Abstract
Background
Syncytin-1, a cell membrane-localizing fusogen, is abnormally expressed in several cancers, including endometrial cancer, breast cancer, and leukemia. Although abnormal syncytin-1 expression has been detected in two-thirds of leukemia blood samples, its expression profile in acute leukemia patients has not yet been analyzed.
Material/Methods
Bone marrow samples from 50 acute myelogenous leukemia (AML) cases and 14 B-cell acute lymphocytic leukemia (B-cell ALL) patients were subjected to flow cytometry to assess leukocyte type distributions and leukocytic syncytin-1 surface expression. RT-PCR was applied to assess leukocytic syncytin-1 mRNA expression. Statistical analysis was applied to compare syncytin-1 expression between AML and B-cell ALL patients across blasts, granulocytes, lymphocytes, and monocytes as well as to determine clinical factors statistically associated with changes in syncytin-1 expression.
Results
The leukocyte type distributions of the AML and B-cell ALL cohorts highly overlapped, with an observable difference in blast distribution between the 2 cohorts. The AML cohort displayed significantly greater syncytin-1 surface and mRNA expression (p<0.05). Syncytin-1 surface and mRNA expression was significantly increased across all 4 leukocyte types (p<0.05). The percentage of syncytin-1-expressing blasts was significantly greater in AML patients (p<0.05), with blasts showing the largest fold-change in syncytin-1 expression (p<0.05). M5, M5a, and M5b AML patients displayed significantly higher syncytin-1 surface expression relative to all other AML French-American-British (FAB) classifications (p<0.05).
Conclusions
These findings suggest leukocytic syncytin-1 expression may play a role in the development and/or maintenance of the AML phenotype and the acute monocytic leukemia phenotype in particular.
MeSH Keywords: Leukemia, Biphenotypic, Acute; Leukemia, Experimental; Leukemia, Myeloid, Acute
Background
Acute leukemias account for approximately 20 000 diagnoses and 10 000 fatalities in the United States annually [1]. According to the International Classification of Diseases for Oncology (Third Edition, ICD-O-3), acute lymphocytic leukemia (ALL, with B-cell and T-cell subtypes) and acute myelogenous leukemia (AML) are the 2 most common variants of acute leukemia [1]. The pathogenesis of these acute leukemias is a complex process, as inherited genetic factors, viral infection, and radiation exposure have all been associated with the onset of acute leukemias [2–4]. With respect to viral infection, no specific virus has been conclusively shown to be a direct cause of acute leukemia [5]. Rather, acute leukemias are more likely to be the result of a dysregulated immune response to common viral infections (e.g., influenza) in certain susceptible patients [5].
Human endogenous retroviruses (HERVs) are believed to be modern remnants of archaic retroviral infections in ancestral germ-line cells [6]. HERV-W is one such HERV with multiple copies present in the human genome that is still capable of being translated into functional proteins [6]. The 538-amino acid residue envelope glycoprotein of HERV-W, termed syncytin-1, is still expressed in modern humans [7]. Previous functional studies have shown that syncytin-1 localizes to the cell membrane and effectively functions as a fusogen, as it induces cell-cell fusion across various cell lines in a receptor-dependent manner [8,9]. Although syncytin-1 has been shown to be expressed in human reproductive tissues, including the endometrium, ovary, and testis, as well as fusing myoblasts [9,10], syncytin-1 is most notably expressed in placental tissue, where it plays a key role in the fusion of single-nucleated trophoblasts to form the placental syncytium [9,11]. The syncytin-1 protein possesses a conserved immunosuppressive domain (amino acid residues 373–397) [12], suggesting that syncytin-1 is involved in suppressing maternal immune responses against the developing fetus.
Similar to placental tissue, exosomes are also produced by lymphocytes, macrophages, and immature dendritic cells, and there are active exchanges of exosomes among these cells during immune responses [9,13]. Moreover, syncytin-1 has been found to be improperly expressed in several human cancers, including endometrial cancer, breast cancer, and leukemia [6]. With respect to leukemia, Sun et al. found abnormal syncytin-1 mRNA and protein expression in more than two-thirds of leukemia blood samples investigated, but neither syncytin-1 transcripts nor protein was detected in blood samples from healthy donors [6]. More recently, Maliniemi et al. detected syncytin-1 expression in malignant lymphocytes in half of mycosis fungoides cases under study, but found no syncytin-1 expression in inflammatory dermatosis (lichen ruber planus) with skin-homing, non-malignant T-lymphocytes [14].
These combined findings suggest that syncytin-1 may play a role in leukemia. However, syncytin-1’s expression profile in acute leukemia patients has not yet been analyzed. Therefore, this study aimed to comparatively assess leukocytic syncytin-1 expression in AML and B-cell ALL patients.
Material and Methods
Ethics statement
This study was approved by the Ethics Committee (IRB) of the First People’s Hospital of Yunnan Province (Kunming, China). All subjects recruited for this study provided written informed consent prior to participation.
Patient selection, sample collection, and leukemia diagnosis
A total of 64 acute leukemia patients were consecutively recruited for this prospective study from the Hematology Clinic at the First People’s Hospital of Yunnan Province. No patient was undergoing treatment for their leukemia at the time of recruitment. From each patient, a bone marrow sample (~0.5 ml) was extracted from the iliac or the anterior superior iliac spine, and a non-fasting peripheral blood sample (2 ml) was drawn into EDTA-K2 anti-coagulated tubes and stored at room temperature. Using these bone marrow and peripheral blood samples, diagnoses of acute leukemia cases were conducted according to the World Health Organization (WHO) guidelines applying a 20% blast threshold [6,15], and the morphological classification of blast cells was performed according to the French-American-British (FAB) criteria [6,16]. The percentage of blasts for each patient was derived from analysis of bone marrow morphology.
