Abstract
Background Infections of wild birds with highly pathogenic avian influenza (AI) subtype H5N1 virus were reported for the first time in the European Union in 2006.
Objectives To capture epidemiological information on H5N1 HPAI in wild bird populations through large‐scale surveillance and extensive data collection.
Methods Records were analysed at bird level to explore the epidemiology of AI with regard to species of wild birds involved, timing and location of infections as well as the applicability of different surveillance types for the detection of infections.
Results In total, 120,706 records of birds were sent to the Community Reference Laboratory for analysis. Incidents of H5N1 HPAI in wild birds were detected in 14 EU Member States during 2006. All of these incidents occurred between February and May, with the exception of two single cases during the summer months in Germany and Spain.
Conclusions For the detection of H5N1 HPAI virus, passive surveillance of dead or diseased birds appeared the most effective approach, whilst active surveillance offered better detection of low pathogenic avian influenza (LPAI) viruses. No carrier species for H5N1 HPAI virus could be identified and almost all birds infected with H5N1 HPAI virus were either dead or showed clinical signs. A very large number of Mallards (Anas platyrhynchos) were tested in 2006 and while a high proportion of LPAI infections were found in this species, H5N1 HPAI virus was rarely identified in these birds. Orders of species that appeared to be very clinically susceptible to H5N1 HPAI virus were swans, diving ducks, mergansers and grebes, supporting experimental evidence. Surveillance results indicate that H5N1 HPAI virus did not establish itself successfully in the EU wild bird population in 2006.
Keywords: Avian influenza, European Union, H5N1 HPAI, surveillance, wild birds
Introduction
Wild birds have been known to be a reservoir of low pathogenic avian influenza (LPAI) viruses. 1 From 1961 when the first highly pathogenic avian influenza (HPAI) infection in wild birds (terns) in South Africa was recorded 2 until almost 45 years later, when in connection with the H5N1 HPAI panzootic in Asia more frequent reports of H5N1 HPAI in wild birds occurred, reports of HPAI infections in wild birds were extremely limited and linked to outbreaks in poultry. However, since the start of outbreaks of H5N1 HPAI in Asia, the reports of infections with this subtype in wild birds have become more frequent. 3 The report of an H5N1 HPAI outbreak in wild birds at lake Qinghai in 2005 where over 5000 Bar headed geese (Anser indicus) died of the infection demonstrated that wild birds may be affected on a larger scale by the ongoing panzootic in Asia and that these epidemics may have a large impact on some species. 4 The continuing north‐western spread of the panzootic and existing evidence that certain species of ducks are able to shed avian influenza (AI) viruses without showing signs of disease 5 has led to the hypothesis that H5N1 HPAI infection could possibly be carried over long distances by wild migrating birds. 3 Nevertheless, all existing reports of this infection in wild birds in the field have indicated a high pathogenicity and mortality even in duck species.
The main value of surveillance in wild birds conducted in the European Union (EU) before the emergence of the H5N1 HPAI panzootic in Asia was the detection of LPAI viruses of H5 and H7 subtypes which when infecting poultry can potentially mutate into HPAI viruses.
The first official EU (15 Member States [MS] at that time) surveys for AI in wild birds were carried out under Commission Decision 2002/649/EC 6 on a voluntary basis, although wild bird surveillance was already in place in several EU MS prior to this time. 7 , 8 , 9 , 10 In that year 11 MS participated in these surveys based on the EU guideline recommendation of targeting 70% waterfowl, 20% shorebirds and 10% other wild bird species. Between 2003 and 2004 relatively low numbers of wild birds were tested for AI in some of the EU MS (3829 birds in 2003 from 11 EU MS resulting in nine AI positives [no H5, six H7 positive], 8943 specimens in 2004 yielding 214 positives [15 H5 and seven H7 positives]). The number of sampled birds increased sixfold to 47 232 tested birds in 2005 when all EU MS conducted wild bird surveillance. In that year 1315 samples tested positive for AI virus of which 156 were positive for subtype H5 and nine for H7. 11
In 2005, due to the continued evolution of the H5N1 HPAI epidemic in Asia, the EU intensified wild bird surveillance and amended the more general existing guidelines (Decision 2005/464/EC) 12 with more specific surveillance guidelines (Decision 2005/726/EC). 13 Surveillance was then recommended to consist of active and passive surveillance programmes and to be risk‐based targeting 15 bird species considered of higher risk (HRS) of introducing the virus to poultry holdings based on their migratory movements and likelihood of contact with poultry. Sampling of HRS was recommended to be enhanced in key locations on migratory flyways of species proceeding from countries outside the EU where outbreaks in wild birds and poultry occurred and where mixing of several species takes place, as well as in the vicinity of poultry farms. Up to 2006 the data collection and analysis of wild bird AI surveillance data at EU level had been very limited.
In response to outbreaks with the ‘Asian’ lineage H5N1 HPAI viruses in several EU MS, surveillance in wild birds became compulsory in February 2006 by Decision 2006/101/EC. 14 In May 2006, the European Food Safety Authority (EFSA) published an updated list of HRS 15 to improve the targeting of H5N1 HPAI surveillance. This list was established by evaluating migratory species of the orders Anseriformes (ducks, geese and swans) and Charadriiformes (waders and gulls) against several criteria: gregariousness during migration/wintering periods (group size and group density), degree of mixing during migration or wintering periods, main habitat during migration or wintering periods, and the degree of mixing with other species. The list included 14 of the 15 species listed in Decision 2005/464/EC, 12 as well as 13 additional species, and was integrated into the new EU guidelines for AI surveillance in 2007. 16 In addition, extended data collection was implemented by the European Commission (EC) and a database was established to capture epidemiological information on collected birds such as location, species of bird and status at the time of sampling. The objectives of the analysis of the available data were to explore the epidemiology of AI with regard to species of wild birds involved, timing and location of infections as well as the applicability of different surveillance types for the detection of infections. Here we present the 2006 surveillance results and discuss the implications for surveillance and early detection.
Materials and methods
Selection and sampling of birds
Although national surveillance programmes in the EU are very diverse, in principle four types of wild bird surveillance are in place:
-
•
active surveillance, focusing on the testing of live caught birds, mostly targeted towards HRS and/or higher risk areas;
-
•
active surveillance of hunted birds (mainly waterfowl);
-
•
passive surveillance through the sampling of birds found dead or diseased that may be in association with incidents of unusually high mortality and/or morbidity;
-
•
sentinel surveillance, most frequently using ducks kept in higher risk areas that were submitted to regular testing.
Details of national survey programmes are available at http://ec.europa.eu/food/animal/diseases/eradication/2006_314_ec.pdf. As the relevant legislation came into effect in February 2006, we report here on data collected from 1 February to 31 December 2006.
