Abstract
Plants are important to humans not only because they provide commodities such as food, feed and raw materials, but increasingly because they can be used as manufacturing platforms for added-value products such as biopharmaceuticals. In both cases, liquid plant extracts may need to be clarified to remove particulates. Optimal clarification reduces the costs of filtration and centrifugation by increasing capacity and longevity. This can be achieved by introducing charged polymers known as flocculants, which cross-link dispersed particles to facilitate solid-liquid separation. There are no mechanistic flocculation models for complex mixtures such as plant extracts so empirical models are used instead. Here a design-of-experiments procedure is described that allows the rapid screening of different flocculants, optimizing the clarification of plant extracts and significantly reducing turbidity. The resulting predictive models allow the identification of robust process conditions and sets of polymers with complementary properties, e.g. effective flocculation in extracts with specific conductivities. The results presented for tobacco leaf extracts can easily be adapted to other plant species or tissues and will thus facilitate the development of more cost-effective downstream processes for commodities and plant-derived pharmaceuticals.
Keywords: Plant Biology, Issue 110, Consumables cost reduction, design of experiments (DoE), downstream processing, flocculation, plant extract clarification, plant-derived pharmaceuticals
Introduction
Plants are widely used to produce food commodities such as fruit juices, but they can also be developed as platforms for the manufacture of higher-value biopharmaceutical products 1-3. In both cases, downstream processing (DSP) often begins with the extraction of liquids from tissues such as leaves or fruits, followed by the clarification of particle-laden extracts 4,5. For the manufacture of biopharmaceuticals, the costs of DSP can account for up to 80% of the overall production costs 6,7 and this in part reflects the high particle burden present in extracts prepared by disruptive methods such as blade-based homogenization 8,9. Although the rational selection of filter layers to match the particle size distribution in the extract can increase filter capacity and reduce costs 10,11, the improvement can never exceed the ceiling of absolute capacity defined by the number of particles that must be retained per unit of filter area to achieve clarification.
The ceiling can be lifted if fewer particles reach the surface of the finest filters in the filtration train, and this can be achieved if dispersed particles are mixed with polymers known as flocculants that promote aggregation to form large flocs 12. Such flocs can be retained further upstream by coarser and less expensive bag filters, reducing the particle burden reaching the finer and more expensive depth filters. The polymers must have safety profiles suitable for their applications, e.g. for biopharmaceuticals they must be compliant with good manufacturing practice (GMP), and typically they must have a molar mass >100 kDa and can either be neutral or charged 13. Whereas neutral flocculants generally act by cross-linking dispersed particles causing their aggregation and the formation of flocs with diameters >1 mm 11, charged polymers neutralize the charge of dispersed particles, reducing their solubility and thus causing precipitation 14.
Flocculation can be improved by adjusting parameters such as buffer pH or conductivity, and the polymer type or concentration, to match the properties of the extract 15,16. For tobacco extracts pretreated with 0.5-5.0 g L-1 polyethylenimine (PEI), a greater than 2-fold increase in depth filter capacity was reported in a 100-L pilot-scale process. The cost of this polymer is less than €10 kg-1 so its introduction into the process resulted in cost savings of about €6,000 for filters and consumables per batch 16 or even more when combined with cellulose-based filter aids 17. Even so, predictive models are required to evaluate the a priori economic benefits of flocculants because their inclusion can require hold steps of 15-30 min 16,18, resulting in further investment costs for storage tanks. However, there are currently no mechanistic models available that can predict the outcome of such experiments due to the complex nature of flocculation. Therefore, a more appropriate design-of-experiments (DoE) approach 19 was developed as described in this article. A protocol for the general DoE procedure has recently been published 20.
Small-scale devices are now available for the high-throughput screening of flocculation conditions 21. However, these devices may not realistically simulate conditions during the flocculation of plant extracts because the dimensions of the reaction vessel (~7 mm for wells on a 96-well plate) and the particles or flocs can be less than an order of magnitude apart. This can affect mixing patterns and thus the predictive power of the model. Furthermore, it can be difficult to scale down processes involving precipitation due to non-linear changes in the mixing behavior and precipitate stability 22. Therefore, this article outlines a bench-top-scale screening system with a throughput of 50-75 samples per day, yielding results that are scalable from the initial 20 ml reaction volume to a 100 L pilot-scale process 16. When combined with a DoE approach, this allows the predictive models to be used for process optimization and documentation as part of a quality-by-design concept.
The method described below may also be adapted to biopharmaceuticals produced in cell culture-based processes, where flocculants are also being considered as a cost-saving tool 23. It can also be used to model the precipitation of target proteins from a crude extract as part of a purification strategy, as demonstrated for β-glucuronidase produced in canola, maize and soybean 24,25. A detailed description of flocculant properties can be found elsewhere 16,26 and it is important to ensure that the polymer concentrations are either non-toxic or below harmful levels in the final product 11.
Protocol
1. Develop an Adequate Experimental Strategy
- Identify the environmental and process parameters that are relevant for the flocculation procedure to be established or optimized, i.e. which factors have the strongest effect on flocculation. Typically, there are several such parameters so a DoE approach as recently described 20 is necessary due to the lack of mechanistic models.
- Select parameters (factors) based on literature data 12, prior knowledge and experience with the system. Typical factors include buffer pH, buffer conductivity, incubation time and temperature as well as polymer type and concentration 15,16,27.
- Use the same references (see 1.1) to define meaningful ranges for each numeric factor and levels for categorical factors.
- Define the experimental outcomes (responses) to be monitored and used to evaluate the efficiency of flocculation. Depending on the system, this can be filtrate or supernatant turbidity, settling velocity, aggregate size or the capacity of a subsequent filter 16,23,28-30.