Flow cytometric gating
Briefly, EDTA-anticoagulated bone marrow aspirates were stained for flow cytometry within 24 hours of specimen collection. Multiple-color flow cytometry was performed using a FACS Canto II flow cytometer (BD Biosciences, San Jose, CA, USA). Amplification and compensation were performed according to standard procedures. Additional details regarding the flow cytometry instrumentation and analysis are provided in Supplementary Table 1.
First, flow cytometric gating to determine syncytin-1 surface expression in each leukocyte subset (i.e., blasts, granulocytes, lymphocytes, and monocytes) was performed with 2 test tubes: a negative control and an isotype control. For the negative control, 10 μl phycoerythrin-Cy5-conjugated anti-human CD45 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added to PBS-diluted bone marrow cells (100 μl of 4–10×109 cells/l) and incubated in the dark for 15–20 min. A hemolytic agent was added to the sample until the liquid cleared and washed with PBS prior to flow cytometry. For the isotype control, 10 μl phycoerythrin-Cy5-conjugated anti-human CD45 and 10 μl monoclonal anti-syncytin-1 (Santa Cruz Biotechnology) was added to PBS-diluted bone marrow cells (100 μl of 4–10×109 cells/l) and incubated in the dark for 15–20 min. A hemolytic agent was added to the sample until the liquid cleared and washed with PBS. Then, 10 μl FITC-labeled goat anti-rabbit IgG (Kirkegaard and Perry Laboratories (KPL), Gaithersburg, MD, USA) was added for incubation in the dark for 15–20 min. The sample was then washed with PBS prior to flow cytometry. At least 10 000 cell events were collected for all samples. An orthogonal side-scatter (SS) versus CD45 plot was used to segregate 4 lymphocytic subsets: blasts, granulocytes, lymphocytes, and monocytes. Applying the isotype controls, voltage, and compensation, the flow cytometer was configured so the leukocyte subsets were appropriately positioned on the dot plots. The mean fluorescence intensities (MFIs) for syncytin-1 in each leukocyte subset were calculated from the leukocyte distribution pattern in the SS versus CD45 plot. The raw flow cytometry images are provided in Supplementary Figure 1.
RT-PCR for Syncytin-1 mRNA Expression
To assess syncitin-1 mRNA expression in the leukocyte subsets, the 4 subsets were isolated through the SSC versus CD45 plot with a FACSCanto II flow cytometer (BD Biosciences), and reverse transcription polymerase chain reaction (RT-PCR) was then conducted as previously described by Ferrer et al. with minor modifications [17]. Briefly, TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA) was applied to each isolated leukocyte subset, and total RNA was extracted with an RNeasy Mini Kit (Qiagen, Beijing, China). RNA quality was assessed through 1% agarose gel electrophoresis. RNA concentration was determined by 260-nm photoabsorption. Genomic DNA remnants were removed from the RNA samples using a buffered DNase I (#EN0521, Fermentas Life Sciences, Hanover, MD, USA). Then, in an ABI 7000 (Applied Biosystems, USA), real-time RT-PCR was performed using a One-Step SYBR RT-PCR Kit according to the kit’s instruction (TaKaRa Biotechnology Co., Ltd., Dalian, China). Three reactions were run in parallel for each sample: (i) a RT-PCR reaction with 0.75 μg DNase I-treated RNA template + reverse transcriptase, (ii) a control lacking the RNA template, and (iii) a control lacking reverse transcriptase. The syncytin-1 (gi.21326140) and GADPH (gi.182860) primers used for RT-PCR amplification have been described previously with the expected PCR products of 494 bp (syncytin-1) and 358 bp (GADPH) [6,18]. The RT-PCR reaction was run in the following sequence: 42°C for 15 min, then 95°C for 2 min followed by 35 cycles of 95°C for 5 s and 60°C for 34 s. Dissociation curves and agarose gel electrophoresis were used to analyze RT-PCR amplification. Expression of syncytin-1 mRNA transcripts (relative to the housekeeping gene GAPDH) were calculated by the 2−ΔC′T method as previously described by Livak and Schmittgen [6,19]. GAPDH was selected as a housekeeping gene, as it has been shown to be an optimal control for human leukocyte studies and displays constant expression in human leukocyte samples [20].
Statistical analysis
The statistical analysis was performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Results were reported as means with associated standard deviations (SDs). The Wilcoxon rank-sum test was applied for pairwise comparisons, while the Kruskal-Wallis test was applied for comparisons across multiple groups. Spearman’s correlation was applied for correlation analyses.
Results
The clinical characteristics of the 50 AML patients and the 14 B-cell ALL patients are detailed in Tables 1 and 2, respectively. There were 40 males (62.5%) and 24 females (37.5%), with a median age of 37 years (range: 4–78 years). The median WBC count was 14.9×109/l (range: 0.96–293.7×109/l), and the median percentage of blasts was 72.4% (range: 0.41–98.5%).
Table 1.
