Abstract
HIV/AIDS has had a profound impact on children around the world since the start of the epidemic. There are currently 3.4 million children under the age of 15 years living with HIV globally, and more than 450,000 children currently receiving lifesaving antiretroviral treatment. This article describes efforts supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) to expand access to treatment for children living with HIV in high-burden countries. The article also highlights a series of case studies that illustrate the impact that the PEPFAR initiative has had on the pediatric HIV epidemic. Through its support of host governments and partner organizations, the PEPFAR initiative has expanded HIV testing and treatment for pregnant women to reduce vertical transmission of HIV, increased access to early infant diagnosis for HIV-exposed infants, improved training and resources for clinicians who provide pediatric care and antiretroviral treatment, and, through public–private partnerships with pharmaceutical manufacturers, helped increase the number of medications available for the treatment of HIV-infected children in resource-limited settings.
Keywords: PEPFAR, pediatric, treatment, HIV
INTRODUCTION
In 2000 at the 13th International AIDS Conference in Durban, South Africa, Nkosi Johnson, a 12-year-old boy living with HIV infection, issued a challenge to the global community. “Care for us,” he said, “and accept us—we are all human beings. We are normal. We have hands. We have feet. We can walk, we can talk, we have needs just like everyone else—don’t be afraid of us—we are all the same!”1 He spoke for the millions of people in the world who were succumbing to the ravages of the AIDS epidemic, and his small, frail body and strained voice provided a vivid image of pediatric HIV disease. At the time, HIV had emerged as a major threat to child health worldwide and particularly in sub-Saharan Africa where an estimated 2 million children were living with HIV infection.2 In 2000, more than 1600 children were newly acquiring HIV infection each day, primarily through mother-to-child perinatal transmission, and 3.8 million pediatric deaths had already been attributed to HIV/AIDS.2
In the initial 20 years after the first cases of AIDS were described in children,3 clinicians and scientists became well acquainted with manifestations of pediatric HIV infection and the particular challenges of providing effective care. In the absence of antiretroviral treatment (ART), HIV infection is a rapidly progressive and fatal disease in infants: 50% of HIV-infected babies in Africa died before reaching their second birthday.4 Many of those who survived the first years of life suffered a broad range of multiorgan manifestations including high rates of encephalopathy, developmental delays, short stature, failure to thrive, pulmonary compromise, frequent infections, and other debilitating complications for which treatments were rarely available.5-9 Children with HIV most often came from families where HIV both infected and affected multiple family members, devastating the core of family life and leaving many orphaned and emotionally fragile children.10 In particular, pregnant women with advanced HIV disease were at high risk for death and for perinatal transmission of rapidly progressive disease to their infants.11-13 And compared with children born to HIV-uninfected women, even those HIV-exposed infants who escaped perinatal HIV infection were at substantially higher risk for common childhood illnesses and mortality.14-16
Furthermore, characteristics of pediatric HIV infection posed and continue to pose challenges to the establishment of effective HIV care and treatment services. Although most children acquire HIV infection through perinatal transmission, not all infants born to HIV-positive mothers become infected.17 Distinguishing the infected from the uninfected infant requires sophisticated laboratory tests [such as HIV DNA polymerase chain reaction (PCR)], which exceeded the laboratory capacity of most resource-constrained settings.18 Without early diagnosis and initiation of ART, HIV-infected infants remain at high risk for severe disease manifestations and early death.4,19 Children with HIV are also particularly vulnerable to other health threats such as malaria, tuberculosis, and malnutrition, which both weaken the child’s natural responses to fight HIV and complicate treatment efforts.20-24 ART options for children, particularly young children, are also complicated by limited availability of palatable, suitable, child-friendly formulations and drug–drug interactions with other commonly used medications such as antimyco-bacterial agents.25-27 In countries with the highest concentration of children living with HIV, health systems accustomed to providing acute episodic health care services are ill equipped to offer the chronic, continuous multidimensional care across the developmental spectrum from infancy through childhood and adolescence that is necessary to effectively treat pediatric HIV disease. Finally, similar to adults with HIV infection, children with HIV and their families struggle to maintain strict adherence to complex medication regimens and to cope with and manage the impact of stigma and discrimination.28
Against this backdrop, the global community mobilized to address the HIV pandemic and expand access to HIV services for children and families in sub-Saharan Africa and other high-burden, low-resource settings. We describe the efforts of the President’s Emergency Program for AIDS Relief (PEPFAR) to expand HIV services to infants, children, and adolescents living with HIV infection.
PEPFAR RESPONSE
PEPFAR was initiated by the US Government (USG) in 2003 to help combat the global HIV/AIDS epidemic. The program was founded to help countries scale-up HIV care and treatment programs and to undertake expanded efforts to prevent new HIV infections. In 2011, PEPFAR directly supported HIV testing and counseling for more than 40 million people and provided ART for more than 3.9 million men, women, and children.29 These efforts have been accomplished through bilateral support to countries, and through the work of implementing government agencies and PEPFAR-funded international and local nongovernmental partner organizations, which have provided technical support to host governments, implemented service programs, and assisted in the development of new international and national guidelines.
To rapidly scale-up HIV care and treatment for children, PEPFAR initially funded and partnered with ministries of health (MOHs) and partner organizations30,31 to provide a family-based approach to pediatric HIV service delivery in its 15 focus countries. Table 1 describes the burden of pediatric HIV in these 15 focus countries32-34 and shows that, in 2010, PEPFAR support was responsible for provision of ART to 83.8% of the estimated number of children receiving treatment in these countries.
TABLE 1.