- Ensure the assays used to measure the responses are quantitative, robust and repeatable/reproducible so that the resulting high-quality data can be augmented with experiments performed later or by another operator.
- Choose a DoE type suiting the number of factors to be investigated and the degree of knowledge already accumulated about the system. Use available literature to identify a suitable DoE type 20.
- Select a screening design if there is little information about the flocculation system to be investigated, a large number of parameters must be screened or little is known about meaningful ranges for the parameters. Typical screening designs are full and fractional factorial designs. Include center points in the design if the parameters are expected to have a non-linear impact, e.g. saturation above a certain threshold 19.
- Select a response surface methodology (RSM), e.g. central composite design (CCD) 31 or optimal 32,33, if only a few factors with well-known ranges need to be characterized precisely.
Set up the DoE with appropriate software, ensuring a priori quality criteria such as the fraction of design space are met 20.
2. Prepare the Flocculation Experiments
 Figure 1: Plant extract flocculation workflow: process scale (left) and benchtop scale (right). Following protein extraction with aqueous buffers, dispersed particles of cell debris are aggregated by the addition of flocculants. The aggregates are then removed by a cascade of bag and depth filtration and the capacity of these filters along with the filtrate turbidities can be used directly to measure the efficiency of flocculation.
Figure 1: Plant extract flocculation workflow: process scale (left) and benchtop scale (right). Following protein extraction with aqueous buffers, dispersed particles of cell debris are aggregated by the addition of flocculants. The aggregates are then removed by a cascade of bag and depth filtration and the capacity of these filters along with the filtrate turbidities can be used directly to measure the efficiency of flocculation.
- Develop a General Experimental Work Flow (Figure 1).
- Use an extraction device that results in the same particle size distribution expected (or already observed) at the final application scale, e.g. a specialized homogenizer. If possible, design a scale-down model of the extractor as described for the homogenization of tobacco leaves 10.
- Define the extract volumes used during flocculation experiments (here 20 ml). Select a volume that allows a representative number of particles to be present in the reaction vessel, e.g. flocculation experiments in 20 ml aliquots yielded reproducible and scalable results for tobacco extracts containing ~7% [w/v] solids 34 and particle sizes of ~0.5 µm to ~3 mm 16.
- Design all monitoring and post-flocculation operations so that they are representative of the final application scale, e.g. select filters with the same particle retention behavior as those used at the production scale.
- Cultivate the Plants from Which the Extracts Will be Derived.
- Use the same plant line, here Nicotiana tabacum cv. Petit Havana SR1, and cultivation conditions that will be used during production as previously described 33.
- If other feed stocks will be processed, prepare these feed stocks so that they are representative of the final application scale, e.g. use authentic buffers, take into account any dilution steps during the process and stick to the anticipated pH and conductivity, here pH 7.5 and conductivity 30 mS cm-1.
- Prepare Filtration Utilities, Turbidity-Monitoring Devices and Sampling Vessels.
- Cut the filter materials to the required sizes, here 15 x 15 cm2, if they are not ready-to-use modules. Ensure all monitoring and analysis devices are functional and label all sample tubes.
- Even though these are simple tasks, prepare these items in time to ensure that they will not cause interruptions during the actual flocculation experiments. Avoid delays because they can interfere with the results given that flocculation is a time-dependent process.
- Prepare Flocculant Stock Solutions. CAUTION: Wear appropriate personal protection equipment when handling flocculants, e.g. gloves. The materials can be dangerous (hazard labels according to Europe UE 67/548/CEE, 1999/45/CE include N, Xi or Xn). Avoid dust and refer to the material safety data sheets. Work under a fume hood.
- Choose a stock concentration for each flocculant, here 80.0 g L-1 for the two PEI flocculants that were used. Select concentrations as high as possible to avoid sample dilution when adding the flocculant, which would reduce particle concentration and thus affect flocculation efficiency.
- Account for any pre-dilution that reflects the manufacturer's formulation of the polymer, e.g. if the polymer is already supplied as an aqueous 50% [w/v] solution. Flocculant stock solutions of 4-8% [w/v] were found to be the most suitable so far.
- Bearing in mind the points discussed above, make sure the flocculant concentration does not generate a highly viscous solution that inhibits pipetting, potentially causing errors because the final flocculant concentration is not adjusted correctly.
- Use the same stock concentration for all flocculants if possible because this will facilitate the setup of a pipetting scheme (2.5) thus reducing the likelihood of errors.
- Adjust the pH and conductivity of each flocculant stock solution to match the conditions of the extracts, here pH 4−10 and conductivity 15−55 mS cm-1. Prepare individual stocks for a single flocculant if more than one set of pH and/or conductivity conditions is tested for that polymer.
- Prepare flocculant stock solutions freshly, no longer than 48 h before use. Even though flocculation can be induced with polymer stocks stored for more than 4 weeks, the efficiency may decline due to polymer hydrolysis at high or low pH values. See the manufacturer's documentation for further details.
- Make sure that the parameters selected for the DoE can be adjusted precisely for each sample during the experiment, e.g. ensure there are heating/cooling baths available to adjust incubation temperatures, here 4 °C, 20 °C and 37 °C. If mixing during flocculation is part of the experiment, ensure that the mixing device is representative of the final application scale in terms of critical parameters such as power input.
- Convert the DoE Schedule into a Pipetting Scheme.
- Convert the different flocculant concentrations to be tested into volumes of stock solution that will be added to the extract samples: divide the final flocculant concentrations by the stock concentration and multiply by the sample volume used in the flocculation experiments. Identify the largest resulting final volume, e.g. if 20 ml of sample is mixed with a maximum of 2 ml flocculant stock solution this will be 22 ml.