Data capture
For each sampled bird the following information was requested; type of samples collected (tracheal/cloacal swab, tissue or faeces), date and location of sample collection, bird species, location of sampling in relation to EU veterinary control measures, namely the control/monitoring areas established around positive findings in wild birds required in Decision 2006/563/EC 17 or protection/surveillance zones on account of outbreaks in poultry based on Decision 2006/415/EC 18 or outside restricted areas. In addition, the state of the bird at the time of sampling (diseased, hunted, live or found dead), laboratory test results including PCR, virus isolation and AI subtype identity, and, for H5/H7 subtypes, pathogenicity characteristics were recorded.
Laboratory testing
All laboratory tests were carried out in accordance with the EU AI diagnostic manual established in Commission Decision 2006/437/EC, 19 recommending initial testing with the M gene real‐time RT‐PCR (to detect presence of influenza A virus) and rapid testing of positives for H5/H7 using real‐time RT‐PCR as well as analysis of the haemagglutinin cleavage site following conventional PCR and nucleotide sequencing according to standard methods to determine the pathogenicity of the AI virus. 19 Testing strategies and approaches varied between MS with targeted testing for the detection of H5 and H7 subtypes only in some countries, as only these subtypes have been shown to mutate to higher pathogenicity following introduction to domestic poultry. In these circumstances M gene screening by PCR was only subjected to follow‐up testing for H5 and H7. In other programmes M gene‐positive samples were subjected to full testing to identify the virus subtype. If alternative tests were used, the test validation data were first approved by the European Community Reference Laboratory (CRL). All tests were carried out at National Reference Laboratories (NRL) for AI or by other laboratories authorized by competent authorities and under the control of the NRL.
Data analysis
The results presented are restricted to data that were collected and submitted to the EC under Decision 2006/101/EC 14 in the required format. Data reported in an inconsistent format were excluded. Therefore differences to other reporting systems such as the Animal Disease Notification System (ADNS: http://ec.europa.eu/food/animal/diseases/adns/index_en.htm) occur for some countries. For example, Germany reported 270 H5N1 HPAI‐infected birds in addition to the 71 H5N1 HPAI‐infected birds reported here. The additional German H5N1 HPAI‐positive samples originated from 41 different species: The majority (51%, 137 birds) of these H5N1 HPAI infections were found in swans (Cygnus spp.) Of the remaining H5N1 HPAI‐positive cases 10% were buzzards (Buteo spp.), 9·6% diving ducks (Aythya spp.), 7% dabbling ducks (Anas spp.) and 3·7% gulls (Larus spp.) All data records submitted to the CRL were checked for data quality and completeness. The data presented in this paper were submitted from the following EU MS: Austria (AT), Belgium (BE), Bulgaria (BG), Cyprus (CY), Czech Republic (CZ), Denmark (DK), Estonia (EE), Finland (FI), France (FR), Germany (DE), Greece (EL), Hungary (HU), Ireland (IE), Italy (IT), Latvia (LV), Lithuania (LT), Luxemburg (LU), Malta (MT), The Netherlands (NL), Poland (PL), Portugal (PT), Sweden (SE), Slovak Republic (SK), Slovenia (SI) and the United Kingdom (UK).
Calculated proportions for H5N1 HPAI were restricted to denominator data for the period February to May 2006 in accordance with the duration of European incidents. A test‐positive bird is defined here as a bird from which at least one sample tested positive on PCR or virus isolation.
The list of HRS used is based on the species identified by EFSA 15 and recommended in the 2006 survey guidelines that were subsequently incorporated in Decision 2007/268/EC. 16 The proportion test function in the statistical software package R 20 was used to test for differences between positive proportions obtained from different surveillance activities with P‐values below 0·05 considered significant. Maps were produced using the Arcmap function of ArcGIS, version 9.1. 21 Display of sampling density at ADNS (using administrative territorial region) level was limited to countries for which at least 85% of the records contained such information. If this was not the case, sampling densities were displayed at an averaged national level. Missing spatial data and/or date information for H5N1 HPAI‐positive cases was supplemented through the use of data from other sources such as the ADNS and OIE reports. A circular statistic approach was applied to evaluate the observed prevalence according to time. The Rayleigh test was applied to the mean vector value. 22 To verify whether monthly prevalence differs between southern and northern Europe, Watson F‐tests were applied. 22
Results
The complete surveillance report containing detailed information regarding all species sampled and MS specific information is available at: (http://ec.europa.eu/food/animal/diseases/controlmeasures/avian/annrepres_surv_wb_02‐12‐2006_en.pdf).
Sampling
Between February and December 2006, results of tests on 120 706 birds were reported to the EU CRL in the required data format, a threefold increase on the 2005 total. The number of birds sampled per 100 km2 for active and passive surveillance is displayed in 1, 2 respectively. Over half of the birds (55%) were sampled between February and May 2006.
Figure 1.
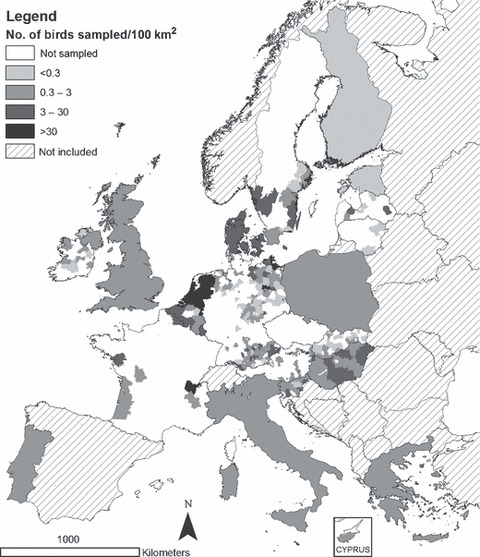
Number of wild birds sampled through active surveillance of live caught or hunted birds per 100 km2 in 2006.
Figure 2.
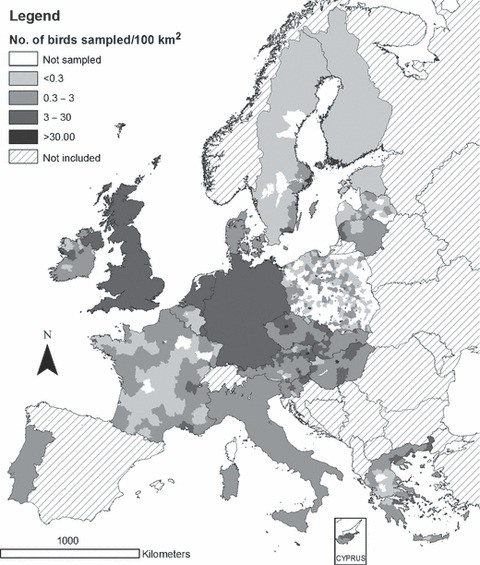
Number of wild birds sampled through passive surveillance of dead or diseased birds per 100 km2 in 2006.
