Abstract
Importance
Early phase trials with monoclonal antibodies targeting PD-1/PD-L1 have demonstrated durable clinical responses in patients with NSCLC, however, current assays for the prognostic/predictive role of tumor PD-L1 expression are not standardized with respect to either quantity or distribution of expression.
Objective
In this study, we demonstrate PD-L1 protein distribution in NSCLC tumors using both conventional immunohistochemistry (IHC) and quantitative immunofluorescence (QIF), and compare results obtained using two different PD-L1 antibodies.
Design
PD-L1 was measured using two rabbit monoclonal antibodies (E1L3N and SP142) in 49 NSCLC whole tissue sections and a corresponding tissue microarray with the same 49 cases. Mel624 cells stably transfected with PD-L1, as well as Mel624 parental cells and human term placenta were used as controls and for antibody validation. PD-L1 protein expression in tumor and stroma was assessed using chromogenic IHC and the AQUA® method of QIF. Tumor-infiltrating lymphocytes (TILs) were scored in hematoxylin/eosin stained slides using current consensus guidelines. The association between PD-L1 protein expression, TILs, and clinico-pathological features were determined.
Setting
NSCLC resections were all performed at Yale New Haven Hospital.
Participants
NSCLC resection cases from 2011–2012 were collected retrospectively from the Yale Thoracic Oncology Program Tissue Bank in Yale Pathology based on tissue availability.
Main Outcome Measure
PD-L1 expression discordance or heterogeneity using DAB and QIF was the main outcome measure selected prior to performing the study.
Results
Using chromogenic IHC, both antibodies showed fair to poor concordance. QIF showed that PD-L1 expression using both PD-L1 antibodies was heterogeneous. Using QIF, the scores obtained with E1L3N and SP142 for each tumor were significantly different according to nonparametric-paired test (p <0.001). Assessment of 588 serial section fields of view by QIF showed discordant expression at a frequency of 25%. Expression of PD-L1 using both E1L3N and SP142 was correlated with high TILs (p = 0.007 and p = 0.021).
Conclusions
Objective determination of PD-L1 protein levels in NSCLC reveals heterogeneity within tumors and prominent inter-assay variability or discordance. This could be due to different antibody affinities, limited specificity, or distinct target epitopes. Efforts to determine the clinical value of these observations are underway.
Introduction
Programmed Death Ligand-1 (PD-L1) expression is a major immune suppressive mechanism via engagement of the PD-1/PD-L1 axis in non-small cell lung cancer (NSCLC). After antigen recognition and activation of T cells through a TCR/MHC peptide-based interaction, PD-L1 can act as a co-regulatory signal through binding of the inhibitory PD-1 receptor, ultimately leading to inactivation of lymphocytes and other immune cells.[1–3] Under certain circumstances such as viral infections, this mechanism can act as a checkpoint to limit the immune response and avoid tissue damage.[4, 5] This mechanism can also mediate immune tolerance as seen by placental trophoblastic expression thereby preventing autoimmune-based destruction of this new immunologically foreign organ.[6–9] Similarly, tumor cells can evade the immune response through upregulation of PD-L1, with diverse human malignancies showing elevated levels of PD-L1 protein, including non-small cell lung cancer (NSCLC).[10–14]
Blockade of the PD-1/PD-L1 interaction using monoclonal antibodies produces durable clinical responses in patients with diverse advanced tumor types.[15–18] While some studies have shown minimal predictive value for PD-L1 expression[19, 20], others have shown significantly increased response rates in expressers over non-expressers.[15, 16, 21] Summarizing these initial studies, tumor PD-L1 protein expression by any assay with any distribution predicts a three-fold increase in response to therapy as compared to non-expressers.[15–21] Most studies, however, also demonstrate a substantial response rate in tumors lacking PD-L1. Yet, initial drug labels for anti-PD-1/anti-PD-L1 therapies including Pembrolizimab and Nivolumab currently do not require measurement of PD-L1 prior to administration of the drug.