Estimated Number of Children Aged 0–14 Years Living With HIV in PEPFAR Focus Countries and Estimated Number of Children Receiving ART From All Sources and From PEPFAR
| PEPFAR Focus Country | No. Children Living With HIV, 2009* | No. Children in Need of ART, 2010† | No. Children Receiving ART, 2010† | No. Children Receiving ART Through PEPFAR Support, 2010‡ |
|---|---|---|---|---|
| Botswana | 16,000 | 11,000 | 10,048 | 2300 |
| Côte d’Ivoire | 52,000§ | 37,000 | 4550 | 3700 |
| Ethiopia | 92,000 | Not available | 14,056 | 12,300 |
| Guyana | Not available | Not available | 177 | 187 |
| Haiti | 12,000 | 8200 | 1558 | 1400 |
| Kenya | 180,000 | 170,000 | 36,096 | 45,100 |
| Mozambique | 130,000 | 91,000 | 17,395 | 12,200 |
| Namibia | 16,000 | 10,000 | 9009 | 8600 |
| Nigeria | 360,000 | 280,000 | 20,401 | 17,900 |
| Rwanda | 22,000 | 17,000 | 7541 | 4900 |
| South Africa | 330,000 | 300,000 | 108,682 | 79,400 |
| Tanzania | 160,000 | 110,000 | 19,854 | 18,700 |
| Uganda | 150,000 | 120,000 | 20,017 | 19,400 |
| Vietnam | Not available | Not available | 2668 | 2100 |
| Zambia | 120,000 | 98,000 | 25,388 | 21,000 |
| Total | 1,640,000∥ | 1,252,200¶ | 297,440 | 249,187 |
United Nations Children’s Fund. Children and AIDS: Fifth Stocktaking Report, 2010.
Joint United Nations Programme on HIV/AIDS. Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Toward Universal Access. Progress report 2011.
Unpublished data, Office of the Global AIDS Coordinator, PEPFAR. Numbers >1000 are rounded to the nearest hundred.
United Nations Children’s Fund. Children and AIDS: Fourth Stocktaking Report, 2009. The estimated number of children living with HIV reported for Côte d’Ivoire is for 2007. No data are available for 2009.
The total does not include data for Vietnam or Guyana as these numbers were not reported.
The total does not include data for Ethiopia, Vietnam, or Guyana as these numbers were not reported.
In efforts to promote pediatric HIV training, routine HIV testing of children, pediatric HIV care and treatment, and laboratory capacity building, PEPFAR leveraged the resources of adult HIV care and treatment programs. PEPFAR promoted the integration of pediatric HIV services into maternal and child health settings to strengthen pediatric HIV prevention, early infant diagnosis, and care for HIV-exposed and HIV-infected infants. Working through its governmental and nongovernmental partners, PEPFAR introduced pilot pediatric HIV programs in Guyana and 9 countries in sub-Saharan Africa in 200435; by 2006, all 15 focus countries had established programs to support pediatric HIV care and ART.
The earliest sites providing pediatric services were located in large urban hospitals offering specialized pediatric clinics or “pediatric days” providing services only to children. PEPFAR supported partner organizations as they established pediatric HIV centers of excellence in countries such as Zambia, Rwanda, and Uganda. These large urban health care facilities served as regional referral sites for HIV-infected children and as training centers for clinicians and allied health workers.
PEPFAR also directly contributed to the expansion of pediatric HIV services by supporting the development of clinical and psychosocial training curricula and national and international pediatric HIV guidelines and policies (see Case Study I: Pediatric Provider-Initiated Testing and Counseling in Zambia). By funding public health program evaluations, PEPFAR has helped build an evidence base to inform best practices and guideline development.36-39 After recognizing the need for simple job aids for health care workers in resource-limited settings providing pediatric care, PEPFAR helped expand the use of the first weight-band dosing charts for pediatric ART.40,41 USG staff also designed a series of early infant diagnosis (EID) manuals and an instructional video to strengthen the capacity of nurses and clinicians, national HIV program managers, and laboratory staff to implement high-quality EID programs.42 PEPFAR’s international partners were also instrumental in developing pediatric HIV guidance documents, toolkits, and educational materials, such as the Handbook on Paediatric AIDS in Africa developed by the African Network for the Care of Children Affected by AIDS (ANECCA; see Case Study II: Regional Capacity).
By expanding pediatric HIV training, supporting simplification of pediatric ART regimens, and advocating for task shifting from pediatricians to general physicians, nurses, and clinical officers, PEPFAR promoted decentralization of EID and pediatric HIV care and treatment to primary health facilities. Decentralization of pediatric HIV services has resulted in a dramatic increase in the number of children receiving care and starting treatment43 (see Case Study III: National EID Program in Mozambique). Between 2006 and 2011, there was more than a 6-fold increase in the number of children receiving ART in the 15 PEPFAR focus countries, from 44,800 children in 2006 to 289,600 in 2011 (Fig. 1).
FIGURE 1.
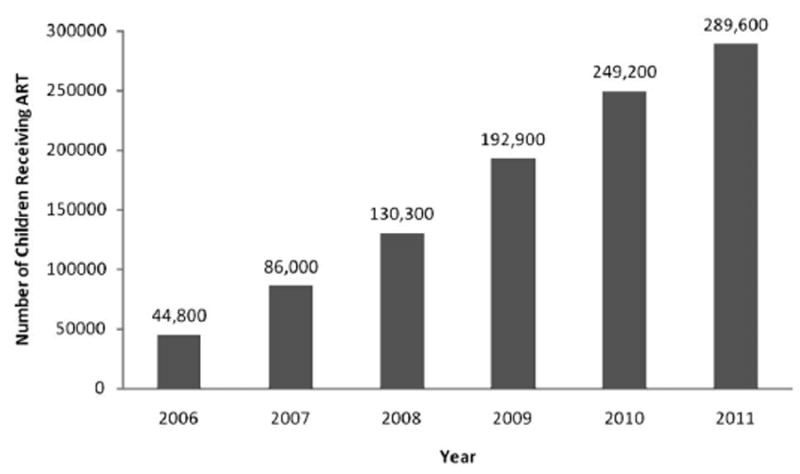
Provision of ART for children aged 0–14 years in PEPFAR focus countries, 2006–2011 (source: Office of the Global AIDS Coordinator).
