Abstract
Background Highly pathogenic avian influenza (HPAI) H5N1 viruses have recently displayed increased virulence for wild waterfowl.
Objectives To study the effect of host age on the shedding and tissue dissemination of a HPAI H5N1 virus in infected Pekin ducks.
Methods Pekin ducks in two age‐matched groups (n = 18), 8 and 12 weeks old (wo) were each infected with 106 EID50/0·1 ml of HPAI A/turkey/Turkey/1/05 (H5N1, clade 2·2). Each day for 5 days, birds were monitored clinically, and cloacal and oropharyngeal swabs collected, before three birds from each group were selected randomly for post‐mortem examination. Tissue samples were collected for examination by real‐time RT‐PCR, histopathology and immunohistochemistry (IHC).
Results Severe clinical signs, including incoordination and torticollis were observed in the 8 wo group resulting in 100% mortality by 4 dpi. Mild clinical signs were observed in the 12 wo group with no mortality. Real‐time RT‐PCR and IHC results demonstrated the systemic spread of H5N1 virus in birds of both age groups. Higher levels of virus shedding were detected in oropharyngeal swabs than in cloacal swabs, with similar levels of shedding detected in both age groups. Variations in level and temporal dissemination of virus within tissues of older ducks, and the presence of the virus in brain and heart were observed, which coincided with the appearance of clinical signs preceding death in younger birds.
Conclusions These results are consistent with reports of natural infections of wild waterfowl and poultry possibly indicating an age‐related association with dissemination and clinical outcome in ducks following infection with H5N1 HPAI virus.
Keywords: Age ducks, H5N1 HPAI, pathogenesis, Pekin
Introduction
Wild aquatic birds, Anseriformes (ducks, swans, geese) and Charadriiformes (shorebirds, gulls, terns), are traditionally thought to act as reservoirs of avian influenza (AI) viruses covering all sixteen haemagglutinin (HA) and nine neuraminidase glycoprotein combinations. The transmission of AI to species of the order Galliformes (chickens and turkeys) results in two pathotypes – highly pathogenic AI (HPAI) and low pathogenicity AI (LPAI). Highly pathogenic AI, which to date has only been caused by viruses of H5 and H7 subtypes, is a severe, systemic disease that can result in up to 100% mortality in infected poultry populations. Prior to 2002, the only documented infection of significant numbers of wild birds infected with an HPAI virus occurred in common terns (Sterna hirundo) in South Africa, in 1961 – when large numbers died as a result of infection with A/tern/South Africa/1961 (H5N3). 1 However, an H5N1 HPAI variant emerged in Penfold and Kowloon waterfowl parks, Hong Kong in December 2002 that, although genetically related to the progenitor isolates A/goose/Guangdong/1/1996 (H5N1) and A/Hong Kong/156/1997 (H5N1), showed increased lethality for various wild waterfowl, including geese, ducks and swans. 2 Although sporadic outbreaks in wild‐bird and poultry populations, with associated mortality because of this H5N1 HPAI virus were reported between 2002 and 2005, a virus representing a distinct sub‐clade of this Asian‐lineage was identified on Qinghai Lake, China in 2005, which displayed higher lethality and transmissibility for a wide range of wild birds. 3 The presence of a large population of migratory birds on Qinghai Lake is thought to have contributed to the spread of this highly lethal variant, now designated as a ‘Eurasian‐lineage’ clade 2.2 H5N1, 4 throughout Asia and into Europe and Africa 3 , 5 causing significant deaths in wild bird and poultry populations. In addition, there have been over 400 human HPAI H5N1 infections with a mortality rate of approximately 60%. 6 , 7
Viruses of the 2.2 clade have also been reported to cause severe disease in commercial ducks, 8 , 9 , 10 which is unusual for HPAI viruses. 11 , 12 Although the H5N1 viruses isolated in 1997 and subsequent 1997‐like viruses isolated up to 2002 did not replicate efficiently in ducks, 13 , 14 recent H5N1 viruses were found to replicate in multiple organs, particularly the brain, instead of ‘conventional’ gastrointestinal tract replication, and consequently caused death in geese, ducks and swans. 15 , 16 , 17 , 18 , 19 , 20 , 21 However, there is some evidence that HPAI viruses may enter the brain in sub‐clinical infections of ducks. 22 Several recent infection studies involving Pekin ducks performed at the Veterinary Laboratories Agency (VLA), United Kingdom have resulted in observations that the age of the ducks at infection may affect the outcome of disease. Similar age‐related effects on clinical response have also been observed recently in pre‐Qinghai lake, clade 1 isolates of AI. 23 To investigate whether a similar presentation is seen with infections of clade 2.2 AI, known to be lethal for wild waterfowl, 16 Pekin ducks (Anas platyrhynchos) of eight and 12 weeks of age were infected with A/turkey/Turkey/1/05 H5N1 clade 2.2 HPAI virus to study effect of age on the pathogenesis, presentation of clinical signs and disease outcome.