The limited prognostic and predictive role of tumor PD-L1 protein expression is most likely due to the challenging nature of the assay. Contradictory results have been published suggesting that different assay methods yield discordant results.[22–25] Currently, nearly every aspect of defining PD-L1 positivity using IHC is subject to lack of standardization and subjective interpretation. Additionally, most clinical trials have utilized PD-L1 assays that are not yet available to the research community. For instance, some trials measured PD-L1 in the epithelial cells[15, 16, 19] or even just the epithelial cell membrane[21], while others included measurement of PD-L1 in immune cells of the peritumoral stroma [26, 27]. The variability in the assays has been further complicated by the multiplicity of the reagents used to measure PD-L1. Diverse commercially available anti-PD-L1 antibodies have been used without thorough validation resulting in a contradictory literature.[14, 28] This is most often not due to antibodies that do not recognize PD-L1, but rather due to antibodies that recognize PD-L1 and, through cross-reactivity, other ill-defined proteins. Further complicating this situation, companies producing companion diagnostic tests have generated their own proprietary antibodies and testing platforms in preparation for FDA submission without external validation or peer review. As a result, the interpretation of the literature and the data surrounding the predictive value of PD-L1 is challenging.
In addition to challenging assays and associated intellectual property limitations, PD-L1 has also been recognized to have a complex processing and heterogeneous expression. PD-L1 protein is expressed in a wide range of cell types, and is stimulated by variable and incompletely understood mechanisms.[2, 3, 9, 29] PD-L1 may also be expressed by lymphocytes, macrophages or dendritic cells and this may account for the observed stromal localization of expression.[26, 27, 30] It is also possible that PD-L1 could be detected in macrophages after its ingestion of the cell on which it was expressed.
In light of the confusing and often contradictory literature on the expression of PD-L1, we have begun a systematic effort to better understand its expression in lung cancer. Here, we use two carefully validated PD-L1 antibodies (E1L3N and SP142) to assess both reproducibility/concordance and heterogeneity of PD-L1 protein expression using both quantitative and conventional methods.
Materials and Methods
Patient cohorts and control preparations
Retrospectively collected formalin fixed, paraffin embedded (FFPE) whole tissue sections (WTS) from 49 NSCLC cases from the Yale Thoracic Oncology Program Tissue Bank were obtained from Yale Pathology archives. These specimens represented only resections and were processed in the routine manner in the Yale Surgical Pathology suite. These patients were not treated with PD-L1 axis therapies, but rather selected as representative lung cancer cases for measurement of expression using multiple antibodies and detection systems for definition of heterogeneity. Cases were also represented in a tissue microarray (TMA) termed YTMA246. Clinico-pathologic information from patients was collected from the clinical records and pathology reports. WTS and TMA preparations were serially cut from tissue blocks, as described. A control TMA termed YTMA245 containing positive and negative control specimens was constructed for reagents titration, PD-L1 assay validation, and reproducibility assessment, as described.[14] All cases obtained had signed consent for tissue use under approved human investigation committee protocols 9503008219 and/or 1010007459.
PD-L1 antibodies
PD-L1 expression in FFPE WTS and TMA was performed using both chromogenic IHC and automated quantitative immunofluorescence (QIF) with two commercially available, validated PD-L1 antibodies: E1L3N, a monoclonal rabbit antibody from Cell Signaling Technology (CST) (cat # 13684S) and SP142, a monoclonal rabbit antibody from Spring Bioscience (cat # MM4420). Each antibody was validated for immunohistochemistry analysis by proving expression in the syncytial trophoblast layer of the placenta, but not in the stroma, and by showing expression in PD-L1 transfected Mel624 cells but not the parental Mel624 lines (see eFigure 1 in the Supplement).
