Abstract
Importance
Vascular leiomyosarcomas (vLMS) are a rare subtype of leiomyosarcomas (LMS) most commonly affecting the inferior vena cava and accounting for 5% of all LMS. These tumors are aggressive malignancies for which adjuvant modalities have not shown increased efficacy compared over surgery. Our study evaluates potential molecular markers that should be evaluated in prospective studies to determine their prognostic and therapeutic utility.
Objective
To evaluate the outcomes of patients with vLMS and associations with immunohistochemical prognostic markers.
Design
Retrospective chart review
Setting
Single institution
Participants
A cohort of 77 patients that presented to MDACC from 1993–2012 was analyzed. All of the cases had a confirmed diagnosis of vascular leiomyosarcoma. Immunohistochemical studies for biomarkers were performed on a tissue microarray that included 26 primary vLMS specimens.
Main Outcomes and Measures
Demographic, and clinical factors were evaluated to assess clinical course, patterns of recurrence and survival outcomes for patients with primary vLMS. Univariate Cox proportional hazards model was utilized to correlate DSS and time to recurrence with potential prognostic indicators.
Results
Five year disease-specific survival (DSS) rates after tumor resection was 65%. Median time to local recurrence was 43 months, versus 25 months for distant recurrence versus 15 months for concurrent local and distant recurrences; p=0.04. Strong cytoplasmic β-catenin (p=0.06) and IGF-1R (p=0.04) expression were associated with inferior DSS.
Conclusions and Relevance
vLMS are aggressive malignancies, with high recurrence rates. Expression of β-catenin and IGF-1R were associated with poor DSS. Prospective studies should evaluate their clinical and therapeutic utility.
Keywords: vascular leiomyosarcomas, β-catenin, IGF-1R
INTRODUCTION
Leiomyosarcomas (LMS) are the most common malignancy affecting the vascular system and are characterized by smooth muscle differentiation and complex genomics with evidence of genetic instability 1,2. Vascular leiomyosarcomas (vLMS) more commonly arise from the venous, rather than arterial, vessels. The inferior vena cava (IVC) is the most commonly involved vein, and is the site of origin in with over 50% of cases 3,4. Currently, the published literature predominately consists of small, single institution retrospective studies focused on IVC LMS, with reported survival rates ranging from 31 – 62% 3,5–12, and 10-year survivals as low as 22% 13. Incomplete resection correlates with a poor prognosis 7. While other retroperitoneal sarcomas are plagued by local recurrences, vascular LMS most often recur at distant sites, with reported metastatic rates of ~50% 3,5–10,14, with the lung and liver being the most common sites. Factors relating to mortality vary between the published studies, and there is no standardized therapeutic approach for this complex and rare malignancy.
Surgical resection remains the mainstay of treatment for vascular LMS. Unfortunately, the benefits of systemic therapy remain to be defined, and only minimal advancement in the treatment of this disease has been made over the past two decades. For retroperitoneal sarcoma, the benefit of adjuvant chemotherapy following resection is unclear, with conflicting results in multiple randomized trials 15,16. A recent meta-analysis suggested that anthracycline/ifosfamide-containing regimens may have some benefit, although this analysis did not include several European studies with differing results 17. Therefore, there is a need for greater understanding of the molecular biology of vascular LMS to provide insight into novel approaches to systemic treatment in the future.
The aims of our current study were (1) to describe the natural history and clinical outcome of primary vascular LMS patient cohort (n = 63) treated at a tertiary cancer center in order to identify clinical and pathologic factors relating to prognosis, patterns of recurrence/metastasis, and information on survival; and (2) to identify molecular targets commonly deregulated in human vascular LMS specimens utilizing a tissue microarray (TMA) to inform potential future therapeutic options.