Clinical characteristics of AML patients (n=50).
| Leukemia diagnosis | Age (yrs) | Sex | WBC count (109/l) | Blast percentage in bone marrow (%) | Syncytin-1 mRNA expression (syncytin-1/GAPDH, means ±SD) |
|---|---|---|---|---|---|
| M0 AML | 4 | M | 45.9 | 39 | 13.3±6.9 |
| M1 AML | 49 | M | 3.19 | 3 | 3.7±1.2 |
| M2 AML | 51 | M | 10.4 | 52 | 33.7±6.5 |
| M2 AML | 52 | M | 20.2 | 0.8 | 21.6±3.3 |
| M2 AML | 17 | F | 11.5 | 24.5 | 17.4±6.4 |
| M2 AML | 64 | F | 9.78 | 10.6 | 11.4±7.7 |
| M2 AML | 43 | M | 11.2 | 18 | 11.8±2.7 |
| M2 AML | 54 | M | 6.12 | 45 | 1.11±2.5 |
| M3 AML | 23 | F | 18 | 93.5 | 27.7±10.9 |
| M3 AML | 30 | M | 45.8 | 96 | 0.6±0.2 |
| M3 AML | 72 | M | 0.96 | 65 | 27.3±12.9 |
| M3 AML | 78 | F | 124 | 93.5 | 2.3±0.4 |
| M3 AML | 46 | F | 1.6 | 45 | 29.8±7.4 |
| M3 AML | 33 | M | 20.2 | 80.4 | 37.8±5.5 |
| M3 AML | 35 | F | 28.1 | 95 | 15.7±7.8 |
| M3 AML | 18 | F | 1.17 | 76.5 | 22±4.7 |
| M3 AML | 37 | M | 10.4 | 52 | 22.1±8.2 |
| M3 AML | 78 | M | 23.5 | 94 | 16.1±7.9 |
| M3 AML | 35 | F | 11 | 3 | 15.1±4.9 |
| M3 AML | 32 | M | 82.2 | 77 | 15.8±8.3 |
| M3 AML | 17 | M | 11.9 | 72.7 | 39.6±10.6 |
| M3 AML | 56 | M | 33.5 | 89.5 | 29.4±5.6 |
| M4 AML | 37 | M | 4.33 | 39.5 | 4.2±2.7 |
| M4 AML | 53 | M | 1.75 | 66.5 | 76.3±0.9 |
| M4 AML | 48 | F | 22.3 | 72 | 7±2.7 |
| M4 AML | 37 | F | 2.73 | 59.5 | 83.7±24.7 |
| M4 AML | 12 | F | 2.02 | 64.3 | 27±11.0 |
| M4 AML | 25 | M | 292 | 84 | 2.5±0.8 |
| M4 AML | 39 | F | 17.3 | 30.12 | 21.1±6.4 |
| M4 AML | 42 | M | 29.8 | 76.5 | 7.9±0.9 |
| M4 AML | 25 | M | 7.86 | 34 | 0.37±0.2 |
| M5 AML | 48 | M | 4.81 | 96.7 | 66.3±9.8 |
| M5 AML | 16 | M | 43.7 | 91.5 | 82.2±13.5 |
| M5 AML | 13 | F | 86.6 | 91.5 | 2.4±0.6 |
| M5 AML | 54 | F | 18.5 | 68 | 63.7±12.9 |
| M5 AML | 61 | F | 23.7 | 89 | 16.9±2.3 |
| M5 AML | 59 | M | 3.44 | 93.5 | 18.8±6.4 |
| M5a AML | 46 | M | 75.7 | 54.2 | 77.8±15.5 |
| M5a AML | 44 | F | 28.1 | 27.8 | 83.6±12.8 |
| M5a AML | 29 | F | 18.5 | 45 | 53.7±16.6 |
| M5b AML | 36 | M | 47.9 | 94.5 | 81.2±10.5 |
| M5b AML | 58 | F | 95.9 | 81 | 76.8±20.3 |
| M5b AML | 18 | M | 163 | 85 | 87±12.9 |
| M5b AML | 37 | F | 25.6 | 89.5 | 34.6±6.6 |
| M5b AML | 72 | M | 148 | 87.5 | 37.6±13.7 |
| M6 AML | 28 | M | 27.5 | 51.8 | 3.8±1.3 |
| CML→AML | 20 | M | 1.71 | 34 | 1±0.7 |
| CML→AML | 29 | M | 25.5 | 20.5 | 3.7±1.7 |
| CML→AML | 27 | M | 2.54 | 57.5 | 5.87±1.3 |
| AML (NOS) | 72 | M | 2.83 | 3.5 | 12.3±2.8 |
Table 2.
Clinical characteristics of B-Cell ALL patients (n=14).
