Abstract
Sigma receptors belong to a class of small molecule-regulated, primarily endoplasmic reticulum (ER) membrane-associated receptors, of which there are two subtypes: the Sigma-1 receptor (S1R) and the Sigma-2 receptor (S2R). Both S1R and S2R bind to a number of drugs including antipsychotic, haloperidol, and the opioid analgesic, (+)-pentazocine. Sigma receptors are implicated in multiple disease pathologies associated with the nervous system including diseases affecting motor control such as Amyotrophic Lateral Sclerosis (ALS) and Alzeimher's disease. This unit describes methods for the pharmacological characterization of S1R and S2R using radioligand-binding assays. In the first section, radioligand saturation binding assay to determine receptor densities and competitive inhibition assays to characterize affinities of novel compounds are presented for S1R using the selective S1R ligand, [3H]-(+)-pentazocine. The second section describes radioligand saturation binding assay and competitive inhibition assays for the S2R using a non-selective S1R and S2R ligand, [3H]-1,3-di(2-tolyl)guanidine ([3H]-DTG).
Keywords: Sigma-1 receptor, sigma-2 receptor, saturation of radioligand binding assays, competitive inhibition of radioligand assays, [3H]-(+)-pentazocine, [3H]-(+)-DTG, haloperidol
INTRODUCTION
Identification of the S1R and its differentiation from G protein-coupled opioid receptors (Walker et al., 1990) was initially based on radioligand binding (Kavanaugh et al., 1989; Su, 1982). Over several decades, a large number of synthetic and endogenous small molecules both agonists and antagonists have been reported to bind to S1R and S2R (Abate et al., 2010; Abate et al., 2011; Ablordeppey et al., 2000). Several of these have been useful in radiolabeled forms to identify S1Rs and S2Rs biochemically (Fontanilla et al., 2008; Pal et al., 2008; Pal et al., 2007). However, the biochemical and functional characterization of sigma receptors present unique challenges given their paucity and membrane environment.
Cloning of the S1R (Hanner et al., 1996; Kekuda et al., 1996) identified a 223 amino acid protein (~25 kDa as assessed by SDS PAGE analysis) with two transmembrane sequences that place the N- and C- termini on the same side of the membrane. The S1R occurs in homo oligomeric states that likely regulate its functions (Chu et al., 2013; Gromek et al., 2014). The S1R knockout mouse was developed in 2003 and showed no overt phenotype which suggests a compensatory mechanism that may be mediated by other proteins including the S2R (Langa et al., 2003). Interestingly, the S1R knockout mice failed to respond to N,N-dimethyltryptamine (DMT), a hallucinogen, in a behavioral assay measuring motor coordination (Fontanilla et al., 2009). In addition, the S1R knockout mouse swam faster than wild-type animals (Mavlyutov et al., 2010) although the precise molecular mechanism(s) are unclear. These results corroborated with previous work suggesting a role of the S1R in coordinator motor response including earlier observations that sigma receptors are involved in dystonia and motor activities in response to drugs of abuse such as cocaine and methamphetamine (Hayashi et al., 2010; Kaushal and Matsumoto, 2011; Menkel et al., 1991; Robson et al., 2012; Ujike et al., 1996). The S2R has yet to be cloned but selective radiolabeled photoaffinity approaches using various mammalian tissues (including the S1R knockout tissues) and cultured cells have identified this receptor as an 18-20 kDa protein (Bowen et al., 1989; Hellewell and Bowen, 1990; Hellewell et al., 1994).
The S1R has been functionally characterized as a molecular chaperone (Hayashi and Su, 2007) with an impressive list of multitasking functions including chaperoning of many types of voltage gated ion channels (Kourrich et al., 2013) and G-protein coupled receptors (Navarro et al., 2013). The goal of the methods outlined below is to provide practical approaches for identification and quantification of S1Rs and S2Rs in membrane preparations and in intact cells. Detailed protocols for characterization of the Sigma receptors and the small molecule compounds that bind to these receptors using radioligand binding are described in Chapter 1 and photoaffinity labeling and immunofluorescence strategies are presented in Chapter 8.
The radioligand saturation-binding assay to assess the S1R characteristics in membranes can be performed using [3H]-(+)-pentazocine, a selective S1R ligand (see Basic Protocol 1). These experiments can be performed with tissue membrane homogenates, cell membranes, and/or E.coli expressed and purified protein to either compare the levels of S1R expression in these samples and/or characterize the ligand binding function of purified proteins that carry mutations.
The inhibition of [3H]-(+)-pentazocine binding assay is mainly used to determine the inhibition constant (KI) of potential S1R ligands (see Basic Protocol 2). These assays are performed with a single concentration of [3H]-(+)-pentazocine at a concentration near its KD and increasing concentrations of non-radioactive ligand.
It should be mentioned that while there are a number of selective S2R ligands that have been developed over the past two decades, the standard method to assess S2R activities is the utilization of radioactive [3H]-DTG, a nonselective S1R and S2R ligand, in the presence of nonradioactive (+)-pentazocine to mask S1R binding sites. The radioligand saturation-binding assay to assess the S2R activity and densities in tissues and/or cells is presented in Section II, Basic Protocol 3.
The inhibition of [3H]-DTG binding assay is mainly used to determine the inhibition constant (KI) of potential S2R ligands (Section II, Basic Protocol 4). These assays are performed with a single concentration [3H]-DTG at a concentration near its KD for the S2R and an increasing concentrations of non-radioactive ligand in the presence of (+)-pentazocine to mask S1R binding sites.
SECTION I: RADIOLIGAND BINDING ASSAYS FOR SIGMA-1 RECEPTOR
S1R are highly expressed in liver tissues of many species. The use of guinea pig liver (GPL) membranes for S1R research has provided useful data in assessing the in vitro characteristics of S1R ligands and radioligands. S1R protein levels are highest in GPL compared to other tissues and from other biological sources such as cells. The preparation of tissue membranes is outlined in Support Protocol 1 and can readily be used for preparation of mouse and other species liver membrane preparations (Fontanilla et al., 2008; Fontanilla et al., 2009; Pal et al., 2007). Basic Protocol 1 describes the radioligand saturation-binding assay with a selective S1R ligand, [3H]-(+)-pentazocine, to determine Bmax and KD. Basic Protocol 2 describes the inhibition of [3H]-(+)-pentazocine to determine KI of novel compounds.
BASIC PROTOCOL 1: RADIOLIGAND SATURATION-BINDING ASSAYS TO DETERMINE Bmax AND KD AT S1R
Saturation-binding studies of S1R binding (Basic protocol 1) describes the direct assessment of S1R to determine receptor densities (Bmax) and the dissociation equilibrium constant (KD) of radioligands for that site. [3H]-(+)-pentazocine ([3H]-(+)-PTZ) is the preferred radioligand since it is a selective S1R ligand.
Materials
Assay Buffer (see Reagents and Solutions section)
0.5 μg/μL GPL membranes (see Support Protocol 1) diluted in Assay Buffer
[3H]-(+)-pentazocine, 34 Ci/mmol (Perkin Elmer, Akron, OH)
100 μM haloperidol (Sigma-Aldrich, St. Louis, MO) dissolved in Assay Buffer
1 mL 96-well deep well polypropylene plates (e.g., USA Scientifics, Orlando, FL)
Glass fiber filter papers (Whatman GF/B, Whatman, Maidstone, UK)
0.5% Polyethyleneimine (Morris et al.) (Sigma-Aldrich, St. Louis, MO)
Filtration system (Brandel Cell Harvester, Gaithersburg, MD)
10 mL scintillation vials (e.g., Fisher Scientific, Waltham, MA)
Scintillation fluid (e.g., Ultima Gold, Perkin Elmer, Waltham, MA)
Scintillation counter (e.g., Packard model 1600CA, Packard Instrument Co., Downers Grove, IL)
Graphpad Prism (Graphpad Software, San Diego, CA)
Procedures
- Prepare sufficient protein samples at a concentration of 0.4 mg/mL in Assay Buffer. Each well contains 80 μL of protein samples (or 0.032 mg) which will be added to binding reaction to a final volume of 100 μL. It should be noted that 0.032 mg protein per sample was determined empirically in our laboratory to give the best results for guinea pig liver membranes. S1R densities vary among different tissues and cells and researchers are advised to use more or less protein accordingly.Example calculations for an assay containing 48 wells are shown below:
-
(1)48 wells × 80 μL/well = 3840 μL or approximately 4000 μL (4 mL) of 0.4 mg/mL membranes is needed
-
(2)4 mL × 0.4 mg/mL = (10 mg/mL) × __V__ μL
- V = 0.16 mL or 160 μL of 10 mg/mL GPL membranes added to 3840 μL of Assay Buffer to make 4 mL of 0.4 mg/mL
-
(1)
Prepare 10X stock concentrations of [3H]-(+)-pentazocine following Table 1. The concentrations should range from 3 to 3000 nM [3H]-(+)-pentazocine (final concentrations ranging from 0.3 – 300 nM). We recommend using several concentrations above and below the KD of [3H]-(+)-pentazocine for the S1R which we have previously determined to be ~ 10 nM.