*Annual Reports to Congress on the President’s Emergency Plan for AIDS Relief (PERFAR). Available online at http://www.pepfar.gov/progress/index.htm.
As part of PEPFAR activities, USG agencies, including the Centers for Disease Control and Prevention and the US Agency for International Development, promoted the expansion of high-quality pediatric care and treatment programs through a variety of activities. In 2005, a PEPFAR technical working group devoted to prevention of mother-to-child transmission (PMTCT) and pediatric HIV care and treatment was created. Among other activities, this group was charged with developing technical guidance documents and identifying areas of emphasis for pediatric HIV programs. These documents have guided country-level technical working groups and program mangers as they developed national guidelines, program priorities and implementation plans, and educational curricula. Key areas have included the following: scaling up cotrimoxazole prophylaxis; developing guidance for a preventive care package for HIV-exposed children44; adapting World Health Organization (WHO) treatment guidelines to the country level; early identification of HIV infection in infants; integrating HIV and maternal, neonatal, and child health45; improving retention in care; and strengthening laboratory and supply chain systems to support drugs, laboratory reagents, and blood drawing equipment for children.
Several management tools are used by PEPFAR to advance pediatric care and treatment. To promote of ART for young infants, PEPFAR requires reporting of treatment results disaggregated by age groups, including the group of <1 year of age. In generalized epidemics, PEPFAR targets call for 65% coverage of HIV-exposed children with EID, and treatment of pediatric populations proportional to their representation in the overall population of HIV-infected people in the country.46 Finally, PEPFAR developed budget codes that cover pediatric care and treatment to better track resources devoted to supporting HIV-infected children.
PEPFAR launched several innovative initiatives to accelerate the scale-up of pediatric care and treatment. As part of the US Food and Drug Administration’s expedited review process for antiretroviral (ARV) medications for use in PEPFAR, a number of generic pediatric ARV formulations, including zidovudine, stavudine, lamivudine, didano-sine, nevirapine, and abacavir, were “tentatively approved,” allowing the use of PEPFAR funds for purchase.27,47 PEPFAR also established a public–private partnership to bring together innovator and generic pharmaceutical companies, civil society organizations, and USG agencies to promote scientific and technical discussions on pediatric HIV treatment, formulations, and access.48 The partnership has helped expedite drug registration, expand the number of pediatric ARV formulations, improve forecasting and supply of ARV drugs, advance EID, and coordinate provider education and training. Since 2005, 9 fixed-dose combination pediatric ARV formulations have been approved by the Food and Drug Administration and/or prequalified by the WHO, leading to expanded and simplified treatment options for children.27
CASE STUDY I: PEDIATRIC PROVIDER-INITIATED TESTING AND COUNSELING IN ZAMBIA
A critical step to the successful scale-up of effective HIV care and treatment has been the early identification of HIV-infected children.4,49-52 In 2005 with PEPFAR funding, the University Teaching Hospital of Zambia (UTH) Pediatric HIV/AIDS Center of Excellence (COE) partnered with Columbia University’s ICAP to provide comprehensive HIV care and treatment for children and to document and disseminate evidence of effective program models. A key area of focus for the COE was in developing strategies to increase the number of children engaged in care. An innovative provider-initiated testing and counseling (PITC) program that offered routine HIV testing and counseling to all children admitted to the hospital and to their parents/caregivers and siblings was piloted at UTH starting in 2006.
In the first 18 months of the PITC program, 13,239 (85%) parents/caregivers of children admitted to UTH with unknown HIV status received counseling, and over 11,571 (87%) children were tested. Testing rates increased over time from 76% in the first quarter of the project to 88% in year 2. Of the children tested, 29% were found to be seropositive.53
The evaluation of the pilot demonstrated that PITC for hospitalized children in high–HIV prevalence settings is a highly effective strategy to identify large numbers of HIV-infected children who could then be linked to care and treatment services. Based on these findings, the MOH in collaboration with COE staff developed national guidelines to expand the use of PITC in pediatric care settings. Using the experience gained during the pilot program, UTH developed a pediatric PITC training curriculum and program for health workers in Zambia.54 Hundreds of health care workers have rotated through the COE to participate in this didactic and experiential training course. The PITC guidelines and training materials have also been adapted for use in multiple African countries.
CASE STUDY II: REGIONAL CAPACITY
Responding to the escalating numbers of children with HIV infection and the limited experience with pediatric HIV programming in sub-Saharan Africa, a small group of experienced clinicians formed ANECCA in 2001.55 The organization was established to advocate for increased access to quality pediatric HIV prevention, care, and treatment services; develop pediatric HIV technical resource materials; build capacity of health workers to provide high-quality care; and produce scientific evidence for effective pediatric HIV programming in Africa.