| Leukemia diagnosis | Age (yrs) | Sex | WBC count (109/l) | Blast percentage in bone marrow (%) | Syncytin-1 mRNA expression (syncytin-1/GAPDH, means ±SD) |
|---|---|---|---|---|---|
| B-cell ALL | 12 | M | 5.5 | 9.5 | 9.3±0.2 |
| B-cell ALL | 22 | F | 2.16 | 98.5 | 0.5±0.3 |
| B-cell ALL | 67 | M | 5.75 | 74 | 4±1.9 |
| B-cell ALL | 22 | M | 9.73 | 68.5 | 3.7±0.4 |
| B-cell ALL | 23 | M | 4.8 | 92.5 | 19.1±8.1 |
| B-cell ALL | 18 | M | 2.36 | 75 | 10±2.8 |
| B-cell ALL | 6 | F | 4.38 | 84.5 | 11.2±0.8 |
| B-cell ALL | 17 | F | 4.5 | 0.41 | 0.17±0.1 |
| B-cell ALL | 50 | M | 42.7 | 96 | 0.32±0.2 |
| B-cell ALL | 25 | M | 4.54 | 83.5 | 0.99±0.4 |
| CML→B-cell ALL | 46 | M | 51.5 | 89.5 | 1.2±0.5 |
| CML→B-cell ALL | 39 | F | 16.8 | 80.5 | 3.7±0.5 |
| CML→B-cell ALL | 41 | M | 13 | 74.5 | 3.1±0.6 |
| CML→B-cell ALL | 46 | F | 293.7 | 67.8 | 21.4±9.2 |
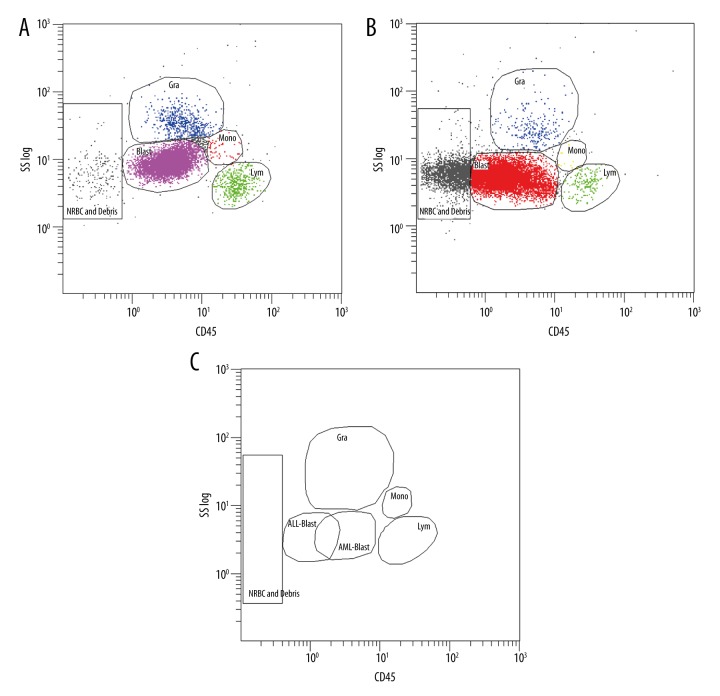
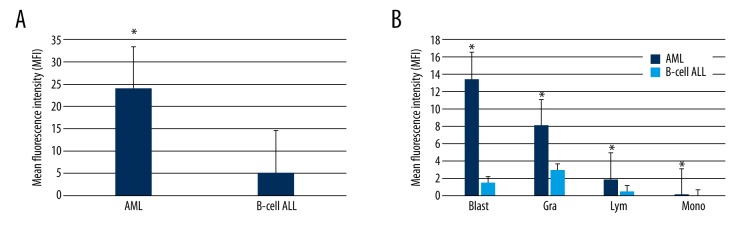
Using flow cytometry, we first assessed leukocyte type distributions in the AML and B-cell ALL cohorts (Figure 1A, 1B). The leukocyte distributions of the AML and B-cell ALL cohorts highly overlapped, but there was an observable difference in blast distribution between the 2 cohorts (Figure 1C). Next, we compared leukocytic syncytin-1 surface expression in the AML and B-cell ALL cohorts and found that the AML cohort displayed significantly greater leukocytic syncytin-1 surface expression relative to the B-cell ALL cohort (p<0.05, Figure 2A). To further investigate this difference, we then analyzed leukocytic syncytin-1 surface expression in the AML and B-cell ALL cohorts by leukocyte type (i.e., blasts, granulocytes, lymphocytes, and monocytes) (Figure 2B). We found that leukocytic syncytin-1 surface expression was significantly increased in all 4 leukocyte types, with blasts contributing the largest proportion of syncytin-1 surface expression and showing the largest fold-change in syncytin-1 surface expression (p<0.05, Figure 2B). To determine whether the difference in leukocytic syncytin-1 surface expression between the AML and B-cell ALL cohorts was a result of higher absolute levels of syncytin-1-expressing leukocytes and/or higher syncytin-1 surface expression per leukocyte, we next compared the percentages of syncytin-1-expressing blasts, granulocytes, lymphocytes, and monocytes between the AML and B-cell ALL cohorts (Table 3). We found that the percentage of syncytin-1-expressing blasts in AML patients was significantly greater than that in B-cell ALL patients (p<0.05; Table 3). However, the percentages of syncytin-1-expressing granulocytes, syncytin-1-expressing lymphocytes, and syncytin-1-expressing monocytes were not significantly different between these 2 cohorts (p>0.05; Table 3). These findings indicate that the higher leukocytic syncytin-1 surface expression in AML patients relative to B-cell ALL patients is a result of an increased syncytin-1-expressing blast cell percentage in combination with upregulated syncytin-1 surface expression on all 4 leukocyte cell types (i.e., blasts, granulocytes, lymphocytes, and monocytes).
Figure 1.
Leukocyte type distributions in AML and B-cell ALL cohorts. Flow cytometry was applied to segregate and analyze leukocyte cell types from AML and B-cell ALL bone marrow samples. (A) An orthogonal side-scatter (SS) versus CD45 plot displaying blast (Blast), granulocyte (Gra), lymphocyte (Lym), and monocyte (Mono) selection in AML samples. (B) An SS versus CD45 plot displaying Gra, Blast, Lym, and Mono selection in B-cell ALL samples. (C) Differences in leukocyte type distributions between AML and B-cell ALL samples.
Figure 2.
