Abstract
Background Human bocavirus (HBoV) was first discovered in Sweden in 2005 and has now been found worldwide; however its role in clinically relevant diseases has not yet been clearly defined.
Objectives To gain new insight into HBoV infection among children hospitalized with acute respiratory infections in Rome.
Methods Between November 2004 and May 2007, 415 nasal washings were tested for the presence of an extensive range of respiratory viruses using molecular methods.
Results Viral pathogens were detected in 214 children (51·6%), 28·9% being respiratory syncytial virus (RSV) and 9·6% being rhinovirus positive. Of the 34 children (8·2%) who tested positive for HBoV, 21 (61·8%) were co‐infected with another respiratory virus, mainly RSV. Human bocavirus was the only pathogen identified in four pneumonia and six bronchiolitis cases in March 2005 and January 2007, respectively. Human bocavirus was also detected in one child hospitalized with gastroenteritis and in another with erythema.
Conclusions In the examined population, HBoV was the third most common virus detected but with a high rate of co‐infection with other respiratory viruses. Human bocavirus appeared to be the etiological agent in some pneumonia and bronchiolitis cases in which tests for all likely respiratory pathogens were negative.
Keywords: Bocavirus, bronchiolitis, Polymerase chain reaction, pneumonia, respiratory tract infections
Introduction
A number of novel respiratory viruses have been identified since 2001, of which human metapneumovirus and the new human coronaviruses, SARS, NL63 and HKU1, have been shown to contribute significantly to the burden of acute respiratory infections (ARIs).
In 2005, a new member of the Parvoviridae family, named human bocavirus (HBoV) because of its similarity to bovine and canine parvovirus, was discovered during random polymerase chain reaction (PCR)/cloning techniques in respiratory samples. Human bocavirus has been found in respiratory specimens by many groups of investigators worldwide, with prevalence ranging from 1·5% to 18%. 1 , 2 , 3 However, it is not yet clear to what extent HBoV causes clinically relevant diseases as most studies were retrospective, examining archival respiratory samples previously tested for respiratory viruses using different techniques, and HBoV is frequently found as a co‐infection.
As a part of a larger study to characterize respiratory virus infections, hospitalized children were tested for HBoV and 13 other viruses to define the prevalence of HBoV in Rome; an analysis was then undertaken of the distribution and demography of those who were HBoV positive.
Methods
Specimen collection and nucleic acid extraction
Children (below 14 years of age) admitted to the Umberto I Hospital Pediatric Department (Sapienza University, Rome) with diagnoses of asthma, bronchiolitis, bronchopneumonia, laryngo‐tracheo bronchitis were enrolled in the study after parental consent had been obtained. The study was approved by the institution’s Ethics Committee. The exclusion criterion was any underlying medical problem (e.g. premature birth, congenital disease, cystic fibrosis). A total of 415 children were tested between November 2004 and May 2007; all patients were admitted to hospital (median length of stay: 3 days) and were not subjected to intensive care.
Nasal washings were obtained at the time of hospital admission – 3 ml of sterile physiological saline solution were injected into each nostril and collected with a syringe. 4 , 5 All samples were delivered on ice within 1–2 hours to the virology laboratory and on arrival, if needed, they were vortexed with beads to solve mucus; 200 μl of each specimen were then subjected to nucleic acid extraction using a Total Nucleic Acid Isolation Kit (Roche Diagnostics, Mannheim, Germany).
PCR‐based assays for respiratory viruses
Reverse transcription‐PCR and PCR assays were performed on the nucleic acid extracted from the nasal washes to detect HBoV and 13 other respiratory viruses; nasal washings collected between November 2004 and October 2006 were tested for HBoV retrospectively.
The specific assays for influenza A and B, respiratory syncytial virus (RSV), coronaviruses OC43 and 229E, adenovirus, rhinovirus, parainfluenza viruses (PIV) 1–3, metapneumovirus, and coronaviruses NL63 and HKU1 have been described previously. 4 For HBoV detection, two published PCR assays 1 , 6 amplifying, respectively, the NS1 and NP1 genes were used. Amplification products from positive samples were cloned using a Topo TA Cloning Kit (Invitrogen, Carlsbad, CA, USA), for use as positive controls in PCR.
