Abstract
Purpose of review
Localization of focal epileptic brain is critical for successful epilepsy surgery and focal brain stimulation. Despite significant progress, roughly half of all patients undergoing focal surgical resection, and most patients receiving focal electrical stimulation, are not seizure free. There is intense interest in high-frequency oscillations (HFOs) recorded with intracranial electroencephalography as potential biomarkers to improve epileptogenic brain localization, resective surgery, and focal electrical stimulation. The present review examines the evidence that HFOs are clinically useful biomarkers.
Recent findings
Performing the PubMed search ‘High-Frequency Oscillations and Epilepsy’ for 2013–2015 identifies 308 articles exploring HFO characteristics, physiological significance, and potential clinical applications.
Summary
There is strong evidence that HFOs are spatially associated with epileptic brain. There remain, however, significant challenges for clinical translation of HFOs as epileptogenic brain biomarkers: Differentiating true HFO from the high-frequency power changes associated with increased neuronal firing and bandpass filtering sharp transients. Distinguishing pathological HFO from normal physiological HFO. Classifying tissue under individual electrodes as normal or pathological. Sharing data and algorithms so research results can be reproduced across laboratories. Multicenter prospective trials to provide definitive evidence of clinical utility.
Keywords: biomarker, electroencephalography, epilepsy, high-frequency oscillations
INTRODUCTION
High-frequency oscillations (HFOs: 65–600 Hz) are local field potentials recorded with intracranial electroencephalography (iEEG), and have received intense interest as potential electrophysiological biomarkers to improve focal epileptic brain mapping, see reviews [1,2,3▪]. For patients with drug-resistant focal epilepsy (DRE) resective surgery provides the best chance for seizure freedom. But despite recording seizures with intracranial EEG (iEEG), considered the ‘gold standard’ for localizing focal epileptogenic brain and the seizure onset zone (SOZ), epilepsy surgery is often unsuccessful [4,5]. In addition, despite rapid progress and class-I evidence for efficacy, focal brain stimulation as currently implemented rarely yields seizure freedom for patients [6▪▪].
Ictal HFOs spanning high-gamma (65–100 Hz), ripple (100–250 Hz), and fast ripple (250–600 Hz) frequency bands have been implicated in seizure generation in human focal epilepsy [7–12]. Research from multiple groups also report increased rates of interictal HFOs in the SOZ, including fast ripples [13–16], ripple [4,17–20], and high-gamma HFO [11]. In addition, increased HFOs are reported to correlate with disease severity, seizure frequency [19,21], and resection of their generators with seizure-free outcome [20,22,23▪]. In addition, recent studies have demonstrated the feasibility of seizure forecasting [24,25], and HFO show promise as a biomarker of the preictal state [26,27].
KEY POINTS.
Human brain generates a wide dynamic range of local field potential oscillations that include high-frequency oscillations (HFO: 65–600 Hz).
Pathological HFO (pHFO) can be distinguished and separated from normal, physiological HFO (nHFO).
pHFOs are electrophysiological biomarkers of epileptogenic brain.
There is a critical need for data and computer code sharing to create reproducible research and advance the use of HFO biomarkers in brain mapping.
Classification of tissue under each electrode as pathological or normal is required for clinical translation and requires additional research.
Thus, HFOs have emerged as promising electrophysiological biomarkers of epileptogenic tissue (see reviews [1,2,28,29]), and HFO-guided brain mapping would appear poised to translate into clinical practice. There remain, however, significant barriers to clinical translation including: distinguishing true HFO from high frequency power associated with increased neuronal firing [30–32] and bandpass filtering of interictal epileptiform sharp waves and nonspecific transients [29,33]; Distinguishing pathological HFO (pHFO) [34–36] from normal HFO (nHFO) associated with physiological functions [37–42]; Classification of tissue under individual electrodes as pathological or normal - To date, most investigations simply report increased HFO when summed across all SOZ electrodes compared to all non-SOZ electrodes, which is not sufficient for guiding epilepsy surgery [1]; Generating reproducible results - To date it has not been possible to compare results from different laboratories. Most studies analyze iEEG data that are not available and rely on expert visual review, or proprietary detectors, to detect HFO. Finally, most studies have analyzed variably selected, relatively short (~10–30 min) datasets, from relatively small numbers of patients, (e.g., N =9 [13], N =19 [15], N =23 [11], N =6 [4], N =7 [17], N =10 [18], N =5 [43], N =9 [44], N =20 [20], N =30 [45], N =35 [36]). Whether these positive results can be achieved in wider practice is unclear. Multicenter prospective trials will be required to demonstrate the clinical utility of HFO bio-markers. In this article, we review research efforts into the gaps identified above and suggest potential avenues for translation of HFO electrophysiological biomarkers to clinical practice.