Determine the actual [3H]-(+)-pentazocine in the reaction mixture, count 10 μL of each 10X stock concentrations and determine the measured radioligand concentration in assay using the specific activities of [3H]-(+)-pentazocine of 34 Ci/mmol (see Table 2).
- Set up binding assays in a 96-well plate below. For one saturation binding experiment, we use 48 wells or ½ of a 96-well plate.
Total binding (μL) Non-specific (μL) Assay buffer 10 - 10X [3H]-(+)-pentazocine (concentrations vary) 10 10 100 μM haloperidol - 10 GPL (0.4 mg/ml) 80 80 Total 100 100 For each concentration of [3H]-(+)-pentazocine in “total binding” prepare a “non-specific” sample by adding 10 μM haloperidol. As an example, use wells A1 to B12 for “total binding” and C1 – D12 for “non-specific.” It is recommended that a ligand with a chemical structure different from that of the radioligand be used to define nonspecific binding. - Incubate the 96-well plate for 90 min at 37°C.Note: The approximately time to reach equilibrium has been determined to be 60 minute for the sigma-1 receptor (Ramachandran et al., 2007).
Soak a glass fiber filter paper in 0.5% PEI at room temperature.
Terminate the reaction by rapid filtration with Assay Buffer through GF/B filters using a Brandel cell harvester. Rinse three times with ~500 μL of ice-cold Assay Buffer.
Remove glass filter paper discs and insert in liquid scintillation vials. Allow the filter paper to dry at room temperature for 1 hour before adding 3 mL of scintillation fluid into each vial and incubate scintillation vials overnight at room temperature.
Determine the bound radioactivity (in DPM) the following day using the scintillation counter.
Table 1.
Sample dilution scheme of [3H]-(+)-pentazocine for saturation-binding experiments
| Dilution # | Final [3H]-(+)-PTZ concentration1 (nM) | 10X stock [3H]-(+)-PTZ concentrations (nM) | Dilution factor | Dilution volumes |
|---|---|---|---|---|
| 1 | 300 nM | 3000 nM | 10 | 102 μL stock + 898 μL of Assay Buffer |
| 2 | 100 nM | 1000 nM | 28 | 34 μL of stock + 966 μL of Assay Buffer |
| 3 | 30 nM | 300 nM | 10 | 100 μL of dilution #1 + 900 μL of Assay Buffer |
| 4 | 10 nM | 100 nM | 10 | 100 μL of dilution #2 + 900 μL of Assay Buffer |
| 5 | 3 nM | 30 nM | 10 | 100 μL of dilution #3 + 900 μL of Assay Buffer |
| 6 | 1 nM | 10 nM | 10 | 100 μL of dilution #4 + 900 μL of Assay Buffer |
| 7 | 0.3 nM | 3 nM | 10 | 100 μL of dilution #5 + 900 μL of Assay Buffer |
Final radiolgiand concentration is the concentrations of [3H]-(+)-pentazocine in the binding assay.
2 The stock concentration of [3H]-(+)-pentazocine is supplied at 34 Ci/mmol (e.g., Perkin Elmer). The calculated concentrations of this stock [3H]-(+)-petnazocine is counted and determined be approximately 29.4 μM (1/34 Ci/mmol × 1000) which is the concentrations used to prepare the 10X stocks radioligand.
Table 2.
Sample conversion of total [3H]-(+)-pentazocine counts into nanomolar concentrations in assay
| Targeted [3H]-(+)-PTZ concentration (nM) | Measured total radioactivity (DPM) | Measured total [3H]-(+)-PTZ (mmol) | Measured [3H]-(+)-PTZ concentration in assay (nM) |
|---|---|---|---|
| 300 | 2037960 | 2.70E-08 | 270.0 |
| 100 | 679420 | 9.00E-09 | 90.0 |
| 30 | 204684 | 2.71E-09 | 27.1 |
| 10 | 66382 | 8.79E-10 | 8.8 |
| 3 | 21047 | 2.79E-10 | 2.8 |
| 1 | 6573 | 8.71E-11 | 0.9 |
| 0.3 | 2234 | 2.96E-11 | 0.3 |
The first column displays targeted concentrations of [3H]-(+)-pentazocine calculated from the stock vial (34 Ci/mmol or ~29.4 μM). Second column contains radioactivity counts in DPM of the targeted concentration of [3H]-(+)-pentazocine. Third column shows radioactivity values transformed from DPM into millimoles of radioligand (Note: the conversion factor used was 2.22 × 1012 DPM per Ci). The fourth column shows the actual concentrations of [3H]-(+)-pentazocine as calculated from the DPM values obtained.
Data Analyses
-
10.Calculate the average of triplicate total and nonspecific binding values for each concentration of [3H]-(+)-pentazocine tested and convert DPM into pmol/mg protein (see Equation 1), results are displayed in Table 3.
-
11.
Determine the “specific binding” by subtracting “nonspecific binding” (in pmol/mg) from “total binding) (see Table 4).
-
12.Using nonlinear regression analysis, fit the specific binding data to Equation 2 below:
where L and KD are the radioligand concentration and dissociation constant, respectively, and Bmax is the maximum binding (Bmax is the receptor densities).Our laboratory uses Graphpad Prism 5.0 to perform data analysis and graphing the results. An example showing saturation binding of [3H]-(+)-pentazocine is shown in Figure 1. Bound [3H]-(+)-pentazocine data are plotted against measured concentrations of [3H]-(+)-pentazocine. Both the Bmax and KD can be obtained from the software package.
Table 3.
Sample transformation of bound radioactivity data from DPM into pmol/mg protein
| Average bound | |||
|---|---|---|---|
| Measured [3H]-(+)-PTZ concentration (nM) | Bound (DPM) | Bound (pmol) | Bound (pmol/mg protein) |
| 270.0 | 18260 | 0.241918389 | 7.559949656 |
| 90.0 | 17936 | 0.237625861 | 7.425808161 |
| 27.1 | 12433 | 0.164719131 | 5.14747284 |
| 8.8 | 7799 | 0.103325384 | 3.228918256 |
| 2.8 | 4559 | 0.060400106 | 1.887503312 |
| 0.9 | 1866 | 0.024721781 | 0.772555644 |
| 0.3 | 1253 | 0.016600424 | 0.518763249 |
The first column displays measured [3H]-(+)-pentazocine calculated from counting an aliquot of each [3H]-(+)-pentazocine concentrations (see Table 2 for detail calculations). The second column shows the mean from triplicates in DPM and the third column displays transformation of the DPM values into radioactivity bound expressed as pmol [Note: radioactivity bound is determined using the conversion factor 2.22 × 1012 DPM per Ci and the specific activity of [3H]-(+)-pentazocine of 34 Ci/mmol]. The last column shows bound radioligand levels normalized to the total protein added to the assay which is 0.032 mg per well.
Table 4.