Materials and methods
Infection of 8 and 12 weeks old Pekin ducks with A/turkey/Turkey/1/05 H5N1 HPAI virus
Commercial high‐health status Pekin ducks were acquired from a single source ‐ Cherry Valley Farms Ltd, United Kingdom. Before experimental infections were performed, oropharyngeal and cloacal swabs, and blood samples were taken to determine current infection with AI virus and/or previous exposure to H5 AI by matrix gene real‐time RT‐PCR (RRT‐PCR) and haemagglutination inhibition (HI) tests respectively. 24 Two ducks were also randomly selected, killed humanely and organs collected for negative controls for immunohistochemistry (IHC). All experiments were performed in Biosafety Level 3 + (BSL‐3 + ) [UK Department of Environmental, Food and Rural Affairs (Defra) Specific Animal Pathogen Order (SAPO) 4; Advisory Committee on Dangerous Pathogens (ACDP) 3] facilities at VLA, Weybridge. The HPAI virus, A/turkey/Turkey/1/05 (H5N1), was selected for use as a contemporary ‘Eurasian lineage’ H5N1 virus, representing the westward movement of these viruses from their origins in South East Asia into Europe. Virus stocks for infections were prepared by growth in 9‐ to 11‐day‐old embryonated fowls’ eggs. Each bird in two groups of eighteen 8 and 12 wo Pekin ducks was infected with a total volume of 0·1 ml infective allantoic fluid, containing 106 EID50 A/turkey/Turkey/1/05 HPAI H5N1 virus, divided equally between the intranasal and intraocular routes. Clinical signs were recorded twice daily. When individual ducks were deemed moribund, such that they were unable to eat or drink, they were killed humanely and recorded as a mortality for that day. Each day, for a period of 5 days, cloacal and oropharyngeal swabs were taken before three animals were selected randomly and killed humanely for post‐mortem examination. Samples of tissues (brain, heart, spleen, lung, liver, kidney, intestine, caecal tonsil, trachea, breast muscle, thigh muscle and wing feathers) were collected for RRT‐PCR. Tissue samples collected for histopathology and IHC examination included those selected for RRT‐PCR as well as nasal turbinates, air sacs, gizzard, proventriculus, pancreas, duodenum, thymus, bursa, adrenals, thyroid, ovary/testes and sciatic nerves. Tissues for RRT‐PCR were collected in brain–heart infusion (BHI) (15% w/v) media containing antibiotics (penicillin G 10000 U/ml; amphotericin B 20 μl/ml; gentamycin 1000 μg/ml). Tissue samples for histology were fixed in 10% v/v buffered formalin for a minimum period of 48 h. All tissue samples for RRT‐PCR and histopathology examination were collected within an hour of the death of the animal.
Viral RNA isolation and detection by matrix gene RRT‐PCR
A 10% w/v suspension was made for each tissue in 1 ml BHI media with antibiotics. The oropharyngeal and cloacal swabs were transferred into 1 ml of BHI with antibiotics and briefly vortexed. A volume of 140 μl from tissue and swab suspensions was loaded with 420 μl of AVL Buffer (QIAGEN, Crawley, UK) in a QIAGEN S‐block for automated viral RNA extraction by the Universal Biorobot system (QIAGEN, Crawley, UK).