Syncytin-1 surface expression on AML and B-cell ALL leukocytes. Flow cytometry was applied to assess syncytin-1 surface expression on leukocytes extracted from AML and B-cell ALL bone marrow samples. (A) Graph of syncytin-1 surface expression across all leukocytes in the AML and B-cell ALL cohorts. The AML cohort displayed significantly higher leukocytic syncytin-1 surface expression over the B-cell ALL cohort. (B) Graph of syncytin-1 surface expression broken down by leukocyte subset, i.e., blast (Blast), granulocyte (Gra), lymphocyte (Lym), and monocyte (Mono). * p<0.05 relative to B-cell ALL group.
Table 3.
Leukocyte type distributions in AML and B-Cell ALL patients.
| Leukocyte type | AML cohort (n=50) | B-cell ALL cohort (n=14) | P-value |
|---|---|---|---|
| Syncytin-1-expressing granulocyte % | 40.95±23.08 | 45.39±27.48 | 0.57 |
| Syncytin-1-expressing blast % | 29.09±27.51 | 6.33±6.95 | <0.001* |
| Syncytin-1-expressing lymphocyte % | 23.79±13.68 | 20.79±10.22 | 0.45 |
| Syncytin-1-expressing monocyte % | 5.10±2.87 | 5.65±3.42 | 0.53 |
P<0.05
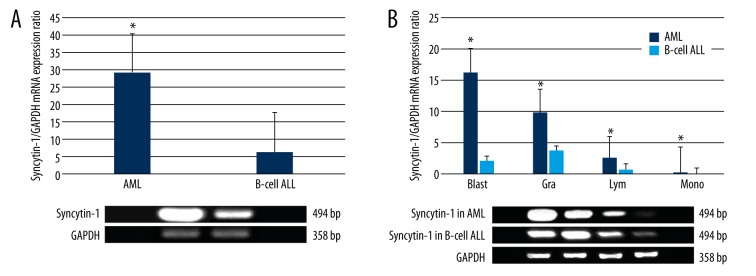
To further validate our flow cytometric findings, we next compared leukocytic syncytin-1 mRNA expression in the AML and B-cell ALL cohorts. In concordance with our flow cytometry findings, we found that the AML cohort displayed significantly greater leukocytic syncytin-1 mRNA expression relative to the B-cell ALL cohort (p<0.05, Figure 3A). We then analyzed leukocytic syncytin-1 mRNA expression in the AML and B-cell ALL cohorts by leukocyte type (i.e., blasts, granulocytes, lymphocytes, and monocytes) (Figure 3B). Consistent with our flow cytometry findings, we found that leukocytic syncytin-1 mRNA expression was significantly increased in all 4 leukocyte types, with blasts showing the largest fold-change in syncytin-1 mRNA expression (p<0.05, Figure 3B).
Figure 3.
Syncytin-1 mRNA expression in AML and B-Cell ALL Leukocytes. RT-PCR was applied to assess syncytin-1 mRNA expression in leukocytes extracted from AML and B-cell ALL bone marrow samples. Data have been presented graphically as the relative fold-change in syncytin-1 expression in relation to the housekeeping gene GAPDH. (A) Graph of syncytin-1 mRNA expression across all leukocytes in the AML and B-cell ALL cohorts. The AML cohort displayed significantly higher leukocytic syncytin-1 mRNA expression over the B-cell ALL cohort. (B) Graph of syncytin-1 mRNA expression broken down by leukocyte subset, i.e., blast (Blast), granulocyte (Gra), lymphocyte (Lym), and monocyte (Mono). * p<0.05 relative to B-cell ALL group.
We finally analyzed potential associations and correlations between key clinical factors and leukocytic syncytin-1 surface expression in the AML and B-cell ALL cohorts. There were no significant findings for the B-cell ALL cohort (p>0.05). However, the AML cohort displayed a significant association between leukocytic syncytin-1 surface expression and FAB classification (p<0.05, Table 4). Upon further analysis, M5, M5a, and M5b AML patients displayed significantly higher leukocytic syncytin-1 surface expression relative to all other AML FAB classifications (p<0.05, Figure 4). However, there were no significant differences in leukocytic syncytin-1 surface expression detected between M5, M5a, and M5b AML patients (p>0.05, Figure 4).
Table 4.
Clinical factor analysis for leukocytic syncytin-1 surface expression in AML and B-Cell ALL patients.
| Factor | AML cohort (n=50) | B-cell ALL cohort (n=14) | ||||
|---|---|---|---|---|---|---|
| Spearman’s coefficient | Kruskal-Wallis H-stat | P-value | Spearman’s coefficient | Kruskal-Wallis H-stat | P-value | |
| FAB classification | – | 21.61 | 0.017* | – | 0.13 | 0.72 |
| Age | 0.076 | – | 0.60 | −0.13 | – | 0.65 |
| Sex | – | 0.94 | 0.33 | – | 0.44 | 0.50 |
| WBC count | 0.070 | – | 0.63 | 0.055 | – | 0.85 |
| Blast % | 0.23 | – | 0.11 | −0.18 | – | 0.53 |
P<0.05.
Figure 4.
