/JCO.2005.05.694. [DOI] [PubMed] [Google Scholar]
- 38.Paun O, Lazarus HM. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first complete remission: Have the indications changed? Curr Opin Hematol. 2012;19:95–101. doi: 10.1097/MOH.0b013e32834ff54b. [DOI] [PubMed] [Google Scholar]