Abstract
Introduction
Paclitaxel and docetaxel were two epoch-making anticancer drugs and have been successfully used in chemotherapy for a variety of cancer types. In 2010, a new taxane, cabazitaxel, was approved by FDA for use in combination with prednisone for the treatment of metastatic hormone-refractory prostate cancer. Albumin-bound paclitaxel (nab™-paclitaxel; abraxane) nanodroplet formulation was another notable invention (FDA approval 2005 for refractory, metastatic, or relapsed breast cancer). Abraxane in combination with gemcitabine for the treatment of pancreatic cancer was approved by FDA in 2013. Accordingly, there have been a huge number of patent applications dealing with taxane anticancer agents in the last five years. Thus, it is a good time to review the progress in this area and find the next wave for new developments.
Area covered
This review article covers the patent literature from 2010 to early 2015 on various aspects of taxane-based chemotherapies and drug developments.
Expert opinion
Three FDA-approved taxane anticancer drugs will continue to expand their therapeutic applications, especially through drug combinations and new formulations. Inspired by the success of abraxane, new nano-formulations are emerging. Highly potent new-generation taxanes will play a key role in the development of efficacious tumor-targeted drug delivery systems.
Keywords: abraxane, cabazitaxel, cancer, chemotherapy, docetaxel, drug combination, formulation paclitaxel, taxane, taxoid
1. Introduction
1.1 Scope of the review and coverage
For the collection of patent literatures and relevant review articles, the authors ran the search using the SciFinder database, first, from 2010 to early 2015. Initially, 379 patents/patent applications, as well as 894 review articles on taxanes and taxoids were identified. The contents were analyzed from titles/abstracts and the literatures were sorted out based on the subject areas, which clearly indicated trends in the clinical and preclinical research. Then, the scope of the review was set on the basis of active research/development and significance, as shown below. Thus, 379 patents/patent applications were funneled down to ca. 200, and 894 reviews to ca. 120 through preferential selections of more comprehensive and newer review articles on the same subject, as well as avoidance of duplication. Those selected review articles guided the further selections of patents/patent applications. There were numbers of patent applications and reviews on drug combinations with taxanes in clinical development, which were examined thoroughly, and those that have solid clinical study records with clear indications are shown in the final list. Naturally, there were even larger numbers of drug combination studies and inventions in the preclinical stage, but only those that have shown the proof of concept for efficacy using in vivo animal models were chosen for the compilation in the final list. Consequently, originally 1,273 combined literatures were funneled down to 164 including references earlier than 2010 added as necessary background information. The authors are confident that those selections and the scope of this review properly reflect the trends and significant developments in the field.
1.2 First-generation taxane anticancer agents
The “taxanes” are a class of anticancer drugs that act by binding to tubulins/microtubules which have a key role in cell division [1, 2]. The binding of taxanes to β-tubulin promotes the assembly of microtubules and simultaneously inhibits disassembly, thereby stabilizing microtubule dynamics [1, 2]. Suppression of microtubule dynamics results in the blockade of cell mitosis, leading to apoptosis [1, 2].