The matrix gene RRT‐PCR was performed using one‐step RT‐PCR kits (QIAGEN, Crawley, UK) with 0·4 μm PCR primers Sep1 (5′‐AGATGAGTCTTCTAACCGAGGTCG‐3′) and Sep2 (5′‐TGCAAAAACATCTTCAAGTCTCTG‐3′) with 0·3 μm of hydrolysis probe SePRO: ([FAM]‐TCAGGCCCCCTCAAAGCCGA‐[TAMRA]) (Sigma, Poole, UK) as described in the EU Diagnostic Manual for avian influenza, 2006. 24 For the M gene RRT‐PCR, a positive extraction control of A/chicken/Scotland/59 (H5N1) RNA of known C t value from inactivated, freeze‐dried, egg‐grown material was included. Quantitative standards of five 10‐fold dilutions of extracted RNA from infective allantoic fluid of a 106 EID50 dose of A/turkey/Turkey/1/05 were also included.
Histopathology and detection of influenza A nucleoprotein (NP) by IHC
Samples for histology were routinely processed through graded alcohols and chloroform and embedded in paraffin wax (routine standard process). Four‐micrometre thick sections, cut on a rotary microtome, were stained with haematoxylin and eosin or used for immunohistochemical detection of influenza A NP as described. 25 Briefly, sections for IHC were dewaxed in xylene and passed through graded alcohols to Tris buffered saline solution (TBS) (0·005 m Tris, pH7·6, 0·85% w/v NaCl). Endogenous peroxidase activity was quenched with a methanol/hydrogen peroxide block (BDH) for 15 minutes and treated with Protease XXIV for 10 minutes at room temperature. Slides were assembled into Shandon coverplates to facilitate IHC using the Sequenza system (Thermo Fischer Scientific, Runcorn, UK) and primary antibody cross‐reactivity with tissue constituents was prevented using a normal immune serum block. Samples were subsequently incubated with an anti‐influenza A NP primary antibody (Statens Serum Institute, Copenhagen, Denmark) for 1 hour, and Dako ENVISIONTM polymer for 30 minutes, at room temperature. Sections were washed three times with TBS between incubations. The immunohistochemical signal was visualised using 3,3‐diaminobenzidine (Sigma‐Aldrich, Poole, UK), and sections were counterstained in Mayer’s haematoxylin (Surgipath, Peterborough, UK), dehydrated in absolute alcohol, cleared in xylene and mounted using Dibutyl Phthalate Xylene and glass coverslips.
Results
Clinical signs and gross pathology
Prior to infection, all ducks were shown to be free from current infection with AI virus and/or previous exposure to H5 AI by matrix gene RRT‐PCR and HI tests respectively. Severe clinical signs, including depression, reluctance to feed, tremors, loss of balance and torticollis were observed in the 8 wo group resulting in 100% mortality by 4 dpi. However, only mild clinical signs, mainly conjunctivitis, were observed in the 12 wo group with no appearance of nervous signs or mortality over an observation period of 7 dpi. Gross pathological observations of the 8 wo ducks, included lesions and haemorrhages in the pancreas. At post‐mortem examination, inoculated 8 wo ducks showed mild rhinitis from 1 dpi, with increased severity from 3 dpi. Multifocal necrotizing pancreatitis was observed from 2 dpi, with severity and extension of lesions progressing with disease advancement, and occasional multifocal haemorrhages. Air sacculitis was evident from 2 dpi onwards. Myocarditis was observed at 3 dpi in one of three birds.
In 12 wo ducks, a mild rhinitis was observed from 1 dpi, with a similar presentation through the course of the experiment. Mild pancreatitis and airsacculitis was observed from 3 dpi. The appearance of gross lesions occurred later than in 8 wo ducks with the severity of the lesions milder, more limited in extent, and with a slower progression.
Histological lesions
The presence of histopathological lesions was observed in the 8 wo challenged ducks from 2 dpi. Lesions included mild acute rhinitis, multifocal necrotising pancreatitis, myocardial necrosis and heterophilic myocarditis, non‐suppurative encephalitis and neuritis, air sacculitis and myositis. From 3 dpi lymphoid necrosis and depletion in thymus, lymphoid depletion in the Bursa, and necrotising adrenalitis and thyroiditis were observed. The severity and extent of the lesions progressed throughout the infection.
In the 12 wo group, acute rhinitis was observed from 2 dpi, mild air sacculitis and non‐suppurative encephalitis from 3 dpi. Necrotising pancreatitis and adrenalitis, acute myocarditis and myosistis, lymphocytic necrosis in thymus and lymphoid depletion in bursa were observed from 4 dpi.
Lesions were milder and less frequent in the 12 wo ducks than in ducks in the 8 wo group.