- Calculate volumes of buffer required to maintain the same final volume in all flocculation aliquots based on the largest volume of flocculant stock to be added, e.g. if 0.75 ml of flocculant stock must be added to a sample then 1.25 ml of buffer is required to maintain the final sample volume of 22 ml (2.5.1).
- Sum up the volumes of flocculant stock solution for each polymer and flocculation condition to calculate the absolute volumes of stock solution that are required for the DoE.
- Harvest Tobacco Leaves and Prepare the Extract.
- Remove the top six leaves (or as many as indicated by in the process instructions) from tobacco plants of an appropriate age, e.g. 6 weeks old, and transfer to a suitable extraction device such as a homogenizer or screw press.
- Add three volumes of extraction buffer per gram biomass, e.g. 300 ml per 100 g, and blend for 8 min.
- Prepare individual extracts with the appropriate buffers if different pH and/or conductivities are being tested. Here, this involves homogenizing the plant material for 3 x 30 sec in a blender or a juicer 34.
- Alternatively, prepare the extract in a manner that is representative of the process under investigation.
- Aliquot the Extract and Add the Buffer.
- Manually and thoroughly stir the extract during the entire procedure to ensure the samples are homogeneous with an even distribution of particles.
- Precisely distribute the extract among the pre-labeled reaction tubes by decanting, here 20 ml in each vessel. Self-standing 50 ml tubes simplify handling and are ideal for 20 ml samples.
- Add the volume of each extraction buffer required to maintain the fixed final volume for each individual reaction tube using a suitable pipette.
3. Flocculate the Plant Extracts with Different Polymers
- Pipette the required volume of flocculant stock solution, here 0.1−2.0 ml, to the samples sequentially as indicated by the randomized run order of the DoE. Thoroughly mix each sample immediately after the addition of flocculant by intense manual shaking for precisely 20 sec.
- If required, adjust the shaking time for other feed stocks to ensure thorough mixing, but strictly ensure consistent mixing times for all samples. Bear in mind that prolonged mixing may cause the irreversible disruption of flocs and inconsistently applied force during shaking can distort the flocculation results.
- Optional: Modify the procedure described above for the simultaneous application of two or more flocculants.
- Option 1: Instead of a single polymer add a mixture of two or more flocculants, here PEI and chitosan. Use the flocculant combinations defined in the DoE. Incorporate the ratio and absolute concentrations of the polymers as individual parameters during the DoE setup (1.1).
- Option 2: Add two or more polymers sequentially to the extract.
- Use the individual polymer concentrations, their type, and the incubation time between each addition to the extract, as additional factors during the DoE setup.
- Also use this approach to test whether the repeated addition of a single polymer can improve flocculation. Use a stepwise addition to simulate the slow addition of a flocculant, e.g. four steps, each adding 0.25 ml 80 g L-1 flocculant stock to a 20 ml volume over 4 min can mimic the addition of flocculant with a flow rate of 0.25 ml min-1.
- In all cases, identify a new maximum final sample volume for this setup by adding the maximum volumes of all flocculants to the sample volume, here still 22 ml, and recalculate the required volumes of flocculant stock solutions and buffers for volume adjustment if necessary.
Incubate the samples for the times defined in the DoE, typically 3-30 min, to allow floc formation. Make sure all other incubation conditions, e.g. temperature, are set according to the DoE.
Observe and document floc formation. Record the progress of floc formation as required, e.g. as mm of floc settling per min by measuring the height of the settled solids. If necessary, prolong flocculation for an extended time period such as overnight.
- Filter the Flocculated Extract.
- Use the filter materials prepared earlier (2.3) to clarify the flocculated extract after the appropriate incubation time by decanting the flocculated samples through the filter material and into a clean vessel or reaction tube.
- Do not re-suspend the settled flocs before filtration and apply the extract with at a rate of ~300 ml min-1 to the filter, corresponding to 3−4 sec for a 20 ml sample.
- Confirm the performance of the filtration step if a different material is used in the bench-top experiments compared to the final process in terms of particle retention, e.g. by measuring the particle size distribution in both sample types 11.
- Analyze the filtrate in terms of turbidity and/or particle size distribution as required using appropriate devices, e.g. a turbidimeter.
- Optional: repeat the analysis after extended incubation times, e.g. 12−24 hr, to investigate the formation or reformation of unstable flocs.
- Analyze the Samples in Terms of the Pre-defined Responses (1.1.3).
- Take samples from the filtrates and analyze them for additional response parameters, e.g. the concentrations of different target proteins or valuable products.
- Optional: Analyze the retentate (mostly solids) for the same parameters to close the mass balance of the process. In particular, quantify the effect of flocculants on liquid recovery by comparing the solids mass remaining after samples are treated or not treated with flocculant.
- Confirm the data quality and transfer the results to the DoE software.
- Look for extreme values in the collected response data, e.g. unexpectedly high values.
- Ensure that all response data are correctly aligned with their corresponding experimental conditions.
- Transfer the results into the DoE software and make sure the standard and randomized run orders are not mixed up.
4. Evaluate the DoE
- Use the built-in data analysis tools of the DoE software to develop a predictive model as previously described 20.
- Select a proper data transformation mode if required to facilitate model building, here log10. A ratio larger than 10 for largest vs. smallest response value indicates that a data transformation may be required. Identify the most suitable transformation using the appropriate statistical tools, e.g. a Box-Cox plot 35.