During the year the focus of surveillance shifted from passive surveillance (testing dead and diseased birds) to active surveillance (testing live and hunted birds) (Figure 3). This shift is probably explained through several factors such as the general targeting of active surveillance to autumn migration, decreased mortalities of wild birds after cold spells declined and possibly decreased awareness of the public after the incidents of H5N1 HPAI ceased. Overall, proportions of birds sourced through active surveillance varied between MS from 0% (CZ) to 95% (BE).
Figure 3.

Number and proportion of total birds sampled by origin and season.
Samples were collected from at least 330 bird species of 22 orders. The order most frequently sampled was Anseriformes (53%) followed by Charadriiformes (10%) (Table 1). Of the EU total, 51% were HRS with national proportions ranging from 4% (BG) to 70% (UK). Sampling frequency was skewed between species and, of the EU total; almost 50% of sampled birds comprised 10 species (Table 2).
Table 1.
Number of birds sampled and positive in EU MS in 2006 according to order of birds
| Order | Number sampled in 2006 | Number sampled February to May | Number (proportion) positive H5N1 HPAI | Number (proportion) positive LPAI |
|---|---|---|---|---|
| Anseriformes | 64 487 | 30 481 | 535 (1·8) | 1428 (2·2) |
| Charadriiformes | 12 527 | 5581 | 5 (0·1) | 99 (0·8) |
| Passeriformes | 8961 | 6278 | 1 (0·02) | 8 (0·1) |
| Falconiformes | 6845 | 6203 | 18 (0·3) | 8 (0·1) |
| Ciconiiformes | 4550 | 3825 | 5 (0·1) | 6 (0·1) |
| Columbiformes | 4043 | 2684 | 0 | 6 (0·2) |
| Gruiformes | 3714 | 2759 | 2 (0·1) | 15 (0·4) |
| Galliformes | 3099 | 1143 | 0 | 0 |
| Pelecaniformes | 1504 | 1055 | 2 (0·2) | 3 (0·2) |
| Strigiformes | 1023 | 924 | 4 (0·4) | 4 (0·4) |
| Podicipediformes | 310 | 248 | 4 (1·6) | 3 (1) |
| Phoenicopteriformes | 308 | 15 | 0 | |
| Piciformes | 72 | 54 | 0 | 0 |
| Procellariiformes | 44 | 37 | 0 | 0 |
| Coraciiformes | 40 | 22 | 0 | 0 |
| Apodiformes | 28 | 10 | 0 | 0 |
| Gaviiformes | 23 | 12 | 0 | 0 |
| Threskiornithidae | 18 | 18 | 0 | 0 |
| Cuculiformes | 12 | 3 | 0 | 0 |
| Psittaciformes | 6 | 2 | 0 | 0 |
| Accipitriformes | 4 | 3 | 0 | 0 |
| Caprimulgiformes | 3 | 3 | 0 | 0 |
| Unknown | 9085 | 5312 | 15 (0·3) | 35 (0·4) |
| Total | 120 706 | 66 672 | 591 | 1615 |
Values within parenthesis are expressed in percentage.
Table 2.
The 10 most frequently sampled species in the 2006 EU wild bird avian influenza survey
| Rank | Species | Number sampled | Proportion of total birds sampled, % |
|---|---|---|---|
| 1 | Mallard (Anas platyrhynchos) | 28 313 | 23·5 |
| 2 | Mute Swan (Cygnus olor) | 8239 | 6·8 |
| 3 | Black‐headed Gull (Larus ridibundus) | 4303 | 3·6 |
| 4 | Common Buzzard (Buteo buteo) | 3597 | 3 |
| 5 | White fronted Goose (Anser a. albifrons) | 3058 | 2·5 |
| 6 | Barnacle Goose (Branta leucopsis) | 2671 | 2·2 |
| 7 | Grey Heron (Ardea cinera) | 2670 | 2·2 |
| 8 | Coot (Fulica atra) | 2494 | 2·1 |
| 9 | Wigeon (Anas penelope) | 2485 | 2·1 |
| 10 | Pheasant (Phasianus colchicus) | 2292 | 1·9 |
Those species with the highest proportions of their biogeographical population actively or passively sampled were Mute (Cygnus olor) or Whooper Swans (Cynus Cygnus) with over 6% of the British Mute Swan population and 2·3% of the north‐west and central European populations sampled. The proportion of the biogeographical population sampled was also high for northern mainland and Icelandic Whooper swans and the Central European White‐fronted Geese (Anser a. albifrons). No other species were sampled for influenza at a rate exceeding 1% of the estimated population. The species sampled in the highest numbers (28 313) was the Mallard (Anas platyrhynchos), with over 0·5% (24 297) of the NW European population tested.
H5N1 HPAI
The first outbreaks of H5N1 HPAI in wild birds in the EU occurred in early February 2006, with the first case being detected in a dead mute swan in EL. The early outbreaks were widespread geographically and affected a low number of birds and species. However, following subsequent north‐west spread, detections were closer in time and space and in some areas they persisted longer (Figure 4), especially in the area of the borders between DE and AT and in the Baltic seas area in the north‐east of DE. It is thought that harsh weather conditions with very low temperatures had forced wild birds off their normal flyways and that the epidemic was propagated through a higher density of wild birds in waters that remained unfrozen.
Figure 4.
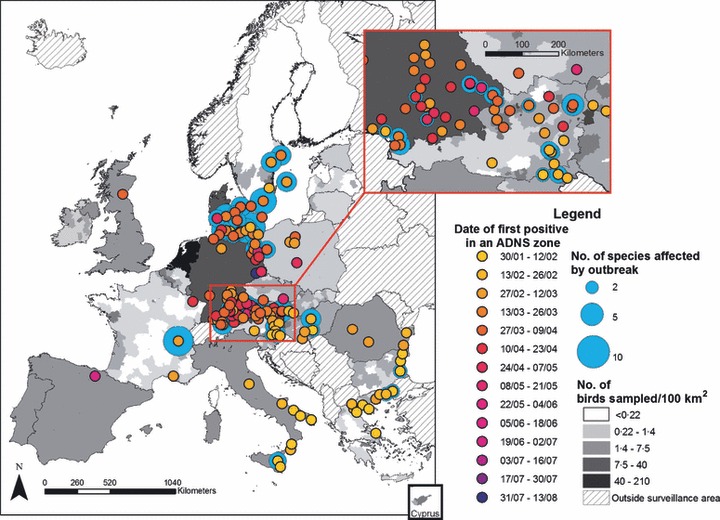
Location, number of species involved and date of H5N1 HPAI wild bird incidents in 2006.