MATERIALS AND METHODS
Clinical database
With the approval of the Institutional Review Board (IRB) of The University of Texas MD Anderson Cancer Center (UTMDACC), we identified all patients diagnosed with primary vLMS from January 1993 to April 2010. A clinical database was constructed including patient, tumor, treatment, and outcome data. The diagnosis of vascular LMS was confirmed by a UTMDACC sarcoma pathologist and in the context of multidisciplinary tumor board review. All leiomyosarcomas included in the study originated from vascular structures. Additionally, to avoid including cases of vascular IVC that could represent metastatic spread from uterine primary, all patients with history of surgery for another tumor or hysterectomy were excluded. Local recurrence was considered as any recurrence at the primary site without metastasis. Clinicopathologic variables included: (1) patient factors: age at diagnosis (≤55 or >55 years); (2) tumor factors, e.g. site, microscopic margins (R0/R1 vs R2), grade (low, intermediate, high) and size (< 5 or ≥5 cm); (3) surgical procedure; (4) use of chemotherapy or radiation treatment. Tumor location within the IVC was described using the previously published segmental classification3,13: level I (infrarenal), level II (inter- and supra-renal up to but not including the main suprahepatic veins), or level III (suprahepatic with possible intracardiac extension).
Tissue microarray (TMA) construction and immunohistochemical studies
Immunohistochemical studies were performed on a previously constructed tissue microarray containing 50 vascular leiomyosarcoma specimens from 39 patients 18. For the survival analysis, only primary tumor tissue was used for analysis (26 patients). Commercially available antibodies (eTable 1) were used following standard protocols (eTable 2). Horseradish-peroxidase labeled secondary antibodies or biotinylated systems (4 plus system Biocare Medical, Concord, CA) were utilized. Scoring was performed by 2 independent pathologists (AJL and EGD). The biomarkers included in our microarray analysis were selected due to the known importance in apoptosis and cell death (Bcl-2, p53, survivin); cell survival and stress response (EGFR, IGF-1R, MET, PDGF-a, PDGF-b, PDGFR-b, β-catenin, AXL); proliferation (Ki-67); cell cycle regulation (cyclin D1, p16, Rb); tumor microenvironment (MMP2, MMP9); and angiogenesis (VEGF). ER, PR, Ki67, and cyclin D1 were scored by percent nuclear expression, as low (< 10 % of positive tumor nuclei/ sample), or high (greater or equal to 10 % positive tumor nuclei), regardless of stain intensity. All other markers were scored on intensity as 0 (absent, or staining in <10% of tumor cells), 1 (low), 2 (moderate), or 3 (high). Samples were grouped based on expression intensity: 0–1 (low), and 2–3 (high) for statistical consideration. If the scores where discordant by only one category (0 versus 1 or 2 versus 3), the scores were averaged. If they were discordant by 2 or more categories (0 versus 3, 0 versus 2 or 1 versus 3) both pathologists looked at them together to come to an accommodation.
Statistics
Local recurrence-free survival (LRFS) was calculated as the time from the initial treatment to recurrence. Deaths due to disease were treated as a disease-specific survival (DSS) endpoint; other deaths were considered as censored observations. The distributions of LRFS and DSS were estimated by the Kaplan-Meier method and compared using the log-rank test. Univariate Cox proportional hazards model was used to correlate DSS and time to distant recurrence with potential prognostic indicators. All computations were carried out using SPSS Statistic software (version 22, IBM).
RESULTS
Patients and Tumor Variables
Seventy-seven patients presented to MDACC with vascular LMS from 1993 – 2012. Of these, 12 patients presented with synchronous metastatic disease and 2 were found to have metastatic disease at the time of referral to our institution; all 14 were excluded from further analysis. The remaining 63 patients with localized disease who underwent surgical resection formed the study population and were utilized for subsequent outcomes analysis (Table 1). The median age at diagnosis was 58 years (range 22–78). The majority of patients were female (65%) and Caucasian (81%).
TABLE 1.