IHC/immunofluorescence
WTS and TMA slides were deparaffinized then subjected to antigen retrieval. For E1L3N, the antigen retrieval solution was sodium citrate buffer (Sigma-Aldrich, St Louis, Mo, USA) with 1 M citric acid (pH = 6.0) and boiling for 20 min at 97°C in a pressure-boiling container (PT module, Lab Vision). SP142 antigen retrieval was performed as recommended in the data sheet using tris-EDTA buffer (Sigma-Aldrich, St Louis, MO, USA) with 1 M sodium hydroxide (pH = 8.0) and boiling for 10 min at 97°C in a pressure-boiling container (PT module, Lab Vision). Slides were then incubated with methanol and hydrogen peroxide (30%) for 30 min at room temperature followed by BSA blockade for 30 min at room temperature.
For IHC, slides were incubated overnight at 4°C with a solution containing the respective primary PD-L1 antibody (E1L3N 1:1600 dilution and SP142 1:500 dilution). Sections were placed for 1h at room temperature with rabbit EnVision amplification reagent (K4003, Dako), followed by incubation for 5 min at room temperature with diaminobenzidine (DAB) (K3468, Dako) prepared 1:50 in DAB substrate buffer. Slides were then counterstained for 7 min at room temperature with Hematoxylin (Automation Hematoxylin Histological Staining Reagent, Dako) and dehydrated for 1 min in graded ethanol washes (70%, 85%, 95%, 100%, and 100%) followed by 5 min in Xylene. Cytoseal (8310-4, Richard-Allan Scientific, Kalamazoo, MI, USA) was used as mounting media. Control slides were run for reproducibility alongside each experimental slide staining run.
For QIF, slides were incubated overnight at 4°C with a solution containing the same primary PD-L1 antibody concentrations and mouse monoclonal anti-human pancytokeratin antibody at 1:100 dilution (clone AE1/AE3, M3515; Dako Corp., Carpinteria, CA, USA). Sections were incubated for 1h at room temperature with Alexa 546-conjugated goat anti-mouse secondary antibody (A21089; Invitrogen Molecular Probes, Eugene, OR, USA) diluted 1:100 in rabbit EnVision amplification reagent (K4003, Dako). Cyanine 5 (Cy5) directly conjugated to tyramide (FP1117; Perkin-Elmer) at a 1:50 dilution was used for target antibody detection. Prolong gold mounting medium (ProLong Gold, P36931; Life Technologies, Eugene, OR, USA) with 4’,6-diamidino-2-phenylindole (DAPI) was used to stain nuclei. Control slides were run alongside each slide staining experiment.
Determination PD-L1 positivity by IHC
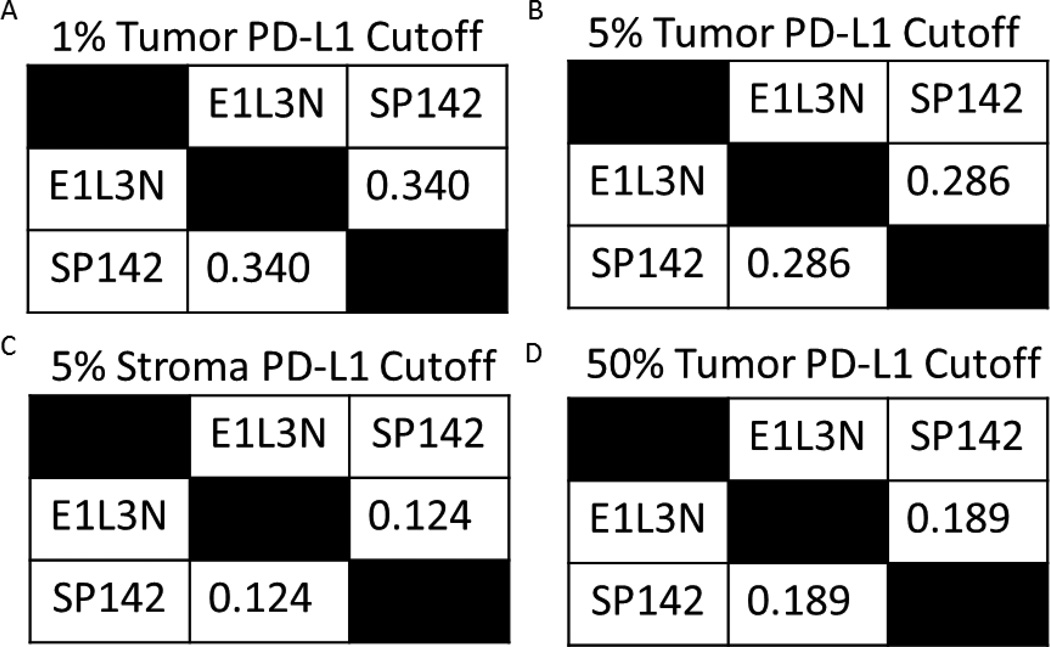
The scoring of WTS slides for PD-L1 positivity with each PD-L1 antibody (E1L3N and SP142) using chromogenic IHC was performed by a pathologist (KAS) using the various cutoffs used in clinical trials.[15–18, 21, 26, 27] PD-L1 positivity was defined by the presence of membranous and cytoplasmic staining in the tumor cells using different cutoffs (1%, 5%, and 50%) and/or in the stroma using 5% as threshold. Cases that could not be appropriately evaluated for technical reasons (e.g., folded tissue, low tumor area, etc) were designated as not evaluable.