Between February and May 13 MS reported 861 cases of H5N1 HPAI to the CRL (591 cases in the present study) and the ADNS from at least 32 species (the species/order of 7·5% of these birds was not known). Two additional single cases of H5N1 HPAI occurred during the summer months: one in a Great‐Crested Grebe (Podiceps cristatus) in northern Spain and another in a captive Black Swan (Cygnus atratus) at Dresden Zoo, DE. 23
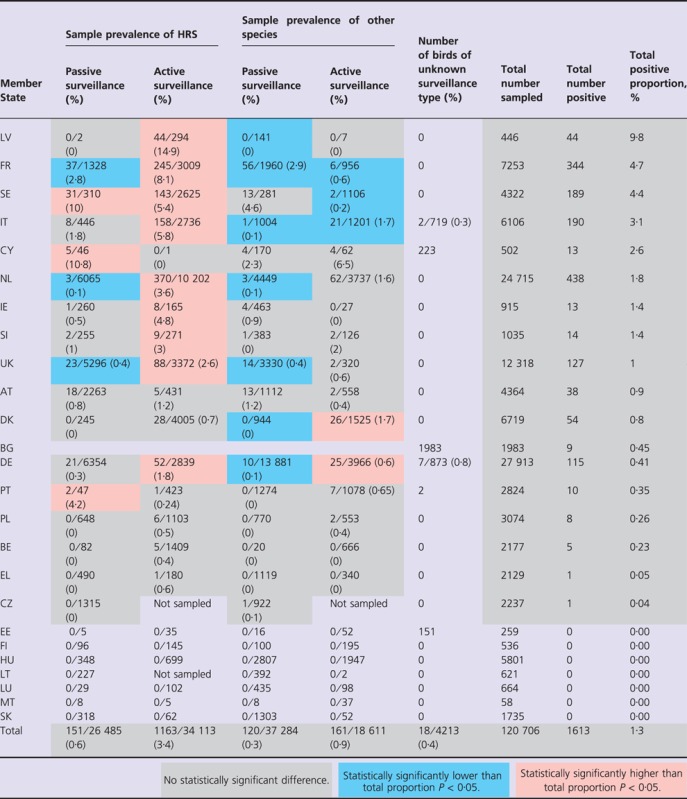
In MS's where H5N1 HPAI incidents occurred, the national proportion of birds that tested positive for H5N1 HPAI between February and May varied from 0·01% in the UK to 7·4% in SI (Table 3). With the exception of 39 Mute Swans (Cygnus olor) in PL and one Herring Gull (Larus argentatus) in DK, all H5N1 HPAI‐positive birds were either found dead or diseased. In contrast, only 17% (271/1615) of birds testing positive for influenza A viruses other than H5N1 HPAI were found dead or showed signs of disease. The differences in the sample prevalence of H5N1 HPAI as detected by different methods of surveillance are highlighted in Table 3.
Table 3.
Number of birds sampled and positive for H5N1 HPAI according to surveillance type and MS between February and May 2006
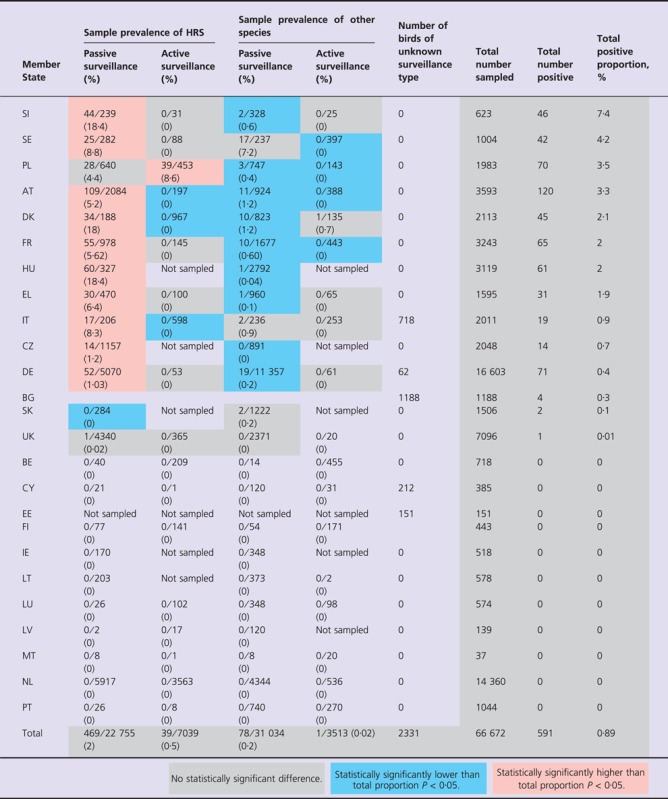
Most H5N1 HPAI infections were detected in Anseriformes (Table 1). Other orders with comparatively high prevalence amongst the sampled birds were grebes (Podicipediformes) (4/248 – 1·61%), owls (Strigiformes) (4/924 – 0·43%) and falcons (Falconiformes) (18/6203 – 0·29%). Although shorebirds (Charadriiformes) and perching birds (Passeriformes) were sampled in relatively high numbers, only a very low proportion tested positive [5/5581 (0·09%) and 1/6278 (0·02%) respectively, see Table 1].
In all EU MS with outbreaks of H5N1 HPAI (with the exception of SK, where only two H5N1 HPAI cases occurred and the single cases in DE and Spain in the summer) swans (Cygnus spp.) tested positive for this subtype and, with very few exceptions, swans were the first infected wild birds to be detected in the incidents. Over 70% (416/591) of all H5N1 HPAI positive cases were identified in swans and 6·8% (416/6102) of sampled swans tested positive for H5N1 HPAI at the time of the incidents. Tufted ducks (Aythya fuligula) were sampled in varying numbers (range = 1–132) in 14 MS (Table 4). Of this species, 19·8% (48/242) tested positive for H5N1 HPAI between February and May. The proportion of H5N1 HPAI‐infected Tufted ducks was especially high in DK (26/39 – 72%) and SE (19/79 – 24%) where Tufted ducks accounted for 57% (26/45) (DK) and 45% (19/42) (SE) of all H5N1 HPAI infections. These results were much higher than in other MS, where swans (Cygnus spp.) were the predominantly affected genus. Sawbills of the genus Mergus, which are not HRS, were tested in very low numbers (99) between February and May but a high proportion of positives were found: 13% (12/92) in Goosander and 29% in Smew (2/7) (Table 5).
Table 4.