Patient and Tumor Demographics for Patients with Localized Disease at Presentation and Patients Included in the Tissue Microarray (TMA) with Vascular Leiomyosarcoma.
| Characteristics | Localized at diagnosis | TMA Patients |
|---|---|---|
| Number | 63 | 26 |
| Age at Diagnosis, median (range) | 58 (22–78) | 55 (22–77) |
| Gender, n (%) | ||
| Male | 22 (35) | 9 (35) |
| Female | 41 (65) | 17 (65) |
| Race, n (%) | ||
| White | 51 (81) | 18 (69) |
| African-American | 7 (11) | 5 (19) |
| Hispanic | 5 (8) | 3 (12) |
| Tumor Size, n (%) | ||
| < 5cm | 12 (21) | 1 (4) |
| ≥ 5cm | 46 (79) | 25 (96) |
| Unknown | 5 (--) | -- |
| Tumor Grade, n (%) | ||
| Low | 3 (11) | 1 (11) |
| Intermediate | 7 (26) | 2 (22) |
| High | 17 (63) | 6 (67) |
| Unknown | 36 (--) | 17 (--) |
| Location, n (%) | ||
| Inferior Vena Cava | 42 (67) | 17 (65) |
| Segment I | 13 (31) | 11 (65) |
| Segment II | 26 (62) | 6 (35) |
| Segment III | 3 (7) | 0 |
| Saphenous/Femoral Vein | 9 (14) | 2 (8) |
| Renal Vein | 4 (6) | 4 (15) |
| Upper Extremity Vein | 3 (5) | -- |
| Other | 5 (8) | 3 (12) |
| Primary Surgery | ||
| At outside institution | 36 (56) | 26 (93) |
| At MDACC | 26 (41) | 2 (7) |
| No surgery | 2 (3) | -- |
| Resection Status | ||
| R0/R1 | 37 (80) | 21 (81) |
| R2 | 9 (20) | 4 (19) |
| Not recorded | 15 (--) | 1 (--) |
| Treatment | ||
| Surgery Alone | 33 (52) | 11 (42) |
| Surgery + Chemotherapy | 12 (19) | 9 (35) |
| Preoperative | 6 | 3 |
| Postoperative | 7* | 7 |
| Surgery + Radiation | 9 (14) | 0 |
| Preoperative | 2 | -- |
| Postoperative | 7 | -- |
| Surgery + Chemotherapy + | 9 (14) | 6 (23) |
| Radiation | ||
| Multivisceral Resection | 16 (25) | |
| Kidney | 13 (21) | 8 (31) |
| Adrenal | 11 (17) | 5 (19) |
| Liver | 1 (2) | 1 (4) |
| Intestine | 2 (3) | 1 (4) |
| Local Recurrence only, n (%) | 6 (10) | 3 (12) |
| Distant Recurrence only, n (%) | 16 (25) | 6 (23) |
| Local & Distant Recurrence (%) | 20 (32) | 6 (23) |
1 patient got preoperative and postoperative chemotherapy
vLMS were most often large and high grade, with 79% being ≥ 5 cm and 89% of the tumors considered intermediate/high grade. The most common anatomic site of involvement was the IVC (67%, n = 42) with 9 tumors arising from the saphenous or femoral veins, 4 from the renal vein, 3 from upper extremity veins, and 5 arising from other sites including the inferior mesenteric vein (IMV), aorta, and pulmonary artery (Table 1).
Treatment
Treatment patterns and surgical management were assessed in the 63 patients who presented with localized disease and underwent surgical resection (Table 1). For the IVC vLMS cases, 12 patients (29%) had ligation of the IVC while 30 (71%) had reconstruction of the IVC with primary repair, vein patch or graft. Fifteen patients (24%) had preoperative/neoadjuvant therapy, including systemic chemotherapy alone (n=6), radiation (n=2), or combined chemoradiation therapy (n=7). An additional 16 patients received postoperative adjuvant therapy, including chemotherapy alone (n=7), radiation alone (n=7) or combined chemoradiation (n=2). Neoadjuvant chemotherapy was used in cases where the possibility of R0/R1 resection was questionable, while postoperative chemotherapy was used in cases at high risk of recurrence (large tumors, high grade sarcomas, or close margins (<1 cm)). 18 patients received radiation therapy including 10 extremity lesions and 8 intra-abdominal lesions. For the purposes of statistical analyses, adjuvant and neoadjuvant therapies were included as one group. The chemotherapy regimens varied; 41% (n=9) were treated with some combination of adriamycin and ifosfamide and 31% (n=7) were treated with gemcitabine and taxotere.