Sample saturation of [3H]-(+)-pentazocine binding data
| Bound [3H]-(+)-pentazocine (pmol/mg) | |||
|---|---|---|---|
| Measured [3H]-(+)-PTZ concentration (nM) | Total | Nonspecific | Specific |
| 270.0 | 7.56 | 2.43 | 5.13 |
| 90.0 | 7.43 | 2.39 | 5.04 |
| 27.1 | 5.15 | 0.82 | 4.33 |
| 8.8 | 3.23 | 0.28 | 2.95 |
| 2.8 | 1.88 | 0.11 | 1.77 |
| 0.9 | 0.77 | 0.09 | 0.68 |
| 0.3 | 0.52 | 0.04 | 0.48 |
Total binding is defined as [3H]-(+)-pentazocine bound while nonspecific binding is the counts obtained for samples that contain 10 μM haloperidol. Specific binding is defined here as total – nonspecific.
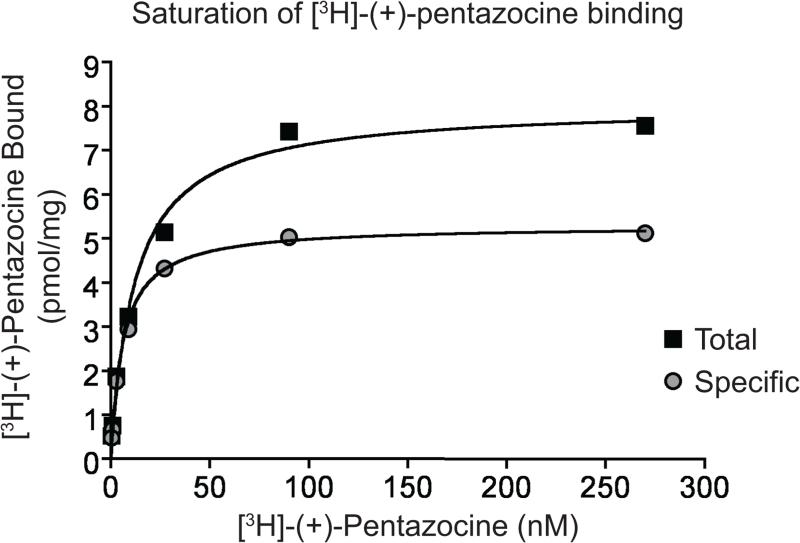
Figure 1. Sample saturation binding of [3H]-(+)-pentazocine.
A representative saturation binding of [3H]-(+)-pentazocine to assess activities and S1R protein levels in GPL membranes. Total refers to total radioactivity bound and Specific refers to Total binding subtract nonspecific binding.
BASIC PROTOCOL 2: INHIBITION OF [3H]-(+)-PENTAZOCINE TO DETERMINE INHIBITION BINDING CONSTANTS (KI) OF POTENTIAL SIGMA-1 RECEPTOR LIGANDS
Basic Protocol 2 describes inhibition of [3H]-(+)-pentazocine with an unlabeled ligand to determine its inhibition constant (KI) (Chu et al., 2011; Fontanilla et al., 2008; Fontanilla et al., 2009; Pal et al., 2007). The biological source use for this example is GPL membranes since guinea pig liver expresses high levels of S1R.
Materials
Assay Buffer (see Reagents and Solutions section)
0.5 μg/μL GPL membranes (see Support Protocol 1) diluted in Assay Buffer
[3H]-(+)-pentazocine (Perkin Elmer, Akron, OH)
Nonradioactive test compounds/inhibitors
100 μM haloperidol (Sigma-Aldrich, St. Louis, MO) dissolved in Assay Buffer
1 mL 96-well deep well polypropylene plates (e.g., USA Scientifics, Orlando, FL)
Glass fiber filter papers (Whatman GF/B, Whatman, Maidstone, UK)
0.5% Polyethyleneimine (Morris et al.) (Sigma-Aldrich, St. Louis, MO)
Filtration system (Brandel Cell Harvester, Gaithersburg, MD)
10 mL scintillation vials (e.g., Fisher Scientific, Waltham, MA)
Scintillation fluid (e.g., Ultima Gold, Perkin Elmer, Waltham, MA)
Scintillation counter (e.g., Packard model 1600CA, Packard Instrument Co., Downers Grove, IL)
Graphpad Prism (Graphpad Software, San Diego, CA)
Procedures
- Prepare sufficient protein samples at a concentration of 0.4 mg/mL in Assay Buffer. Each well contains 80 μL of protein samples (or 0.032 mg) which will be added to binding reaction to a final volume of 100 μL. It should be noted that 0.032 mg proteins per sample was determined empirically in our laboratory to give the best results for guinea pig liver membranes. S1R densities vary in different tissue and cell types, researchers are advised to adjust the protein concentrations according when another biological source is used.Example calculations for an assay containing 48 wells are shown below
-
(1)48 wells × 80 μL/well = 3840 μL or approximately 4000 μL (4 mL) of 0.4 mg/mL membranes is needed
-
(2)4 mL × 0.4 mg/mL = (10 mg/mL)× __V__ μL
- V = 0.16 mL of 10 mg/mL GPL membranes added to 3.840 mL of Assay Buffer to make 4 mL of 0.4 mg/mL
-
(1)
- Prepare 100 nM [3H]-(+)-pentazocine.We have previously determined the KD of (+)-pentazocine for the S1R to be approximately 10 nM. It is ideal to use the concentration of the radioligand equal to or slightly above the KD. To determine the actual [3H]-(+)-pentazocine in the reaction mixture, count 10 μL of the 100 nM [3H]-(+)-pentazocine (10X stock) and determine the measured radioligand concentration in assay taking into account the specific activities of [3H]-(+)-pentazocine (for this example, the specific activity of 100 nM [3H]-(+)-pentazocine was 34 Ci/mmol and the conversion factor is 2.22 × 1012 DPM/Ci).
Prepare stock concentrations of inhibitors following Table 6. It is suggested that researchers prepare a stock 1.00E-2 M (or 100 mM) of inhibitor and use this master stock to prepare 1.00E-3 M (or 1 mM) and 3.00E-4 M (or 0.3 mM). These are used to prepare subsequent dilutions for the binding assay.
- Set up binding assays in a 96-well plate as shown below in quadruplicates. For one competition experiment, we use 48 wells or ½ of the 96-well plate which allows for one quadruplicate set (4 wells) of “total binding,” one quadruplicate set (4 wells) of “non-specific binding” (4 wells), and 10 concentrations of inhibitor (40 wells) for a total of 48 wells.
Total binding (μL) Non-specific (μL) Inhibitor (μL) GPL (0.4 mg/ml) 80 80 80 100 nM [3H]-(+)-pentazocine 10 10 10 100 μM haloperidol - 10 - 10X Inhibitor (concentrations vary, see Table 6) - - 10 Assay buffer 10 - - Total 100 100 100 - Incubate the 96-well plate for 90 min at 37°C.Note: Time to reach equilibrium depends on the physical and chemical properties of the inhibitor. We typically incubate the assay for 90 min but researchers are advised to make adjustments appropriately (see Unit 1.3 for background on estimation of time to reach steady state).
Soak a glass fiber filter paper in 0.5% PEI at RT.
Terminate the reaction by rapid filtration with Assay Buffer through GF/B filters using a Brandel cell harvester. Rinse three times with ~500 μL of ice-cold Assay Buffer.
Remove glass filter paper discs and insert in liquid scintillation vials. Allow the filter paper to dry at room temperature for 1 hour before adding 3 mL of scintillation fluid into each vial and incubate scintillation vials overnight at room temperature.
Determine the bound radioactivity (in DPM) the following day using the scintillation counter.
Table 6.