High-frequency oscillations, high-frequency power, and high-frequency activity
There are a range of important technical issues when recording and analyzing wide bandwidth electrophysiology that have been reviewed elsewhere, for example, appropriate data sampling frequency and distinguishing between increased high-frequency power (HFP), HFO and artifacts [29,33]. The distinction between HFO and HFP has been extensively reviewed, but the terms are often conflated or their distinction ignored in the literature. The term high-frequency activity (HFA) was recently suggested to encompass both HFO and HFP [46▪▪]. These terms (HFO, HFP, HFA) and others (e.g., fast and very fast activity or oscillations) are variably used in the literature to describe different frequency ranges and types of high-frequency cerebral electrical activity [38]. Therefore, defining the frequency range of interest, for example (~65–500 Hz), and what type of high-frequency activity is being analyzed is critical when reporting results. Fourier spectral decomposition of a rapidly changing iEEG signal, for example a sharp data transient, epileptiform spikes and sharp waves, all produce an increase in HFP [29,33]. Furthermore, recent studies show that filtered extracellular action potentials ‘contaminate’ high-frequency activity [30–32]. The HFP from these sources is due to the high-frequency Fourier components required to represent the raw data, and should not be confused with actual data oscillations or true HFO. In the extreme case of a data discontinuity (e.g., a square wave signal), the Fourier component sums at the discontinuity do not die out as higher frequency terms are added, a phenomenon referred to as ‘Gibbs’ artifact’ [47].
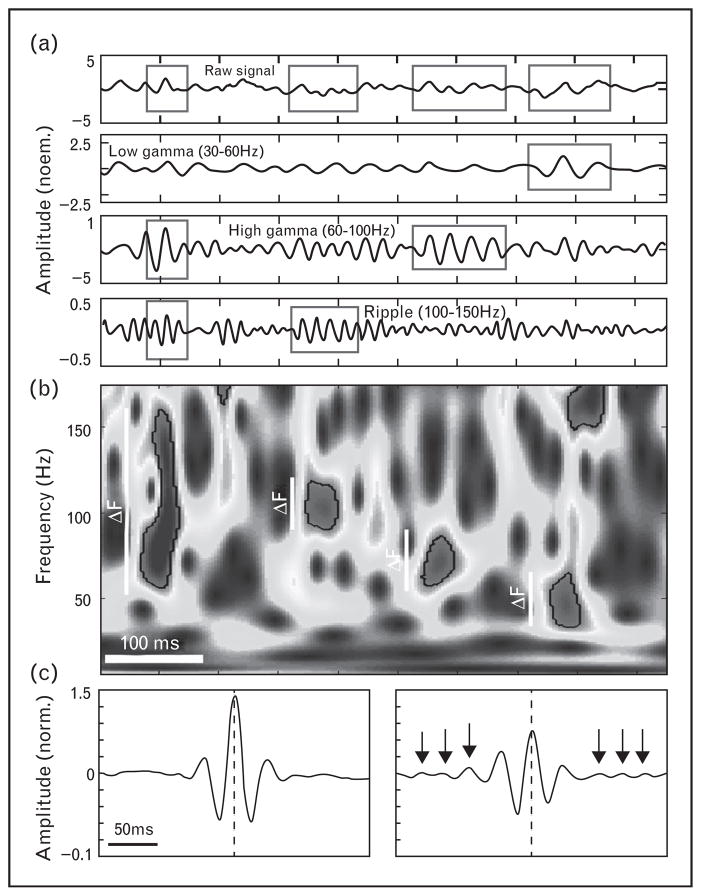
Gibbs’ phenomenon and how to distinguish HFO from HFP in iEEG has been extensively discussed [29,33]. Electrophysiological data are often filtered in particular frequency bands for analysis, and care should be taken to distinguish between HFP and HFO (Fig. 1, unpublished data). When investigating electrophysiological brain recordings the term HFO should be used to describe true high-frequency local field potential oscillations in the iEEG, that is oscillations visible in the raw recording and not the high-frequency Fourier components from a bandpass filter. In the analysis of focal epilepsy ignoring the distinction between HFO and HFP may distort results [49▪], because in focal epilepsy the HFP associated with epileptiform sharp waves (rapidly changing voltage transient [50]) are more widely distributed than HFO. In the future, research may benefit from defining specific HFO and HFP electrophysiological events in order to avoid heterogeneous signals that could degrade the performance of electrophysiological biomarkers.
FIGURE 1.