vLMS-Specific Recurrence, Metastasis and Survival
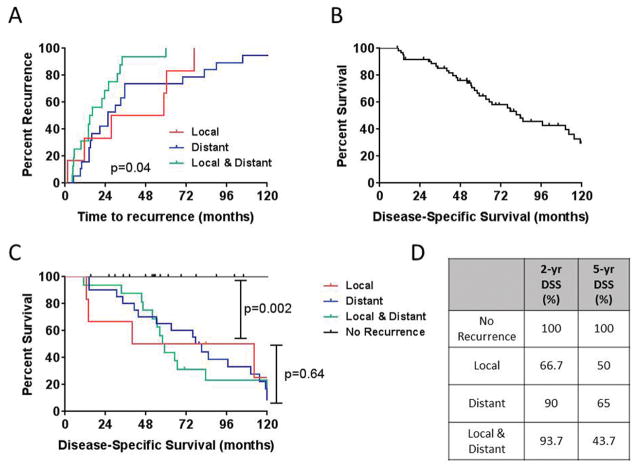
At a median follow-up of 5.1 years (range 0.87–13.9) years, 42 patients (65%) developed recurrent disease (local and/or distant) at a median time to recurrence of 33 months. Six patients developed a local recurrence only, 20 patients developed distant recurrences only and 16 patients developed synchronous local and distant recurrences (Table 1). The median time to local recurrence was 43 months compared to 25 months for distant recurrences (Fig. 1A). Interestingly, patients that presented with synchronous local and distal disease had a median time of 15 months (p=0.04) (Fig. 1A). The majority of distant metastases were to the lung (43%), liver (31%), bone (23%), abdomen (18%), and other sites (21%), including brain and soft tissue.
Figure 1. Time to recurrence and sarcoma–specific survival stratified by recurrence type.
Kaplan-Meier estimate of (A) Time to recurrence stratified by site of recurrence. (B) Disease-specific survival for all patients with primary vascular leiomyosarcoma. (C) Disease-specific survival for patients with vascular leiomyosarcoma stratified by site of recurrence. (D) 2-year & 5-year disease-specific survival estimates for patients with vascular leiomyosarcoma stratified by site of recurrence. DSS=disease-specific survival.
The DSS for the 63 patients who presented with localized disease was 96% at 1 year and 65% at 5 years (Fig. 1B). Patients without recurrent disease demonstrated improved sarcoma-specific survival compared to patients with local failure (Fig. 1C & D). However, DSS was similar regardless of the type of recurrence (local versus distant). Univariate analyses of patient, tumor, and treatment factors failed to demonstrate any significant difference in DSS, although there was a trend towards improved survival in the patients treated with surgery and radiation (HR 0.43; 95%CI 0.19 – 1.01; p=0.052). The limited number of patients in each group precluded any definite conclusion regarding the site of origin. Therefore, the subset analysis based on site or origin was omitted.
Immunohistochemical Analysis of Tissue Microarrays
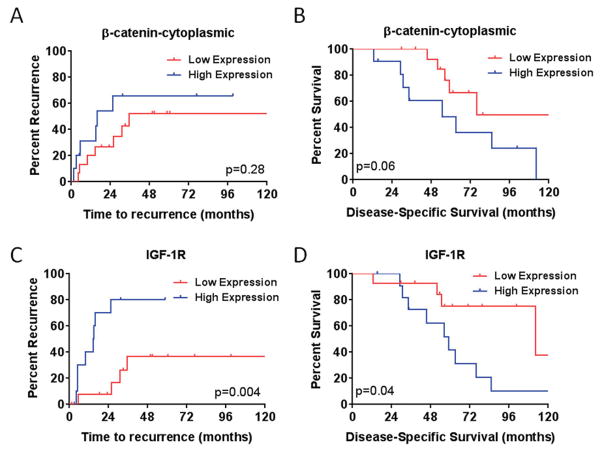
The association between several biomarkers, distant recurrence, and DSS was evaluated (eTable 1 & 2). High expression of cytoplasmic β-catenin was associated with higher distant recurrence and reduced disease-specific survival (Figure 2A & B; Table 2). Expression of elevated levels of IGF-1R was associated with reduced sarcoma-specific survival and shorter time to distant recurrence (Fig. 2C & D; Table 2).