Automated quantitative fluorescence
QIF enables objective and sensitive measurement of targets within user-defined tissue compartments. QIF measurements were performed using the AQUA® method, as described.[31] Briefly, the QIF score of PD-L1 signal for each antibody in the tumor and in the stroma was calculated by dividing the target PD-L1 pixel intensities in the area of the tumor and stroma compartment defined by the cytokeratin/DAPI positivity. Stroma (TIL) was defined as cells that have DAPI stain but lack CK positivity. Scores were normalized to the exposure time and bit depth at which the images were captured, allowing scores collected at different exposure times to be comparable. For each WTS, between 5 to 24 fields of view (FOV) representative of the tumor were selected based on amount of viable tissue available such that the two PD-L1 antibodies were examined in serial section for each FOV. Each FOV was 0.7 mm by 0.7 mm in size. All FOVs were visually evaluated and FOVs were systematically excluded when there were staining artifacts or presence of less than 2% tumor area.
Evaluation of tumor-infiltrating lymphocytes
The scoring of TILs was performed in hematoxylin & eosin stained WTS independently by two pathologists (DCH and VP) using a recently reported system for standardized evaluation of tumor-infiltrating lymphocytes in breast cancer.[32] Cases that could not be appropriately evaluated for technical reasons (e.g., folded tissue, low tumor area, etc) were designated as not evaluable. Spots with discordance in TIL scoring between pathologists were reviewed jointly and a single consensus score was established.
Statistical analysis
For each antibody, the PD L1 QIF scores were compared between groups divided by clinical and pathologic characteristics using a two-sided t test with P=0.05 considered as statistically significant. The concordance of PD-L1 positivity using chromogenic staining with different PD-L1 antibodies and various cutoffs in tumor (1%, 5%, and 50%) and stroma (5%) were evaluated using weighted Cohen’s kappa coefficient. Coefficients of variance were calculated for each individual case for both E1L3N and SP142. Total, mean, and maximum values of PL-L1 scores for each tissue sample were compared between the two antibodies based on: (1) Pearson and Spearman correlation coefficients, and (2) nonparametric paired tests including sign test and Wilcoxon signed-rank test. PD-L1 QIF scores for each antibody and TILs were compared using a two-sided t test with P=0.05 considered as significant. Statistical analyses were performed using GraphPad Prism (version 6.03, 2014) and SAS (version 9.4).