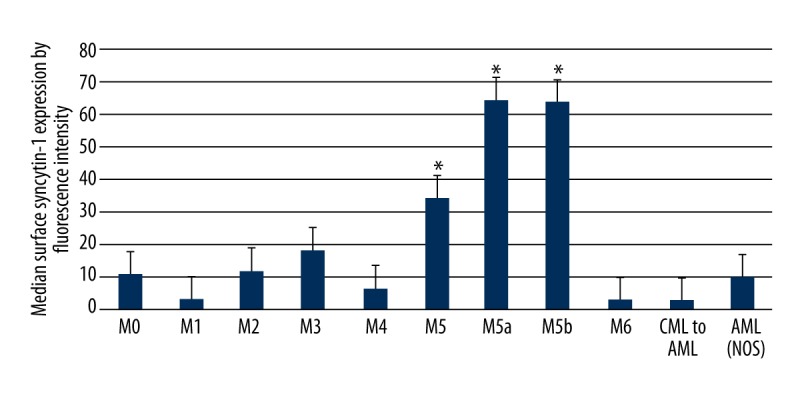
Syncytin-1 surface expression on AML leukocytes by FAB classification. Graph of leukocytic syncytin-1 surface expression in the AML cohort broken down by FAB classification. The M5, M5a, and M5b AML patients displayed significantly higher leukocytic syncytin-1 surface expression over all other AML patients. However, there were no significant differences in leukocytic syncytin-1 surface expression detected between M5, M5a, and M5b AML patients. * p<0.05 relative to non-M5 FAB classifications.
Discussion
Here, using flow cytometry and RT-PCR, we comparatively assessed leukocytic syncytin-1 expression in cohorts of AML and B-cell ALL patients. We found that AML patients displayed significantly greater leukocytic syncytin-1 expression relative to B-cell ALL patients, which is attributable to an increased syncytin-1-expressing blast cell percentage combined with upregulated syncytin-1 expression on blasts, granulocytes, lymphocytes, and monocytes in AML patients. Moreover, we found a significant association between leukocytic syncytin-1 surface expression and FAB classification, with acute monocytic leukemia (i.e., M5, M5a, and M5b AML) patients displaying significantly higher leukocytic syncytin-1 surface expression relative to all other AML FAB classifications. These findings suggest leukocytic syncytin-1 expression may play a role in the development and/or maintenance of the AML phenotype and the acute monocytic leukemia phenotype in particular.
Based on the classical model of hematopoietic commitment and blood lineage development, hematopoietic stem cells (HSCs) can differentiate along 1 of 2 cell lineages: a myeloid lineage (which produces granulocytes, monocytes, erythrocytes, and platelets) or a lymphoid lineage (which produces B-cells and T-cells) [21,22]. Malignant transformation of myeloid lineage progenitors produces AML blasts, while malignant transformation in lymphoid linage progenitors produces ALL blasts [18,19]. As a result, AML blasts display surface antigens that are also expressed on normal immature myeloid cells (e.g., CD13, CD33, and CD34) as well as other surface antigens including monocytic surface markers (e.g., CD4, CD11b, and CD14), erythroid surface markers (e.g., CD36, CD71), and megakaryocytic surface markers (e.g., CD41a and CD61) [23]. In terms of the pathoetiology of AML, mounting research has identified several common genetic mutations that can be linked to AML development, including nucleophosmin 1 (NPM1), DNA methyltransferase 3A (DNMT3A), FMS-like tyrosine kinase 3 (FLT3), isocitrate dehydrogenase (IDH), and ten-eleven translocation 2 (TET2) [23]. Notably, the acute monocytic leukemia phenotype (i.e., M5, M5a, and M5b AML) has been more strongly associated with NPM1 and FLT3 mutations [23,24]. As our current study found that acute monocytic leukemia patients displayed significantly higher leukocytic syncytin-1 surface expression relative to all other AML types, future studies in this field should focus on the relationship (if any) between NPM1 and FLT3 mutations and leukocytic syncytin-1 upregulation in AML patients.
Pathophysiologically, the relationship between leukocytic syncytin-1 upregulation and the AML phenotype may be associated with syncytin-1’s immunosuppressive properties. Although little is known about syncytin-1’s role in immunity, it is noteworthy that syncytin-1 and HIV-1’s gp160, both of which are envelope proteins with immunosuppressive properties, share several common structural features. First, the syncytin-1 precursor 73-Kd gPr73 is cleaved into a 50-Kd surface subunit (SU) and a 24-Kd transmembrane subunit (TM), while HIV-1 gp160 is cleaved into gp120 (a surface subunit) and gp41 (a transmembrane subunit) [6,7,25]. Second, structural analysis of syncytin-1 has revealed an immunosuppressive peptide domain (amino acid residues 373–397), while the HIV-1 gp41 peptide also has a 17-amino acid segment with similar immunosuppressive functions [6,12,25]. On this basis, similar to the aforementioned role of syncytin-1 in the placenta [9,26], we speculate that heightened exosome-mediated syncytin-1 expression on the cell surface of AML blasts may contribute to immune tolerance in AML patients. Moreover, on account of syncytin-1’s fusion activity [9,11], heightened syncytin-1 secretion may also be conducive to enhanced AML blast migration. Consistent with this hypothesis, the acute monocytic leukemia (i.e., M5, M5a, and M5b AML) phenotype, which displays significantly higher syncytin-1 upregulation, has been more strongly linked to the development of extramedullary disease [24]. On this basis, future studies in this field should focus on the relationship (if any) between leukocytic syncytin-1 upregulation and AML blast migratory capability.