Number of wild birds tested and proportion positive for H5N1 HPAI and other subtypes by species and MS for higher risk species (HRS)
| HRS species | Number (proportion) of | ||||||
|---|---|---|---|---|---|---|---|
| MS | Birds | ||||||
| H5N1 HPAI positive | Other positive | Sampled 2006 | H5N1 HPAI positive (%) | Other positive (%) | Sampled 2006 | Feb–May sampled | |
| Mute Swan (Cygnus olor) | 11 | 9 | 21 | 372 (6·9) | 44 (0·5) | 8239 | 5379 |
| Tundra Swan (Cygnus columbianus) | 0 | 1 | 6 | 0 (0) | 1 (1·9) | 52 | 3 |
| Whooper Swan (Cygnus cvgnus) | 4 | 5 | 14 | 44 (6·1) | 22 (1·3) | 1741 | 720 |
| Bean Goose (Anser fabalis) | 0 | 3 | 9 | 0 (0) | 13 (1·9) | 679 | 85 |
| Pink‐footed Goose (Anser brachyrhynchus) | 0 | 2 | 4 | 0 (0) | 4 (0·9) | 427 | 198 |
| White‐fronted Goose (Anser albifrons) | 2 | 3 | 12 | 0 (0) | 38 (1·2) | 3058 | 953 |
| Greylag Goose (Anser anser) | 3 | 3 | 17 | 3 (0·4) | 6 (0·3) | 1769 | 732 |
| Canada Goose (Branta Canadensis) | 2 | 3 | 9 | 2 (0·4) | 7 (0·4) | 1854 | 538 |
| Barnacle Goose (Branta leucopsis) | 0 | 0 | 8 | 0 (0) | 0 (0) | 2671 | 2105 |
| Brent Goose (Branta bernicla) | 0 | 1 | 6 | 0 (0) | 1 (0·4) | 271 | 224 |
| Red‐breasted Goose (Branta ruficollis) | 1 | 1 | 3 | 1 (50) | 1 (16·7) | 6 | 2 |
| Wigeon (Anas Penelope) | 0 | 6 | 13 | 0 (0) | 36 (1·4) | 2485 | 578 |
| Common Teal (Anas crecca) | 0 | 8 | 18 | 0 (0) | 88 (4·6) | 1900 | 371 |
| Mallard (Anas platyrhynchos) | 6 | 16 | 24 | 34 (0·3) | 988 (3·5) | 28 313 | 12 725 |
| Pintail (Anas acuta) | 1 | 4 | 11 | 1 (0·3) | 8 (1·4) | 565 | 323 |
| Garganey (Anas querquedula) | 0 | 3 | 14 | 0 (0) | 13 (9·1) | 143 | 41 |
| Shoveler (Anas clypeata) | 0 | 5 | 10 | 0 (0) | 26 (12·9) | 202 | 44 |
| Red‐crested Pochard (Netta rufina) | 0 | 0 | 4 | 0 (0) | 0 (0) | 178 | 8 |
| Pochard (Aythya ferina) | 1 | 2 | 14 | 1 (0·8) | 2 (0·6) | 310 | 130 |
| Tufted Duck (Aythya fuligula) | 3 | 5 | 14 | 48 (19·8) | 29 (5·9) | 493 | 242 |
| Coot (Fulica atra) | 1 | 5 | 22 | 1 (0) | 11 (0·4) | 2494 | 2145 |
| Golden Plover (Pluvialis apricaria) | 0 | 1 | 4 | 0 (0) | 1 (0·3) | 344 | 164 |
| Lapwing (Vanellus vanellus) | 0 | 1 | 11 | 0 (0) | 1 (0·3) | 349 | 101 |
| Ruff (Philomachus pugnax) | 0 | 0 | 8 | 0 (0) | 0 (0) | 53 | 8 |
| Black‐tailed Godwit (Limosa limosa) | 0 | 0 | 2 | 0 (0) | 0 (0) | 2 | 2 |
| Black‐headed Gull (Larus ridibundus) | 1 | 4 | 23 | 1 (0) | 21 (0·5) | 4303 | 2199 |
| Common Gull (Larus canus) | 0 | 2 | 12 | 0 (0) | 3 (0·3) | 1048 | 348 |
Table 5.
Number of wild birds tested and proportion positive for H5N1 HPAI and other subtypes by species and MS for species in which H5N1 HPAI was detected in 2006 and which are not listed as HRS
| Non‐HRS H5N1 HPAI‐positive species | Number (proportion) of | ||||||
|---|---|---|---|---|---|---|---|
| MS | Birds | ||||||
| H5N1 HPAI positive | Other positive | Sampled 2006 | H5N1 HPAI positive (%) | Other positive, (%) | Sampled 2006 | Feb–May sampled | |
| Great Crested Grebe (Podiceps cristatus) | 3 | 1 | 18 | 4 (1·84) | 3 1·1 | 261 | 217 |
| Cormorant (Phalacrocorax carbo) | 2 | 1 | 18 | 2 (0·2) | 1 0·1 | 1036 | 827 |
| Grey Heron (Ardea cinerea) | 3 | 3 | 22 | 4 (0·2) | 4 0·1 | 2670 | 2564 |
| White Crane (Ardea spp.) | 1 | 0 | 10 | 1 (0·1) | 0 0·0 | 870 | 804 |
| Teal Duck (Cygnus spp.) | 2 | 3 | 14 | 4 (0·5) | 9 0·7 | 1257 | 772 |
| Embeden Goose (Anser sp.) | 2 | 1 | 13 | 2 (0·6) | 1 0·1 | 822 | 357 |
| Snow Goose (Anas strepera) | 1 | 2 | 9 | 1 (1·4) | 6 2·2 | 275 | 69 |
| Dabbling Ducks (Anas spp.) | 2 | 7 | 20 | 4 (0·1) | 52 1·1 | 4889 | 2987 |
| Greater Scaup (Aythya marila) | 1 | 1 | 4 | 4 (36·4) | 1 7·7 | 13 | 11 |
| Smew (Mergus albellus) | 2 | 0 | 4 | 2 (28·6) | 0 0·0 | 8 | 7 |
| Common merganser (Mergus merganser) | 3 | 1 | 9 | 11 (12·0) | 5 4·8 | 105 | 92 |
| Muscovy Duck (Cairina moschata) | 1 | 0 | 2 | 1 (6·3) | 0 0·0 | 18 | 16 |
| Goshawks (Accipiter spp.) | 1 | 0 | 6 | 1 (0·1) | 0 0·0 | 747 | 737 |
| Common Buzzard (Buteo buteo) | 5 | 0 | 17 | 13 (0·4) | 0 0·0 | 3597 | 3514 |
| Rough‐legged Hawk (Buteo lagopus) | 1 | 0 | 3 | 1 (5·9) | 0 0·0 | 18 | 17 |
| Common kestrel (Falco tinnunculus) | 1 | 3 | 15 | 1 (0·2) | 6 0·9 | 687 | 593 |
| Peregrine Falcon (Falco peregrinus) | 1 | 0 | 9 | 1 (2·1) | 0 0·0 | 56 | 48 |
| Steppe Buzzard (Falco spp.) | 1 | 0 | 11 | 1 (0·9) | 0 0·0 | 148 | 107 |
| Purple Gallinule (Porphyrio porphyrio) | 1 | 0 | 1 | 1 (100) | 0 0·0 | 1 | 1 |
| Herring Gull (Larus argentatus) | 2 | 3 | 12 | 3 (0·5) | 38 2·4 | 1579 | 608 |
| Seagull (Larus spp.) | 1 | 3 | 18 | 1 (0·1) | 11 0·7 | 1541 | 1164 |
| Bengal Eagle Owl (Bubo bubo) | 2 | 1 | 9 | 4 (13·8) | 2 4·0 | 50 | 29 |
| Common Magpie (Pica pica) | 1 | 1 | 20 | 1 (0·3) | 0 0·0 | 465 | 324 |
| Unknown species | 3 | 7 | 23 | 15 (0·3) | 35 0·4 | 9085 | 5312 |
Although Great‐Crested Grebes (Podiceps cristatus) were tested in very low numbers [n = 217 (range 1–76)], 2·3% (5/217) were found H5N1 HPAI positive in three MS between February and May (Table 5). Mallards (Anas platyrhynchos), that made up a third of all birds sampled, accounted for only 5·6% (33/591) of all positive H5N1 HPAI cases, while accounting for 61% (988/1613) of all non‐H5N1 HPAI AI detections. Overall only 0·26% (34/12 725) [range in MS with positives from <0·5% (DE, IT, SE) to 1·7% (AT)] of the Mallards sampled between February and May and 0·3% (34/10 826) of Mallards sampled through passive surveillance tested positive for H5N1 HPAI. All 34 H5N1 HPAI‐infected Mallards were found dead.