Results
Assessment of PD-L1 expression
PD-L1 protein expression with two antibodies using chromogenic IHC (DAB) and quantitative immunofluorescence (QIF) was heterogeneous. Representative cases are shown from different parts of the same tumor (WTS shown in H&E), with both antibodies using DAB demonstrating positive staining in some regions of the tumor but negative in other regions (Figure 1). Distribution of expression was noted, but could not be definitively or reproducibly defined as either leading edge or non-leading edge expression but was frequently present near stromal tumor interfaces (see eFigure2 in Supplemental). Coefficients of variation between FOVs for individual cases ranged from 6.75% to 75.24% for E1L3N, and from 12.17% to 109.61% for SP142 using QIF (see eTable 1 in the Supplement).
Figure 1. PD-L1 protein heterogeneity using DAB.
Intra-tumor PD-L1 protein heterogeneity with DAB using E1L3N and SP142. Bars in DAB panels represent 100 µm.
Associations of PD-L1 protein using QIF with clinico-pathological characteristics and TILs
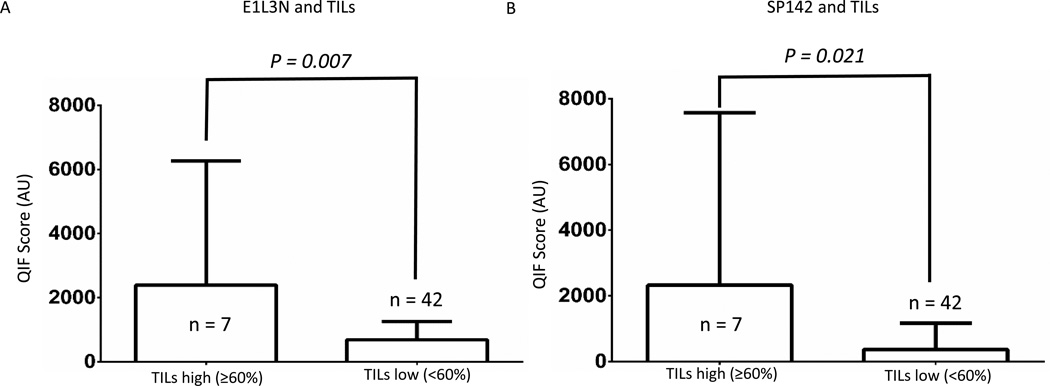
The majority of patients were female, smokers with early stage (stage I) lymph node negative lung adenocarcinomas (Table 1). PD-L1 protein QIF scores separated by clinico-pathological features did not correlate with gender, age, smoking status, histological subtype, stage, or primary tumor size with either of the antibodies (Table 1). SP142 had significantly higher QIF scores in lymph node positive patients than lymph node negative patients (p = 0.03) (Table 1). While E1L3N did not have significantly higher QIF scores in lymph node positive compared to negative patients, it trended in that direction (p = 0.058) (Table 1). The majority of tumors had low TILs (defined as < 60%; n = 42) (Figure 2). E1L3N and SP142 had significantly higher QIF scores in tumors with high TILs than low TILs (p = 0.007 and p = 0.021, respectively).
Table 1.
Clinico-pathological characteristics of NSCLC cohort.