Viral detection by IHC
Immunolabelling against influenza NP (1, 2) was detected in lung and spleen at 1 dpi in the 8 wo challenged group. Multiple organs displayed viral antigen in their parenchymal cells at 2 dpi including nasal turbinates, lung, airsacs, spleen, thymus, bursa, liver, heart, brain, sciatic nerve, skin and feather follicles, pancreas, adrenal and skeletal muscle. Thyroid follicle epithelium was immunolabelled from 3 dpi. The highest number of immunolabelled cells was observed in lung, heart, brain and pancreas, and were numerous in most tissues, maintaining their number or increasing throughout the infection. However, a decrease in number of immunolabelled cells was observed in spleen and lung tissues in the last days of the experiment.
Figure 1.
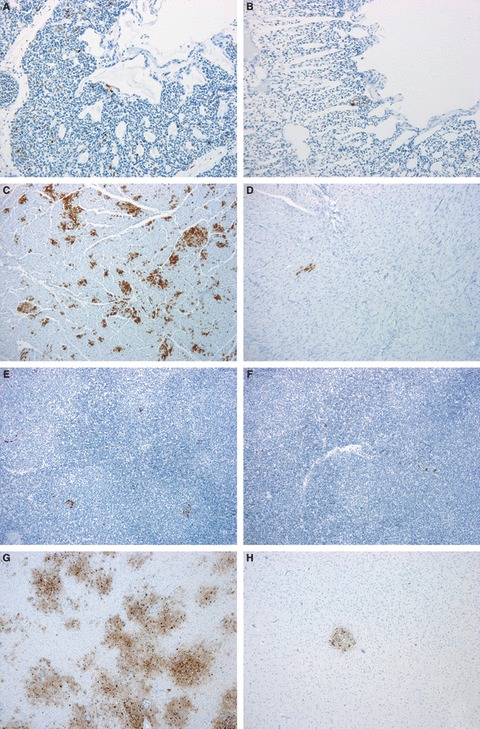
Immunohistochemical detection of Influenza A nucleoprotein in 8‐ (A, C, E and G) and 12‐week‐old Pekin ducks (B, D, F and H) challenged with A/turkey/Turkey1/2005 HPAI H5N1. ENVISION. (A) Lung, 3 dpi, immunolabelling of air capillary epithelial cells, 20×. (B) Lung, 3 dpi, 20×. (C) Heart, 3 dpi, multifocal labelling of myocardiocytes, 10×. (D) Heart, 3 dpi, focal labelling of myocarddiocytes, 10×. (E) Spleen, 2 dpi, small groups of macrophages expressing viral antigen in their cytoplasm, 10×. (F) Spleen, 2 dpi, 10×. (G) Brain, 3 dpi, abundant neuronal and glial labeling, 10×. (H) Brain, 3 dpi, focal area of viral antigen detection, 10×.
Figure 2.
Immunohistochemical detection of Influenza A nucleoprotein in 8‐ (A, C, E and G) and 12‐week‐old Pekin ducks (B, D, F and H) challenged with A/turkey/Turkey1/2005 HPAI H5N1. ENVISION. (A) Thymus, 3 dpi, abundant thymic epithelium and lymphocyte immunolabelling, 10×. (B) Thymus, 3 dpi, scarce labelling in thymic epithelial cells, 10×. (C) Skeletal muscle, 3 dpi, abundant immunolabelled myocytes, 5×. (D) Skeletal muscle, 3 dpi, focal area of immunolabelling of few myocytes, 20×. (E) Pancreas, 4 dpi, multifocal labelling in pancreatic acinary cells, 10×. (F) Pancreas, 4 dpi, focal labeling, 10×. (G) Feather follicle, 4 dpi, multiple areas of immunolabelling in feather shaft and pulp, 5×. (H) Feather follicle, 4 dpi, substantially reduced immunolabelling in feather shaft and pulp, 5×.
Viral antigen detection in 12 wo infected ducks occurred from 2 dpi in nasal cavity, lung, air sacs, spleen, thymus, bursa, liver, heart, brain, skin and feather follicles and skeletal muscle in a small amount of cells, without a marked increased in their numbers throughout infection, except in the heart and brain where moderate increases were observed. Immunolabelled cells were observed in the pancreas and adrenal glands from 4 dpi and in the thyroid gland at 5 dpi.