- Chose a base model that (i) fits to the selected DoE (1.2) and (ii) agrees with previous knowledge about the system under investigation based on the analysis of variance (ANOVA) tools of the DoE software, e.g. a second-order polynomial often fits better to the observed effect of incubation time than a first-order polynomial.
- Remove insignificant model factors, e.g. p >0.05, or factor interactions iteratively by deselecting them, thereby reducing the model complexity and increasing its predictive power.
- Confirm the model quality by comparing R2, adjusted R2 and predicted R2 along with the normal probability plot of the studentized residuals, residuals-vs-run, predicted-vs-actual and Box-Cox plots 36. All R2 values should be within a 0.2 range.
- Make sure that the final model agrees with basic assumptions of physics and thermodynamics, e.g. no negative concentrations are predicted.
- Use the model to predict conditions that are most favorable for the system under investigation, here a low turbidity.
- Select the most important responses and their preferred states, e.g. minimal turbidity. Combine these selections by maximizing a desirability function or similar tools, which are built-in features of several DoE software packages 36.
- Depending on the intended application of the model, select confirmation runs to verify the optimal conditions and/or the predictive power of the model in general, e.g. if the conditions predicted to be most favorable were not part of the initial DoE select them as follow-up experiments.
5. Improve the Model and Verify the Predictive Power
Narrow down the initial design space to the most desirable operation conditions, e.g. low turbidity in the 0−1,000 nephelometric turbidity units (NTU) range, based on the model predictions (4.2).
Set up a new DoE within these ranges, including only those factors that have been identified as significant or relevant (4.1.2)). NOTE: A factor can be significant in terms of model analysis but irrelevant for a process, i.e. its effect on a response is <1% compared to that of other factors.
Repeat steps 1-5.2 until the quality of the model predictions matches the requirements, e.g. the standard deviation of the model as indicated by the software is sufficient for the intended application. Modify the experimental strategy if required for these iterations, e.g. use only one flocculant in the refinement.
Transfer and confirm the results from the scale-down model to the pilot scale or final production scale.
Representative Results
Flocculation of tobacco extract with different polymers
The method described above was successfully used to develop a process for the flocculation of tobacco extracts during the manufacture of a monoclonal antibody (the HIV-neutralizing antibody 2G12) and a fluorescent protein (DsRed) (Figure 1) 16, and has since been transferred to other proteins including lectins, malaria vaccine candidates and fusion proteins (unpublished data). Typically, the application of flocculants reduced the turbidity of bag-filtered plant extract from ~6,000 NTU (10,000 NTU after extraction) to ~1,000 NTU. In an initial screening experiment, a 91-run IV-optimal design was used to test 18 different polymers in three different concentrations (because this factor affects flocculation efficiency 13,27) and observed flocculation over a ~12 hr incubation period (Figure 2A and B). The long incubation period can be important to identify meaningful time frames for the flocculation process. Also pH values of 4-8 were tested because these may be relevant in future processes due to the properties of specific target proteins 13,25,27,37. Among the 18 tested polymers, six were found to reduce extract turbidity after bag filtration in typical extracts with a conductivity of 25 mS cm-1.
The model was refined by excluding all ineffective polymers in two iterations and then including additional process parameters, such as conductivity in the 15-45 mS cm-1 range, an incubation time of 5-75 min and temperatures of 4-30 °C, to generate models suitable for a wider range of process conditions. The predictive power of the model increased after each iteration, resulting in a highly reliable model (Figure 3A).
After four iterations, the highly-charged cationic and branched polymer PEI was found to be the most efficient for the aggregation of dispersed particles in tobacco extracts. However, the efficiency of this polymer declined with increasing extract conductivity. The properties molecule size, charge, structure (branched or linear), charge density and degree of amine substitution (primary, secondary, tertiary or quaternary) were tested as factors in a DoE and the last two parameters had the largest effect. The details have been reported elsewhere 16. Based on this knowledge of polymer properties from the DoE results, five other polymers were selected with molecular characteristics similar to PEI (charge density >meq g-1 and quaternary amine). One of these five polymers demonstrated greater flocculation efficiency at higher conductivities (Figure 3B) 11.
As part of the DoE approach, it was confirmed that none of the PEIs affected product recovery under any of the tested conditions. Indeed the capacity of depth filters used subsequently to remove remaining dispersed solids increased by a factor of 3.2-5.7, reaching ~110 L m-2 depending on the filter type. These results were also confirmed in a 100 L pilot-scale process, in for which the application of flocculants reduced the clarification-related production costs by >50% and the total production costs by ~20%.