With support from PEPFAR and other local and international donors, ANECCA developed a variety of resources to support the scale-up of pediatric HIV care and ART. The Handbook on Paediatric AIDS in Africa is an easily accessible and practical resource developed by African experts with substantial experience caring for children.56 More than 15,000 copies have been distributed in over 35 countries, and it is a well-used and valuable tool for clinicians throughout the African continent. ANECCA also developed the Comprehensive Paediatric HIV/AIDS Care Training Curriculum, a training package for health workers that has been adapted and implemented as the national curriculum in over 12 African countries.57 ANECCA’s Clinical Mentorship Program and the Clinical Mentorship Toolkit for Paediatric HIV Care in Africa have equipped more than 2000 HIV clinicians to mentor others providing health care to children with HIV infection. ANECCA has greatly contributed to building pediatric HIV expertise in Africa, and this will continue to serve as a model for the next generations of health care workers.
More than 1000 African practitioners from more than 20 African countries are currently members of ANECCA, and the organization continues to identify critical emerging issues in the field and advocate for scientifically-informed mechanisms to scale-up high-quality care for children. Regional consultations bring together a broad range of stakeholders invested in care of children and provide an opportunity for exchange and innovation. For example, the proposal to include HIV exposure status on the child health card originated during an ANECCA forum. This proposal has already resulted in policy change and has the potential to improve child health outcomes throughout the continent.
CASE STUDY III: NATIONAL EID PROGRAM IN MOZAMBIQUE
During the early years of the scale-up of HIV services in Mozambique, most pediatric patients starting ART were older children with established HIV infection. The number of infants and young children on treatment was disproportionately low and particularly problematic given the risk of early disease progression and death in the first year of life.4 Failure to identify and treat more young children was attributed to limited laboratory capacity for specialized virological testing required for HIV diagnosis among infants.
In 2005, with support from PEPFAR partners, EID testing with HIV DNA PCR was established at the MOH National Institute of Health laboratory. An initial pilot conducted at the Pediatric Day Hospital in Maputo validated the use of dried blood spots (DBS) to conduct PCR testing and informed the EID national roll-out over the subsequent 2 years. DBS facilitated both specimen collection (not necessitating venipuncture) and transport (using filter paper rather than specimen tubes).
Intensive nationwide training of nurses on DBS collection and medical officers and doctors on pediatric HIV treatment was conducted. Three additional laboratories were equipped and laboratory technicians trained to perform PCR testing and strategically placed to ensure access to EID throughout the country. By 2007, 70 health facilities collected 5893 DBS samples. In 2011, DBS samples were collected at 270 facilities and 35,970 samples were tested.
A DBS sample referral network using Internet communication and general packet radio service technology, a mobile data service, was established to ensure timely provision of EID results to clinic facilities. Implementation of the system has resulted in measurable decreases in the number of lost results and the time from specimen collection to return of results.
By the end of 2011, 23,053 of the estimated 130,000 HIV-infected children living in Mozambique had received ART. Over the past 3 years, the proportion of infants on ART rapidly increased because of the EID scale-up and improved follow-up of HIV-exposed infants. As demonstrated in Figure 2, the proportion of HIV-infected infants initiating ART in 142 clinics supported by PEPFAR through the Elizabeth Glaser Pediatric AIDS Foundation in 4 provinces in Mozambique increased from 39% to 72% (Fig. 2). This noteworthy change can be attributed to improved capacity to identify, diagnose, and treat infants and young children in Mozambique.
FIGURE 2.
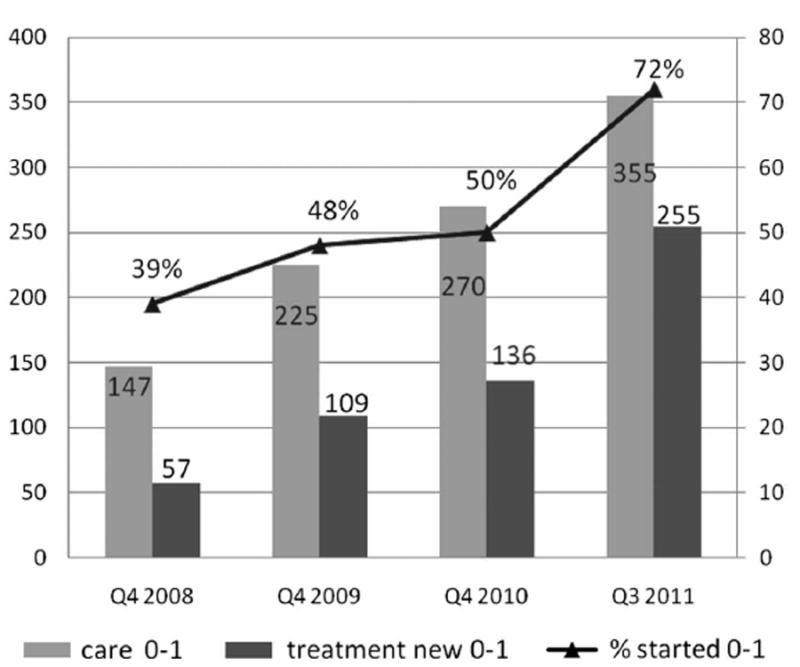
Rapid increase in the proportion of HIV-infected infants receiving ART as a result of the expansion of EID at 142 facilities in Maputo, Gaza, Nampula, and Cabo Delgado provinces, Mozambique, 2008–2011 (courtesy of Elizabeth Glaser Pediatric AIDS Foundation, Mozambique).
CASE STUDY IV: YOUTH-FRIENDLY SERVICES IN BOTSWANA
With improvements in pediatric ART and scale-up of treatment programs, children with HIV are living into adolescence and adulthood. It is estimated that over 2 million youth aged 10–19 years worldwide are living with HIV as a result of mother-to-child transmission and behaviorally acquired infection.32 Providing care for adolescents living with HIV has unique challenges with regard to testing and disclosure, adherence to treatment, and retention in care.58-60 In Botswana, where 2.4% of men and 5% of women aged 15–19 years are living with HIV, over 13,000 adolescents aged 10–19 years are estimated to be HIV infected, there is an urgent need for targeting services to this population.61 The Botswana MOH and international and local organizations have been working to provide a multifaceted response to this need.