The detection of viral antigen by IHC in 8 wo infected ducks occurred in a higher number of cells than in 12 wo ducks for almost all tissues examined (1, 2). Organ distribution of viral antigen was similar in 8 and 12 wo ducks at the end of the study but temporal differences in the detection of virus in certain tissues were observed in lung and spleen (1 dpi in 8 wo, 2 dpi in 12 wo) and the pancreas and adrenal glands (2 dpi in 8 wo, 4 dpi in 12 wo). The increase in the number of immunolabelled cells in conjunction with disease progression was more marked in 8 wo birds.
Detection of viral RNA by RRT‐PCR in swabs and tissues
Viral RNA present in tissues and swabs was detected by RRT‐PCR and measured as relative equivalent units of RNA against a 10‐fold dilution series of RNA purified from infective allantoic fluid containing 106 EID50/0·1 ml A/turkey/Turkey/1/2005 (H5N1). Although these units measured the amount of viral RNA present and not infectivity, it may be inferred from the linear relationship with the dilution series that they are proportionate to the amount of infectious virus present. Higher levels of viral shedding were detected in oropharyngeal swabs than in cloacal swabs in both 8 and 12 wo age groups, with similar levels of shedding detected in both age groups (Figure 3). In keeping with clinical signs, virus detection in the various tissues tested, including trachea, caecal tonsil, thigh muscle, heart and brain, showed much higher levels in 8 wo ducks, compared with older ducks, in which virus detection was usually minimal (Figure 4). In contrast, substantially higher levels of virus were detected in the liver, kidney, intestine and spleen tissue of 12 wo ducks, compared with 8 wo ducks (Figure 4). Interestingly, the only organ to show similar dissemination of virus in both age groups was the lung. In the skeletal muscle tissues, similarly high levels of virus were detected in the breast muscle of 8 wo birds with minimal detection in tissues of 12 wo ducks. An age dependency for the temporal dissemination of virus within these skeletal muscle tissues was also observed with the delayed infiltration into tissues of older ducks (Figure 5). In the heart samples from birds in the 12 wo group, little virus was detected, but higher levels of virus were detected in 8 wo ducks peaking at 3 dpi (Figure 6). Generally, the highest levels of viral RNA was detected in the brain samples from 8 wo ducks on days 3 and 4 pi, with an increase in levels in the brain samples from 12 wo ducks only on day 5 dpi (Figure 6). Although the mean viral titres in the brain samples of 12 wo ducks increased sharply at 5 dpi, this was because of a single individual bird that showed substantial amounts of virus present without the presentation of clinical signs. A similar age dependency for temporal viral dissemination, as seen for skeletal muscles, was also observed in brain and heart tissues which coincided with the appearance of clinical signs preceding death in younger birds.
Figure 3.
Matrix gene RRT‐PCR results for oropharyngeal and cloacal swabs taken over 5 dpi with A/turkey/Turkey/1/05 H5N1. Viral RNA was detected by RRT‐PCR (y‐axis) and quantified as relative equivalent units (REU) of RNA against a 10‐fold dilution series of RNA purified from infective allantoic fluid of a 106 EID50/0.1 ml dose of A/turkey/Turkey/1/2005 H5N1 and are therefore a guide to the amount of infectious virus present. Error bars indicate SE.
Figure 4.
Detection of viral RNA by RRT‐PCR in tissues of lung, kidney, caecal tonsil and feathers. Viral RNA detected by RRT‐PCR (y‐axis) was measured as a relative equivalent unit (REU) of RNA against a 10‐fold dilution series of RNA purified from infective allantoic fluid of a 106 EID50/0.1 ml dose of A/turkey/Turkey/1/2005 H5N1. Error bars indicate SE. Note y‐axes are not presented on the same scale.
Figure 5.
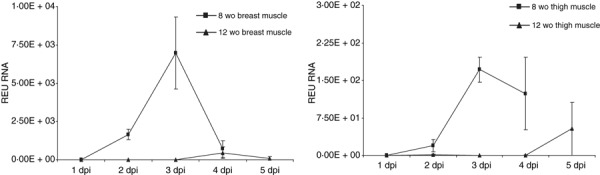
Detection of viral RNA by RRT‐PCR in tissues of thigh and breast muscle. Viral RNA detected by RRT‐PCR (y‐axis) was measured as a relative equivalent unit (REU) of RNA against a 10‐fold dilution series of RNA purified from infective allantoic fluid of a 106 EID50/0.1ml dose of A/turkey/Turkey/1/2005 H5N1. Error bars indicate SE. Note y‐axes are not presented on the same scale.