Distinguishing of HFO and HFP activity based on their spectral properties. (a) Discrete bursts of power increases can be detected using a sensitive spectral analysis method [48▪▪] and grouped according to their frequency span (ΔF) into broad and narrow band detections (boxed data segments). Top panels show the original signal together with its three bandpass filtered traces below; the bottom spectrogram visualizes four detections of increased power delineated by the black outline. (b) Average raw signal waveforms of the two event types detected at 45 Hz aligned to the peak of the filtered oscillation (center dashed line). Note consistent oscillations around peak amplitude of the narrowband events (right panel: arrows point to peaks of each cycle) in contrast to the broadband power increases (left panel).
Physiological and pathological high-frequency oscillations
Distinguishing normal physiological HFO (nHFO) [37–41,48▪▪] from pathological, epileptiform HFO (pHFO) [34,35,36,51] remains a fundamental challenge in clinical epileptology [52▪▪,53,54▪▪,55▪]. Classic examples of physiological and pathological HFOs are ripple frequency oscillations [37,38]. The sharp–wave–ripple complex is a physiological HFO that results from phasic inhibitory input on the soma of pyramidal cells [56]; whereas the ripple and fast ripple oscillations superimposed on interictal epileptiform sharp waves are largely generated by synchronized pyramidal cell burst firing [54▪▪,55▪]. Although currently it is unclear how to definitively differentiate pHFO from nHFO in clinical iEEG recordings, one approach is to simply classify HFO associated with epileptiform sharp waves as pHFO and event-related HFO associated with physiological tasks as nHFO [35,40]. Using this approach, the characteristics (e.g., spectral properties, amplitude, duration, and so on) of HFO associated with physiological motor and memory tasks, that is nHFO, can be directly compared to pHFO associated with epileptiform sharp waves [35]. Using this approach, a study of event-related evoked nHFO in human motor cortex had lower amplitudes than pHFO associated with epileptiform sharp waves [35]. The amplitude of LFP, however, is highly variable and sensitively depends on the distance between recording electrodes and the local HFO generators. Of course, HFO frequency would be a more attractive measure as it would not be expected to depend on the distance to the generator [57], but multiple studies in humans report a wide range of overlapping pHFO and nHFO frequencies [11,17,44,52▪▪,58].
An exception is found in the Kainic rodent epilepsy model in which fast ripple HFOs are distinctly pathological oscillations [14,59]. Whether HFOs in the fast ripple frequency range (>250–600 Hz) are uniquely pathological oscillations in humans remains an open question, but increased rates of high-gamma [11], ripple [17], and fast ripple HFO have consistently been described in human epileptogenic tissue. In addition, ripple frequency HFOs are recorded in dentate gyrus of epileptic rats, but not in control rodents. Thus, the anatomic location may identify what is pathological [34].
Classification of seizure onset zone and epileptogenic tissue
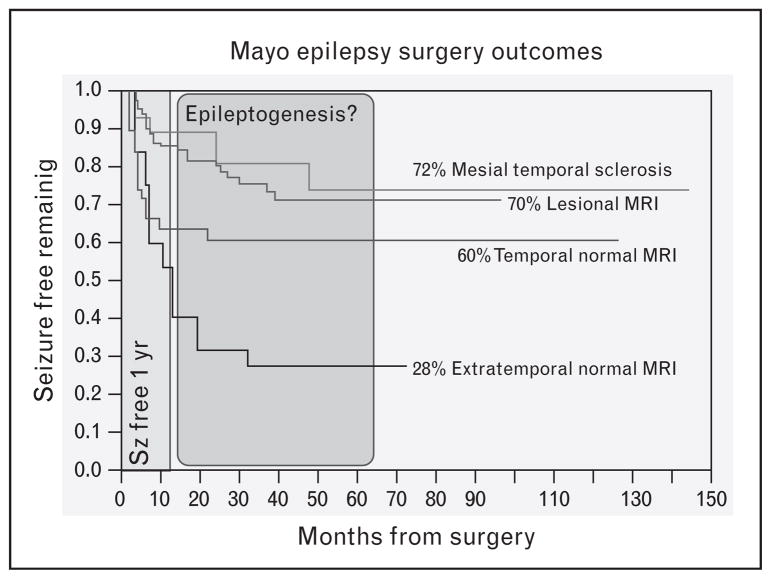
Although there is strong evidence that HFOs are biomarkers of epileptogenic brain, whether the signal is adequate for individualized patient care remains unclear. In order to guide surgical resection, biomarkers must be able to classify tissue under each individual electrode as pathological or normal. In addition to identifying the tissue generating seizures currently, the optimal biomarker would identify tissue at risk for generating seizures in the future, that is tissue undergoing epileptogenesis. Late seizure recurrence after a year or more of post-surgical resection seizure freedom supports the hypothesis that previously quiescent tissue undergoes epileptogenesis in some patients [5]. Interestingly, patients with seizure recurrence are often rendered seizure free after repeated operations that extend the prior resections [60], suggesting that the surgical margins of the initial resection should have been extended. Ultimately, the clinical goal is to identify electrophysiological biomarkers that not only improve SOZ localization, but can also predict the tissue and networks at risk for epileptogenesis (hypothesized to be the cause of late epilepsy recurrence in Fig. 2 [61–63]). There is evidence in rodent epilepsy models that pHFOs (200–600 Hz) are a biomarker of epileptogenesis [59,64▪▪]. This is a critically important topic for epilepsy surgery, given that many patients that are initially seizure free suffer late recurrence of their seizures.