Figure 2. Time to recurrence and sarcoma–specific survival stratified by cytoplasmic β-catenin or IGF-1R expression levels.
Kaplan-Meier estimate of (A & B) time to distant recurrence (A) and Disease-specific survival (B) for patients with primary vascular leiomyosarcoma stratified by site β-catenin status. Kaplan-Meier estimate of (C & D) time to distant recurrence (C) and Disease-specific survival (D) for patients with primary vascular leiomyosarcoma stratified by site IGF-1R status.
TABLE 2.
Prognostic factors for leiomyosarcoma-specific mortality and distant recurrence in 26 primary tumors treated with surgical resection.
| RFS | DSS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | N | Median Time to Recurrence (months) | HR | (95% CI) | p-value | Median Survival (months) | HR | (95% CI) | p-value |
| β-catenin cytoplasmic | |||||||||
| Negative | 15 | 35.4 | -- | 0.69 | 75.9 | -- | 0.055 | ||
| Positive | 11 | 15.9 | 1.37 | (0.29-6.54) | 54.6 | 5.33 | (0.97-29.3) | ||
| IGF-1R | |||||||||
| Negative | 14 | NR | -- | 0.024 | 112.2 | -- | 0.024 | ||
| Positive | 12 | 15.1 | 2.74 | (1.14-6.56) | 59.2 | 2.74 | (1.14-6.56) | ||
CI, confidence interval
DISCUSSION
The current study includes a homogeneous group of patients with vascular leiomyosarcoma treated at a single institution. The majority of patients in this cohort developed recurrent disease, most commonly distant metastases. The time to recurrence varied based on the type of recurrence with distant recurrences occurring earlier than local recurrences (p=0.04). Expression of β-catenin and IGF-1R was associated with reduced sarcoma-specific survival and high IGF-1R expression was associated with reduced time to distant recurrence.
The population in this study is consistent with previously published studies demonstrating a preponderance of large tumors in female patients in their 6th decade, and a large proportion of high grade tumors 10. The large majority of our patients had tumors arising in the IVC, as seen in other published series 6,7,9. Reported 5-year survival rates range from 31–63% 3,5–10. Our result were similar, with 5-year disease-specific survival of 65% and the 5-year recurrence-free survival of 35%. Of the patients who presented with localized disease, 52% of patients were treated with surgery alone. Of patients receiving multi-modality treatment, approximately half received their adjuvant treatment prior to surgical resection. There was a trend towards improved survival with the addition of adjuvant therapy (chemotherapy and/or radiation) to macroscopially margin-negative surgery (HR 2.13, p = 0.054), which echo the findings of other small case series 6. However, definitive support for the routine use of radiation in this patient population is lacking and our study was limited in its ability to establish possible radiation therapy benefits in vLMS by small sample size. These findings need to be explored in larger multi-institutional patient cohorts. Currently, the utility of preoperative radiation in patients with retroperitoneal sarcomas is being examined in the STRASS study, which includes patients with LMS (NCT01344018) as well as a variety of other histologies and seeks to address the question of the benefit of radiation in these unique tumors in a randomized, prospective fashion.
We found that the 5-year RFS for patients with local vLMS disease was 35%. This was slightly lower than the RFS observed in patients with uterine LMS reported previously from our institution (5-year RFS = 42%) 22. However,, similar to other series, we observed that patients with vLMS treated with multimodality therapy, including radical resection and radiation had a better 10-year survival than patients with uterine leiomyosarcoma ( 41% versus 27% respectively)6,22.