Sample dilution scheme for a binding site inhibitor tested in a competition binding assay
| Dilution # | Final unlabeled inhibitor concentration (M) | 10X Stock unlabeled inhibitor concentrations (M) | Dilution factor | Dilution volumes |
|---|---|---|---|---|
| 1 | 1.00E-05 | 1.00E-04 | 10 | 100 μL of 1.00E-03 M stock + 900 μL of Assay Buffer |
| 2 | 3.00E-06 | 3.00E-05 | 10 | 100 μL of 3.00E-04 M stock + 900 μL of Assay Buffer |
| 3 | 1.00E-06 | 1.00E-05 | 10 | 100 μL of dilution #2 + 900 μL of Assay Buffer |
| 4 | 3.00E-07 | 3.00E-06 | 10 | 100 μL of dilution #3 + 900 μL of Assay Buffer |
| 5 | 1.00E-07 | 1.00E-06 | 10 | 100 μL of dilution #4 + 900 μL of Assay Buffer |
| 6 | 3.00E-08 | 3.00E-07 | 10 | 100 μL of dilution #5 + 900 μL of Assay Buffer |
| 7 | 1.00E-08 | 1.00E-07 | 10 | 100 μL of dilution #6 + 900 μL of Assay Buffer |
| 8 | 3.00E-09 | 3.00E-08 | 10 | 100 μL of dilution #7 + 900 μL of Assay Buffer |
| 9 | 1.00E-09 | 1.00E-08 | 10 | 100 μL of dilution #8 + 900 μL of Assay Buffer |
| 10 | 3.00E-10 | 3.00E-09 | 10 | 100 μL of dilution #9 + 900 μL of Assay Buffer |
The final concentration of unlabeled inhbitor is the concentration in the well (column 2). The 10X stock unlabeled inhibitor concentrations is the stock concentration prior to dilution (column 3).
Data Analyses
-
10.
Inhibition binding data are typically expressed simply as radioligand binding (CPM or DPM) or % specific binding against a logarithmic molar concentrations of inhibitor. Data should be fit with an unrestricted Hill slope (see Unit 1.3 for background).
-
11.We typically report our results as % specific binding and these values can be determined using Equation 3
-
12.To determine the KI values for tested compounds, we fit the data to a “one-site competition” function in GraphPad Prism (see Figure 2). From the IC50 value (concentration of inhibitor causing a 50% reduction in radioligand specific binding), a KI (inhibition constant; the concentration of inhibitor that would occupy 50% of the receptors in the absence of radioligand) is calculated using the Cheng-Prusoff Equation (Equation 4).IC50 = inhibition concentration at 50%, which is determined from the graph [L] = concentrations of [3H]-(+)-pentazocine, we use 10 nM (researchers should count a small aliquot of radioactive [3H]-(+)-pentazocine to determine the actual concentration) KD = equilibrium dissociation constant of [3H]-(+)-pentazocine, we use 10 nM which can be determined in a saturation binding of [3H]-(+)-pentazocine (see Basic Protocol 1).
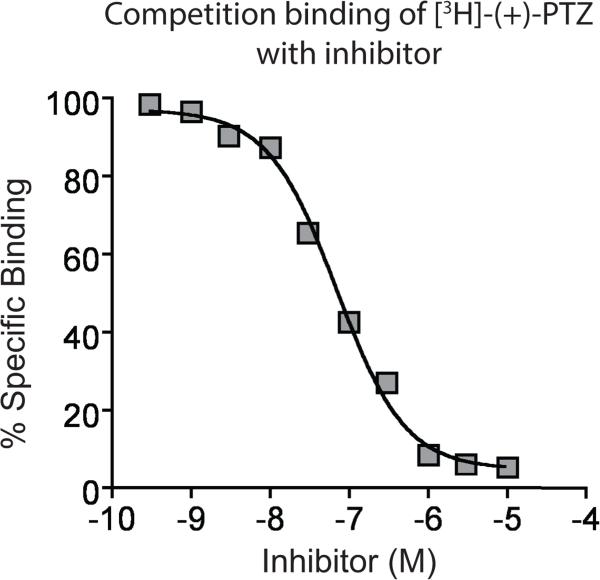
Figure 2. Sample inhibition of [3H]-(+)-pentazocine binding to the S1R.
A representative inhibition of [3H]-(+)-pentazocine to determine KI of a test compound. Points are mean of quadruplicates. Percent specific binding is calculated using Equation 3 (see text).
SECTION II: RADIOLIGAND BINDING ASSAYS FOR SIGMA 2 RECEPTORS
Section II first describes a saturation equilibrium binding of [3H]-1,3-Di-o-tolylguanidine ([3H]-DTG) to directly assess the receptor densities (Bmax) and dissociation constant (KD) for this receptor (Basic Protocol 3) in different tissues and cell types. Basic Protocol 4 outlines a competitive inhibition of [3H]-DTG to determine the dissociation constant (KI) of compounds that bind to the S2R. DTG is a non-selective ligand for both the S1R and S2R thus requiring the addition of non-radioactive (+)-pentazocine, a selective S1R ligand, to each reaction to mask the S1R sites. The S2R protein levels in RL are 3 folds higher than the S1R protein level (e.g. the ratio of S2R to S1R is approximately 75% to 25% respectively (unpublished data). In addition, the absolute levels of the S2R is highest in RL as compared to other tissues/cells thus RL is a preferred biological source of S2R for testing potential S2R compounds.
BASIC PROTOCOL 3: RADIOLIGAND SATURATION-BINDING ASSAYS TO DETERMINE KD and Bmax AT S2R
Basic Protocol 3 describes the direct assessment of S2R levels using saturation equilibrium binding of [3H]-DTG. It should be noted that the emphasis of this protocol is on the measurement of S2R, data are presented for [3H]-DTG binding to both S1R and S2R.
Materials
Assay Buffer (see Reagents and Solutions section)
0.5 μg/μL GPL membranes (see Support Protocol 1) diluted in Assay Buffer
[3H]-DTG, 40 Ci/mmol (Perkin Elmer, Akron, OH)
100 μM haloperidol (Sigma-Aldrich, St. Louis, MO) dissolved in Assay Buffer
Nonradioactive 1 μM (+)-pentazocine (Sigma-Aldrich, St. Louis, MO) prepared in Assay Buffer
1 mL 96-well deep well polypropylene plates (e.g., USA Scientifics, Orlando, FL)
Glass fiber filter papers (Whatman GF/B, Whatman, Maidstone, UK)
0.5% Polyethyleneimine (Morris et al.) (Sigma-Aldrich, St. Louis, MO)
Filtration system (Brandel Cell Harvester, Gaithersburg, MD)
10 mL scintillation vials (e.g., Fisher Scientific, Waltham, MA)
Scintillation fluid (e.g., Ultima Gold, Perkin Elmer, Waltham, MA)
Scintillation counter (e.g., Packard model 1600CA, Packard Instrument Co., Downers Grove, IL)
Graphpad Prism (Graphpad Software, San Diego, CA)
Procedures
- Prepare sufficient protein samples at a concentration of 0.4 mg/mL in Assay Buffer. Each well contains 70 μL of protein samples (or 0.028 mg) which will be added to binding reaction to a final volume of 100 μL. It should be noted that 0.028 mg proteins per sample was determined empirically in our laboratory to give the best results for rat liver membranes. S2R densities vary among different tissues and cells and researchers are advised to use more or less proteins accordingly.Example calculations for an assay containing 72 wells are shown below:
-
(1)72 wells × 70 μL/well = 5040 μL or round up to 5200 μL (5.2 mL) of 0.4 mg/mL membranes is needed
-
(2)5.2 mL × 0.4 mg/mL = (10 mg/mL)× __V__ μL
- V = 0.208 mL of 10 mg/mL RL membranes added to 4.992 mL of Assay Buffer to make 5.2 mL of 0.4 mg/mL
-
(1)
Prepare 10X stock concentrations of [3H]-DTG following the examples shown in Table 9. The concentrations should range from 3 to 3000 nM [3H]-DTG (final concentrations ranging from 0.3 – 300 nM). We recommend using several concentrations above and below the KD of [3H]-DTG for the S2R which we have determined to be 35 – 60 nM.
To determine the actual [3H]-DTG in the reaction mixture, count 10 μL of each 10X stock concentration and determine the measured radioligand concentration in assay using the specific activities of [3H]-DTG of 40 Ci/mmol (see Table 10).
- Set up binding assays in a 96-well plate below. For one saturation binding experiment, we use 72 wells or ¾ of a 96-well plate.
Total binding (μL) S2R Binding (μL) Non-specific (μL) RL membranes (0.4 mg/ml) 70 70 70 10X [3H]-DTG (concentrations see Table 9) 10 10 10 1 μM (+)-pentazocine - 10 10 100 μM haloperidol - - 10 Assay buffer 20 10 - Total 100 100 100 As an example, use A1 to B12 for “total binding,” C1 – D12 for “S2R binding,” and E1 – F12 for “non-specific.” It is recommended that a ligand with a chemical structure different from that of the radioligand be used to define nonspecific binding and haloperidol is a standard molecule used since it binds to both S1R and S2R. - Incubate the 96-well plate for 90 min at 37°C.Note: The approximately time to reach equilibrium has been determined to be 60 minute for the S2R (Ramachandran et al., 2007).