Most amplified fragments were purified and sequenced; any amplification products not sequenced were confirmed as true positives by testing against an aliquot of the sample. The sequences obtained from HBoV positive samples were aligned with clustal w (EMBL‐EBI, European Bioinformatics Institute, Heidelberg, Germany).
Statistical methods
A chi‐squared test was applied to compare the age distribution of virus‐positive children, significance being fixed at the 5% level. The analysis was performed using spss v.13·0 (SPSS Inc., Chicago, Illinois, USA) for Windows.
Results
Respiratory virus detection
Nasal washings were collected from 415 children hospitalized for ARIs between November 2004 and May 2007; the children’s median age was 4·5 months (range 0–169 months). The results and percentages of virus detection are shown in Table 1; the most common agent was RSV, identified in 120 samples (28·9%), followed by rhinovirus (found in 40 samples, 9·6%).
Table 1.
Detection rates of viral infections in 415 children hospitalized for acute respiratory infections (ARI) (November 2004–May 2007)
| Cases ARI | n = 415(%) |
|---|---|
| Virus‐positive | 214 (51·6) |
| Co‐infections | 30 (7·2) |
| RSV | 120 (28·9) |
| Rhinovirus | 40 (9·6) |
| HBoV | 34 (8·2) |
| PIV1‐3 | 15 (3·6) |
| Influenza A | 14 (3·4) |
| Other viruses | 21 (5·1) |
Human bocavirus was detected in 34 respiratory samples (8·2%), 21 (61·8%) of which harbored an additional respiratory virus. Human bocavirus was identified in specimens taken from autumn to early summer throughout the study period (Fig. 2), with single infection cases grouped in March 2005 and January 2007.
Figure 2.
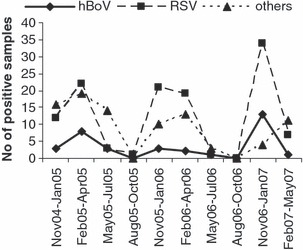
Temporal distribution of human bocavirus (HBoV) infection cases. Distribution of HBoV, respiratory syncytial virus (RSV), and other respiratory‐virus‐positive cases over the study period.
Figure 1 shows the age distribution in percentage of the virus‐positive children: RSV was most frequently detected in 0‐to 3‐month‐old children (P < 0·01 for comparison in chi‐squared test with every virus); rhinovirus, PIV1‐3 and influenza rates of infection were similar in all age groups. Human bocavirus infection showed an age distribution characterized by the highest infection rates at 4–12 months (case distribution by patient age in chi‐squared test: P < 0·05 HBoV versus RSV, rhinovirus and influenza A; P = 0·09 HBoV versus PIV1–3).
Figure 1.

Age distribution of respiratory virus infection cases. Respiratory syncytial virus (RSV), Rhinovirus, human bocavirus (HboV), parainfluenza viruses (PIV1–3) and influenza A positive cases, expressed in percentages, divided by age groups.
HBoV and respiratory diseases
During the study period, 121/204 (59·3%) children hospitalized for bronchiolitis tested positive for at least one virus; as expected, the main agent was RSV, found in 90 children (44·1%). Rhinovirus was present as the sole pathogen in 16 cases (7·8%) of bronchiolitis; HBoV was found in 22 samples (10·8% of total bronchiolitis cases), of which 15 (68·2%) were co‐infected with RSV. Interestingly, six of the seven cases of bronchiolitis in which HBoV was the only agent identified occurred in January/February 2007.
In 67 (44·1%) of the 152 children hospitalized for pneumonia, a viral pathogen was identified; again, the most common agent was RSV (23 children; 15·1%), with rhinovirus ranked second (14 children; 9·2%). Human bocavirus was detected in nine children (5·9%); of these, five (55·6%) were co‐infected with other viruses and four were single infections that occurred in March 2005. In addition, a single HBoV infection was detected in an atopic child with an episode of wheezing bronchitis.
Sequence data from HBoV identified in the single infections of pneumonia and bronchiolitis (March 2005 and January/February 2007, respectively) were aligned and compared with each other and with other HBoV infections found in other periods but no differences were observed in the amino acid sequences.
Between November 2006 and May 2007, in the framework of an ongoing study of immune response to respiratory viruses, venous samples were taken from all children hospitalized with a clinical diagnosis of bronchiolitis. Plasma samples from eight HBoV‐infected children (five single infections and three co‐infected with RSV) were tested by HBoV PCR but none was positive.