| Parameter | Number of Patients |
E1L3N QIF mean +/− standard deviation, AU |
P Value |
SP142 QIF mean +/− standard deviation, AU |
P Value |
|---|---|---|---|---|---|
| All Patients | 49 (100%) | ||||
| Age (Years) | 0.36 | 0.14 | |||
| < 70 | 24 (49%) | 719.2 +/− 131.6 | 194.0 +/− 45.7 | ||
| ≥ 70 | 25 (51%) | 1140.0 +/− 425.3 | 1083.0 +/− 583.3 | ||
| Gender | 0.29 | 0.28 | |||
| Male | 19 (38.8%) | 627.2 +/− 128.5 | 911.8 +/− 489.9 | ||
| Female | 30 (61.2%) | 1128.0 +/− 358.3 | 230.1 +/− 46.9 | ||
| Smoking Status | 0.93 | 0.42 | |||
| Smoker | 43 (87.8%) | 686.6 +/− 84.5 | 275.4 +/− 49.1 | ||
| Never | 4 (8.2%) | 662.4 +/− 212.6 | 141.7 +/− 73.4 | ||
| Unknown | 2 (4%) | ||||
| Histology | 0.85 | 0.57 | |||
| Adenocarcinoma | 36 (73.5%) | 894.0 +/− 299.9 | 665.5 +/− 390.0 | ||
| Squamous | 10 (20.4%) | 1002.0 +/− 202.2 | 235.7 +/− 108.0 | ||
| Other | 3 (6.1%) | ||||
| Stage | 0.3 | 0.21 | |||
| I | 32 (65.3%) | 760.4 +/− 119.4 | 380.3 +/− 154.5 | ||
| II – IV | 17 (34.7%) | 1261.0 +/− 616.4 | 1199.0 +/− 872.1 | ||
| Tumor Size | 0.41 | 0.48 | |||
| < 3 cm | 29 (59.2%) | 764.1 +/− 139.7 | 450.7 +/− 189.8 | ||
| ≥ 3 cm | 20 (40.8%) | 1142.0 +/− 476.4 | 889.0 +/− 636.8 | ||
| Lymph Node Status | 0.06 | 0.03 | |||
| Negative | 40 (81.6%) | 731.6 +/− 98.4 | 339.2 +/− 128.1 | ||
| Positive | 9 (18.4%) | 1833.0 +/− 1156.0 | 2018.0 +/− 1531.0 |
Figure 2. PD-L1 protein heterogeneity using QIF.
Intra-tumor PD-L1 protein heterogeneity with QIF in different cases using E1L3N (A) and SP142 (B). H&E with heat maps using QIF of representative cases for each antibody showing PD-L1 protein intra-assay heterogeneity were generated, with intensity of QIF scores ranging from gray (low) to high (red). Black squares represent FOV in which QIF scores were not calculated (lack of tumor, poor quality, etc.). Representative positive and negative FOV from each heat map case are shown using IF. PD-L1 protein represented in the red channel, DAPI (nuclear) in the blue channel, and cytokeratin (tumor) in the green channel.
PD-L1 comparison using different PD-L1 antibodies and IHC
Cohen’s Kappa coefficients were calculated between the antibodies at cutoffs of 1%, 5%, and 50% in tumor and 5% in stroma, which have all been previously defined as positive cutoffs used in clinical trials. Kappa concordance between antibodies was low, irrespective of the cutoff utilized (see eTable 2 in the Supplement).
PD-L1 comparison using different PD-L1 antibodies and QIF
PD-L1 QIF scores were obtained from 5-24 FOV for each WTS, with nearly every case having at least 10 FOV. Distribution of QIF shown for each of the 49 different cases is shown. The majority of cases showed a wide variation in QIF scores and differences between PD-L1 protein expression within the same case. When QIF scores for 588 FOVs were compared using E1L3N vs their serial sections using SP142, we found 26.6% discordance, including 8.6% positive by SP142 and negative by E1L3N and 18.8% positive by E1L3N and negative by SP142. These FOVs included both epithelial and stroma FOVs.
The total, mean, and maximum QIF scores measured in the tumor for each case and QIF scores from the corresponding TMA were compared between the PD-L1 antibodies (see eFigure3 in the Supplement). The Pearson correlation coefficients were calculated to be 0.81 (p<0.001), 0.91 (p<0.001), and 0.83 (p<0.001) for the total, mean, and maximum scores, respectively, between the antibodies E1L3N and SP142 but is driven by few high values. The Spearman Rank correlation coefficients, however, were estimated to be 0.21 (p<0.001), 0.14 (p=0.344), and 0.13 (p=−.367) for total, mean, and maximum scores, respectively. This indicates that the significance in the Pearson correlation coefficients was mainly driven by outliers (see eFigure3 in the Supplement). The majority of the measurements from the two antibodies were not significantly correlated according to the Spearman correlation as a rank-based metric. For paired means, medians, and maximums from the two antibodies, both the sign test and the Wilcoxon signed-rank test led to p-values less than 0.001 for the total, mean and maximum scores, indicating significant discrepancy between measurements from the two antibodies. QIF scores were also measured in the stroma, and compared to scores measured in the tumor for each antibody. QIF scores between the stroma and tumor showed a high association for each PD-L1 antibody (data not shown).