The number of birds tested during 2006, the test results by species and MS for HRS and other species in which H5N1 HPAI infection was detected are displayed in 4, 5 respectively.
Other AI subtypes
Infections with subtypes other than H5N1 HPAI were found in 1615 birds of at least 61 species (for 6·1% of the birds there was no information at species level) of 10 orders in 18 MS. LPAI of subtype H5 was detected in 136 birds (8·4% of positives for subtypes other than H5N1 HPAI) and LPAI H7 was identified in 26 birds (1·6% of positives for subtypes other than H5N1 HPAI).
In contrast to the results for H5N1 HPAI, the LPAI sample prevalence was much higher in active (live or hunted birds) than in passive surveillance (dead or diseased birds) (Table 6). Amongst all LPAI‐positive birds, 82% (1324/1615) were either caught live or hunted. Sweden, CY and PT were exceptions, where a higher proportion of positives were found through passive surveillance of dead or diseased birds (Table 6). However, many of these infections in SE with a pending subtype result at the time of reporting occurred during the time of the H5N1 HPAI incidents, originated from dead or diseased diving ducks in the Protection Zone and are therefore more likely to be H5N1 HPAI‐affected birds. The high proportion of positive samples from passive surveillance in CY were all of the LPAI H1N1 subtype and were collected from birds that died from an outbreak of botulism during the summer.
Table 6.
Number of birds sampled and positive for subtypes other than H5N1 HPAI according to surveillance type and MS between February and December 2006
Results of the application of the circular statistic showed that the prevalence of LPAI subtypes was significantly (P < 0·01) concentrated around September and the standard deviation included both August and October. The observed trend is in agreement with the known AI virus ecology in dabbling ducks (Anas spp). 10 , 24 , 25
The monthly prevalence of northern and southern Europe has a significant concentration on the circular statistic, but when compared (Watson F‐test) no statistical differences could be demonstrated.
Similar to the sample prevalence of H5N1 HPAI, the highest proportion of LPAI‐infected birds was found in Anseriformes (2·2%; 1427/64 487), followed by Podicipediformes (0·97%, 3/310). In contrast to H5N1 HPAI, however, Charadriiformes also showed a comparatively high proportion of LPAI positives (98/12 527 – 0·79%). In Passeriformes, the very low prevalence of LPAI (8/8961– 0·09%) was similar to that found for H5N1 HPAI.
Amongst all LPAI infections, 75% (1211/1615) were found in dabbling ducks (Anas spp.). Among dabbling ducks LPAI infections were most frequently identified in mallards (A. platyrhynchos). The overall sample prevalence for mallards was 3·5% (988/28 313; MS range 0·4–25%) (Table 4). Furthermore, a high LPAI sample prevalence was detected in Shovelers (Anas clypeata) (26/202 – 12·9%) and Teal (Anas crecca) (88/1900 = 4·6%; MS range 0·8–26%) (Table 4). However, only very low numbers of Shovelers were sampled, limiting the conclusions that can be made.
Discussion
The presence of incidents of H5N1 HPAI infection in wild birds during 2006 in many MS in the absence of reports of disease in poultry suggests a likely introduction of the virus into the EU via wild birds. There has been previous debate as to the potential for wild birds to carry HPAI infections over large distances while shedding the virus without showing impact of the infection and thereby acting as silent carriers. 25 , 26 , 27 In this survey almost all H5N1 HPAI‐infected birds were found dead or diseased and no ‘reservoir’ species could be detected despite intensive live bird surveillance, which was frequently targeted to HRS and involved very large sample numbers. However, there were two findings of infection in wild birds that did not exhibit apparent clinical signs: a group of Mute swans (Cygnus olor) in PL and a Herring Gull (Larus argentatus) in DK. While these could be seen as indications of the existence of a possible carrier state, it is more likely that these cases were detected before they started showing clinical signs especially as the group of mute swans in PL was detected initially through the finding of a dead swan and subsequent confinement and testing of the remainder detected birds that were not clinically affected. In recent experimental infections with H5N1 HPAI virus, Mute swans shed virus for up to 7 days without clinical signs before very quickly succumbing to death. 28 Another possible explanation for the absence of clinical signs in the Polish swans could be that they had prior exposure to AI virus as in another experiment Mute swans that had been previously exposed to another AI subtype did not display clinical signs when infected with H5N1 HPAI. 29
The relatively short duration of the wild bird incidents and often limited number of birds affected in them portray a different picture than the outbreak at lake Qinghai in China, where a substantial mortality in bar headed geese occurred. 4 Mortality in the 2006 H5N1 HPAI outbreaks in the EU was relatively low when compared with Qinghai, even in areas of high bird density. Assuming that the majority of birds are clinically susceptible to the infection, the comparatively low number of infections and mortalities even in areas of high bird density suggest that the transmission rate, although likely to have been enhanced through the cold weather conditions and associated higher density of wild birds, was also quite low. The transmission rate is mainly influenced by the availability and density of susceptible hosts, virus survival, concentration in the environment and the duration of virus excretion. Therefore if passive surveillance is only initiated through the occurrence of large‐scale mortalities, incidents of H5N1 HPAI in wild birds could be missed especially in areas of lower wild bird density.