Technical difficulties associated with complete tumor eradication with local treatment alone encourage the development of novel systemic treatment regimens for LMS. The use of doxorubicin for metastatic leiomyosarcoma has been associated with an improvement in progression-free survival 19, although in other studies of leiomyosarcomas lower response rates to doxorubicin- or epirubicin-containing regimens have been reported 20. Additionally, gemcitabine plus docetaxel has shown some efficacy in patients with unresectable LMS, with a 53% response rate 21. However, given the variability in the literature and the lack of effective adjuvant treatments, there is still the need to identify new targets of therapy.
In order to understand the underlying molecular biology of vascular leiomyosarcomas, an immunohistochemical vLMS microarray was constructed as previously described 22 and used to analyze 26 primary tumor samples from patients that underwent surgical resection. Increased expression of cytoplasmic β-catenin within the primary tumor sample correlated with poorer disease-specific survival in univariate analysis (HR 5.33, 95% CI 0.97 – 29.3, p = 0.055). β-catenin is a downstream signaling protein in the canonical Wnt pathway, which is involved in both embryogenesis and oncogenesis and may also mediate effects via modulation of cell-cell adhesion through the cadherin family of transmembrane proteins 23. Moreover, β-catenin mediated signaling is known to play a role in other mesenchyma tumors, including desmoid fibromatosis and synovial sarcoma 24. High levels of nuclear β-catenin and total β-catenin have been seen in a number of high grade sarcomas, including leiomyosarcomas 25–27. Targeted therapy against Wnt signaling is an active area of oncologic investigation 28, and monoclonal anti-Wnt1 antibody therapy has demonstrated cytotoxic effects in in vitro models of sarcoma 29. There are a small number of molecules indirectly targeting the Wnt signaling pathway under evaluation for use in human disease, however no trials of targeted therapies are ongoing in sarcoma. Further studies of the role of β-catenin in vLMS and potential for targeted therapy in this context are required.
We found that high level of IGF-1R expression in vascular LMS was associated with worse DSS survival and time to distant recurrence (Table 2). Similarly, it has been reported that about half of leiomyosarcomas from skin, stomach, intestine, and abdominal wall express IGF-II at a high level compared to normal myometrium and leiomyomas 30. Taken together, these findings suggest a role for IGF-mediated signaling in progression of leiomyosarcoma, and a potential therapeutic target for aggressive tumors. Of note, several phase I and phase II clinical trials have examined the role of IGF-1R blockade in other complex karyotype soft tissue sarcomas (STS) 31,32. Recently a phase I & phase II trial of combined IGF-1R antibody blockade in combination with mTOR blockade has been examined given the complementary mechanisms of action of these targeted therapies 33,34.
Overexpression of β-catenin in uterine LMS has been shown to be associated to local failure 22. In vLMS, β-catenin expression was also associated with recurrence. Contrary to uterine LMS, survivin was not found to be associated with recurrence and bcl-2 was not found to be a prognostic factor of DSS. These molecular differences may reflect subtle unique molecular variants between uterine LMS and vLMS.
This study has several limitations including the small sample size, retrospective design, long treatment interval, and the variability in the use of chemotherapy and radiation therapy. However, we feel that these limitations did not greatly affect the results of this study.
In conclusion, we report on the largest series of vLMS yet reported. Tumors frequently behaved in an aggressive fashion with early distant metastases. Although we were unable to find significance in many treatment variables, likely due to disease heterogeneity, we did find correlation between β-catenin and IGF1R and worse outcomes which suggest further avenues of investigation into disease biology and targeted therapy”
Supplementary Material
Acknowledgments
Funding for this research was provided in part by NIH/NCI K08CA160443 (KET), The Sally M. Kingsbury Sarcoma Research Foundation (KET), Marty Lindley Foundation (KET) and Amschwand Foundation (supporting CDM), and NIH/NCI Cancer Center Support Grant P30CA016672 (GMB). CLR and KET had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. None of the funding organizations above had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Disclosures: There are no conflicts of interest with any financial organization regarding the material discussed in this article.