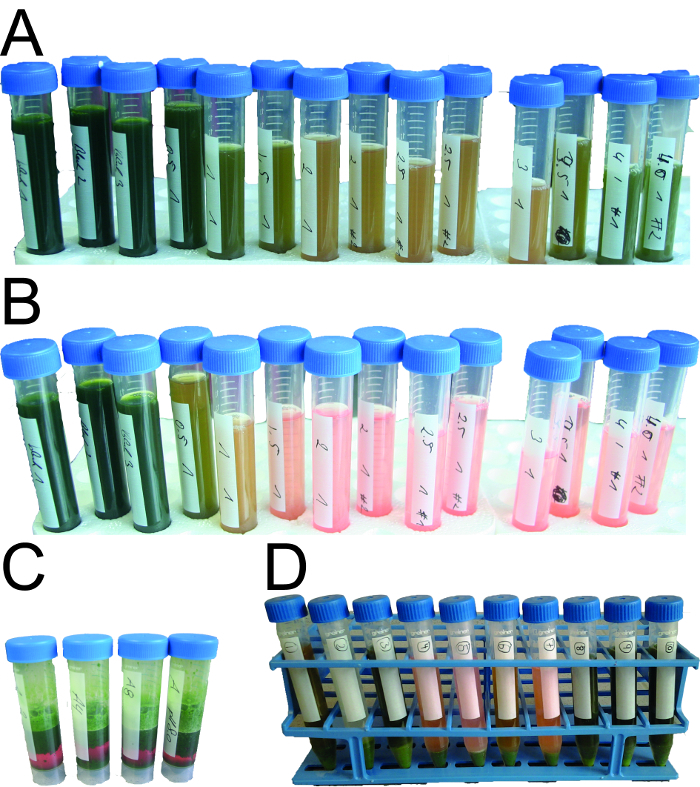 Figure 2: Efficiency of different flocculants under diverse process conditions. (A)Extract samples directly after flocculation and bag filtration can still appear turbid. (B) After settling for several hours, the turbidity of the same samples can be reduced significantly. However, turbidity values obtained immediately after filtration are often preferable because extended hold times may not be possible in large-scale manufacturing processes. (C) Flocculation is also effective when applied to plant extracts generated with a screw press instead of a blender as indicated by the clear red liquid at the bottom of the 50 ml tubes (the red color is due to the presence of the fluorescent protein DsRed). (D) Mixtures of different flocculants can also induce flocculation.
Figure 2: Efficiency of different flocculants under diverse process conditions. (A)Extract samples directly after flocculation and bag filtration can still appear turbid. (B) After settling for several hours, the turbidity of the same samples can be reduced significantly. However, turbidity values obtained immediately after filtration are often preferable because extended hold times may not be possible in large-scale manufacturing processes. (C) Flocculation is also effective when applied to plant extracts generated with a screw press instead of a blender as indicated by the clear red liquid at the bottom of the 50 ml tubes (the red color is due to the presence of the fluorescent protein DsRed). (D) Mixtures of different flocculants can also induce flocculation.
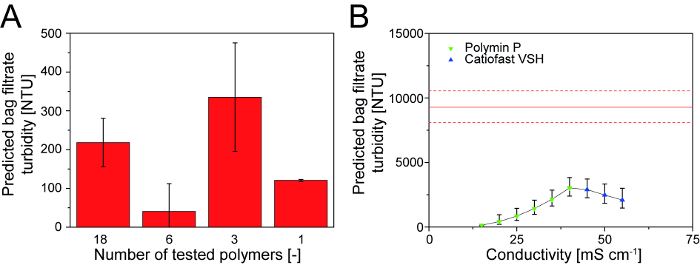 Figure 3: Modeling flocculation using a DoE approach. (A) The accuracy of the model predictions increased as the number of polymers in the model was reduced from initial screening to refinement even though the number of process parameters increased from two to five. (B) Switching the polymer type (here from one PEI to another) as a consequence of a change in process parameters (here conductivity) maintains efficient particle flocculation and corresponding low filtrate turbidity compared to non-treated control extract (solid red line). Error bars in A and B indicate standard deviations of model predictions. Dashed red lines indicate standard deviations of the non-treated extract (n = 10). Please click here to view a larger version of this figure.
Figure 3: Modeling flocculation using a DoE approach. (A) The accuracy of the model predictions increased as the number of polymers in the model was reduced from initial screening to refinement even though the number of process parameters increased from two to five. (B) Switching the polymer type (here from one PEI to another) as a consequence of a change in process parameters (here conductivity) maintains efficient particle flocculation and corresponding low filtrate turbidity compared to non-treated control extract (solid red line). Error bars in A and B indicate standard deviations of model predictions. Dashed red lines indicate standard deviations of the non-treated extract (n = 10). Please click here to view a larger version of this figure.
Flocculation of tobacco extracts prepared with a screw-press
The flocculation results were also transferred from tobacco extracts prepared with a homogenizer to those prepared with a screw-press, which generated fewer dispersed particles in the mm size range but more particles in the µm size range. In a 29-run IV-optimal design, it was shown that PEI is also effective for this type of extract in a similar concentration range and that the recovery of target proteins is not affected (Figure 2C). This shows (i) that flocculation conditions identified for one type of feed stock can be to some extent transferred to other feed stocks, saving time during process development, and (ii) that the DoE strategy can be used to confirm this transferability not only for individual process conditions but over the entire design space.
Flocculation experiments with flocculant mixtures
Combinations of flocculants can be more effective than single polymers, e.g. due to more enhanced bridging between particles 12. Therefore, the method described above was adapted to accommodate the addition of two polymers (3.2) 26. Three non-synthetic polymers were tested alone, in combination with each other or combined with PEI. The most efficient flocculation of tobacco extracts was achieved with PEI alone, but a combination of PEI and chitosan or polyphosphates can reduce the concentration of PEI required. Furthermore, the DoE approach allowed us to identify the most effective polymer combinations when omitting PEI (with or without chitosan and polyphosphates), thus helping to define optimal flocculation conditions in processes where PEI is incompatible with the target protein, e.g. due to precipitation, as reported for βglucuronidase 24,25. Furthermore, the DoE was able to characterize a complex design space for which no mechanistic model was available (Figure 2D). Using the ANOVA tools of the DoE software it was possible to distinguish between reliable predictive models and poorly-evaluated counterparts (Figure 4).