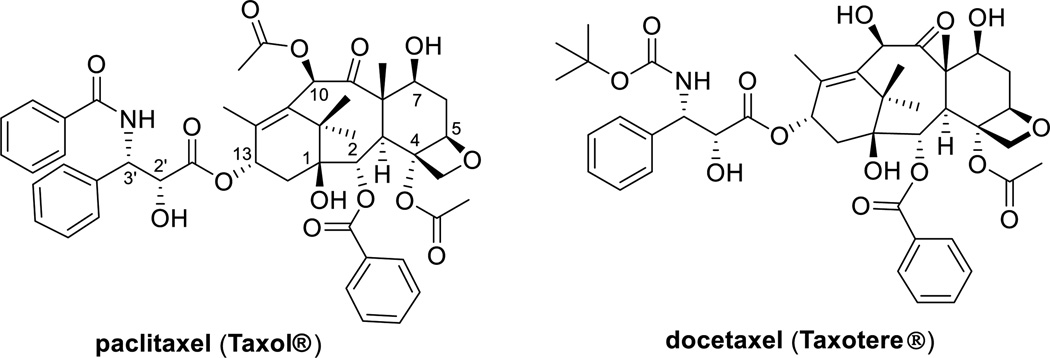
Paclitaxel, a diterpenoid natural product (Figure 1), was discovered by Wall and Wani in 1966, as the primary active component in extracts from the bark of the Pacific yew (Taxus brevifolia Nutt). The chemical structure of paclitaxel was established in 1971 [3]. Injectable paclitaxel (Taxol®) was approved by the U.S. Food and Drug Administration (FDA) for the treatment of refractory ovarian cancer in 1992, refractory or anthracycline-resistant breast cancer in 1994, Kaposi’s sarcoma in 1997, and non-small cell lung cancer in 1998 [4].
Figure 1.
First-generation taxanes
Docetaxel (Taxotere®) (Figure 1), a semi-synthetic analogue of paclitaxel, exhibited excellent efficacy better than paclitaxel in some cases. Docetaxel was approved by the FDA for the treatment of advanced breast cancer in 1996 [5], non-small-cell lung cancer (NSCLC) in 1999 [6], metastatic hormone-refractory prostate cancer (HRPC) in 2004, and head and neck cancer in 2006 [7]. (Note: the terms, hormone-refractory or hormone-resistant prostate cancer (HRPC) and castration-resistant or castrate-resistant prostate cancer (CRPC) are commonly used for the same meaning. In this review, the term “hormone-refractory” prostate cancer (HRPC) is used for consistency.)
It should be noted that the patents for paclitaxel and docetaxel have expired and thus the generic drug market is activated for their manufacturing and clinical use.
Although paclitaxel and docetaxel have been serving as two of the most important drugs for the treatment of various cancers, drug resistance imposes limitations to the efficacy of these drugs. A nemesis for these two drugs is multidrug resistance (MDR) since both drugs have high affinity for multidrug-resistance proteins, in particular the ATP-dependent drug efflux pump P-glycoprotein (Pgp) [1, 8]. Expression of Pgp by cancer cells can be responsible for both constitutive and acquired resistance to taxanes. Overexpression of class III β-tubulin was also identified as the cause of taxane resistance [9]. Accordingly, the drug discovery and development of taxane anticancer agents has been focusing on addressing these limitations of the first-generation taxanes.
Although the primary mechanism of taxanes is to induce microtubule stabilization, mitotic arrest, and apoptotic cell death, but recently it has been shown that taxanes also affect androgen receptor (AR) signaling, i.e., taxanes inhibit ligand-induced AR nuclear translocation and downstream transcriptional activation of AR-targeted genes such as prostate-specific antigen (PSA) [10]. A significant correlation between clinical response to taxane chemotherapy and AR cytoplasmic sequestration was found in the circulating tumor cells (CTC) isolated from hormone-refractory prostate cancer (HRPC) patients. These findings suggest that clinical responses to taxane chemotherapy might be assessed by monitoring AR subcellular localization in the CTCs of HRPC patients [10–12]. Accordingly, the application and diagnosis of taxane chemotherapy for HRPC have been receiving substantial attention.