References
- 1.Demicco EG, Maki RG, Lev DC, Lazar AJ. New therapeutic targets in soft tissue sarcoma. Advances in anatomic pathology. 2012 May;19(3):170–180. doi: 10.1097/PAP.0b013e318253462f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kevorkian J, Cento DP. Leiomyosarcoma of large arteries and veins. Surgery. 1973 Mar;73(3):390–400. [PubMed] [Google Scholar]
- 3.Dzsinich C, Gloviczki P, van Heerden JA, et al. Primary venous leiomyosarcoma: a rare but lethal disease. Journal of vascular surgery. 1992 Apr;15(4):595–603. [PubMed] [Google Scholar]
- 4.Burke AP, Virmani R. Sarcomas of the great vessels. A clinicopathologic study. Cancer. 1993 Mar 1;71(5):1761–1773. doi: 10.1002/1097-0142(19930301)71:5<1761::aid-cncr2820710510>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Dew J, Hansen K, Hammon J, McCoy T, Levine EA, Shen P. Leiomyosarcoma of the inferior vena cava: surgical management and clinical results. The American surgeon. 2005 Jun;71(6):497–501. doi: 10.1177/000313480507100609. [DOI] [PubMed] [Google Scholar]
- 6.Hines OJ, Nelson S, Quinones-Baldrich WJ, Eilber FR. Leiomyosarcoma of the inferior vena cava: prognosis and comparison with leiomyosarcoma of other anatomic sites. Cancer. 1999 Mar 1;85(5):1077–1083. [PubMed] [Google Scholar]
- 7.Hollenbeck ST, Grobmyer SR, Kent KC, Brennan MF. Surgical treatment and outcomes of patients with primary inferior vena cava leiomyosarcoma. Journal of the American College of Surgeons. 2003 Oct;197(4):575–579. doi: 10.1016/S1072-7515(03)00433-2. [DOI] [PubMed] [Google Scholar]
- 8.Ito H, Hornick JL, Bertagnolli MM, et al. Leiomyosarcoma of the inferior vena cava: survival after aggressive management. Annals of surgical oncology. 2007 Dec;14(12):3534–3541. doi: 10.1245/s10434-007-9552-z. [DOI] [PubMed] [Google Scholar]
- 9.Kieffer E, Alaoui M, Piette JC, Cacoub P, Chiche L. Leiomyosarcoma of the inferior vena cava: experience in 22 cases. Annals of surgery. 2006 Aug;244(2):289–295. doi: 10.1097/01.sla.0000229964.71743.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mingoli A, Cavallaro A, Feldhaus RJ, di Marzo L, Morelli MM, Sciacca V. Inferior vena cava leiomyosarcoma: establishment of an international registry. European journal of vascular surgery. 1994 May;8(3):380–381. doi: 10.1016/s0950-821x(05)80166-5. [DOI] [PubMed] [Google Scholar]
- 11.Wachtel H, Jackson BM, Bartlett EK, et al. Resection of primary leiomyosarcoma of the inferior vena cava (IVC) with reconstruction: A case series and review of the literature. Journal of surgical oncology. 2015 Mar;111(3):328–333. doi: 10.1002/jso.23798. [DOI] [PubMed] [Google Scholar]
- 12.Dull BZ, Smith B, Tefera G, Weber S. Surgical management of retroperitoneal leiomyosarcoma arising from the inferior vena cava. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2013 Dec;17(12):2166–2171. doi: 10.1007/s11605-013-2385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laskin WB, Fanburg-Smith JC, Burke AP, Kraszewska E, Fetsch JF, Miettinen M. Leiomyosarcoma of the inferior vena cava: clinicopathologic study of 40 cases. The American journal of surgical pathology. 2010 Jun;34(6):873–881. doi: 10.1097/PAS.0b013e3181ddf569. [DOI] [PubMed] [Google Scholar]
- 14.Gronchi A, Miceli R, Allard MA, et al. Personalizing the Approach to Retroperitoneal Soft Tissue Sarcoma: Histology-specific Patterns of Failure and Postrelapse Outcome after Primary Extended Resection. Annals of surgical oncology. 2015 Oct 10;22(5):1447–1454. doi: 10.1245/s10434-014-4130-7. [DOI] [PubMed] [Google Scholar]
- 15.Bramwell V, Rouesse J, Steward W, et al. Adjuvant CYVADIC chemotherapy for adult soft tissue sarcoma--reduced local recurrence but no improvement in survival: a study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1994 Jun;12(6):1137–1149. doi: 10.1200/JCO.1994.12.6.1137. [DOI] [PubMed] [Google Scholar]
- 16.Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. The lancet oncology. 2012 Oct;13(10):1045–1054. doi: 10.1016/S1470-2045(12)70346-7. [DOI] [PubMed] [Google Scholar]