In early 2010, the Botswana MOH established a technical working group dedicated specifically to the needs of adolescents living with HIV. This group is currently developing youth-focused treatment guidelines and an “adolescent clinical care package,” which when released later this year will recommend minimum adolescent-focused services for all tiers of the national health care system.
Similar to many programs throughout sub-Saharan Africa, at the Baylor-Botswana Children’s Clinical COE, Gaborone’s largest pediatric HIV referral center, the number of youth in HIV care has increased dramatically from 23 adolescents in 2003 to over 800 in 2010. A focus of the COE has been to develop tools and programs to support the transition of adolescents into adult care, including use of an algorithm to identify when patients are ready to transition, and peer programs, including “Young Adult Club,” which support youth through the transition process.62
Finally, to improve adherence to treatment and encourage retention in care for adolescents, the Kgakololo Project uses instant messaging technology to deliver adherence messages. The program leverages existing cell phone coverage and engages local youth in information technology skill building. The success of the effort, led by PING (Positive Innovation for the Next Generation), has resulted in plans to expand the project to several southern African countries.63
ACHIEVEMENTS, CHALLENGES, AND NEXT STEPS
Efforts to prevent mother-to-child transmission have expanded over the past decade and are now escalating in response to the global challenge to meet ambitious targets toward elimination of new perinatal infections.64 It is estimated that since 1995, more than 350,000 pediatric HIV infections have been averted with the provision of ARV medications for PMTCT. Although the number of new pediatric infections annually remains unacceptably high, at 390,000 in 2010, this number is 30% lower than the 560,000 new pediatric infections reported in 2002 and in 2003.32 By the end of 2010, an estimated 3.4 million children <15 years of age were living with HIV infection, primarily in sub-Saharan Africa. More than 450,000 children were receiving ART by the end of 2010, a 29% increase from 2009.32 The 250,000 pediatric deaths attributed to HIV in 2010 represents a 20% decline from 2005, suggesting a measurable benefit of efforts to identify, engage, and treat children with HIV infection.32
Despite these noteworthy successes, millions of children are still in need of HIV care and treatment. Coverage estimates for children remain low when compared with adults: in 2010, 51% of adults in need of ART were receiving treatment versus only 23% of children. Currently 1–1.5 million children in need of ART have not yet initiated treatment, and each day as many as 1000 children acquire HIV.32 Furthermore, major challenges threaten expansion of services and the stability of current successful programs. Incomplete PMTCT and EID coverage, low HIV testing rates during childhood and adolescence, poor rates of linkages to and retention within treatment programs, and shortages of skilled health workers competent to treat children represent a subset of the many issues that will need to be addressed to expand HIV services for pediatric populations.65-67 As the international community shifts focus and funding to reach ambitious global goals to prevent new pediatric infections, it will be critical to continue to address the care and treatment needs of children and adolescents living with HIV infection.
Many innovations have successfully been tested within the PEPFAR program (as described in more detail in the articles in this journal), and PEPFAR’s continued support of pediatric HIV care and treatment programs will be vital for continued success. In particular, continued efforts to train nonphysician health workers to provide pediatric care will be critical as will efforts to integrate pediatric HIV care with other community-based or clinic-based services.68–70 Furthermore, because pediatric ARV medications contribute only a small proportion of the global ARV market, the supply chain is often threatened with delayed production, stock outs, and potential discontinuation. It will be critical to rationalize and consolidate national pediatric HIV formularies to medications on the WHO essential medicines list to ensure a continued supply of pediatric medications.27,71,72 Continued work to strengthen public–private partnerships with pharmaceutical companies to support development and production of existing and promising new accessibly priced pediatric drugs and formulations is needed.
We can also expect that with the expansion of treatment and maturation of existing programs, more children will survive into adolescence and adulthood, requiring a comprehensive array of services needed to address complex biomedical (treatment failure, inadequate adherence, ART-related complications, retention) and psychosocial (disclosure, sexuality and contraception, peer relations) issues associated with this disease. The growing number of youth living with HIV will also need targeted support as they transition into adult HIV care and treatment services (see Case Study IV: Youth-Friendly Services in Botswana).
PEPFAR remains a driving force to expand lifesaving services for HIV-infected children. Through its partnerships with MOHs, the international community and implementing partners, PEPFAR can continue to provide the needed momentum to expand pediatric HIV services. In closing words at the 13th International AIDS Conference, Nelson Mandela echoed Nkosi Johnson’s call to the global community. He spoke of the importance of the conference as “… a gathering of human beings concerned about turning around one of the greatest threats humankind has faced, and certainly the greatest after the end of the great wars of the previous century.” He articulated what would become to represent the PEPFAR and global response to the epidemic. He said “… Others will not save us if we do not primarily commit ourselves. Let us, however, not underestimate the resources required to conduct this battle. Partnership with the international community is vital. A constant theme in all our messages has been that in this inter-dependent and globalized world, we have indeed again become the keepers of our brother and sister. That cannot be more graphically the case than in the common fight against HIV/AIDS.”1
Acknowledgments
The authors gratefully acknowledge all of the individuals who worked tirelessly to improve health outcomes for children and families living with HIV. They also thank specific individuals who assisted in the compilation of data and information for this article, including Jordana DeLeon, Office of the Global AIDS Coordinator, Department of State; Haruna Jibril, pediatric clinical advisor, Masa ARV Programme at the Botswana MOH; Marape Marape, associate director for research, Botswana-Baylor Children’s Clinical COE; and Silvia Matitimele, Elizabeth Glaser Pediatric AIDS Foundation, Mozambique.