FIGURE 2.
Mayo Clinic Surgery Outcomes. Kaplan-Meir curves show 5-year seizure freedom for patients with unilateral mesial temporal sclerosis (MTS) is 72%, lesional MRI excluding MTS is 70%, and normal MRI temporal lobe epilepsy and extratemporal lobe epilepsy is ~60% [62,63] and 28% [64▪▪], respectively. Normal MRI patients have worse long-term outcomes, and for all patients the risk of seizure recurrence extends well beyond 1 year. A hypothesis for the recurrence after 1 year is epileptogenesis in tissue at the margin of prior resection.
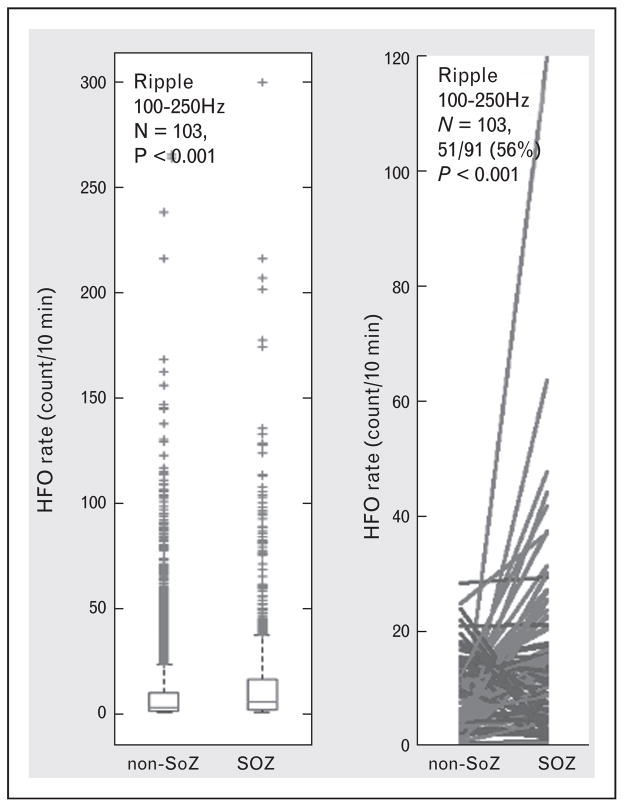
Despite the clear need for classifying tissue under individual electrodes, most investigations to date only report group results showing that HFOs are increased when summed across all SOZ compared with non-SOZ electrodes (e.g., reviews [1,2,65]). This type of group analysis supports that HFOs are interictal biomarkers of SOZ, and even that resection of electrodes with increased HFO in aggregate is associated with seizure freedom [20], but falls far short of clinical utility. When considering individual patients the rates of HFOs are often highly variable and less specific for epileptic brain localization [44]. For example, we analyzed HFO rates in 91 patients with focal epilepsy and 12 control patients without epilepsy undergoing motor cortex stimulation for intractable facial pain (Fig. 3, unpublished data). There is strong evidence from the group analysis that increased rates of HFO are associated with SOZ, but when considering individual patients HFOs were increased in the SOZ of only 56% (51/91, P <0.01) patients overall. These results, like many in the literature, are confounded by nHFO, and false detections, but have the advantage of reproducibility, given they are generated by automated detectors on data that are freely available (http://msel.mayo.edu/data.html). A significant remaining challenge is to extend localization analysis to a-priori classification of individual electrodes [66▪]. Although the vast majority of research has focused on group analysis (all electrodes in SOZ versus all electrodes in non-SOZ), there are a few exceptions that report seizure-free outcomes after resection of single electrodes generating fast ripple HFO in short intra operative recordings [22,23▪].
FIGURE 3.