Figure 6.
Detection of viral RNA by RRT‐PCR in tissues of brain and heart tissues coinciding with the appearance of clinical signs at 3–4 dpi, preceding death in younger birds. Viral RNA detected by RRT‐PCR (y‐axis) was measured as a relative equivalent unit (REU) of RNA against a 10‐fold dilution series of RNA purified from infective allantoic fluid of a 106 EID50/0.1 ml dose of A/turkey/Turkey/1/2005 H5N1. Error bars indicate SE.
Discussion
Although ducks and some other aquatic birds had been demonstrated to be ‘resistant’ to most HPAI viruses, which caused only asymptomatic infections, they have recently been shown to be highly susceptible to certain HPAI H5N1 isolates. 8 , 9 , 10 , 25 , 26 This susceptibility, although probably species and virus strain related, is also dependent upon the age of the bird at the time of infection. Age‐related effects on clinical presentation in infected ducks have been observed recently for pre‐Qinghai, clade 1 isolates of HPAI H5N1 virus. 23 However, to our knowledge, there have been no reports on the age‐related susceptibility of ducks to the more recent and more widely distributed clade 2.2 HPAI H5N1 viruses. In the present study, an age‐related association with viral loads excreted and the tissue distribution of virus (as detected by IHC and RRT‐PCR) over time post‐infection was identified. This association also coincided with the appearance of clinical signs and mortality. Recently reported experimental infections of six species of wild duck, including mallards, with A/turkey/Turkey/1/2005 (H5N1) virus, showed age‐related observations consistent with those obtained in the present study. 26 In this wild bird study, mallards (Anas platyrhynchos) were 8–11 months old and showed ‘abundant virus excretion without clinical or pathologic evidence of debilitating disease’. 26 Similarly, no clinical or pathological evidence of debilitating disease was observed in 12 wo Pekin ducks in the present study, but ‘abundant excretion’ levels were not observed. The distribution and severity of the histological lesions and widespread immunohistochemical detection of virus antigen in tissues were similar to previous reports of ducks and waterfowl that succumbed to disease when infected naturally or experimentally with H5N1 viruses isolated after 2002, 10 , 16 , 23 , 25 , 27 with a similarly marked neuro‐ and cardiotropism representative of the increased pathogenicity for ducks. Although the nature and location of histological lesions observed in both age groups of ducks were similar, the severity and prevalence of these lesions were lower in older ducks, which is consistent with the reduced clinical disease at 12 wo. Pantin‐Jackwood et al., 23 have reported no mortality in Pekin ducks as young as 5 wo. However, this is likely to be because they used pre‐Qinghai Lake (2005) clade 1 isolates, and is evidence for the increased virulence for ducks of the clade 2.2 H5N1 variant that apparently emerged in the Qinghai Lake region of China in 2005. 3 Interestingly, virus was detected in wing feathers of both age groups, which has been demonstrated previously, 25 , 28 , 29 and is suggested as a possible sampling method for HPAI surveillance in wild bird populations. However, in older ducks in the present study, detection of virus was shown to be related to the level of calcification of the feather shaft and therefore the age of the bird sampled may affect the sensitivity of feather sampling as a method of surveillance. Also of interest was the higher viral loads detected in liver, spleen and intestine tissue in older ducks, the significance of which remains unknown.