Soak 2 glass fiber filter paper in 0.5% PEI at RT.
Terminate the reaction by rapid filtration with Assay Buffer through GF/B filters using a Brandel cell harvester. Rinse three times with ~500 μL of ice-cold Assay Buffer.
Remove glass filter paper discs and insert in liquid scintillation vials. Allow the filter paper to dry at room temperature for 1 hour before adding 3 mL of scintillation fluid into each vial and incubate scintillation vials overnight at room temperature.
Determine the bound radioactivity (in DPM) the following day using the scintillation counter.
Table 9.
Sample dilution scheme of [3H]-DTG for saturation-binding experiments
| Dilution # | 1Final [3H]-DTG concentration (nM) | Stock [3H]-DTG concentrations (nM) | Dilution factor | Dilution volumes |
|---|---|---|---|---|
| 1 | 300 nM | 3000 nM | 8.3 | 120 μL stock2 + 880 μL of Assay Buffer |
| 2 | 100 nM | 1000 nM | 25 | 40 μl of stock + 960 μL of Assay Buffer |
| 3 | 30 nM | 300 nM | 10 | 100 μL of dilution #1 + 900 μL of Assay Buffer |
| 4 | 10 nM | 100 nM | 10 | 100 μL of dilution #2 + 900 μL of Assay Buffer |
| 5 | 3 nM | 30 nM | 10 | 100 μL of dilution #3 + 900 μL of Assay Buffer |
| 6 | 1 nM | 10 nM | 10 | 100 μL of dilution #4 + 900 μL of Assay Buffer |
| 7 | 0.3 nM | 3 nM | 10 | 100 μL of dilution #5 + 900 μL of Assay Buffer |
Final radiolgiand concentration is the concentrations of [3H]-(+)-pentazocine in the binding assay.
The stock concentration of [3H]-DTG is supplied at 40 Ci/mmol (e.g., Perkin Elmer). The calculated concentrations of this stock [3H]-DTG is counted and determined be approximately 25 μM (1/40 Ci/mmol × 1000) which is the concentrations used to prepare the 10X stocks radioligand.
Table 10.
Sample conversion of total [3H]-DTG counts into nanomolar concentrations in assay
| Targeted [3H]-DTG concentration (nM) | Measured total radioactivity (DPM) | Measured total [3H]-DTG (mmol) | Measured [3H]-DTG concentration in assay (nM) |
|---|---|---|---|
| 300 | 2337238 | 2.63E-08 | 310 |
| 100 | 693785.5 | 7.81E-09 | 92 |
| 30 | 204121.8 | 2.30E-09 | 27 |
| 10 | 67841.83 | 7.64E-10 | 9 |
| 3 | 20148.28 | 2.27E-10 | 2.7 |
| 1 | 6984 | 7.86E-11 | 0.8 |
| 0.3 | 2223 | 2.50E-11 | 0.25 |
The first column displays targeted concentrations of [3H]-DTG calculated from the stock vial (40 Ci/mmol or ~30 μM). Second column contains radioactivity counts in DPM of the targeted concentration of [3H]-DTG. Third column shows radioactivity values transformed from DPM into millimoles of radioligand (Note: the conversion factor used was 2.22 × 1012 DPM per Ci). The fourth column shows the actual concentrations of [3H]-DTG as calculated from the DPM values obtained.
Data Analyses
-
10.
Calculate the average of triplicate total and nonspecific binding values for each concentration of [3H]-DTG tested and convert DPM into pmol/mg protein (see Basic Protocol 1, Equation 1). To simplify the display of data, only specific binding values are presented (Table 11).
-
11.Using nonlinear regression analysis, fit the specific binding data to Equation 2 (Basic Protocol 1).Our laboratory uses Graphpad Prism 5.0 to perform data analysis and graphing the results. An example showing saturation binding of [3H]-DTG is shown in Figure 3. Bound [3H]-DTG data are plotted against measured increasing concentrations of measured free [3H]-DTG. Both the Bmax and KD can be obtained from the software program.
Table 11.
Sample transformation of bound radioactivity data from DPM into pmol/mg protein
| SIR + S2R Binding | S2R Binding | |||
|---|---|---|---|---|
| Measured [3H]-DTG concentration (nM) | Specific Binding* (DPM) | Specific Binding* (pmol/mg) | Specific Binding* (DPM) | Specific Binding* (pmol/mg) |
| 310 | 57234 | 20.14 | 47234 | 16.62 |
| 92 | 52722 | 18.55 | 40345 | 14.19 |
| 27 | 32299 | 11.36 | 22584 | 7.94 |
| 9 | 18545 | 6.52 | 11345 | 3.99 |
| 2.7 | 6280 | 2.21 | 5123 | 1.80 |
| 0.8 | 2676 | 0.94 | 1543 | 0.54 |
| 0.25 | 1253 | 0.44 | 938 | 0.33 |
The first column displays measured [3H]-DTG (see Table 10 for detail calculations). Values in columns two and three represent specific binding* of [3H]-DTG to both S1R and S2R. The second column shows the mean from triplicates in DPM and the third column displays transformation these counts into radioactivity bound normalized to proteins (pmol/mg). The fourth and fifth columns display specific binding* of [3H]-DTG to only the S2R (the S1R sites were masked by the addition of (+)-pentazocine). Column four are mean from triplicates in DPM and the fifth column displays transformation of these counts into radioactivity bound normalized to proteins (pmol/mg). Note: radioactivity bound is determined using the conversion factor 2.22 × 1012 DPM per Ci, the specific activity of [3H]-DTG is 40 Ci/mmol, and total proteins equal 0.032 mg per well (see Equation 1 for details).
Specific binding is defined as total bound – nonspecific binding (in the presence of 10 μM of haloperidol.
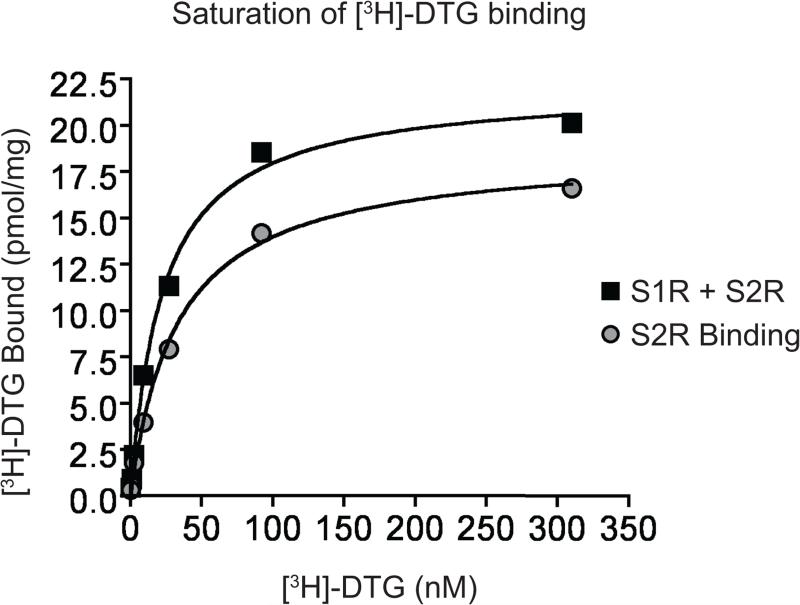
Figure 3. Sample saturation binding of [3H]-DTG to both S1R and S2R.
A representative saturation-binding of [3H]-DTG to assess S1R and S2R proteins in RL membranes. S1R + S2R binding is defined as total [3H]-DTG bound while S2R binding is determined by subtracting the counts from the plus (+)-pentazocine conditions from the total [3H]-DTG binding (without (+)-pentazocine (see Table 11 for data).