Footnotes
Various authors have professional relationships with PEPFAR (either as employees of PEPFAR-supported US Government agencies or as grantees/ contractors) as outlined in the Copyright Transfer Agreement Forms.
The authors have no other funding or conflicts of interest to disclose.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the US Government, or the World Health Organization.
References
- 1. [March 18, 2012];Transcript of speech. Available at: http://www.journaids.org/index.php/essential_information/hivaids_key_people/nkosi_johnson/
- 2.UNAIDS Report on the Global HIV/AIDS Epidemic. Geneva, Switzerland: UNAIDS; 2000. [Google Scholar]
- 3.Shannon KM, Ammann AJ. Acquired immune deficiency syndrome in childhood. J Pediatr. 1985;106:332–342. doi: 10.1016/s0022-3476(85)80320-6. [DOI] [PubMed] [Google Scholar]
- 4.Newell ML, Coovadia H, Cortina-Borja M, et al. Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 5.Pillay T, Adhikari M, Mokili J, et al. Severe, rapidly progressive human immunodeficiency virus type 1 disease in newborns with coinfections. Pediatr Infect Dis J. 2001;20:404–410. doi: 10.1097/00006454-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Spira R, Lepage P, Msellati P, et al. Natural history of human immunodeficiency virus type 1 infection in children: a five-year prospective study in Rwanda. Mother-to-Child HIV-1. Transmission Study Group Pediatrics. 1999;104:e56. doi: 10.1542/peds.104.5.e56. [DOI] [PubMed] [Google Scholar]
- 7.Taha TE, Graham SM, Kumwenda NI, et al. Morbidity among human immunodeficiency virus-1-infected and -uninfected African children. Pediatrics. 2000;106:E77. doi: 10.1542/peds.106.6.e77. [DOI] [PubMed] [Google Scholar]
- 8.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, et al. Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr. 2006;41:504–508. doi: 10.1097/01.qai.0000188122.15493.0a. [DOI] [PubMed] [Google Scholar]
- 9.Chintu C, Bhat GJ, Walker AS, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomized placebo-controlled trial. Lancet. 2004;364:1865–1871. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 10.UNICEF. Africa’s Orphaned and Vulnerable Generations: Children Affected by AIDS. New York: UNICEF, UNAIDS, & PEPFAR; 2006. [Google Scholar]
- 11.Kuhn L, Aldrovandi GM, Sinkala M, et al. Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother-to-child HIV transmission. AIDS. 2010;24:1374–1377. [PMC free article] [PubMed] [Google Scholar]
- 12.Mofenson LM. Epidemiology and determinants of vertical HIV transmission. Semin Pediatr Infect Dis. 1994;5:252–256. [Google Scholar]
- 13.Abrams EJ, Matheson PB, Thomas PA, et al. New York City Perinatal HIV Transmission Collaborative Study Group. Neonatal predictors of infection status and early death among 332 infants at risk of HIV-1 infection monitored prospectively from birth. Pediatrics. 1995;96:451–458. [PubMed] [Google Scholar]
- 14.Rouet F, Sakarovitch C, Msellati P, et al. 049 Ditrame Study Group. Pediatric viral human immunodeficiency virus type 1 RNA levels, timing of infection, and disease progression in African HIV-1-infected children. Pediatrics. 2003;112:e289. doi: 10.1542/peds.112.4.e289. [DOI] [PubMed] [Google Scholar]
- 15.Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health. 2009;14:276–287. doi: 10.1111/j.1365-3156.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 16.Marinda E, Humphrey JH, Iliff PJ, et al. ZVITAMBO Study Group. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26:519–526. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 17.Kourtis AP, Lee FK, Abrams EJ, et al. Mother-to-child transmission of HIV-1: timing and implications for prevention. Lancet Infect Dis. 2006;6:726–732. doi: 10.1016/S1473-3099(06)70629-6. [DOI] [PubMed] [Google Scholar]
- 18.Rogers MF, Ou CY, Rayfield M, et al. Use of the polymerase chain reaction for early detection of the proviral sequences of human immunodeficiency virus in infants born to seropositive mothers. New York City Collaborative Study of Maternal HIV Transmission and Montefiore Medical Center HIV Perinatal Transmission Study Group. N Engl J Med. 1989;320:1649–1654. doi: 10.1056/NEJM198906223202503. [DOI] [PubMed] [Google Scholar]
- 19.Violari A, Cotton MF, Gibb DM, et al. CHER Study Team. Early anti-retroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thea DM, St Louis ME, Atido U, et al. A prospective study of diarrhea and HIV-1 infection among 429 Zairian infants. N Engl J Med. 1993;329:1696–1702. doi: 10.1056/NEJM199312023292304. [DOI] [PubMed] [Google Scholar]
- 21.Walters E, Cotton MF, Rabie H, et al. Clinical presentation and outcome of tuberculosis in human immunodefficiency virus infected children on anti-retroviral therapy. BMC Pediatr. 2008;8:1. doi: 10.1186/1471-2431-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braitstein P, Nyandiko W, Vreeman R, et al. The clinical burden of tuberculosis among human immunodeficiency virus-infected children in Western Kenya and the impact of combination antiretroviral treatment. Pediatr Infect Dis J. 2009;28:626–632. doi: 10.1097/INF.0b013e31819665c5. [DOI] [PubMed] [Google Scholar]
- 23.Malamba S, Hladik W, Reingold A, et al. The effect of HIV on morbidity and mortality in children with severe malarial anaemia. Malar J. 2007;6:143. doi: 10.1186/1475-2875-6-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reitz C, Coovadia A, Ko S, et al. Initial response to protease inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of co-treatment for tuberculosis. J Infect Dis. 2010;201:1121–1131. doi: 10.1086/651454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giaquinto C, Morelli E, Fregonese F, et al. Current and future antiretroviral treatment options in paediatric HIV infection. Clin Drug Investig. 2008;28:375–397. doi: 10.2165/00044011-200828060-00005. [DOI] [PubMed] [Google Scholar]
- 26.Heidari S, Mofenson LM, Hobbs CV, et al. Unresolved antiretroviral treatment management issues in HIV-infected children. J Acquir Immune Defic Syndr. 2012;59:161–169. doi: 10.1097/QAI.0b013e3182427029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waning B, Diedrichsen E, Jambert E, et al. The global pediatric antire-troviral market: analyses of product availability and utilization reveal challenges for development of pediatric formulations and HIV/AIDS treatment in children. BMC Pediatr. 2010;10:74. doi: 10.1186/1471-2431-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vreeman RC, Wiehe SE, Pearce EC, et al. A systematic review of pediatric adherence to antiretroviral therapy in low- and middle-income countries. Pediatr Infect Dis J. 2008;27:686–691. doi: 10.1097/INF.0b013e31816dd325. [DOI] [PubMed] [Google Scholar]
- 29.PEPFAR. [March 18, 2012];Using science to save lives: latest PEPFAR results. 2011 Available at: www.pepfar.gov.