Clinical signs, mortality and gross pathological observations of the 8 wo ducks, were found to be similar to those observed in a previous study using 4 wo ducks. 25 Higher levels of viral shedding were detected in oropharyngeal swabs than in cloacal swabs in both age groups, which is consistent with the reported shift from the traditional enteric system of spread of AI viruses to respiratory spread. 9 , 25 , 30 , 31 Similar levels of viral shedding were also detected in oropharyngeal and cloacal swabs from both age groups, which may have implications for the transmission and geographical spread of these H5N1 clade 2.2 viruses. As wild waterfowl (represented by Pekin ducks as a model in this study) of migratory age, infected with H5N1 virus, appear healthy without the presentation of clinical signs, they could act as long‐distance vectors. Although in our study 12 wo Pekin ducks were able to shed virus without showing clinical signs or succumbing to disease, the viral load shed was low and therefore it is debatable whether this shedding dose would be sufficient to allow transmission to susceptible hosts. The ability of wild waterfowl to act as long‐distance vectors may, therefore, depend on the species infected, with swans and geese as prime candidates, 27 , 32 although Keawcharoen et al., 26 suggest mallards also play a role. The results from the present study confirm the systemic spread and neurotropism of HPAI virus in Pekin ducks and demonstrate an age‐related association with dissemination of virus effecting clinical outcome in infections with ‘Eurasian‐lineage’ clade 2.2 H5N1 viruses. An age‐related association with clinical disease and viral dissemination has also been identified in infections of ducks with Duck Hepatitis B Virus, 33 where the presence of neutralising antibodies contributed to the clearance of virus consequently reducing clinical signs. Although a more mature immune system in older ducks is an obvious candidate for this phenomenon, the fact that viral loads in organs such as liver, spleen and intestine were higher in older ducks, does indicate that a more successful immune response may not be the sole contributing factor. Although the present and other studies have shown the age affect on disease outcome, the exact mechanism of this ‘protection’ remains unknown.
Acknowledgements
The authors thank Shadia Omar, Charles Cocks, Rachel Baker, Christopher Glue and Stuart Smith for their technical assistance. This work was funded by the Department for Environment, Food and Rural Affairs (Defra) ROAME SE0782 and the European Union 6th framework projects – FLUAID and FLUPATH. This work was performed in part for submission towards a higher degree at the University of Reading, United Kingdom.
This article ‘The effect of age on the pathogenesis of a highly pathogenic avian influenza (HPAI) H5N1 virus in Pekin ducks (Anas platyrhynchos) infected experimentally.’ was written by Brandon Z. Löndt of Veterinary Laboratories Agency‐Weybridge. It is published with the permission of the Controller of HMSO and the Queen's Printer for Scotland.
References
- 1. Becker WB. The isolation and classification of tern virus: influenza virus A/tern/South Africa/1961. J Hyg (Lond) 1966; 64:309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sturm‐Ramirez KM, Ellis T, Bousfield B et al. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J Virol 2004; 78:4892–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen H, Smith GJD, Zhang SY et al. H5N1 virus outbreak in migratory waterfowl. Nature 2005; 436:191–192. [DOI] [PubMed] [Google Scholar]
- 4. WHO/OIE/FAO H5N1 Evolution Working Group . Toward a unified nomenclature system for highly pathogenic avian influenza virus (H5N1). Emerg Infect Dis 2008; 14:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu J, Xiao H, Lei F et al. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science 2005; 309:1206. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization, WHO . Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO [web page], 2009. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_01_27/en/index.html (Accessed 26 May 2009).
- 7. World Organisation for Animal Health . Update on highly pathogeneic avian influenza in animals (type H5 and H7) [Web Page], 2009. Available at: http://www.oie.int/downld/AVIAN%20INFLUENZA/A_AI‐Asia.htm (Accessed 26 May 2009).
- 8. Saito T, Watanabe C, Takemae N et al. Pathogenicity of highly pathogenic avian influenza viruses of H5N1 subtype isolated in Thailand for different poultry species. Vet Microbiol 2009; 133:65–74. [DOI] [PubMed] [Google Scholar]
- 9. Sturm‐Ramirez KM, Hulse‐Post DJ, Govorkova EA et al. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J Virol 2005; 79:11269–11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vascellari M, Granato A, Trevisan L et al. Pathologic findings of highly pathogenic avian influenza virus a/duck/Vietnam/12/05 (H5N1) in experimentally infected pekin ducks, based on immunohistochemistry and in situ hybridization. Vet Pathol 2007; 44(5):635–642. [DOI] [PubMed] [Google Scholar]