Discussion
The most important aspect to consider when setting up a DoE to characterize particle flocculation is that the design must in principle be able to detect and describe the anticipated or possible effects 36,38, e.g. the influence of pH, polymer type and polymer concentration 16. Therefore, it is important to evaluate the fraction of design space (FDS) before starting the actual experiments. The FDS is the fraction of the multidimensional experimental space (covered by the design factors, e.g. pH) within which it is possible to detect pre-defined differences between two experimental outcomes given a system of known variability, e.g. detecting a difference in turbidity of 250 NTU given a variability of 125 NTU. The FDS can be increased by augmenting the design with additional runs and should be ≥0.95 for designs intended to guide process control 36. Furthermore, if the number of runs does not permit the entire experiment to be carried out in a single day, blocks should be pre-defined in the DoE to account for batch-to-batch and day-to-day variability. When working with plant material, the inclusion of reference runs in each block (e.g. non-treated controls) helps to compensate for variability, allowing the comparison of data from several runs each normalized to their corresponding reference run. In this context, increasing the number of replicate runs in the DoE is also useful.
When large numbers of polymers are screened, it is advisable to use the individual properties of the flocculants, e.g. charge density and molecular mass, as discrete numeric factors rather than the polymers themselves as categorical factors. This reduces the number of experiments because experimental designs often need to be replicated for categorical factors, whereas additional levels of numeric factors only need a small number of extra runs. The information content of the experiment also increases and allows the identification of polymer properties that improve flocculation, e.g. a high charge density as found in the experiments described here. CCD and RSM experimental designs are useful to establish models with high predictive power, allowing the identification of robust processing conditions (e.g. to guide process control) and are typically used to follow up screening designs. If the number of factors and factors levels under investigation results in DoE with more than 400 individual experiments, it may be advisable to reduce the number of factor levels or switch to other design types because the number of sample that can be easily handled with the technique presented here is limited to ~100 per day.
From an experimental point of view, polymers must remain stable under the selected experimental conditions, e.g. they must not depolymerize at low pH. Careful preparation of the flocculant stocks in terms of concentration is also necessary to obtain reproducible results and high-quality models. In this context, the flocculant may need to be pretreated, e.g. swelling times or pH adjustment for chitin, to ensure complete solubilization, and thus to obtain a homogeneous solution. Highly viscous stocks should be avoided because this can cause pipetting errors when transferring the polymer to the extract. Many polymers can have a strong buffering effect and the stocks have extreme pH values, e.g. pH ~9.5 for 8% [w/v] PEI. This can affect the pH of the extract if the stocks are not pre-adjusted and will distort the experimental results. For example, if flocculation is more effective at high pH and a non-pH adjusted PEI stock is used then a DoE might suggest that high PEI concentrations are more effective. However, this effect will be caused by the higher pH caused by the larger volume of stock that was added, not by the increased polymer concentration per se. The stock concentrations used should also resemble those used in large-scale applications to avoid differing dilution effects between the scales that can affect the particle concentration and thus flocculation. Some clay-based flocculants such as kaolin contain a large number of fine particles themselves which can mask the flocculation effect, e.g. turbidity reduction after initial filtration, and other responses should be selected to evaluate the efficiency of these substances, e.g. downstream filter capacity.
For data analysis it is important to evaluate the collected results in terms of extreme values, misalignments and general consistency, e.g. extreme values can indicate a copy-paste error, a shift in the decimal place or a malfunction of equipment/analytical devices. A thorough analysis will ensure that only high-quality data are used for model building. During model building it is important to constantly assess the broad set of quality indicators provided by the DoE software. The most basic criteria are the R2, adjusted R2 and predicted R2 values, but normal residuals, residuals-vs-run and actual-vs-predicted plots (Figure 4) are even more important because they provide information about each run in an experiment rather than a sum parameter. Furthermore, the coherence of the final model and its predictions with the known mechanisms of flocculation should always be investigated. Major discrepancies between predictions and scientific expectations may occur because DoE models are only descriptive rather than mechanistic, e.g. models may predict extreme values at the edges of a design space reflecting the use of polynomial fitting algorithms.
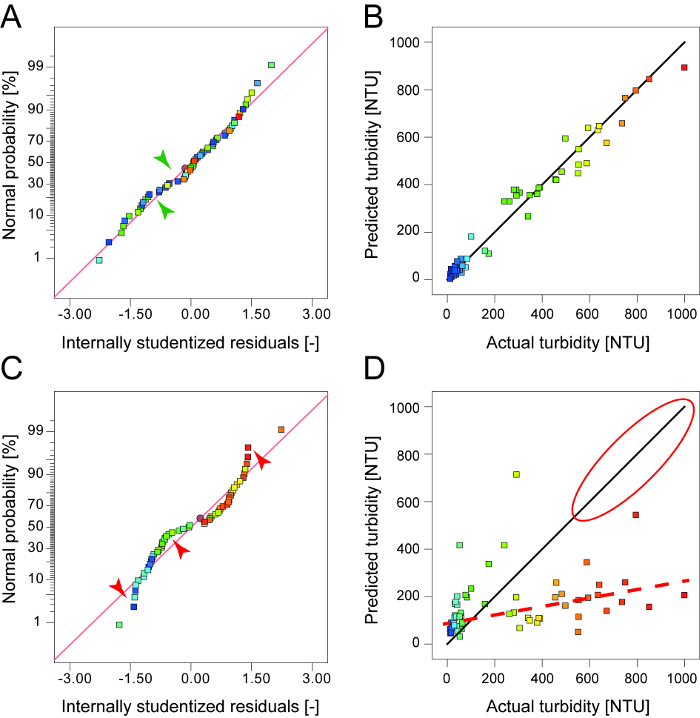 Figure 4: Quality indicators of DoE models. The normal plot of studentized residuals should resemble a straight line as closely as possible (A) with only minor deviations (green arrows) acceptable for high-quality models. A curved appearance (C) with strong deviations (red arrows) from the ideal line (red) indicates a poor model, e.g. due to missing significant factors. Ultimately, the predicted and experimental (actual) values should match (B) and again follow a straight line. Deviations from the ideal line (red circle and dashed line) indicate poor model predictions (D). Please click here to view a larger version of this figure.