During this study, patients were also examined 7–10 days after recovery from respiratory symptoms and second nasal washings were obtained. The second nasal washings from children with HBoV infections in their first specimens tested negative to both HBoV PCRs.
Bocavirus in children without respiratory diseases
During the period from November 2006 to May 2007, nasal washings obtained from 21 children hospitalized for non‐respiratory illness at the same institution were tested for the presence of respiratory viruses; the children’s median age was 3 months (range 0–124 months). Only three children tested positive for respiratory viruses, two for HBoV and one for rhinovirus. One of the HBoV positive children had gastroenteric symptoms and the other had a clinical diagnosis of Exanthema subitum.
Discussion
During the study period, HBoV was the third most frequently found viral agent (Table 1) in children hospitalized for ARI, which is consistent with other recent reports. 7 , 8 The HBoV distribution found in Rome is greater than that previously reported in Italian children with respiratory diseases, 9 but lower than that reported in nearby European countries. 10 , 11 The HBoV infections were equally distributed between male and female children (exactly 1:1) and most occurred (88%) in the first year of life similarly to RSV (Fig. 1); nonetheless, HBoV case distribution significantly differed from the other respiratory viruses tested, in percentages at 4–12 months of age.
Any seasonal prevalence of HBoV infections was not obvious because of the small number of samples collected in summer; nevertheless, there were clusters of cases in winter/early spring (Fig. 2). The samples in which HBoV was the only pathogen identified seem to indicate an etiological role for HBoV in pneumonia and bronchiolitis, as most other respiratory viral agents were excluded.
Various percentages of HBoV co‐infection (11–90%) have been reported previously by other researchers; 8 , 12 , 13 however, co‐infection rates consistent with our study have been reported in studies that used sensitive and comprehensive diagnostic tests. 13 , 14 Undoubtedly, the HBoV rate of co‐infection is higher than for other respiratory viruses, which could, in principle, either be because of the high incidence of HBoV in mild or asymptomatic upper respiratory infections in children or to the long shedding period or to reactivation after prior acute infections. In an attempt to clarify this, HBoV was sought for in HBoV‐infected children after they had recovered from respiratory disease, and in hospitalized children with no respiratory symptoms. Human bocavirus was no longer detectable in the second nasal washings taken 8–12 days after the first in HBoV‐positive children, suggesting a limited period of viral shedding in children with bronchiolitis. In a recent report, 15 persistent HBoV shedding for over a month was observed in patients with significant underlying diseases; however, follow‐up tests were not performed in otherwise healthy children. Differences between studies could, perhaps, be accounted for by the fact that this study did not include children with underlying medical problems that could cause immune suppression and prolonged HBoV shedding.
Several studies reported no or few cases of HBoV infections in asymptomatic populations or differently symptomatic control populations but often without age matching and using different types of specimens. 16 In the present study, children hospitalized for non‐respiratory diseases were tested for HBoV, using the same type of specimen, taken by the same physicians, for several months during the study period. Human bocavirus was detected in 2/21 children; these patients had symptoms (gastroenteritis or exanthema) similar to those described previously in HBoV infections. 17 , 18 , 19 There are a number of possibilities that could account for these non‐respiratory HBoV infections: hospitalization due to an HBoV‐associated disease; HBoV infection before hospitalization, together with other pathogens; HBoV nosocomially acquired, as reported in infants hospitalized since birth. 18
In this study, HBoV was not detectable in the plasma of eight children affected with bronchiolitis, during HBoV acute infection. An Italian paper 9 reported HBoV viremia in only one child with gastroenteric symptoms, while two patients with respiratory disease tested negative. On the other hand, HBoV was detected in 53% of 43 acute‐phase serum samples and in 19% of the 43 convalescent‐phase serum samples from wheezing children; 14 the authors concluded that the occurrence of HBoV viremia was associated with HBoV high viral load in respiratory samples and linked in time with episodes of acute wheezing. More series of samples, with accurate quantitative analysis of HBoV DNA, will be required to clarify the issue of HBoV localized or systemic infection during ARI.
As HBoV was the sole viral pathogen detected in cases of pneumonia and bronchiolitis that had been tested for nearly all respiratory viruses, HBoV is most likely to be the agent responsible for these low‐respiratory tract diseases.