- 30.The US President’s Emergency Plan for AIDS Relief. [March 18, 2012];Partners, 2004-2007. Available at: http://2006-2009.pepfar.gov/partners/index.htm.
- 31.The US President’s Emergency Plan for AIDS Relief. [March 18, 2012];All countries—partner and sub-partner counts by local status, organization type and program area. Available at: http://2001-2009.state.gov/s/gac/progress/other/data/partners/60363.htm.
- 32.WHO. Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 33.UNICEF, UNAIDS, WHO, UNFPA, and UNESCO. [March 18, 2012];Children and AIDS: Fifth Stocktaking Report, 2010. Available at: http://www.unicef.org/aids/files/ChildrenAndAIDS_Fifth_Stocktaking_Report_2010_EN.pdf.
- 34.UNICEF, UNAIDS, WHO, and UNFPA. [March 18, 2012];Children and AIDS: Fourth Stock-taking Report, 2009. Available at: http://www.unicef.org/publications/files/Children_and_AIDS_Fourth_Stocktaking_Report_EN_120209.pdf.
- 35.The US President’s Emergency Plan for AIDS Relief. [March 18, 2012];Focusing on our future: prevention, diagnosis, and treatment of pediatric HIV/AIDS. 2005. Available at: http://www.state.gov/documents/organization/53422.pdf.
- 36.Song R, Menzies H, Vandebriel G, et al. Evaluation of TB screening approaches among HIV-infected children in Rwanda, oral presentation at the 2009. International Workshop for Pediatric HIV, Cape Town, South Africa, and oral presentation at the 40th IUATLD World Conference on Lung Health, Cancun, Mexico. [Google Scholar]
- 37.Sandison TG, Homsy J, Arinaitwe E, et al. Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ. 2011;342:d1617. doi: 10.1136/bmj.d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katrak S, Gasasira A, Arinaitwe E, et al. Safety and tolerability of artemether-lumefantrine versus dihydroartemisinin-piperaquine for malaria in young HIV-infected and uninfected children. Malar J. 2009;8:272. doi: 10.1186/1475-2875-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arinaitwe E, Sandison TG, Wanzira H, et al. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for falciparum malaria: a longitudinal, randomized trial in young Ugandan children. Clin Infect Dis. 2009;49:1629–1637. doi: 10.1086/647946. [DOI] [PubMed] [Google Scholar]
- 40.International Center for AIDS Care and Treatment Programs; Centers for Disease Control and Prevention Global AIDS Program; Baylor International Pediatric AIDS Initiative. [March 18, 2012];Pediatric antiretroviral dosing in resource-limited settings. 2006 Available at: http://www.cdc.gov/globalaids/resources/pmtct-care/pmtct-pediatric-dosing-guide.html.
- 41.Weidle PJ, Abrams EJ, Gvetadze R, et al. A simplified weight-based method for pediatric drug dosing for zidovudine and didanosine in resource-limited settings. Pediatr Infect Dis J. 2006;25:59–64. doi: 10.1097/01.inf.0000195619.76277.3f. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. Guide to implementation of services for early infant diagnosis in resource-limited settings. 2009 Available at: http://www.womenchildrenhiv.org/wchiv?page=ch-09-00-eid.
- 43.Fayorsey R, Saito S, Carter RJ, et al. The role of primary health facilities in expanding pediatric care and treatment services in sub-Saharan Africa. Presented at: 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 10, 2011; Rome, Italy. Available at: http://pag.ias2011.org/abstracts.aspx?aid=1001. [Google Scholar]
- 44.The US President’s Emergency Plan for AIDS Relief. [March 18, 2012];Guidance for a preventive care package for children born to HIV-infected mothers #1. 2006 Available at: http://www.pepfar.gov/guidance/78263.htm.
- 45.The US President’s Emergency Plan for AIDS Relief. [March 18, 2012];PEPFAR guidance on the integration of prevention of mother to child transmission, MNCH (maternal, neonatal and child health) and pediatric HIV services. 2011 Available at: http://www.pepfar.gov/documents/organization/158963.pdf.