The QIF score of each TMA case was compared to the corresponding mean WTS QIF score for the same case (see eFigure4 in the Supplement). The Pearson correlation coefficients were calculated to be 0.75 (p<0.001) for E1L3N and 0.98 (p<0.001) for SP142 but is driven by few high values. The Spearman Rank correlation coefficients, however, were estimated to be 0.20 (p=0.17) for E1L3N and 0.52 (p<0.001) for SP142, respectively. This indicates that the significance in the Pearson correlation coefficients was mainly driven by outliers.
Discussion
Recent studies suggest that determination of PD-L1 status in tumors may help predict response to novel anti-PD-1/anti-PD-LI monoclonal antibody therapy.[15–21, 33] However, each therapeutic study used its own companion diagnostic methods and antibodies without reference to a common standard. Our results here suggest that PD-L1 protein expression is heterogeneous and that different antibodies assays may yield discordant results. As shown in figure 4, two different antibodies showed that over 25% of patients that were positive by one antibody were negative (below the threshold) by the other. This is concerning since PD-L1 antibodies and platforms utilized by pharmaceutical companies for their clinical trials are proprietary and thus there is no opportunity for comparison between methods or reagents. Recently Ventana made their companion diagnostic antibody (SP142) available and it is tested here, but shown not to be concordant with another validated PD-L1 antibody not currently used in any companion diagnostic test.
Figure 4. FOV comparison of two PD-L1 antibodies using QIF.
A. QIF PD-L1 scores for E1L3N and SP142 (A) sorted by E1L3N mean score for individual cases. B. Comparison of identical FOV for all cases using both PD-L1 antibodies. Mean score represented by black bar. Dotted red line represents visual detection threshold for both antibodies. Scores in arbitrary units (AU).
PD-L1 protein expression has been noted to be heterogeneous, though it has not been demonstrated in a quantitative, validated assay.[34] While this heterogeneity is difficult to objectivity demonstrate using traditional IHC methods, QIF allows for more objective measurement. The inherent heterogeneity of PD-L1 may partly explain the contradictory role of PD-L1 as a predictive biomarker to anti-PD-1/anti-PD-L1 antibodies seen this far in clinical trials.[1, 15–21, 26, 27] For instance, some tumors deemed PD-L1 “negative” may be negative at the biopsied site but may be positive at another location. This phenomenon may also partly explain so-called “mixed” responses seen between different tumor sites in clinical trials. Despite the PD-L1 heterogeneity, it is notable that PD-L1 expression, as detected by either antibody is significantly correlated with TILs using QIF.
Inherent differences between validated PD-L1 antibodies have also not been reported. Many of the commercially available PD-L1 antibodies have not been thoroughly validated, leading to conflicting results regarding PD-L1 expression and correlation to overall survival and the presence of TILs.[14, 35–38] Here we compared two different validated PD-L1 antibodies: E1L3N and SP142. Using traditional IHC methods (DAB), we show that concordance between two rigorously validated antibodies is fair to poor. Comparison of both antibodies using QIF demonstrated that the two antibodies have low correlation and are statistically different than each other in identical FOVs in the same cases. While both E1L3N and SP142 reportedly bind to the intracellular domain of PD-L1, the difference between the two antibodies raises concerns and suggests antibody validation data should be shown in future clinical trial reports. The assay performance data has been largely absent in clinical trial reports to date, even those reporting benefit in IHC selected groups[39].
Our analysis has a number of limitations that could not be addressed in this first quantitative study. One major limitation is that it only includes recent retrospectively collected cases and that mature survival information is therefore not yet available. A second is that we selected only 49 cases for analysis. The low number of cases makes it difficult to draw conclusions in terms of associations with clinico-pathological characteristics and outcome, although the cases are representative of an average population of NSCLC and the study is sufficiently powered for the comparison of two antibodies. A third limitation is the lack of data available for response to anti-PD-1/anti-PD-L1 monoclonal antibodies in this patient population. In the future, we hope to be able to do similar studies on material from patients treated with anti-PD-1 therapy.