1.3 Cabazitaxel, most recently FDA-approved taxane anticancer agent
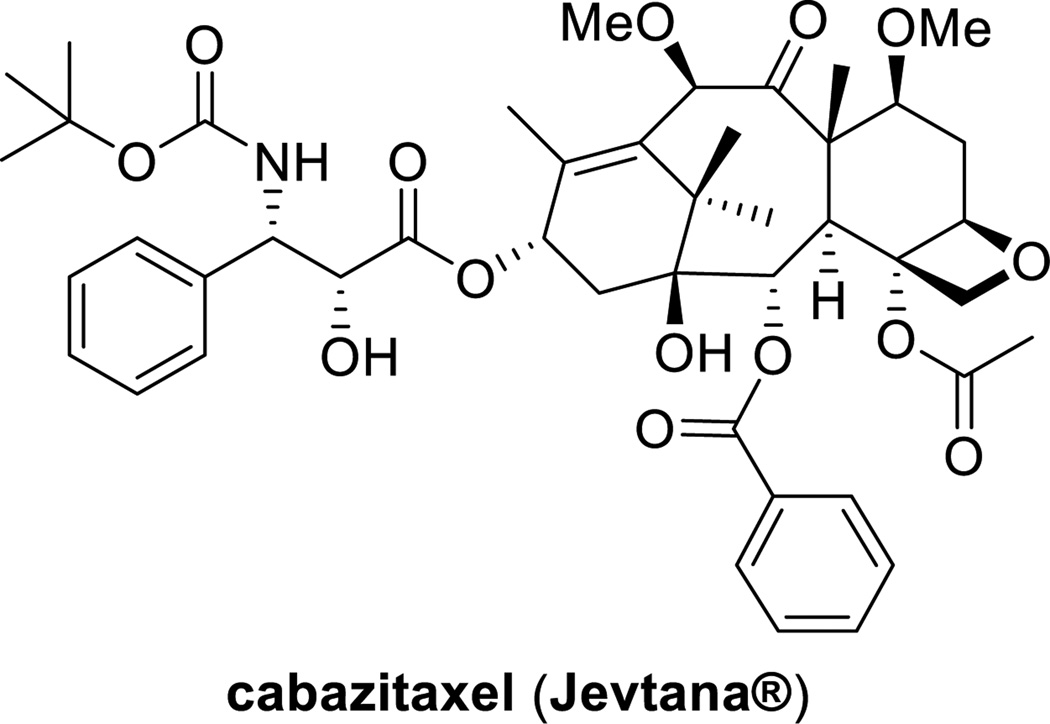
Resistance to taxanes is associated mainly with the increased expression of the multidrug resistance (MDR) 1 gene that encodes P-glycoprotein. Cabazitaxel (Figure 2) was superior to paclitaxel and docetaxel because of its poor affinity to P-glycoprotein due to the presence of methoxy groups at C7 and C10. This improved property makes this drug effective against docetaxel-resistant tumors [13]. The indication of cabazitaxel, in combination with prednisone, is for the treatment of patients with metastatic HRPC previously treated with a docetaxel-prednisone regimen [14]. The extra methoxy groups also provide cabazitaxel with a unique ability to the cross blood-brain barrier (BBB), but the clinical advantages of this property have not been explored yet [15].
Figure 2.
Chemical structure of cabazitaxel
Preclinical study
Cabazitaxel demonstrated significant cytotoxicity in cell lines including P388 (lymphoblastic leukemia), HL60 (promyelocytic leukemia), KB (cervical ademocarcinoma), and Calc18 (breast carcinoma), which are docetaxel-sensitive cell lines [16]. The compound was also active in cancer cell lines with acquired resistance to docetaxel, including P388/DOX, P388/TXT, P388/VCR, HL60/TAX, Calc18/TXT and KBV1 [16]. Resistance factor ratios ranged from 1.8 to 10 for cabazitaxel, whereas comparable values were 4.8–59 for docetaxel. Furthermore, cabazitaxel showed greater cytotoxicity compared to docetaxel against the human colon adenocarcinoma Caco-2 cell line, which exhibits primary resistance to taxanes [17].