High-frequency oscillations (HFOs) are increased in seizure onset zone (SOZ). Data from N =91 patients with focal epilepsy patients and 12 control patients undergoing motor cortex stimulation for drug resistant facial pain. An automated HFOs detection algorithm using a signal line-length threshold was used to detect HFOs (65–600 Hz) events in 2 h of continuous data. (a) HFO (65–600 Hz) rates (#counts/min-channel) are increased in SOZ versus non-SOZ when considering all channels in all patients (total electrodes =5862, N =103 patients). (b) When considering individual patients, however, only 56% (51/91, paired t test P <0.0001) of all (N =91) focal epilepsy patients showed significantly increased HFO rates in the SOZ.
Generating reproducible results
Because of a lack of shared data, algorithms, and computer code comparing research results across laboratories has been impossible. There are multiple reasons for the lack of data sharing in epilepsy electrophysiology research, including patient privacy laws. However, within IRB approved studies using appropriate data, de-identification barriers can be overcome. Recent reports about the lack of biomedical research reproducibility have highlighted the interest in data and computer code sharing [67–69]. The journal Nature recently published a series of articles on research reproducibility (collected at http://www.nature.com/news/reproducibility-1.17552). Reproducible research requires open source data, algorithms, and computer code [70]. The importance of data sharing has spawned EEG databases with freely available or data that can be purchased (examples include http://ieeg.org & http://msel.mayo.edu/data.html & http://epilepsy-database.eu/ & https://epilepsy.uni-freiburg.de/freiburg-seizure-prediction-project/eeg-database), which are facilitating reproducibility and algorithm development. Open access to data, methods, algorithms, and computer code will accelerate research. Recently, data sharing and crowd source analysis was used effectively to explore seizure detection and prediction [25].
Multicenter prospective trials
Most studies published to date have analyzed variably selected, relatively short (~10–30 min) datasets, from relatively small numbers of patients [1]. Although small single site feasibility trials have emerged investigating HFO [22,71], definitive demonstration will require a collaborative effort between multiple epilepsy centers [72]. These types of studies are challenging for multiple reasons, including cost, effort, patient selection, and the difficultly of multiple centers uniformly adopting a protocol. At this time, the superiority of surgery guided by recording seizures, interictal epileptiform spikes, and HFO can be debated, but the definitive data are lacking.
CONCLUSION
There is emerging evidence that pHFO can be differentiated from physiological nHFO, and that pHFO are biomarkers of epileptogenic brain. There is a critical need to share data, algorithms, and computer code in order to realize the opportunity for rapid progress on nHFO and pHFO detection and classification. With high accuracy automated detectors and classification algorithms, the inherent variability associated with visual review can be eliminated and the feasibility of mapping normal and epileptogenic brain with HFO biomarkers can be investigated. The first step is creating a database of freely available, well annotated, wide bandwidth interictal and ictal iEEG data, and clinical metadata (electrode locations, pre and post-operative MRI, long-term seizure outcome) coupled with a commitment to share computer algorithms and code to create reproducible results.
Acknowledgments
The authors would like to acknowledge Karla Crocket, Cindy Nelson, Ben Brinkmann, and Dan Crepeau for assistance with data management.
Financial support and sponsorship
This work was supported by funding from the National Institutes of Health (NIH: UH2-NS095495, R01-NS092882, and R01-NS063039). Czech Republic Grant agency (P103/11/0933), European Regional Development Fund – Project FNUSA – ICRC (CZ.1.05/1.1.00/02.0123).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Worrell G, Gotman J. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: clinical studies. Biomark Med. 2011;5:557–566. doi: 10.2217/bmm.11.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs J, Staba R, Asano E, et al. High frequency oscillations (HFOs) in clinical epilepsy. Prog Neurobiol. 2012;98:302–315. doi: 10.1016/j.pneurobio.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3▪.Staba RJ, Stead M, Worrell GA. Electrophysiological biomarkers of epilepsy. Neurotherapeutics. 2014;11:334–346. doi: 10.1007/s13311-014-0259-0. This is a concise, up to date review of electrophysiological biomarkers in epilepsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urrestarazu E, Jirsch JD, LeVan P, et al. High-frequency intracerebral EEG activity (100–500 Hz) following interictal spikes. Epilepsia. 2006;47:1465–1476. doi: 10.1111/j.1528-1167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 5.Najm I, Jehi L, Palmini A, et al. Temporal patterns and mechanisms of epilepsy surgery failure. Epilepsia. 2013;54:772–782. doi: 10.1111/epi.12152. [DOI] [PubMed] [Google Scholar]