There are several limitations to the current study. First, the sample size of this study (n=50 AML patients, n=14 B-cell ALL patients) was rather limited and was restricted to Han Chinese patients recruited from 1 study site in China. Therefore, future studies in the field should seek to recruit larger cohorts from multiple international study sites to ensure adequate powering and minimization of bias. Second, only untreated acute leukemia patients were recruited for the current study. Therefore, it was not possible to analyze the effects of chemotherapy on syncytin-1 expression. Future studies should seek to recruit both treated and untreated participants in order to analyze the effects of chemotherapy on syncytin-1 expression. Third, as antibodies for syncytin-1 also cross-react with other HERV-Wenv proteins expressed on the plasma membrane [27], this cross-reaction phenomenon may have adversely affected our flow cytometry findings regarding syncytin-1 surface expression. Fourth, although we determined that AML patients display significantly higher leukocytic syncytin-1 expression relative to B-cell ALL patients, the mechanism(s) underlying such leukocytic syncytin-1 upregulation and its effects (if any) on clinical outcomes in AML patients remain unknown.
Conclusions
In conclusion, AML patients display significantly greater leukocytic syncytin-1 expression relative to B-cell ALL patients. Moreover, acute monocytic leukemia (i.e., M5, M5a, and M5b AML) patients display significantly higher leukocytic syncytin-1 surface expression relative to all other AML FAB classifications. These findings suggest leukocytic syncytin-1 expression may play a role in the development and/or maintenance of the AML phenotype and the acute monocytic leukemia phenotype in particular. Future studies in this field should focus on defining the relationships (if any) between leukocytic syncytin-1 upregulation, NPM1 and FLT3 mutations, AML blast migratory capability, and clinical outcomes in AML patients.
Supplementary Material
Raw flow cytometry images.
Supplementary Table 1.
Additional details on flow cytometry
| Flow cytometer manufacturer and model | BD Biosciences FACSCantoTMII flow cytometer (BD Biosciences, San Jose, CA) | |||
|
| ||||
| Flow cell and fluidics | Fixed-alignment cuvette flow cell. The cells and fluidics had not been altered from the original manufacturer | |||
|
| ||||
| Light sources | Two-lasers: 488-nm Coherent® SapphireTM solid state (20 mW) and 633-nm JDS UniphaseTM HeNe air-cooled (17 mW). The light sources had not been altered from the original manufacturer | |||
|
| ||||
| Excitation optics configuration | The configuration had not been altered from the original manufacturer | |||
|
| ||||
| Optical filters | The filters were from the original manufacturer | |||
|
| ||||
| Optical detectors | The detectors were from the original manufacturer | |||
|
| ||||
| Detectors/amps | Parameter | Detector | Voltage | Mode |
| P1 | FSC | 286 | Lin | |
| P2 | SSC | 434 | Lin | |
| P3 | FL1 | 473 | Log | |
| P4 | FL2 | 550 | Log | |
| P5 | FL3 | 530 | Log | |
| P6 | FL4 | 650 | Log | |
|
| ||||
| Threshold Primary parameter: FSC Value: 40 Secondary parameter FL3 Value: 170 | ||||
|
| ||||
| Compensation FL2 – 49% FL1 FL3 – 3.5% FL1 FL4 – 0.0% FL1 FL1 – 0.4% FL2 FL3 – 10.0% FL2 FL4 – 0.0% FL2 FL1 – 0.1% FL3 FL2 – 1.3% FL3 FL4 – 4.3% FL3 FL3 – 0.1% FL4 | ||||
|
| ||||
| Amplifier settings | The amplifier settings were calibrated monthly using BD Cytometer Setup Tracking Beads (no. 641319, BD Biosciences) | |||
|
| ||||
| Data transformation | BD FACSDiva™ software (version 6.1.2) was used to transform the data as follows:
|
|||
|
| ||||
| Gating details | An orthogonal side scatter (SS) versus CD45 plot was used to segregate four lymphocytic subsets: granulocytes, lymphoblasts, lymphocytes, and monocytes. Applying the isotype controls, voltage, and compensation, the flow cytometer was configured so the leukocyte subsets were appropriately positioned on the dot plots. The mean fluorescence intensities (MFIs) for syncytin-1 in each leukocytic subset (i.e, granulocytes, lymphoblasts, lymphocytes, and monocytes) were calculated from the leukocytic distribution pattern in the SS versus CD45 plot | |||
Footnotes
Conflicts of interest
None. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Source of support: This work was supported by the National Natural Science Foundation for the Youth of China (grant no. 81100377) and the Yunnan Applied Basic Research Joint Special Project (grant no. 2013FB203)
References
- 1.Dores GM, Devesa SS, Curtis RE, et al. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood. 2012;119:34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greaves M. Aetiology of acute leukaemia. Lancet. 1997;349:344–49. doi: 10.1016/s0140-6736(96)09412-3. [DOI] [PubMed] [Google Scholar]
- 3.Ou W-B. Correlations between MDM2 gene SNP309 polymorphism and susceptibility to leukemia. Med Sci Monit. 2015;21:213–18. doi: 10.12659/MSM.892919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruan XL, Li S, Geng P, et al. Association between TP53 gene Codon 72 polymorphism and acute myeloid leukemia susceptibility: Evidence based on a meta-analysis. Med Sci Monit. 2015;21:3048–53. doi: 10.12659/MSM.894625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943–55. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, Ouyang DY, Pang W, et al. Expression of syncytin in leukemia and lymphoma cells. Leuk Res. 2010;34:1195–202. doi: 10.1016/j.leukres.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Gong R, Peng X, Kang S, et al. Structural characterization of the fusion core in syncytin, envelope protein of human endogenous retrovirus family W. Biochem Biophys Res Commun. 2005;331:1193–200. doi: 10.1016/j.bbrc.2005.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguilar PS, Baylies MK, Fleissner A, et al. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 2013;29:427–37. doi: 10.1016/j.tig.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Q, Chen H, Li J, et al. Epigenetic and non-epigenetic regulation of syncytin-1 expression in human placenta and cancer tissues. Cell Signal. 2014;26:648–56. doi: 10.1016/j.cellsig.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Bjerregaard B, Talts JF, Larsson L-I, editors. In Cell Fusions. Springer; 2011. pp. 267–75. [Google Scholar]