Clinical study
A Phase I study escalating cabazitaxel from 10 to 25 mg/m2 demonstrated activity in 2 HRPC patients, whereas mitoxantrone and docetaxel failed [17]. In a Phase III study, the efficacy and safety of cabazitaxel in combination with prednisone was evaluated in a randomized, open-label trial involving 755 patients with HRPC who had previously received a treatment regimen that contained doecetaxel [14, 18, 19]. Interestingly, the study was initiated in the absence of a specific Phase II study in patients with HRPC, but was supported by robust preclinical and Phase I data, as well as by the limited treatment options for the disease at that time [20, 21]. Patients receiving cabazitaxel had statistically significant longer overall survival time compared with those receiving mitoxantorone [18, 19]. The median survival time of patients in the cabazitaxel group was 15.1 months compared with 12.7 months for patients in the mitoxantrone group [18, 19]. The investigator-assessed tumor response rate was 14.4 % for patients in the cabazitaxel group compared with 4.4% for patients in the mitoxantrone group [19].
2. Clinical development of taxane anticancer agents
2.1 New formulations of taxane anticancer agents
All formulations of taxane anticancer drugs approved by the FDA are intravenously administered so far. The bulky and fused-ring skeleton of taxanes with lipophilic substituents result in very poor aqueous solubility. Thus, excipients such as Cremophor EL (polyethylated castor oil: CrEL) and ethanol are used for paclitaxel, polysorbate 80 (Tween 80) and ethanol for docetaxel, polysorbate 80, ethanol, and citric acid for cabazitaxel. CrEL and polysorbate 80 can entrap and solubilize the taxanes in water by forming micelles. Citric acid can stabilize the formulation by adjusting the pH, as exemplified in the cabazitaxel formulation. Adverse effects of excipients, such as hypersensitivity, hemolysis, and cholestasis, have been well recognized and reported [22]. To reduce the side effects, patients must be pretreated with an antihistamine, a corticosteroid and a H2 antagonist, before receiving these medications [23].
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel; ABI-007; abraxane) is a novel CrEL-free formulation of paclitaxel. The human albumin-stabilized paclitaxel particles have an average size of approximately 130 nm, which allows intravenous infusion without the risk of capillary blockage [24]. Abraxane can be reconstituted in normal saline at concentrations of 2–10 mg/mL compared with 0.3–1.2 mg/mL for CrEL-paclitaxel; therefore, the volume and infusion time are reduced [25, 26]. In addition, abraxane can be prepared in standard plastic intravenous infusion bags since it is not associated with the risk of leaking plasticizers from the infusion bags or tubing (CrEL-paclitaxel has this risk) [27]. Abraxane was approved by the FDA in 2005 for the treatment of refractory, metastatic, or relapsed breast cancer. Abraxane can be administered over a short period of time (~30 min.), is much better tolerated than CrEL-paclitaxel, and causes much less side effects [22]. Recently, abraxane was also examined for its efficacy against pediatric solid tumors [28], bladder cancer [29], pancreatic cancer [29], hepatocellular carcinoma [30], melanoma [31], and other proliferative diseases [32, 33]. Abraxane in combination with gemcitabine for the treatment of pancreatic cancer was approved by the FDA in 2013 [34].
“One-vial-Taxotere” formulation was approved by the FDA in 2010 [35], which eliminated the need for a tedious initial dilution procedure that needed to be performed by a physician before administration. A stable formulation for taxanes with reduced amounts of excipient and ethanol was also developed by adding alkyl esters of citric acid to the “one-vial” formulation [36]. A one-pot cabazitaxel formulation was also developed by employing an enclosed container and CO2 gas instead of citric acid [37].
Liposomal formulations for taxanes have been extensively investigated and developed in the last decade [38]. For example, a liposomal-paclitaxel, “Lipusu” [39], has been successful in China [40], and a liposome-entrapped paclitaxel (LEP-ETU), as well as a paclitaxel-loaded cationic liposome (EndoTAG-1) are showing promising results in clinical studies [41].