One key unaddressed issue is the potential of a quantitative assay (protein, mRNA, etc) to predict response to anti-PD-1/anti-PD-L1 therapies along with the potential differences between PD-L1 binding antibodies. However, access to tissue from treated patients is still challenging since the PD-1 axis therapies have only recently been approved in lung cancer. Future studies measuring PD-L1 protein quantitatively in patients treated with anti-PD-1/anti-PD-L1 therapies may better address the prognostic/predictive value of these biomarkers. Determination of the optimal assay, PD-L1 antibody and the best cut-point for PD-L1 positivity, will require further rigorous studies including tissues with known response to anti-PD-1/anti-PD-L1 therapies.
Supplementary Material
Figure 3. PD-L1 protein correlation with TILs using QIF.
Correlation of two PD-L1 antibodies (A and B) with tumor infiltrating lymphocytes (TILs). QIF scores measured in arbitrary units (AU).
Acknowledgments
This work was supported by funds from the Yale Cancer Center, a Gift from Gilead Sciences and the Breast Cancer Research Foundation. David Rimm is a paid consultant or advisor to Genoptix/Novartis, Applied Cellular Diagnostics, BMS, Amgen, Optrascan, Biocept, Perkin Elmer, and Metamark Genetics. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; decision to submit the manuscript for publication. The authors Joseph McLaughlin and David L. Rimm had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Joseph McLaughlin, Gang Han, Kurt A. Schalper, and David L. Rimm all conducted and are responsible for the data analysis. All information and materials in this manuscript are original.
Footnotes
The other authors have no conflicts of interest to disclose.
References
- 1.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Keir ME, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56(5):739–745. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 5.Nurieva RI, Liu X, Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol Rev. 2009;229(1):88–100. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 10.Ghebeh H, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8(3):190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamanishi J, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108(1):19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Nomi T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 14.Velcheti V, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94(1):107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topalian SL, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonia S, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients with non-small cell lung cancer treated with nivolumab; IASLC 15th World Conference on Lung Cancer; 2013. Abstract MO18.02. [Google Scholar]
- 20.Garon E, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) ASCO. 2014 Abstract #8020. [Google Scholar]
- 21.Taube JM, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13(18 Pt 1):5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 23.Hino R, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 24.Hasan A, et al. Therapeutic targeting of B7-H1 in breast cancer. Expert Opin Ther Targets. 2011;15(10):1211–1225. doi: 10.1517/14728222.2011.613826. [DOI] [PubMed] [Google Scholar]
- 25.Mu CY, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 26.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbst RS, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. ASCO. 2013 Abstract #3000. [Google Scholar]
- 28.Sundar R, et al. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer. 2014;85(2):101–109. doi: 10.1016/j.lungcan.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 30.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100(9):5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8(11):1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 32.Salgado R, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daud AI HO, et al. AACR. San Diego: AACR; 2014. Antitumor activity of the anti-PD1 monoclonal antibody MK-3475 in melanoma (MEL): Correlation of tumor PD-L1 expression with outcome. [Google Scholar]
- 34.Madore J, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2014 doi: 10.1111/pcmr.12340. [DOI] [PubMed] [Google Scholar]
- 35.Fridman WH, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 36.Konishi J, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 37.Schalper KA, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer (NSCLC) JNCI. 2015;107(3) doi: 10.1093/jnci/dju435. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng H, et al. Expression and significance of gp96 and immune-related gene CTLA-4, CD8 in lung cancer tissues. Zhongguo Fei Ai Za Zhi. 2010;13(8):790–794. doi: 10.3779/j.issn.1009-3419.2010.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garon EB, et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N Engl J Med. 2015 doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.