- 11.Huppertz B, Borges M, editors. In Cell Fusion. Springer; 2008. pp. 135–47. [DOI] [PubMed] [Google Scholar]
- 12.Mi S, Lee X, Li X, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–89. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 13.Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–68. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 14.Maliniemi P, Vincendeau M, Mayer J, et al. Expression of human endogenous retrovirus-w including syncytin-1 in cutaneous T-cell lymphoma. PloS One. 2013;8:e76281. doi: 10.1371/journal.pone.0076281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 16.Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–25. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer DG, Jaldín-Fincati JR, Amigone JL, et al. Standardized flow cytometry assay for identification of human monocytic heterogeneity and LRP1 expression in monocyte subpopulations: Decreased expression of this receptor in nonclassical monocytes. Cytometry A. 2014;85:601–10. doi: 10.1002/cyto.a.22455. [DOI] [PubMed] [Google Scholar]
- 18.Kudo Y, Boyd C, Sargent I, Redman C. Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: Implications for impaired trophoblast syncytialisation in pre-eclampsia. Biochim Biophys Acta. 2003;1638(1):63–71. doi: 10.1016/s0925-4439(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Spinsanti G, Zannolli R, Panti C, et al. Quantitative real-time PCR detection of TRPV1-4 gene expression in human leukocytes from healthy and hyposensitive subjects. Mol Pain. 2008;4:51. doi: 10.1186/1744-8069-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 22.Adolfsson J, Månsson R, Buza-Vidas N, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential: A revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Saultz JN, Garzon R. Acute myeloid leukemia: A concise review. J Clin Med. 2016;5(3) doi: 10.3390/jcm5030033. pii: E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tallman MS, Kim HT, Paietta E, et al. Acute monocytic leukemia (French-American-British classification M5) does not have a worse prognosis than other subtypes of acute myeloid leukemia: A report from the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:1276–86. doi: 10.1200/JCO.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 25.Denner J, Norley S, Kurth R. The immunosuppressive peptide of HIV-1: Functional domains and immune response in AIDS patients. Aids. 1994;8:1063–72. [PubMed] [Google Scholar]
- 26.Tolosa JM, Schjenken JE, Clifton VL, et al. The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta. 2012;33:933–41. doi: 10.1016/j.placenta.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Arru G, Leoni S, Pugliatti M, et al. Natalizumab inhibits the expression of human endogenous retroviruses of the W family in multiple sclerosis patients: A longitudinal cohort study. Mult Scler. 2014;20(2):174–82. doi: 10.1177/1352458513494957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw flow cytometry images.
Supplementary Table 1.
Additional details on flow cytometry
| Flow cytometer manufacturer and model | BD Biosciences FACSCantoTMII flow cytometer (BD Biosciences, San Jose, CA) | |||
|
| ||||
| Flow cell and fluidics | Fixed-alignment cuvette flow cell. The cells and fluidics had not been altered from the original manufacturer | |||
|
| ||||
| Light sources | Two-lasers: 488-nm Coherent® SapphireTM solid state (20 mW) and 633-nm JDS UniphaseTM HeNe air-cooled (17 mW). The light sources had not been altered from the original manufacturer | |||
|
| ||||
| Excitation optics configuration | The configuration had not been altered from the original manufacturer | |||
|
| ||||
| Optical filters | The filters were from the original manufacturer | |||
|
| ||||
| Optical detectors | The detectors were from the original manufacturer | |||
|
| ||||
| Detectors/amps | Parameter | Detector | Voltage | Mode |
| P1 | FSC | 286 | Lin | |
| P2 | SSC | 434 | Lin | |
| P3 | FL1 | 473 | Log | |
| P4 | FL2 | 550 | Log | |
| P5 | FL3 | 530 | Log | |
| P6 | FL4 | 650 | Log | |
|
| ||||
| Threshold Primary parameter: FSC Value: 40 Secondary parameter FL3 Value: 170 | ||||
|
| ||||
| Compensation FL2 – 49% FL1 FL3 – 3.5% FL1 FL4 – 0.0% FL1 FL1 – 0.4% FL2 FL3 – 10.0% FL2 FL4 – 0.0% FL2 FL1 – 0.1% FL3 FL2 – 1.3% FL3 FL4 – 4.3% FL3 FL3 – 0.1% FL4 | ||||
|
| ||||
| Amplifier settings | The amplifier settings were calibrated monthly using BD Cytometer Setup Tracking Beads (no. 641319, BD Biosciences) | |||
|
| ||||
| Data transformation | BD FACSDiva™ software (version 6.1.2) was used to transform the data as follows:
|
|||
|
| ||||
| Gating details | An orthogonal side scatter (SS) versus CD45 plot was used to segregate four lymphocytic subsets: granulocytes, lymphoblasts, lymphocytes, and monocytes. Applying the isotype controls, voltage, and compensation, the flow cytometer was configured so the leukocyte subsets were appropriately positioned on the dot plots. The mean fluorescence intensities (MFIs) for syncytin-1 in each leukocytic subset (i.e, granulocytes, lymphoblasts, lymphocytes, and monocytes) were calculated from the leukocytic distribution pattern in the SS versus CD45 plot | |||