BASIC PROTOCOL 4: INHIBITION OF [3H]-1,3-DI-O-TOLYLGUANIDINE TO DETERMINE INHIBITION BINDING CONSTANTS (KI) OF POTENTIAL S2R LIGANDS
Basic Protocol 4 describes inhibition of [3H]-DTG with an unlabeled ligand to determine its inhibition constant (KI). The biological source use for this example is RL membranes since rat liver expresses the highest levels of S2R as compared to S1R.
Materials
Assay Buffer (see Reagents and Solutions section)
0.5 μg/μL GPL membranes (see Support Protocol 1) diluted in Assay Buffer
0.3 μM or 300 nM [3H]-DTG (Perkin Elmer, Akron, OH)
1 μM (+)-pentazocine (Sigma-Aldrich, St. Louis, MO) dissolved in Assay Buffer
Nonradioactive test compounds/inhibitors
100 μM haloperidol (Sigma-Aldrich, St. Louis, MO) dissolved in Assay Buffer
1 mL 96-well deep well polypropylene plates (e.g., USA Scientifics, Orlando, FL)
Glass fiber filter papers (Whatman GF/B, Whatman, Maidstone, UK)
0.5% Polyethyleneimine (Morris et al.) (Sigma-Aldrich, St. Louis, MO)
Filtration system (Brandel Cell Harvester, Gaithersburg, MD)
10 mL scintillation vials (e.g., Fisher Scientific, Waltham, MA)
Scintillation fluid (e.g., Ultima Gold, Perkin Elmer, Waltham, MA)
Scintillation counter (e.g., Packard model 1600CA, Packard Instrument Co., Downers Grove, IL)
Graphpad Prism (Graphpad Software, San Diego, CA)
Procedures
- Prepare sufficient protein samples at a concentration of 0.4 mg/mL in Assay Buffer. Each well contains 70 μL of protein samples (or 0.028 mg) which will be added to binding reaction to a final volume of 100 μL. It should be noted that 0.028 mg proteins per sample was determined empirically in our laboratory to give the best results for rat liver membranes. S2R densities vary in different tissue and cell types, researchers are advised to adjust the protein concentrations according when another biological source is used.Example calculations for an assay containing 48 wells are shown below:
-
(1)48 wells × 70 μL/well = 3360 μL or approximately 3500 μL (3.5 mL) of 0.4 mg/mL membranes is needed
-
(2)3.5 mL × 0.4 mg/mL = (10 mg/mL) __V__ μL
- V = 0.14 mL of 10 mg/mL RL membranes added to 3.360 mL of Assay Buffer to make 3.5 mL of 0.4 mg/mL
-
(1)
- Prepare 0.3 μM [3H]-DTG.We have previously determined the KD of [3H]-DTG for the S2R to be approximately 30 - 50 nM. It is ideal to use the concentration of the radioligand equal to or slightly above the KD. To determine the actual [3H]-DTG in the reaction mixture, count 10 μL of each 0.3 μM [3H]-DTG and determine the measured radioligand concentration in assay using the specific activities of [3H]-DTG (for this example, the specific activity of 300 nM [3H]-DTG was 40 Ci/mmol and the conversion factor is 2.22 × 1012 DPM/Ci).
Prepare stock concentrations of inhibitors following Table 5. It is suggested that researchers prepare a stock 1.00E-2 M (or 100 mM) of inhibitor and use this master stock to prepare 1.00E-3 M (or 1 mM) and 3.00E-4 M (or 0.3 mM). These are used to prepare subsequent dilutions for the binding assay.
- Set up binding assays in a 96-well plate as shown below in quadruplicates. For one competition experiment, we use 48 wells or ½ of the 96-well plate which allows for one quadruplicate set (4 wells) of “total binding,” one quadruplicate set (4 wells) of “non-specific binding” (4 wells), and 10 concentrations of inhibitor (40 wells) for a total of 48 wells.
Total binding (μL) Non-specific (μL) Inhibitor (μL) RL membranes (0.4 mg/ml) 70 70 70 0.30 μM or 300 nM [3H]-DTG 10 10 10 100 μM haloperidol - 10 - 1 μM (+)-pentazocine 10 10 10 10X Inhibitor (concentrations vary) - - 10 Assay buffer 10 - - Total 100 100 100 - Incubate the 96-well plate for 90 min at 37°C.Note: Time to reach equilibrium depends on the physical and chemical properties of the inhibitor. We typically incubate the assay for 90 min but researchers are advised to make adjustments appropriately (see Unit 1.3 for background on estimation of time to reach equilibrium).
Soak a glass fiber filter paper in 0.5% PEI at RT.
Terminate the reaction by rapid filtration with Assay Buffer through GF/B filters using a Brandel cell harvester. Rinse three times with ~500 μL of ice-cold Assay Buffer.
Remove glass filter paper discs and insert in liquid scintillation vials. Allow the filter paper to dry at room temperature for 1 hour before adding 3 mL of scintillation fluid into each vial and incubate scintillation vials overnight at room temperature.
Determine the bound radioactivity (in DPM) the following day using the scintillation counter.
Table 5.
Typical KD and Bmax values for both [3H]-(+)-pentazocine and [3H]-DTG binding
| [3H]-(+)-pentazocine | [3H]-DTG | |||
|---|---|---|---|---|
| KD (nM) | Bmax (pmol/mg) | KD (nM) | Bmax (pmol/mg) | |
| Guinea Pig Liver | 0.2 | 3.1 | - | - |
| Rat Liver | - | - | 150 | 11 |
| Recombinant SIR* | 35 | 10600 | - | - |
KD and Bmax of [3H]-(+)-pentazocine for recombinant S1R were obtained from Ramachandran et al. 2007.
Data Analyses
-
10.
Inhibition binding data are typically expressed simply as radioligand binding (CPM or DPM) or % specific binding against a logarithmic molar concentrations of inhibitor. Data should be fit with an unrestricted Hill slope (see Unit 1.3 for background).
-
11.
We typically report our results as % specific binding and these values can be determined using Equation 3 (see Basic Protocol 2).
-
12.
To determine the KI values for tested compounds, we fit the data to a “one-site competition” function in GraphPad Prism (see Basic Protocol 2, Figure 2 for an example). From the IC50 value (concentration of inhibitor causing a 50% reduction in radioligand specific binding), a KI (inhibition constant; the concentration of inhibitor that would occupy 50% of the receptors in the absence of radioligand) is calculated using the Cheng-Prusoff Equation (see Basic Protocol 2). We use 30 nM as the concentration of [3H]-DTG and 30 nM as the KD for S2R.
SUPPORT PROTOCOL 1: PREPARATION OF MEMBRANE FRACTIONS FROM TISSUES
S1R and S2R are ubiquitously expressed in many different tissues throughout the body. Although originally found in brain tissues, S1R and S2R are also abundant in liver tissues which are the main tissue membranes used in our laboratory to characterize novel ligands for both receptor subtypes. S1R expression is higher in guinea pig livers compared to rat livers (approximately 80% of [3H]-DTG binding is attributed to S1R in guinea pig livers). In contrast, rat expresses higher levels of S2R (approximately 75% of [3H]-DTG binding in rat liver is attributed to S2R). Described below are ways to prepare crude membranes from these tissues for use in radioligand-binding studies and for photolabeling experiments (Chapter 8). The S1R activities (as assessed both by [3H]-(+)-pentazocine) in guinea pig liver membranes are stable for up to one year. On the contrary, the half-life of the S2R (as assessed by [3H]-DTG binding) is three months. Researchers are advised to prepare fresh membranes as needed.
Materials
Homogenization Buffer (see Regeants and Solutions section)
Fresh or frozen liver tissues (Pel Freez Biologicals, Rogers, AR)
Sharp dissecting scissor
Tissue disrupter (e.g., Brinkmann Polytron homogenizer)
Tissue homogenizer (e.g., Potter-Elvehjem Grinding Chambers and Pestles)
Hi-speed centrifuge and centrifuge tubes or an equivalent centrifuge (e.g., Beckman L7 Ultracentrifuge with 45Ti rotor)
Bradford Protein Assay Reagent (Thermo Scientific, Waltham, MA)
Liquid nitrogen
Procedures
Weigh ~10 – 15 g frozen/fresh liver tissue and thaw on ice in Homogenization Buffer at a buffer to tissue ratio of 5 mL/g liver tissue.