- 6▪▪.Bergey GK, Morrell MJ, Mizrahi EM, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84:810–817. doi: 10.1212/WNL.0000000000001280. This is a landmark paper describing the long-term follow-up of patients receiving focal brain stimulation using the NeuroPace responsive stimulation device. Previously, the class I evidence for efficacy was reported, and here the class IV evidence of a durable, improving, efficacy with focal stimulation. Over the course of 7 years, a significant number of patients had extended periods of seizure freedom, with 23% having at least 1 seizure-free period of more than 6 months and 12.9% with at least 1 seizure-free period more than 12 months, but no participants were seizure-free over the entire follow-up. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher RS, Webber WR, Lesser RP, et al. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol. 1992;9:441–448. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- 8.Alarcon G, Binnie CD, Elwes RD, Polkey CE. Power spectrum and intracranial EEG patterns at seizure onset in partial epilepsy. Electroencephalogr Clin Neurophysiol. 1995;94:326–337. doi: 10.1016/0013-4694(94)00286-t. [DOI] [PubMed] [Google Scholar]
- 9.Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80–200 Hz) during seizures: intracellular correlates. J Neurophysiol. 2003;89:841–852. doi: 10.1152/jn.00420.2002. [DOI] [PubMed] [Google Scholar]
- 10.Traub RD, Whittington MA, Buhl EH, et al. A possible role for gap junctions in generation of very fast EEG oscillations preceding the onset of, and perhaps initiating, seizures. Epilepsia. 2001;42:153–170. doi: 10.1046/j.1528-1157.2001.26900.x. [DOI] [PubMed] [Google Scholar]
- 11.Worrell GA, Parish L, Cranstoun SD, et al. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127(Pt 7):1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- 12.Jirsch JD, Urrestarazu E, LeVan P, et al. High-frequency oscillations during human focal seizures. Brain. 2006;129(Pt 6):1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- 13.Bragin A, Engel JJ, Wilson CL, et al. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Bragin A, Engel JJ, Wilson CL, et al. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia. 1999;40:1210–2121. doi: 10.1111/j.1528-1157.1999.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 15.Bragin A, Wilson CL, Staba RJ, et al. Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002;52:407–415. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- 16.Staba RJ, Wilson CL, Bragin A, et al. Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- 17.Worrell GA, Gardner AB, Stead SM, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131(Pt 4):928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs J, Levan P, Chander R, et al. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs J, Levan P, Châtillon CE, et al. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132(Pt 4):1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs J, Zijlmans M, Zelmann R, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zijlmans M, Jacobs J, Zelmann R, et al. High frequency oscillations and seizure frequency in patients with focal epilepsy. Epilepsy Res. 2009;85:287–292. doi: 10.1016/j.eplepsyres.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu JY, Sankar R, Lerner JT, et al. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010;75:1686–1694. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪.van Klink NE, Van’t Klooster MA, Zelmann R, et al. High frequency oscillations in intra-operative electrocorticography before and after epilepsy surgery. Clin Neurophysiol. 2014;125:2212–2219. doi: 10.1016/j.clinph.2014.03.004. Observations that show fast ripples in intra-operative ECoG, compared to ripples, may be a better biomarker for epileptogenicity. Further studies are required to determine the relation between resection of epileptogenic tissue and physiological ripples generated by the sensorimotor cortex. [DOI] [PubMed] [Google Scholar]
- 24.Cook MJ, O’Brien TJ, Berkovic SF, et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol. 2013;12:563–571. doi: 10.1016/S1474-4422(13)70075-9. [DOI] [PubMed] [Google Scholar]
- 25.Brinkmann B. Crowdsourcing reproducible seizure forecasting in human and canine epilepsy. Brain. 2015 doi: 10.1093/brain/aww045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce A, Wulsin D, Blanco JA, et al. Temporal changes of neocortical high frequency oscillations in epilepsy. J Neurophysiol. 2013;110:1167–1179. doi: 10.1152/jn.01009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarado-Rojas C, Valderrama M, Fouad-Ahmed A, et al. Slow modulations of high-frequency activity (40–140-Hz) discriminate preictal changes in human focal epilepsy. Sci Rep. 2014;4:4545–4543. doi: 10.1038/srep04545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staba RJ, Bragin A. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: underlying mechanisms. Biomark Med. 2011;5:545–556. doi: 10.2217/bmm.11.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Worrell GA, Jerbi K, Kobayashi K, et al. Recording and analysis techniques for high-frequency oscillations. Prog Neurobiol. 2012;98:265–278. doi: 10.1016/j.pneurobio.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray S, Crone NE, Niebur E, et al. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28:11526–11536. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schomburg EW, Anastassiou CA, Buzsáki G, Koch C. The spiking component of oscillatory extracellular potentials in the rat hippocampus. J Neurosci. 2012;32:11798–11811. doi: 10.1523/JNEUROSCI.0656-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldert S, Lemon RN, Kraskov A. Influence of spiking activity on cortical local field potentials. J Physiol. 2013;591(Pt 21):5291–5303. doi: 10.1113/jphysiol.2013.258228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on ‘false’ ripples. Clin Neurophysiol. 2010;121:301–310. doi: 10.1016/j.clinph.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Engel J, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto A, Brinkmann BH, Stead SM, et al. Pathological and physiological high frequency oscillations in focal human epilepsy. J Neurophysiol. 2013;110:1958–1964. doi: 10.1152/jn.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Wang IZ, Bulacio JC, et al. Ripple classification helps to localize the seizure-onset zone in neocortical epilepsy. Epilepsia. 2013;54:370–376. doi: 10.1111/j.1528-1167.2012.03721.x. [DOI] [PubMed] [Google Scholar]
- 37.Buzsaki G, Horvath Z, Urioste R, et al. High-frequency network oscillaiton in hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 38.Buzsáki G, Silva FL. High frequency oscillations in the intact brain. Prog Neurobiol. 2012;98:241–249. doi: 10.1016/j.pneurobio.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buzsaki G. Rhythms of the brain. Oxford University Press; 2006. [Google Scholar]
- 40.Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121(Pt 12):2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- 41.Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–295. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- 42.Kucewicz MT, Worrell GA, Gotman J. Pathologic brain network activity: memory impairment in epilepsy. Neurology. 2013;81:12–13. doi: 10.1212/WNL.0b013e318297ef3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schevon CA, Trevelyan AJ, Schroeder CE, et al. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132(Pt 11):3047–3059. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanco JA, Stead M, Krieger A, et al. Data mining neocortical high-frequency oscillations in epilepsy and controls. Brain. 2011;134(Pt 10):2948–2959. doi: 10.1093/brain/awr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haegelen C, Perucca P, Châtillon CE, et al. High-frequency oscillations, extent of surgical resection, and surgical outcome in drug-resistant focal epilepsy. Epilepsia. 2013;54:848–857. doi: 10.1111/epi.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪▪.Burke JF, Ramayya AG, Kahana MJ. Human intracranial high-frequency activity during memory processing: neural oscillations or stochastic volatility? Curr Opin Neurobiol. 2014 doi: 10.1016/j.conb.2014.09.003. A review of recent data supporting the view that HFA during memory processing is more consistent with an asynchronous signal, and can be used as a biomarker of neural activation to functionally map memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibbs J. Letter to the Editor, Fourier’s series. Nature. 1899:59200. [Google Scholar]
- 48▪▪.Kucewicz MT, Cimbalnik J, Matsumoto JY, et al. High frequency oscillations are associated with cognitive processing in human recognition memory. Brain. 2014;137(Pt 8):2231–2244. doi: 10.1093/brain/awu149. This paper explores the spatial and temporal distribution of nHFO in a visual memory task. The study shows that high frequency oscillations are associated with memory processing and generated along distributed cortical and limbic brain regions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49▪.Jacobs J, Vogt C, LeVan P, et al. The identification of distinct high-frequency oscillations during spikes delineates the seizure onset zone better than high-frequency spectral power changes. Clin Neurophysiol. 2015;127:129–142. doi: 10.1016/j.clinph.2015.04.053. The rate of HFOs showed the best performance in identifying SOZ. [DOI] [PubMed] [Google Scholar]
- 50.Gloor P. Contributions of electroencephalography and electrocorticography to the neurosurgical treatment of the epilepsies. Adv Neurol. 1975;8:59–105. [PubMed] [Google Scholar]
- 51.Le Van Quyen M, Khalilov I, Ben-Ari Y. The dark side of high-frequency oscillations in the developing brain. Trends Neurosci. 2006;29:419–427. doi: 10.1016/j.tins.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 52▪▪.Alvarado-Rojas C, Huberfeld G, Baulac M, et al. Different mechanisms of ripple-like oscillations in the human epileptic subiculum. Ann Neurol. 2014;77:281–290. doi: 10.1002/ana.24324. Many HFOs during interictal discharges and during pharmacologically induced preictal discharges preceding ictal-like events had frequencies more than 250 Hz, but also a significant proportion were spectrally similar to physiological ripples (150–250 Hz). Interestingly, preictal ripples were associated with depolarizing synaptic inputs frequently reaching the threshold for bursting in most pyramidal cells, suggesting that ripple oscillations (150–250 Hz) in human epileptic hippocampus are associated with two distinct population activities that rely on different cellular and synaptic mechanisms. Thus, the frequency of activity is not likely to be a reliable feature to distinguish pathological versus physiological HFO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jefferys JG, Menendez de la Prida L, Wendling F, et al. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012;98:250–264. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54▪▪.Menendez de la Prida L, Staba RJ, Dian JA. Conundrums of high-frequency oscillations (80–800 Hz) in the epileptic brain. J Clin Neurophysiol. 2015;32:207–219. doi: 10.1097/WNP.0000000000000150. This invited review article is focused on the underlying complex neuronal events, that are not well understood, underlying pathological HFOs (80–800 Hz) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪.Gulyás AI, Freund TT. Generation of physiological and pathological high frequency oscillations: the role of perisomatic inhibition in sharp-wave ripple and interictal spike generation. Curr Opin Neurobiol. 2015;31:26–32. doi: 10.1016/j.conb.2014.07.020. This article discusses evidence supporting that although physiological ripple oscillation is primarily the result of phasic perisomatic inhibitory currents, pathological high-frequency ripples are population spikes of partially synchronous, massively bursting, uninhibited pyramidal cells. [DOI] [PubMed] [Google Scholar]
- 56.Chrobak JJ, Buzsaki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci. 1996;16:3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Logothetis NK, Kayser C, Oeltermann A. In vivo measurement of cortical impedance spectrum in monkeys: implications for signal propagation. Neuron. 2007;55:809–823. doi: 10.1016/j.neuron.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 58.Le Van Quyen M, Staba R, Bragin A, et al. Large-scale microelectrode recordings of high-frequency gamma oscillations in human cortex during sleep. J Neurosci. 2010;30:7770–7782. doi: 10.1523/JNEUROSCI.5049-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bragin A, Wilson CL, Almajano J, et al. High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- 60.Siegel AM, Cascino GD, Meyer FB, et al. Resective reoperation for failed epilepsy surgery: seizure outcome in 64 patients. Neurology. 2004;63:2298–2302. doi: 10.1212/01.wnl.0000147476.86575.a7. [DOI] [PubMed] [Google Scholar]
- 61.Bell ML, Rao S, So EL, et al. Epilepsy surgery outcomes in temporal lobe epilepsy with a normal MRI. Epilepsia. 2009;50:2053–2060. doi: 10.1111/j.1528-1167.2009.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burkholder DB, Sulc V, Hoffman EM, et al. Interictal scalp electroencephalography and intraoperative electrocorticography in magnetic resonance imaging-negative temporal lobe epilepsy surgery. JAMA Neurol. 2014;71:702–709. doi: 10.1001/jamaneurol.2014.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noe K, Sulc V, Wong-Kisiel L, et al. Long-term outcomes after nonlesional extratemporal lobe epilepsy surgery. JAMA Neurol. 2013;70:1003–1008. doi: 10.1001/jamaneurol.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64▪▪.Jones RT, Barth AM, Ormiston LD, Mody I. Evolution of temporal and spectral dynamics of pathologic high-frequency oscillations (pHFOs) during epileptogenesis. Epilepsia. 2015;56:1879–1889. doi: 10.1111/epi.13218. This paper in a rat model of epileptogenesis demonstrates that hippocampal pHFOs exhibit a dynamic evolution during the epileptogenic period following status epilepticus, consistent with their role in transitioning to the chronic epilepsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engel J. Biomarkers in epilepsy: introduction. Biomark Med. 2011;5:537–544. doi: 10.2217/bmm.11.62. [DOI] [PubMed] [Google Scholar]
- 66▪.Gliske SV, Irwin ZT, Davis KA, et al. Universal automated high frequency oscillation detector for real-time, long term EEG. Clin Neurophysiol. 2015 doi: 10.1016/j.clinph.2015.07.016. (In press). This paper describes a detector for nterictal HFOs in intracranial EEG. The objective was to automatically remove false HFO detections related to artifacts, facilitating clinical use. These methods provide a strategy for real-time HFO detection in continuous EEG with minimal human monitoring of data quality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 68.Ioannidis JP. Why most published research findings are false. PLoS medicine. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Landis SC, Amara SG, Asadullah K, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Donoho DL. An invitation to reproducible computational research. Biostatistics. 2010;11:385–388. doi: 10.1093/biostatistics/kxq028. [DOI] [PubMed] [Google Scholar]
- 71.Modur PN, Zhang S, Vitaz TW. Ictal high-frequency oscillations in neocortical epilepsy: implications for seizure localization and surgical resection. Epilepsia. 2011;52:1792–1801. doi: 10.1111/j.1528-1167.2011.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van ’t Klooster MA, Leijten FS, Huiskamp G, et al. HFO study group. High frequency oscillations in the intraoperative ECoG to guide epilepsy surgery (‘The HFO Trial’): study protocol for a randomized controlled trial. Trials. 2015;16:422. doi: 10.1186/s13063-015-0932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]