Figure 4: Quality indicators of DoE models. The normal plot of studentized residuals should resemble a straight line as closely as possible (A) with only minor deviations (green arrows) acceptable for high-quality models. A curved appearance (C) with strong deviations (red arrows) from the ideal line (red) indicates a poor model, e.g. due to missing significant factors. Ultimately, the predicted and experimental (actual) values should match (B) and again follow a straight line. Deviations from the ideal line (red circle and dashed line) indicate poor model predictions (D). Please click here to view a larger version of this figure.
The DoE approach can help to characterize flocculation in complex feed stocks such as plant extracts, even if there are no existing data. The flocculation of tobacco extracts was optimized with a work load of 2 weeks and consumables costs of ~ €500. This reduced the number of depth filters required for a single pilot-scale batch involving ~800 L of plant extract by 60%, which achieved a corresponding reduction in consumables costs.
The flocculants were also applied to different plant extracts and to cell culture homogenates. Although the same flocculant was effective for all of these feed stocks, the polymer concentration had to be adjusted in order to accommodate the different concentrations of dispersed particles. Additionally, once an effective polymer has been identified, the filtration and/or centrifugation steps may need to be adjusted to match the different particle size distribution 11.
The method described here can easily be adapted to other feed stocks and is therefore also relevant for scientists and engineers developing clarification strategies for mammalian cell cultures and food/feed production processes. Especially plant-based processes will benefit from the intermediate sample volumes suggested here because plant extracts can contain particles up to 1 mm in diameter which are incompatible with microplate formats 21, e.g. because the mixing dynamics differ due to a particle diameter to vessel diameter ratio that is not representative of the process scale.
Disclosures
The author has no conflicts of interest to disclose.
Acknowledgments
I would like to acknowledge Dr. Thomas Rademacher for providing the transgenic tobacco seeds and Ibrahim Al Amedi for cultivating the tobacco plants. I wish to thank Dr. Richard M Twyman for editorial assistance and Prof. Dr. Rainer Fischer for fruitful discussions. This work was funded in part by the European Research Council Advanced Grant ''Future-Pharma'', proposal number 269110, the Fraunhofer-Zukunftsstiftung (Fraunhofer Future Foundation) and the Fraunhofer-Gesellschaft Internal Programs under Grant No. Attract 125-600164.
References
- Godfray HCJ, et al. Food Security: The Challenge of Feeding 9 Billion People. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- Fischer R, Schillberg S, Buyel JF, Twyman RM. Commercial aspects of pharmaceutical protein production in plants. Curr. Pharm. Des. 2013;19:5471–5477. doi: 10.2174/1381612811319310002. [DOI] [PubMed] [Google Scholar]
- Pastores GM, et al. A Phase 3, multicenter, open-label, switchover trial to assess the safety and efficacy of taliglucerase alfa, a plant cell-expressed recombinant human glucocerebrosidase, in adult and pediatric patients with Gaucher disease previously treated with imiglucerase. Blood Cells Mol. Dis. 2014;53:253–260. doi: 10.1016/j.bcmd.2014.05.004. [DOI] [PubMed] [Google Scholar]
- De Paepe D, et al. A comparative study between spiral-filter press and belt press implemented in a cloudy apple juice production process. Food Chem. 2015;173:986–996. doi: 10.1016/j.foodchem.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Twyman RM, Fischer R. Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol. Adv. 2015;33:902–913. doi: 10.1016/j.biotechadv.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Wilken LR, Nikolov ZL. Recovery and purification of plant-made recombinant proteins. Biotechnol. Adv. 2012;30:419–433. doi: 10.1016/j.biotechadv.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Buyel JF. Process development trategies in plant molecular farming. Curr. Pharm. Biotechnol. 2015;16:966–982. doi: 10.2174/138920101611150902115413. [DOI] [PubMed] [Google Scholar]
- Hassan S, Keshavarz-Moore E, Ma J, Thomas C. Breakage of transgenic tobacco roots for monoclonal antibody release in an ultra-scale down shearing device. Biotechnol. Bioeng. 2014;111:196–201. doi: 10.1002/bit.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S, van Dolleweerd CJ, Ioakeimidis F, Keshavarz-Moore E, Ma JK. Considerations for extraction of monoclonal antibodies targeted to different subcellular compartments in transgenic tobacco plants. Plant Biotechnol. J. 2008;6:733–748. doi: 10.1111/j.1467-7652.2008.00354.x. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Fischer R. Scale-down models to optimize a filter train for the downstream purification of recombinant pharmaceutical proteins produced in tobacco leaves. Biotechnol. J. 2014;9:415–425. doi: 10.1002/biot.201300369. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Fischer R. Downstream processing of biopharmaceutical proteins produced in plants: the pros and cons of flocculants. Bioengineered. 2014;5:138–142. doi: 10.4161/bioe.28061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J, Barany S. Adsorption and flocculation by polymers and polymer mixtures. Adv. Colloid Interface Sci. 2011;169:1–12. doi: 10.1016/j.cis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Franks GV. Flocculation mechanism induced by cationic polymers investigated by light scattering. Langmuir. 2006;22:6775–6786. doi: 10.1021/la060281+. [DOI] [PubMed] [Google Scholar]