Mince liver tissue on ice using dissecting scissors.
Homogenize liver tissue by four 10-sec bursts using a Brinkmann Polytron on setting 6 (setting 10 is maximum).
Sediment the homogenate by centrifuging for 10 min at 17,000 x g and 4°C.
Pour supernatant from step 4 into a separate tube taking care not to disrupt the pellet and centrifuge for 60 min at 100,000 x g and 4°C.
Discard the resultant supernant and resuspend the pellet from step 5 in 10 mL of Homogenization Buffer/g tissue and homogenize tissue pellet using a Potter-Elvehjem Pestles on setting 5 until membranes are homogenous (smooth milky suspension without the presence of detectable particulate).
Determine the protein concentration using Bradford Protein Assay Reagent according to manufacturer's protocol.
Snap-freeze liver membranes in 1 mL aliquots at a stock concentrations of 10 mg/mL and store at −80°C.
REAGENTS AND SOLUTIONS
Use deionized distilled water in all recipes and protocol steps.
Homogenization Buffer
10 mM NaH2PO4 pH 7.4
0.32 M sucrose
1 mM MgSO4
10 μg/mL leupeptin
1 μg/mL pepstatin A
5 μg/mL soybean trypsin inhibitor
1 mM phenylmethylsulfonyl fluoride (PMSF)
0.5 mM EGTA
Assay Buffer
50 mM Tris-HCl pH 8.0
COMMENTARY
Background Information
Receptor-binding assays are straightforward and powerful techniques for determining ligand-receptor interactions. The three main types of binding assays include saturation, inhibiton and kinetic. Saturation binding measures the direct interaction between a radioligand that binds with high affinity (nanomolar concentrations) to a receptor and expresses as dissociation constant (KD). In addition, maximum binding of the radioligand (Bmax) can inform researchers of the receptor densities in the tissue being studied. A second method for determining affinity of compounds for a receptor is compete a fixed concentration of the radioligand off using increasing concentrations of an inhibitor. The affinity for the nonradiolabel inhibitor can be derived from the Cheng-Prusoff correction and expresses as inhibition constant (KI). This method relies on an assumption that the binding site for the radioligand and the inhbitor share some overlapping regions. Finally, kinetic binding studies (not cover in this unit) measure the binding of radiolabel ligand at various time points to determine association and dissociation rates. For more information about the basic principles of receptor binding assays, see Unit 1.3.
Sigma-1 and sigma-2 receptors represent two distinct binding sites in mammalian tissues. [3H]-(+)-pentazocine was established as the radioligand used in the detection of sigma-1 receptor (de Costa et al., 1989) while the accepted radioligand binding techniques for measuring sigma-2 receptor uses [3H]-DTG in the presence of 100 nM of unlabeled (+)-pentazocine (Quirion et al., 1992) (Hellewell and Bowen, 1990; Hellewell et al., 1994; Walker et al., 1992). While a number of radiolabeled compounds have been developed in recent years (Mach and Wheeler, 2009), from a historical stand point [3H]-(+)-pentazocine and [3H]-DTG remain reliable radioligand for the pharmacological assessment of S1R and S2R respectively.
Critical Parameters
Saturation binding of radioligand assays
In our experience, it takes at least 90 min at 37°C for [3H]-(+)-pentazocine to reach equilibrium in tissue membranes, we recommend keeping incubation time to at least 90 min. Although it is possible to use a different compound to measure non-specific binding, in our laboratory haloperidol has been a reliable ligand for non-specific binding measurements.
Inhibition of radioligand
Assuming the inhibition of radioligand binding follows a one-site model, 10 - 12 concentrations of test compound inhibitor should be sufficient to accurately measure the IC50 value for only one site and 20 if there is evidence of a biphasic interaction. We recommend to use the concentration range spanning at least 100-fold above and below the anticipated IC50.
Preparation of tissue membranes
We recommend preparing membranes from fresh tissues when possible, in the case where fresh tissues are not available frozen tissues can be purchased from vendors such as Pel Freez (see Support Protocol 1). When working with fresh tissues harvested from rodents and/or other organisms, consult institutional guidelines for proper method of euthanization. While the S1R binding site in liver membranes is highly stable at −80°C, in our experience, the half-life of S2R binding site (as measured by both [3H]-DTG binding and [125I]-IAF photolabeling (see Chapter 8)) in tissue membranes is about three months, researchers are advised to prepare fresh membranes as needed.
Data analysis
data analyses are outlined in each Basic Protocol, refer to Unit 1.3 for detail theory on receptor pharmacology.
Troubleshooting
| General issues with saturation radioligand-binding assays | ||
|---|---|---|
| Issue | Possible Explanations | Solutions |
| Linearity of binding curve | 1.Insufficient incubation time 2.Radioligand concentrations too low |
1.Increase incubation time 2.Increase radioligand concentrations |
| No binding | Receptor concentrations too low due receptor degradation | Prepare new membranes |
| General issues with inhibition of radioligand-binding assays | ||
|---|---|---|
| Issue | Possible Explanations | Solutions |
| Incomplete inhibition of radioligand | Narrow concentration range of inhibitor | Widen the concentration range of inhibitor |
Anticipated Results
Radioligand binding assays
Figure 1 shows a sample saturation binding of [3H]-(+)-pentazocine to the S1R in GPL membranes. Figure 2 shows sample results for inhibition of [3H]-(+)-pentazocine with a test compound in GPL membranes. Figure 3 shows saturation binding of [3H]-DTG to the S1R and S2R in RL membranes. We did not show an example of competitive inhibition of [3H]-DTG with test compounds but researchers should expect to see similar a binding curve that that reported in Figure 3. A table of KI of compounds that bind to both S1R and S2R is summarized in Table 8.
Table 8.
Typical KI values (nM) for reference compounds at sigma-1 and sigma-2 receptors.
| S1R | S2R | |
|---|---|---|
| BD1047 | 0.931 | 471 |
| BD1063 | 9.21 | 4491 |
| Dimethyltryptamine | 14,7002 | 21,7002 |
| DTG | 35.53 | 39.93 |
| Fenpropimorph | 9.34 | Not yet determined |
| Haloperidol | 0.93 | 7.93 |
| (+)-Pentazocine | 1.63 | 728.43 |
| PRE-084 | 445 | 130916 |
| Progesterone | 2397 | 4417 |
| Rimcazole | 858 | 3868 |
Matsumoto RR et al. (1995) EJP 280: 301-310.
Fontanilla D et al. (2009) Science 323(5916): 934-937.
Lever JR et al. (2006) Synapse 59: 350-358.
Pal A et al. (2007) Molecular Pharmacology 72:921-933.
Su TP et al. (1991) JPET 259(2): 543-550.
Maurice T. et al. (1999) Br J Pharmacol 127(2): 335-342.
Johannnessen M et al. (2011) Am J Physiol Cell Physiol 300(2): C328-337.
Husbands SM et al (1997) J Med Chem 40:4340-4346.
Time Considerations
Radioligand binding assays
Saturation binding assay and inhibition of radioligand binding assays typically require approximately one to three hours to complete if all reagents are prepared in advance. For inhibition of radioligand binding assays the time to complete an experiment may vary depending on the number of compounds tested at one time. We advise limitation of the number of compounds tested to a maximum of three as the number of samples may get too large. While the experiment takes one to three hours to complete, the data are obtained on the second day.
Preparation of tissue membranes
The time required to prepare tissue membranes depends on whether the tissue is fresh or frozen. For one to two tissue samples, it typically requires approximately two hours to prepare.
Table 7.
Sample transformation of competition binding against [3H]-(+)-PTZ
| Final unlabeled inhibitor concentration (M) | Specific [3H]-(+)-PTZ Bound (DPM) | % Specific Binding |
|---|---|---|
| 1.00E-05 | 851 | 5.25 |
| 3.00E-06 | 950 | 6.06 |
| 1.00E-06 | 1250 | 8.51 |
| 3.00E-07 | 3521 | 27.10 |
| 1.00E-07 | 5420 | 42.64 |
| 3.00E-08 | 8210 | 65.47 |
| 1.00E-08 | 10886 | 87.37 |
| 3.00E-09 | 11253 | 90.37 |
| 1.00E-09 | 12011 | 96.57 |
| 3.00E-10 | 12296 | 98.91 |
The first column displays the concentrations of unlabeled inhibitor as determined by serial dilution (Table 6). The second column shows mean of quadruplicate counts (in DPM) measured. Values in the third column are derived using Equation 3 where the nonspecific binding value was 209 DPM and the total binding was 12220 DPM.