It is not known whether the two cases that did occur after May resulted from separate introductions or were the last of the spring incidents. Nevertheless, the fact that, with the exception of H5N1 HPAI‐positive cases in Spain in July and in DE in August, no further such incidents were detected for the remainder of 2006, suggests that H5N1 HPAI did not establish itself efficiently in the EU wild bird population. Alternatively the virus may have been present at a very low prevalence in the EU wild bird population and was not detected. While the latter is possible, most recent H5N1 HPAI outbreaks or wild bird incidents in EU MS in 2007 involved H5N1 HPAI strains that, although closely related, are quite different to and clearly distinguishable from those present in 2006; therefore these most likely represent a new introduction. Detailed genetic analysis of viral isolates from 2006 30 led to the conclusion that in 2006 there were several independent introductions before local spread within wild bird populations occurred. Consequently the above findings suggest that it does not seem likely that the H5N1 HPAI virus could persist and circulate in wild bird populations for extended time periods in the absence of outbreaks in poultry.
Nevertheless, information of H5N1 HPAI outbreaks or incidents needs to be analysed further to improve the knowledge of factors affecting the epidemiology of H5N1 HPAI in wild birds. All surveillance results so far have indicated that H5N1 HPAI is very different from LPAI and that different criteria for its detection need to be applied.
The almost exclusive detection of the H5N1 HPAI incidents in the EU through the finding of dead infected birds highlights the importance of passive surveillance. Unless the existence of a carrier species can be proved and targeted surveillance for such species be implemented, maintaining and supporting large‐scale passive surveillance is of high importance for the early detection of H5N1 HPAI incidents. Passive surveillance may consist of more enhanced activities such as thorough regular patrols by staff on wild bird reserves, but also depends on additional reporting by the public. Therefore informing and maintaining public awareness to encourage reporting, as well as providing motivation or incentives for patrols is essential, particularly in times of outbreaks in neighbouring MS and non‐EU countries in geographical proximity.
To investigate the epidemiology of H5N1 HPAI in wild birds further, it is important to intensify surveillance in the areas where birds infected with H5N1 HPAI were detected. Integrated field investigations of birds (including collection of data and information on the species and populations present) in areas where infection is known to be present will be very useful for this purpose. Such intensified focused surveillance in response to an outbreak in wild birds and poultry is also required to investigate the role of possible bridge species (Species listed as those that may provide contact between risk species and poultry through sharing of wetlands or farmlands with poultry 15 ) and to elaborate effective bioexclusion measures for use by poultry farmers, e.g. as recommended by EFSA 15 and required by Community legislation 2005/734/EC. 31 Swan spp. were shown to have played a special role in the 2006 outbreaks, possibly due to a high susceptibility that has also been observed by experimental infections 28 , 29 combined with an increased probability of being detected after death compared to smaller, less obvious birds, whose carcasses can be rapidly removed by predators and scavengers. In addition, swans are frequently found to live near more inhabited areas, which might further facilitate detection. However, some additional species, such as grebes, mergansers and diving ducks, although tested in very low numbers, were also found to be more frequently infected than other species, which may be attributable to a number of factors including clinical susceptibility, type of exposure and behavioural characteristics. In recent 2007 outbreaks in DE, a colony of Black Necked Grebes (Podiceps nigricollis) was identified as infected, 32 suggesting that further investigation of the role of this order of birds together with diving ducks and mergansers would be valuable.
Raptors were considered to be a good surveillance source for the detection of AI due to contact with many potentially infected dead birds when scavenging upon them. Indeed, diurnal birds of prey (Falconiformes) and Owls (Strigiformes) showed comparatively high positive proportions for H5N1 HPAI and their value as a surveillance source and inclusion into future surveys appears justified, if found dead. In contrast, mallards, while appearing to play an important role in the detection of LPAI, seem to be a less valuable surveillance source for the detection of H5N1 HPAI infection. Very few if any AI viruses were detected in Passeriformes, pigeons and doves and consequently these species appear to have little significance in AI surveillance, consistent with findings in other studies. 33
Several of the HRS were sampled in very low numbers and therefore do not permit any firm conclusions about their potential role in the epidemiology of HPAI based on these results. Most of the infrequently sampled populations of HRS (<0·1% of total populations sampled) were those populations occurring in the Black Sea/Mediterranean flyway, reflecting the smaller national surveillance programmes in that region (Central and Eastern Mediterranean and Black Sea) compared to north‐west Europe.
As the knowledge of the epidemiology of H5N1 HPAI evolves, it would be of high value to design surveillance systems at an international level through multinational collaboration so as to ensure sufficient coverage and strategic placement and enhancement of surveillance activities to aid in the early detection and warning of this virus. Research projects have been funded by the EU and by individual MS that aim at implementing such a transboundary surveillance for early detection of AI. 34 For LPAI, the surveillance results presented here do reflect previous studies: for example, most frequent detection of LPAI viruses in Anseriformes, specifically dabbling ducks. Higher positive proportions were frequently identified in late summer/early autumn, more specifically around September and these findings are in agreement with previous studies in the northern hemisphere. 9 , 10
The collected information has allowed some important conclusions to be made about H5N1 surveillance in wild birds and provides a very valuable data set for further extended analysis and research. There are some aspects though that should be considered when interpreting the results: cluster effects occur and were not accounted for in the analysis. In addition, when small sample numbers are collected the uncertainty around a proportion/apparent prevalence increases. Furthermore, surveillance programmes were quite variable with respect to a number of parameters including sample size, weighting between active and passive surveillance and targeting. Therefore, as with all surveillance data collected through various sources with heterogeneous design, no direct comparisons can be made regarding the prevalence between MS and the apparent prevalence observed in a species cannot be assumed to be the true underlying prevalence.
Acknowledgements
We would like to acknowledge all the immense resources and effort invested by chief veterinary officers, veterinary services, national reference laboratories, ornithologists, bird watching organizations, hunters and all the people who have carefully watched birds and contributed to the collection of samples in the conduction of the survey and compilation of this data set. We would also like to acknowledge the Member Directorate General for Health and Consumers, Environment and Research and Member States for funding. Also many thanks to Theo Knight‐Jones from the RVC, London who produced the maps for this publication.
This article Avian influenza surveillance in wild birds in the European Union in 2006 was written by Dr. Ian Brown of the Veterinary Laboratories Agency. It is published with the permission of the Controller of HMSO and the Queen's Printer for Scotland.