- Runkana V, Somasundaran P, Kapur PC. Mathematical modeling of polymer-induced flocculation by charge neutralization. J. Colloid Interface Sci. 2004;270:347–358. doi: 10.1016/j.jcis.2003.08.076. [DOI] [PubMed] [Google Scholar]
- Hjorth M, Jorgensen BU. Polymer flocculation mechanism in animal slurry established by charge neutralization. Water Res. 2012;46:1045–1051. doi: 10.1016/j.watres.2011.11.078. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Fischer R. Flocculation increases the efficacy of depth filtration during the downstream processing of recombinant pharmaceutical proteins produced in tobacco. Plant Biotechnol. J. 2014;12:240–252. doi: 10.1111/pbi.12132. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Opdensteinen P, Fischer R. Cellulose-based filter aids increase the capacity of depth filters during the downstream processing of plant-derived biopharmaceutical proteins. Biotechnol. J. 2014;10:584–591. doi: 10.1002/biot.201400611. [DOI] [PubMed] [Google Scholar]
- Yasarla LR, Ramarao BV. Dynamics of Flocculation of Lignocellulosic Hydrolyzates by Polymers. Ind. Eng. Chem. Res. 2012;51:6847–6861. [Google Scholar]
- Montgomery DC. Design and Analysis of Experiments. John Wiley & Sons Incorporated; 2007. [Google Scholar]
- Buyel JF, Fischer R. Characterization of complex systems using the design of experiments approach: transient protein expression in tobacco as a case study. J. Vis. Exp. 2014. p. e51216. [DOI] [PMC free article] [PubMed]
- Espuny Garcia Del Real G, Davies J, Bracewell DG. Scale-down characterization of post-centrifuge flocculation processes for high-throughput process development. Biotechnol. Bioeng. 2014;111:2486–2498. doi: 10.1002/bit.25313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore AS, Sofer G. Process Validation in Manufacturing of Biopharmaceuticals, 3rd edn, Vol. 1. Taylor & Francis; 2012. [Google Scholar]
- Kang Y, et al. Development of a Novel and Efficient Cell Culture Flocculation Process Using a Stimulus Responsive Polymer to Streamline Antibody Purification Processes. Biotechnol. Bioeng. 2013;110:2928–2937. doi: 10.1002/bit.24969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkhaus TJ, Eriksson SU, Whitson PB, Glatz CE. Host selection as a downstream strategy: Polyelectrolyte precipitation of beta-glucuronidase from plant extracts. Biotechnol. Bioeng. 2002;77:148–154. doi: 10.1002/bit.10135. [DOI] [PubMed] [Google Scholar]
- Holler C, Vaughan D, Zhang CM. Polyethyleneimine precipitation versus anion exchange chromatography in fractionating recombinant beta-glucuronidase from transgenic tobacco extract. J. Chromatogr. A. 2007;1142:98–105. doi: 10.1016/j.chroma.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Fischer R. Synthetic polymers are more effective than natural flocculants for the clarification of tobacco leaf extracts. J. Biotechnol. 2014;195:37–42. doi: 10.1016/j.jbiotec.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Pearson CR, Heng M, Gebert M, Glatz CE. Zeta potential as a measure of polyelectrolyte flocculation and the effect of polymer dosing conditions on cell removal from fermentation broth. Biotechnol. Bioeng. 2004;87:54–60. doi: 10.1002/bit.20097. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Gruchow HM, Boes A, Fischer R. Rational design of a host cell protein heat precipitation step simplifies the subsequent purification of recombinant proteins from tobacco. Biochem. Eng. J. 2014;88:162–170. [Google Scholar]
- Wang S, Liu C, Li Q. Impact of polymer flocculants on coagulation-microfiltration of surface water. Water Res. 2013;47:4538–4546. doi: 10.1016/j.watres.2013.04.040. [DOI] [PubMed] [Google Scholar]
- Menkhaus TJ, Anderson J, Lane S, Waddell E. Polyelectrolyte flocculation of grain stillage for improved clarification and water recovery within bioethanol production facilities. Bioresour. Technol. 2010;101:2280–2286. doi: 10.1016/j.biortech.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Mune MAM, Minka SR, Mbome IL. Optimising functional properties during preparation of cowpea protein concentrate. Food Chem. 2014;154:32–37. doi: 10.1016/j.foodchem.2013.12.108. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Fischer R. Predictive models for transient protein expression in tobacco (Nicotiana tabacum L.) can optimize process time, yield, and downstream costs. Biotechnol. Bioeng. 2012;109:2575–2588. doi: 10.1002/bit.24523. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Kaever T, Buyel JJ, Fischer R. Predictive models for the accumulation of a fluorescent marker protein in tobacco leaves according to the promoter/5'UTR combination. Biotechnol. Bioeng. 2013;110:471–482. doi: 10.1002/bit.24715. [DOI] [PubMed] [Google Scholar]
- Buyel JF, Fischer R. A juice extractor can simplify the downstream processing of plant-derived biopharmaceutical proteins compared to blade-based homogenizers. Process Biochem. 2014;50:859–866. [Google Scholar]
- Anderson MJ, Whitcomb PJ. DOE Simplified: Practical Tools for Effective Experimentation. Vol. 1. Vol. 1. Taylor & Francis; 2000. [Google Scholar]
- Anderson MJ, Whitcomb PJ. Response Surface Methods Simplified. Productivity Press; 2005. [Google Scholar]
- Buyel JF, Fischer R. Generic chromatography-based purification strategies accelerate the development of downstream processes for biopharmaceutical proteins produced in plants. Biotechnol. J. 2014;9:566–577. doi: 10.1002/biot.201300548. [DOI] [PubMed] [Google Scholar]
- Myers RH, Montgomery DC, Anderson-Cook CM. Response Surface Methodology: Process and Product Optimization Using Designed Experiments. Wiley; 2009. [Google Scholar]