Acknowledgements
This work is supported by NIH MH065503 (AER), NIH NS075820 (AER), Edwin and Dorothy Gamewell UW McPherson Retina Research Foundation Professorship (AER) and a Pharmaceutical Manufacturer's Post Doctoral Fellowship (UBC).
Footnotes
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Abate C, Elenewski J, Niso M, Berardi F, Colabufo NA, Azzariti A, Perrone R, Glennon RA. Interaction of the sigma(2) receptor ligand PB28 with the human nucleosome: computational and experimental probes of interaction with the H2A/H2B dimer. ChemMedChem. 2010;5:268–273. doi: 10.1002/cmdc.200900402. [DOI] [PubMed] [Google Scholar]
- Abate C, Niso M, Lacivita E, Mosier PD, Toscano A, Perrone R. Analogues of sigma receptor ligand 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]pipe razine (PB28) with added polar functionality and reduced lipophilicity for potential use as positron emission tomography radiotracers. J Med Chem. 2011;54:1022–1032. doi: 10.1021/jm1013133. [DOI] [PubMed] [Google Scholar]
- Ablordeppey SY, Fischer JB, Glennon RA. Is a nitrogen atom an important pharmacophoric element in sigma ligand binding? Bioorg Med Chem. 2000;8:2105–2111. doi: 10.1016/s0968-0896(00)00148-6. [DOI] [PubMed] [Google Scholar]
- Bowen WD, Hellewell SB, McGarry KA. Evidence for a multi-site model of the rat brain sigma receptor. Eur J Pharmacol. 1989;163:309–318. doi: 10.1016/0014-2999(89)90200-8. [DOI] [PubMed] [Google Scholar]
- Chu UB, Hajipour AR, Ramachandran S, Ruoho AE. Characterization of Interactions of 4-nitrophenylpropyl-N-alkylamine with Sigma Receptors. Biochemistry. 2011;50:7568–7578. doi: 10.1021/bi2004872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu UB, Ramachandran S, Hajipour AR, Ruoho AE. Photoaffinity labeling of the sigma-1 receptor with N-[3-(4-nitrophenyl)propyl]-N-dodecylamine: evidence of receptor dimers. Biochemistry. 2013;52:859–868. doi: 10.1021/bi301517u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Costa BR, Bowen WD, Hellewell SB, Walker JM, Thurkauf A, Jacobson AE, Rice KC. Synthesis and evaluation of optically pure [3H]-(+)-pentazocine, a highly potent and selective radioligand for sigma receptors. FEBS Lett. 1989;251:53–58. doi: 10.1016/0014-5793(89)81427-9. [DOI] [PubMed] [Google Scholar]
- Fontanilla D, Hajipour AR, Pal A, Chu UB, Arbabian M, Ruoho AE. Probing the steroid binding domain-like I (SBDLI) of the sigma-1 receptor binding site using N-substituted photoaffinity labels. Biochemistry. 2008;47:7205–7217. doi: 10.1021/bi800564j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromek KA, Suchy FP, Meddaugh HR, Wrobel RL, LaPointe LM, Chu UB, Primm JG, Ruoho AE, Senes A, Fox BG. The oligomeric states of the purified sigma-1 receptor are stabilized by ligands. J Biol Chem. 2014;289:20333–20344. doi: 10.1074/jbc.M113.537993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. P.N.A.S. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Justinova Z, Hayashi E, Cormaci G, Mori T, Tsai SY, Barnes C, Goldberg SR, Su TP. Regulation of sigma-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J Pharmacol Exp Ther. 2010;332:1054–1063. doi: 10.1124/jpet.109.159244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bowen WD. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 1990;527:244–253. doi: 10.1016/0006-8993(90)91143-5. [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Kaushal N, Matsumoto RR. Role of sigma receptors in methamphetamine-induced neurotoxicity. Curr Neuropharmacol. 2011;9:54–57. doi: 10.2174/157015911795016930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh MP, Parker J, Bobker DH, Keana JF, Weber E. Solubilization and characterization of sigma-receptors from guinea pig brain membranes. J Neurochem. 1989;53:1575–1580. doi: 10.1111/j.1471-4159.1989.tb08554.x. [DOI] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1). Biochem Biophys Res Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, Bonci A. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell. 2013;152:236–247. doi: 10.1016/j.cell.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa F, Codony X, Tovar V, Lavado A, Gimenez E, Cozar P, Cantero M, Dordal A, Hernandez E, Perez R, Monroy X, Zamanillo D, Guitart X, Montoliu L. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur J Neurosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- Mach RH, Wheeler KT. Development of molecular probes for imaging sigma-2 receptors in vitro and in vivo. Cent Nerv Syst Agents Med Chem. 2009;9:230–245. doi: 10.2174/1871524910909030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Andersen KA, Ziskind-Conhaim L, Ruoho AE. The sigma-1 receptor is enriched in postsynaptic sites of C-terminals in mouse motoneurons. An anatomical and behavioral study. Neuroscience. 2010;167:247–255. doi: 10.1016/j.neuroscience.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkel M, Terry P, Pontecorvo M, Katz JL, Witkin JM. Selective sigma ligands block stimulant effects of cocaine. Eur J Pharmacol. 1991;201:251–252. doi: 10.1016/0014-2999(91)90355-t. [DOI] [PubMed] [Google Scholar]
- Morris DI, Speicher LA, Ruoho AE, Tew KD, Seamon KB. Interaction of forskolin with the P-glycoprotein multidrug transporter. Biochemistry. 1991;30:8371–8379. doi: 10.1021/bi00098a014. [DOI] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Bonaventura J, Brugarolas M, Farre D, Aguinaga D, Mallol J, Cortes A, Casado V, Lluis C, Ferre S, Franco R, Canela E, McCormick PJ. Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PloS one. 2013;8:e61245. doi: 10.1371/journal.pone.0061245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Chu UB, Ramachandran S, Grawoig D, Guo LW, Hajipour AR, Ruoho AE. Juxtaposition of the steroid binding domain-like I and II regions constitutes a ligand binding site in the sigma-1 receptor. The Journal of biological chemistry. 2008;283:19646–19656. doi: 10.1074/jbc.M802192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Hajipour AR, Fontanilla D, Ramachandran S, Chu UB, Mavlyutov T, Ruoho AE. Identification of regions of the sigma-1 receptor ligand binding site using a novel photoprobe. Molecular Pharmacology. 2007;72:921–933. doi: 10.1124/mol.107.038307. [DOI] [PubMed] [Google Scholar]
- Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Lu H, Prabhu U, Ruoho AE. Purification and characterization of the guinea pig sigma-1 receptor functionally expressed in Escherichia coli. Protein expression and purification. 2007;51:283–292. doi: 10.1016/j.pep.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson MJ, Noorbakhsh B, Seminerio MJ, Matsumoto RR. Sigma-1 receptors: potential targets for the treatment of substance abuse. Curr Pharm Des. 2012;18:902–919. doi: 10.2174/138161212799436601. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. The Journal of cell biology. 1983;96:1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharmacol Exp Ther. 1982;223:284–290. [PubMed] [Google Scholar]
- Ujike H, Kuroda S, Otsuki S. sigma Receptor antagonists block the development of sensitization to cocaine. Eur J Pharmacol. 1996;296:123–128. doi: 10.1016/0014-2999(95)00693-1. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Goldstein SR, Roberts AH, Patrick SL, Hohmann AG, DeCosta B. Autoradiographic distribution of [3H](+)-pentazocine and [3H]1,3-di-o-tolylguanidine (DTG) binding sites in guinea pig brain: a comparative study. Brain Res. 1992;581:33–38. doi: 10.1016/0006-8993(92)90340-f. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC. Sigma receptors: biology and function. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]