Other formulations that have been developed in the last 5 years include cyclodextrin-based polymer-taxane conjugates [42], oil-in-water nano-emulsions [43], polyarginine-based nanocarriers [44], cellulose-based nanoparticles [45], nanodispersion [46], a diblock poly(lactic) acid-poly(ethylene)glycol conjugate [47] magnetically responsive nanoparticle carriers [48], silicate-conjugated nanoparticle prodrugs [49], taxane-aggregates of branched homopolymers modified with functional groups [50], orotic acid esters [51], a spray-drying method for oral administration [52], and a combination with cyclosporin and a P-glycoprotein inhibitor for oral formulation [53].
2.2 New taxanes in clinical development
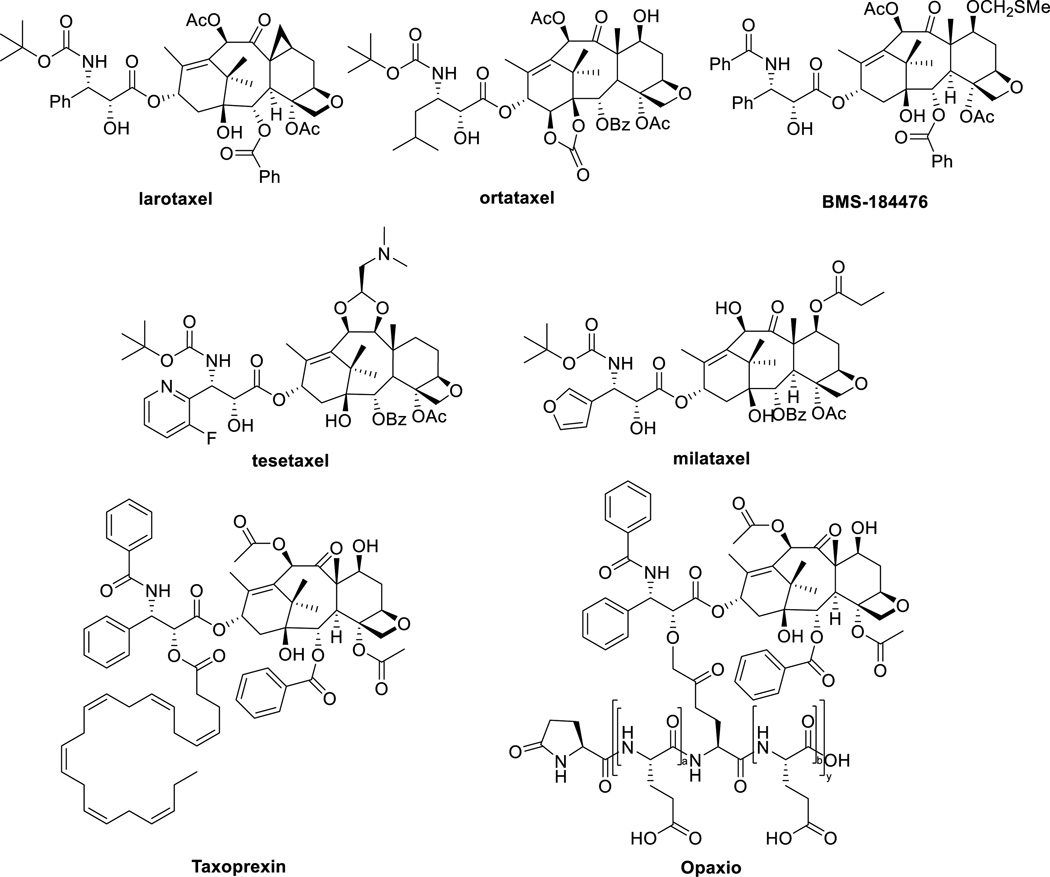
Analogues of paclitaxel which are currently undergoing clinical evaluation include larotaxel, milataxel, ortataxel, and tesetaxel. Larotaxel is under clinical evaluation as a single agent or in combination therapy for urethral bladder cancer, advanced pancreatic cancer, advanced NSCLC and metastatic breast cancer (Figure 3) [54–57]. A Phase III trial of larotaxel with cisplatin for locally advanced/metastatic urothelial tract or bladder cancer suggested that it does not improve outcomes versus cisplatin/gemcitabine [58]. Ortataxel is currently under Phase II evaluation for taxane-refractory NSCLC and metastatic breast cancer [59], as well as recurrent glioblastoma [60]. After failing to demonstrate improved efficacy in a Phase II evaluation in metastatic colorectal cancer, tesetaxel has recently completed Phase I and II trials in solid tumors [61–63]. Milataxel failed to demonstrate efficacy in a Phase II study for advanced previously-treated colorectal cancer, but proved to be effective in a separate study in patients with platinum-refractory NSCLC [64]. BMS-184476 showed evidence of antitumor activity in previously treated NSCLC and was well tolerated at 60 mg/m2 dose [65].