In principle, some cases attributed to HBoV could be due to bacterial co‐infections, as in this study, like in most others on ARI in children, bacteria were not searched for. However, the clinical data of our HBoV‐infected patients and the epidemiology of pneumonia and bronchiolitis (especially below 1 year of age) do not point to bacterial infections.
In order to investigate the possibility of a common source for HBoV infection in those children with pneumonia and bronchiolitis who were temporally related, the sequencing data of NS1 and NP1 amplified fragments were compared. No differences in the amino acid sequences were found, possibly due to low variability in HBoV in NS1 and NP1 genes. In fact, only two closely related genotypes have so far been described and most nucleotide differences are in the VP1/VP2 genes. 18 , 20
In conclusion, these data together seem to indicate that HBoV is a widely circulating respiratory virus that infects patients mainly in the first year of life, probably causing subclinical manifestations and occasionally severe respiratory diseases, such as pneumonia and bronchiolitis. Nevertheless, because of its high prevalence in children, HBoV’s etiological role cannot definitely be proven without isolating the virus and testing for immunity. Future prospective studies using standardized procedures for sampling and quantitative detection of viral particles are essential to study HBoV’s pathogenicity and biological properties, and eventually to establish a correlation between viral load and the severity of clinical symptoms.
Acknowledgement
This study was supported by grants to G. Antonelli from Sapienza Università di Roma (fondi Ricerche Universitarie).
References
- 1. Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung‐Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA 2005; 102:12891–12896. Erratum in: Proc Natl Acad Sci USA 102:15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bastien N, Brandt K, Dust K, Ward D, Li Y. Human Bocavirus infection, Canada. Emerg Infect Dis 2006; 12:848–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kahn J. Human bocavirus: clinical significance and implications. Curr Opin Pediatr 2008; 20:62–66. [DOI] [PubMed] [Google Scholar]
- 4. Pierangeli A, Gentile M, Di Marco P et al. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J Med Virol 2007;79:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Forman MS, Valsamakis A. Specimen collection, transport and processing: Virology In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. (eds). Manual of Clinical Microbiology. 9th edn Washington DC: ASM Press, 2007:1284–1296. [Google Scholar]
- 6. Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol 2006; 78:1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manning A, Russell V, Eastick K et al. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis 2006; 194:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi EH, Lee HJ, Kim SJ et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis 2006; 43:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maggi F, Andreoli E, Pifferi M, Meschi S, Rocchi J, Bendinelli M. Human bocavirus in Italian patients with respiratory diseases. J Clin Virol 2007; 38:321–325. [DOI] [PubMed] [Google Scholar]
- 10. Weissbrich B, Neske F, Schubert J et al. Frequent detection of bocavirus DNA in German children with respiratory tract infections. BMC Infect Dis 2006; 6:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pozo F, Garcia‐Garcia ML, Calvo C, Cuesta I, Perez‐Brena P, Casas I. High incidence of human bocavirus infection in children in Spain. J Clin Virol 2007; 40:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung JY, Han TH, Kim SW, Kim CK, Hwang ES. Detection of viruses identified recently in children with acute wheezing. J Med Virol 2007; 79:1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fry AM, Lu X, Chittaganpitch M et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis 2007; 195:1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allander T, Jartti T, Gupta S et al. Human bocavirus and acute wheezing in children. Clin Infect Dis 2007; 44:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lau SK, Yip CC, Que TL et al. Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. J Infect Dis 2007; 196:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mackay IM. Human bocavirus: multisystem detection raises questions about infection. J Infect Dis 2007; 196:968–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnold JC, Singh KK, Spector SA, Sawyer MH. Human bocavirus: prevalence and clinical spectrum at a children’s hospital. Clin Infect Dis 2006; 43:283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kesebir D, Vazquez M, Weibel C et al. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis 2006; 194:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vicente D, Cilla G, Montes M, Perez‐Yarza EG, Perez‐Trallero E. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis 2007; 13:636–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chieochansin T, Chutinimitkul S, Payungporn S et al. Complete coding sequences and phylogenetic analysis of Human Bocavirus (HBoV). Virus Res 2007; 129:54–57. [DOI] [PubMed] [Google Scholar]