- 11. Alexander DJ, Allan WH, Parsons DG, Parsons G. The pathogenicity of four avian influenza viruses for fowls, turkeys and ducks. Res Vet Sci 1978; 24:242–247. [PubMed] [Google Scholar]
- 12. Alexander DJ, Parsons G, Manvell RJ. Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quail. Av Pathol 1986; 15:647–662. [DOI] [PubMed] [Google Scholar]
- 13. Shortridge KF, Zhou NN, Guan Y et al. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virol 1998; 252:331–342. [DOI] [PubMed] [Google Scholar]
- 14. Tumpey TM, Suarez DL, Perkins LEL et al. Characterization of a highly pathogenic H5N1 avian influenza a virus isolated from duck meat. J Virol 2002; 76:6344–6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bui HH, Peters B, Assarsson E, Mbawuike I, Sette A. Ab and T cell epitopes of influenza A virus, knowledge and opportunities. Proc Natl Acad Sci U S A 2007; 104:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellis TM, Bousfield RB, Bissett LA et al. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Av Pathol 2004; 33:492–505. [DOI] [PubMed] [Google Scholar]
- 17. Isoda N, Sakoda Y, Kishida N et al. Pathogenicity of a highly pathogenic avian influenza virus, A/chicken/Yamaguchi/7/04 (H5N1) in different species of birds and mammals. Arch Virol 2006; 151:1267–1279. [DOI] [PubMed] [Google Scholar]
- 18. Lee CW, Suarez DL, Tumpey TM et al. Characterization of highly pathogenic H5N1 avian influenza a viruses isolated from South Korea. J Virol 2005; 79:3692–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mase M, Eto M, Tanimura N et al. Isolation of a genotypically unique H5N1 influenza virus from duck meat imported into Japan from China. Virology 2005; 339:101–109. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen DC, Uyeki TM, Jadhao S et al. Isolation and characterization of avian influenza viruses, including highly pathogenic H5N1, from poultry in live bird markets in Hanoi, Vietnam, in 2001. J Virol 2005; 79:4201–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou JY, Shen HG, Chen HX et al. Characterization of a highly pathogenic H5N1 influenza virus derived from bar‐headed geese in China. J Gen Virol 2006; 87:1823–1833. [DOI] [PubMed] [Google Scholar]
- 22. Wood GW, Parsons G, Alexander DJ. Replication of influenza A viruses of high and low pathogenicity for chickens at different sites in chickens and ducks following intranasal inoculation. Av Pathol 1995; 24:545–551. [DOI] [PubMed] [Google Scholar]
- 23. Pantin‐Jackwood MJ, Suarez DL, Spackman E, Swayne DE. Age at infection affects the pathogenicity of Asian highly pathogenic avian influenza H5N1 viruses in ducks. Virus Res 2007; 130:151–161. [DOI] [PubMed] [Google Scholar]
- 24. European Union Council Directive 2005/94/EC . European Union Diagnostic Manual for avian influenza [web page], 2006. Available at: http://eur‐lex.europa.eu/LexUriServ/site/en/oj/2006/l_237/l_23720060831en00010027.pdf (Accessed 26 May 2009).
- 25. Londt BZ, Nunez A, Banks J, Nili H, Johnson LK, Alexander DJ. Pathogenesis of highly pathogenic avian influenza A/turkey/Turkey/1/2005 H5N1 in Pekin ducks (Anas platyrhynchos) infected experimentally. Avian Pathol 2008; 37:619–627. [DOI] [PubMed] [Google Scholar]
- 26. Keawcharoen J, Van RielD, Van Amerongen G et al. Wild ducks as long‐distance vectors of highly pathogenic avian influenza virus (H5N1). Emerg Infect Dis 2008; 14:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalthoff D, Breithaupt A, Teifke JP et al. Highly pathogenic avian influenza virus (H5N1) in experimentally infected adult mute swans. Emerg Infect Dis 2008; 14:1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamamoto Y, Nakamura K, Okamatsu M, Miyazaki A, Yamada M, Mase M. Detecting avian influenza virus (H5N1) in domestic duck feathers. Emerg Infect Dis 2008; 14:1671–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamamoto Y, Nakamura K, Okamatsu M, Yamada M, Mase M. Avian influenza virus (H5N1) replication in feathers of domestic waterfowl. Emerg Infect Dis 2008; 14:149–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown JD, Stallknecht DE, Beck JR, Suarez DL, Swayne DE. Susceptibility of North American ducks and gulls to H5N1 highly pathogenic avian influenza viruses. Emerg Infect Dis 2006; 12:1663–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hulse‐Post DJ, Sturm‐Ramirez KM, Humberd J et al. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc Natl Acad Sci U S A 2005; 102:10682–10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown JD, Stallknecht DE, Swayne DE. Experimental infection of swans and geese with highly pathogenic avian influenza virus (H5N1) of Asian lineage. Emerg Infect Dis 2008; 14:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jilbert AR, Wu TT, England JM et al. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J Virol 1992; 66:1377–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]