References
- 1. Alexander DJ. A review of avian influenza in different bird species. Vet Microbiol 2000; 74:3–13. [DOI] [PubMed] [Google Scholar]
- 2. Becker WB. The isolation and classification of tern virus: influenza virus A/Tern/South Africa/1961. J Hyg Camb 1966; 64:309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. FAO Technical Task Force on Avian Influenza . Potential risk of highly pathogenic avian influenza (HPAI) spreading through wild water bird migration. FAO AIDE News 2005(Special Issue);33:1–7. [Google Scholar]
- 4. Chen H, Smith GJ, Zhang SY et al. Avian flu: H5N1 virus outbreak in migratory waterfowl. Nature 2005; 436:191–192. [DOI] [PubMed] [Google Scholar]
- 5. Keawcharoen J, Van Riel D, Van Amerongen G et al. Wild ducks as long‐distance vectors of highly pathogenic avian influenza virus (H5N1). Emerg Infect Dis 2008; 14:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. EC . 2002/649/EC Commission Decision of 5 August 2002 on the implementation of surveys for avian influenza in poultry and wild birds in the Member States. OJEU L213, 09.08.2002:38–42. [Google Scholar]
- 7. Capua I, Grossele B, Bertoli E, Cordioli P. Monitoring for highly pathogenic avian influenza in wild birds in Italy. Vet Rec 2000;147:640. [PubMed] [Google Scholar]
- 8. De Marco MA, Foni E, Campitelli L et al. Influenza virus circulation in wild aquatic birds in Italy during H5N2 and H7N1 poultry epidemic periods (1998 to 2000). Avian Pathol 2005; 34:480–485. [DOI] [PubMed] [Google Scholar]
- 9. Globig A, Starick E, Werner O. Influenza virus infections in migrating waterfowl: results of a two year study in Germany. Berl Munch Tierarztl Wochenschr 2006; 119:132–139. [PubMed] [Google Scholar]
- 10. Munster VJ, Baas C, Lexmond P et al. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog 2007; 3:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cooke MK, Powell LF, Brown IH. A Report of Surveys in Wild Birds in Member States during 2005. Report to the European Commission 2006. http://ec.europa.eu/food/animal/diseases/controlmeasures/avian/res_surv_wb_010605_310106_en.pdf [Accessed 5 December 2007]. [Google Scholar]
- 12. EC . 2005/464/EC Commission Decision of 21 June 2005 on the implementation of survey programmes for avian influenza in poultry and wild birds to be carried out in the Member States. OJEU, L164, 24.06.2005:52–56.
- 13. EC . 2005/726/EC Commission Decision of 17 October 2005 amending Decision 2005/464/EC on the implementation of survey programmes for avian influenza in poultry and wild birds to be carried out in the Member States. OJEU, L273, 19.10.2005:21–24.
- 14. EC . 2006/101/EC Commission Decision of 6 February 2006 on the implementation of survey programmes for avian influenza in poultry and wild birds to be carried out in the Member States in 2006. OJEU, L46, 16.02.2006:40–46.
- 15. EFSA . Scientific report on migratory birds and their possible role in the spread of highly pathogenic avian influenza. Annex to the EFSA Journal 2006; 357:1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. EC . 2007/268/EC Commission Decision of 13 April 2007 on the implementation of surveillance programmes for avian influenza in poultry and wild birds to be carried out in the Member States and amending Decision 2004/450/EC. OJEU, L115, 03.05.2007:3–17.
- 17. EC . 2006/563/EC: Commission Decision of 11 August 2006 concerning certain protection measures in relation to highly pathogenic avian influenza of subtype H5N1 in wild birds in the Community and repealing Decision 2006/115/EC. OJEU, L222, 15.08.2006:11–19.
- 18. EC . 2006/415/EC Commission Decision of 14 June 2006 concerning certain protective measures in relation to highly pathogenic avian influenza of the subtype H5N1 in poultry in the Community and repealing Decision 2006/135/EC. OJEU, L164, 16.06.2006:51–60.
- 19. EC . 2006/437/EC Commission decision of 4 August 2006 approving a diagnostic manual for avian influenza as provided for in Council Directive 2005/94/EC. OJEU, L237, 31.08.2006:1–27.
- 20. R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2005. ISBN 3‐900051‐07‐0, http://www.r‐project.org. [Google Scholar]
- 21. ESRI . ArcMap, Version 9.1. Redlands, CA: Environmental Systems Research Institute, 1999–2005, 2005. [Google Scholar]
- 22. Batschelet E. Circular Statistics in Biology. London: Academic Press Inc., 1981. [Google Scholar]
- 23. OIE . WAHID interface – OIE World Animal Health Information Database. http://www.oie.int/wahid‐prod/public.php?page=disease_immediate_summary&selected_year=2006 [accessed 5 December 2007].
- 24. Guberti V, Scremin M, Busani L, Bonfanti L, Terregino C. A simulation model for low‐pathogenicity avian influenza viruses in dabbling ducks in Europe. Avian Dis 2007; 51(Suppl. 1):275–278. [DOI] [PubMed] [Google Scholar]
- 25. Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus ADME, Fouchier RAM. Global patterns of influenza A virus in wild birds. Science 2006; 312:384–388. [DOI] [PubMed] [Google Scholar]
- 26. Feare CJ. The role of wild birds in the spread of HPAI H5N1. Avian Dis 2007; 51(Suppl. 1):440–447. [DOI] [PubMed] [Google Scholar]
- 27. Yasue M, Feare CJ, Bennun L, Fiedler W. The epidemiology of H5N1 avian influenza in wild birds: why we need better ecological data. Bioscience 2006; 56:1–7. [Google Scholar]
- 28. Brown JD, Stallknecht DE, Swayne DE. Experimental infection of swans and geese with highly pathogenic avian influenza virus (H5N1) of Asian lineage. Emerg Infect Dis 2008; 14:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalthoff D, Breithaupt A, Teifke JP et al. Highly pathogenic avian influenza virus (H5N1) in experimentally infected adult mute swans. Emerg Infect Dis 2008; 14:1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Starick E, Beer M, Hoffmann B et al. Phylogenetic analyses of highly pathogenic avian influenza virus isolates from Germany in 2006 and 2007 suggest at least three separate introductions of H5N1 virus. Vet Microbiol 2007; 128:243–252. [DOI] [PubMed] [Google Scholar]
- 31. EC . 2005/734/EC Commission Decision of 19 October 2005 laying down biosecurity measures to reduce the risk of transmission of highly pathogenic avian influenza caused by influenza virus A subtype H5N1 from birds living in the wild to poultry and other captive birds and providing an early detection system in areas of particular risk. OJEU, L274, 20.10.2005:105–107.
- 32. Friedrich‐Loeffler‐Institut . Bewertung des Risikos zur Einschleppung von hochpathogenem aviaeren Influenzavirus H5N1 in Hausfluegelbestaende in Deutschland. Report of the FLI. http://www.fli.bund.de/fileadmin/user_upload/Dokumente/News/aktuelle_Krankheitsgeschehen/avi_Flu/lb_influenza_071015.pdf [accessed 5 December 2007].
- 33. Schnebel B, Dierschke V, Rautenschlein S, Ryll M, Neumann U. Investigations on infection status with H5 and H7 avian influenza virus in short‐distance and long‐distance migrant birds in 2001. Avian Dis 2007; 51(Suppl. 1):432–433. [DOI] [PubMed] [Google Scholar]
- 34. EC . Catalogue of the European Commission Influenza Research – EU funded Projects 2001–2007. http://ec.europa.eu/research/health/poverty‐diseases/doc/influenza‐research_en.pdf [accessed 5 December 2007].