- 46.The US President’s Emergency Plan for AIDS Relief. [March 18, 2012];The U.S. President’s Emergency Plan for AIDS Relief: five-year strategy. 2009 Available at: http://www.pepfar.gov/strategy/prevention_care_treatment/133372.htm.
- 47.US Food and Drug Administration. [March 18, 2012];Approved and tentatively approved antiretrovirals in association with the President’s emergency plan. Available at: http://www.fda.gov/InternationalPrograms/FDABeyondOurBordersForeignOffices/AsiaandAfrica/ucm119231.htm.
- 48.The US President’s Emergency Plan for AIDS Relief. [March 18, 2012];Building a new public-private partnership for pediatric AIDS treatment. 2006 Available at: http://www.pepfar.gov/documents/organization/125996.pdf.
- 49.Horwood C, Liebeschuetz S, Blaauw D, et al. Diagnosis of pediatric HIV infection in a primary health care setting with a clinical algorithm. Bull World Health Organ. 2003;81:858–866. [PMC free article] [PubMed] [Google Scholar]
- 50.Vetter KM, Djomand G, Zadi F, et al. Clinical spectrum of HIV disease in children in a West African city. Pediatr Infect Dis J. 1996;15:438–442. doi: 10.1097/00006454-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Rogerson SR, Gladstone M, Callaghan M, et al. HIV infection among paediatric in-patients in Blantyre, Malawi. Trans R Soc Trop Med Hyg. 2004;98:544–552. doi: 10.1016/j.trstmh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Reyburn H, Mwakasungula E, Chonya S, et al. Clinical assessment and treatment in paediatric wards in the north-east of the United Republic of Tanzania. Bull World Health Organ. 2008;86:132–139. doi: 10.2471/BLT.07.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kankasa C, Carter RJ, Briggs N, et al. Routine offering of HIV testing to hospitalized pediatric patients at University Teaching Hospital, Lusaka, Zambia: acceptability and feasibility. J Acquir Immune Defic Syndr. 2009;51:202–208. doi: 10.1097/qai.0b013e31819c173f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. [March 19, 2012];Women and Children Web site. Available at: www.womenchildrenhiv.org/
- 55. [March 19, 2012];ANECCA Web site. Available at: http://www.anecca.org/index.html.
- 56. [March 19, 2012];ANECCA Web site. Available at: http://www.anecca.org/publication.html.
- 57. [March 19, 2012];ANECCA Web site. Available at: http://www.anecca.org/clinical.html.
- 58.Gray GE. Adolescent HIV—cause for concern in Southern Africa. PLoS Med. 2010;7:e1000227. doi: 10.1371/journal.pmed.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bakanda C, Birungi J, Mwesigwa R, et al. Survival of HIV-infected adolescents on antiretroviral therapy in Uganda: findings from a nationally representative cohort in Uganda. PLoS One. 2011;6:e19261. doi: 10.1371/journal.pone.0019261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nglazi MD, Kranzer K, Holele P, et al. Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC Infect Dis. 2012;12:21. doi: 10.1186/1471-2334-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Botswana AIDS Impact Survey III (BAIS III), 2008: Preliminary Results. Gaborone, Botswana: Central Statistics Office; 2009. [Google Scholar]
- 62.Botswana-Baylor Children’s Clinical Centre of Excellence. [April 20, 2012];Botswana Teen Club Web site. Available at: http://www.botswanateenclub.word-press.com.
- 63.Positive Innovation for the New Generation. [April 20, 2012];PING Web site. Available at: http://www.pingsite.org.
- 64.WHO. Towards the Elimination of Mother-to-Child Transmission of HIV: Report of a WHO Technical Consultation. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 65.Stinson K, Boulle A, Smith PJ, et al. Coverage of the prevention of mother-to-child transmission programme in the Western Cape, South Africa using cord blood surveillance. J Acquir Immune Defic Syndr. 2012;60:199–204. doi: 10.1097/QAI.0b013e31824d985e. [DOI] [PubMed] [Google Scholar]
- 66.Ciaranello AL, Park JE, Ramirez-Avila L, et al. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9:59. doi: 10.1186/1741-7015-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ekouevi DK, Stringer E, Coetzee D, et al. Health facility characteristics and their relationship to coverage of PMTCT of HIV services across four African countries: the PEARL study. PLoS One. 2012;7:e29823. doi: 10.1371/journal.pone.0029823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brennan AT, Long L, Maskew M, et al. Outcomes of stable HIV-positive patients down-referred from a doctor-managed antiretroviral therapy clinic to a nurse-managed primary health clinic for monitoring and treatment. AIDS. 2011;25:2027–2036. doi: 10.1097/QAD.0b013e32834b6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Janssen N, Ndirangu J, Newell ML, et al. Successful paediatric HIV treatment in rural primary care in Africa. Arch Dis Child. 2010;95:414–421. doi: 10.1136/adc.2009.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Long L, Brennan A, Fox MP, et al. Treatment outcomes and cost-effectiveness of shifting management of stable ART patients to nurses in South Africa: an observational cohort. PLoS Med. 2011;8:e1001055. doi: 10.1371/journal.pmed.1001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Decroo T, Telfer B, Biot M, et al. Distribution of antiretroviral treatment through self-forming groups of patients in Tete province, Mozambique. J Acquir Immune Defic Syndr. 2011;56:e39–e44. doi: 10.1097/QAI.0b013e3182055138. [DOI] [PubMed] [Google Scholar]
- 72.SCMS. [March 18, 2012];Report on the development of a reliable and replicable methodology for forecasting the global demand for paediatric anti-retroviral medicines. 2007 Available at: www.scms.pfscm.org.