Figure 3.
Chemical structures of paclitaxel analogues in clinical trials
The C2’ position has been extensively exploited for the development of prodrugs of paclitaxel to increase aqueous solubility and improved antitumor activity. Docosahexaenoic acid (DHA)-paclitaxel (“Taxoprexin”) and poly(L-glutamic acid) PG-paclitaxel (“Opaxio”) are two examples of such paclitaxel conjugates, which are currently in clinical trials (Figure 3).
DHA is an omega-3 naturally occurring polyunsaturated fatty acid, which is taken up by tumor cells [66]. Preclinical studies of DHA-paclitaxel demonstrated increased activity relative to paclitaxel [67]. Phase I clinical studies demonstrated a well-defined and manageable side effect. In a Phase II trial, 36 patients with metastatic melanoma were treated, 4 patients had a partial response, and 13 had stabilization of the disease [67, 68]. DHA-paclitaxel advanced to Phase III clinical trials for the treatment of metastatic malignant melanoma [67].
Poly(L-glutamic acid) is a water-soluble biodegradable polymer with carboxylic acid side chains, to which paclitaxel can be conjugated. The resulting conjugate is highly water soluble (>20 mg/kg) and does not need CrEL for formulation. In chemotherapy-naïve patients with advanced NSCLC, PG-paclitaxel was compared to gemcitabine or vinorelbine and showed equivalent efficacy with less myelotoxicity, but more neurotoxicity [69]. When compared to docetaxel in the second-line treatment of NSCLC, PG-paclitaxel produced similar survival rates with reduced alopecia, neutropenia, and febrile neutropenia, but increased neurotoxicity rates [69]. PG-paclitaxel was combined with temozolomide for the treatment of high-grade gliomas and showed promising results [70]. A Phase II trial of PG-paclitaxel with concurrent radiation for newly diagnosed glioblastoma without O6-methylguanine-DNA methyltransferase methylation is ongoing. Neoadjuvant concurrent PG-paclitaxel and radiotherapy combination therapy for esophageal carcinoma was well tolerated and yielded CR in 32 % of patients [71]. Phase III trials with NSCLC, ovarian cancer, and glioblastoma are underway [27].