- 17.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008 Aug 1;113(3):573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 18.Demicco EG, Boland GM, Brewer Savannah KJ, et al. Progressive loss of myogenic differentiation in leiomyosarcoma has prognostic value. Histopathology. 2015 Apr;66(5):627–638. doi: 10.1111/his.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penel N, Italiano A, Isambert N, Bompas E, Bousquet G, Duffaud F. Factors affecting the outcome of patients with metastatic leiomyosarcoma treated with doxorubicin-containing chemotherapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010 Jun;21(6):1361–1365. doi: 10.1093/annonc/mdp485. [DOI] [PubMed] [Google Scholar]
- 20.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999 Jan;17(1):150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 21.Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 Jun 15;20(12):2824–2831. doi: 10.1200/JCO.2002.11.050. [DOI] [PubMed] [Google Scholar]
- 22.Lusby K, Savannah KB, Demicco EG, et al. Uterine Leiomyosarcoma Management, Outcome, and Associated Molecular Biomarkers: A Single Institution’s Experience. Annals of surgical oncology. 2013 Jan 20; doi: 10.1245/s10434-012-2834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000 Mar 3;287(5458):1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 24.Ng TL, Gown AM, Barry TS, et al. Nuclear beta-catenin in mesenchymal tumors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005 Jan;18(1):68–74. doi: 10.1038/modpathol.3800272. [DOI] [PubMed] [Google Scholar]
- 25.Vijayakumar S, Liu G, Rus IA, et al. High-frequency canonical Wnt activation in multiple sarcoma subtypes drives proliferation through a TCF/beta-catenin target gene, CDC25A. Cancer cell. 2011 May 17;19(5):601–612. doi: 10.1016/j.ccr.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhnen C, Herter P, Muller O, et al. Beta-catenin in soft tissue sarcomas: expression is related to proliferative activity in high-grade sarcomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2000 Sep;13(9):1005–1013. doi: 10.1038/modpathol.3880181. [DOI] [PubMed] [Google Scholar]
- 27.Kuhnen C, Herter P, Monse H, et al. APC and beta-catenin in alveolar soft part sarcoma (ASPS)--immunohistochemical and molecular genetic analysis. Pathology, research and practice. 2000;196(5):299–304. doi: 10.1016/s0344-0338(00)80059-x. [DOI] [PubMed] [Google Scholar]
- 28.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nature reviews. Clinical oncology. 2011 Feb;8(2):97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 29.Mikami I, You L, He B, et al. Efficacy of Wnt-1 monoclonal antibody in sarcoma cells. BMC cancer. 2005;5:53. doi: 10.1186/1471-2407-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gloudemans T, Pospiech I, Van Der Ven LT, et al. Expression and CpG methylation of the insulin-like growth factor II gene in human smooth muscle tumors. Cancer research. 1992 Dec 1;52(23):6516–6521. [PubMed] [Google Scholar]
- 31.Malempati S, Weigel B, Ingle AM, et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children’s Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Jan 20;30(3):256–262. doi: 10.1200/JCO.2011.37.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macaulay VM, Middleton MR, Protheroe AS, et al. Phase I study of humanized monoclonal antibody AVE1642 directed against the type 1 insulin-like growth factor receptor (IGF-1R), administered in combination with anticancer therapies to patients with advanced solid tumors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013 Mar;24(3):784–791. doi: 10.1093/annonc/mds511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naing A, LoRusso P, Fu S, et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 May 1;18(9):2625–2631. doi: 10.1158/1078-0432.CCR-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quek R, Wang Q, Morgan JA, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011 Feb 15;17(4):871–879. doi: 10.1158/1078-0432.CCR-10-2621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.