2.3. New combinations of taxane anticancer agents with other drugs
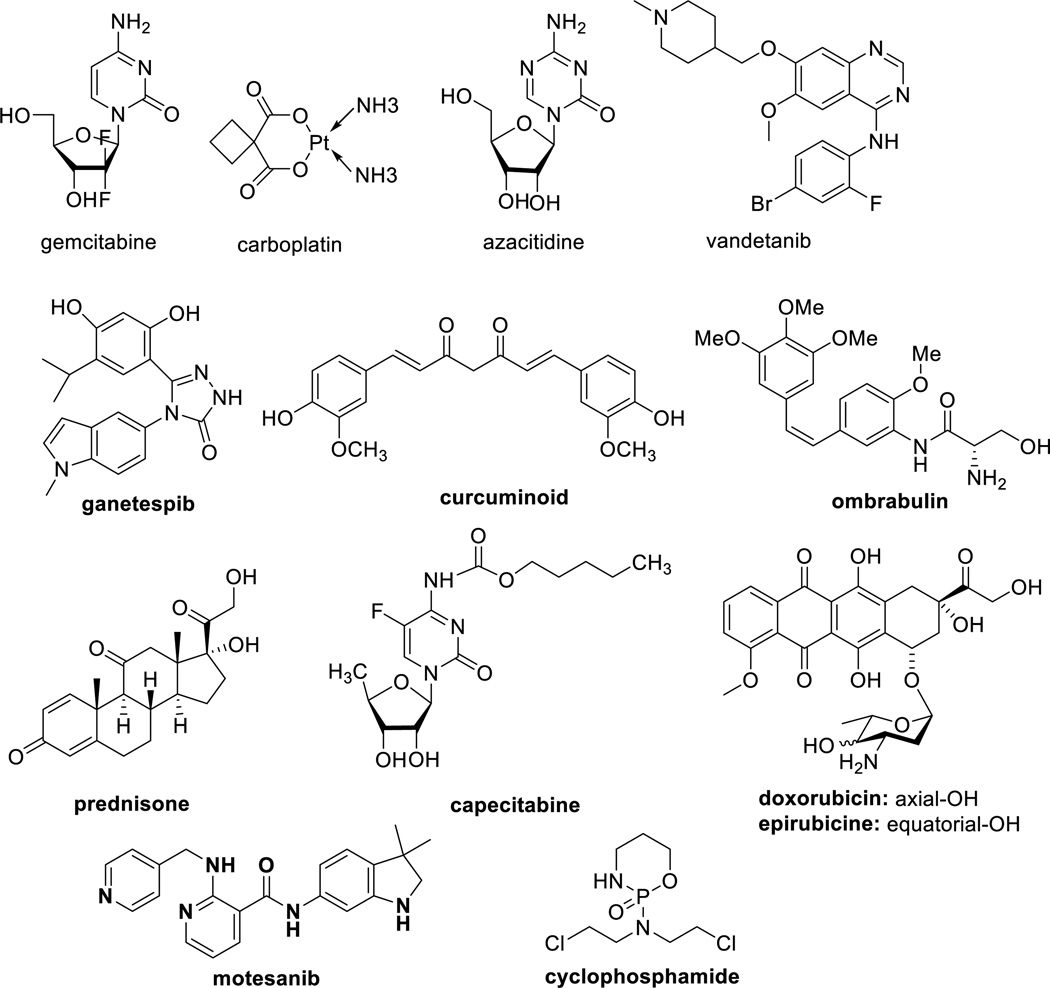
Common paclitaxel toxicities include hypersensitivity reactions (due to CrEL), bone marrow suppression, joint pain, diarrhea, and hair loss, whereas toxicities for docetaxel include edema and shortness of breath [72, 73]. Combination chemotherapy has been applied to minimize these side effects. The rationale for combination chemotherapy is to use drugs that work by different mechanisms, thereby decreasing the probability for cancer cells to develop resistance. When drugs with different modes of actions are combined in the absence of drug-drug interactions, each drug can be used at its optimal dose, without intolerable side effects. The combination chemotherapies of taxanes with various drugs, currently under clinical investigation based on published patents, patent applications and journal articles, are shown in Table 1 and Figure 4.
Table 1.
Drug combinations in clinical development
| Drug combination | Development stage, Indication | References |
|---|---|---|
| abraxane, carboplatin, trastuzumab |
open-label Phase II first-line therapy for HER2-positive metastatic breast cancer |
[79], [80] |
| azacitidine, abraxane | Phase I/II: various solid tumors and leukemia | [33] |
| abraxane, gemcitabine | Phase III: metastatic pancreatic cancer | [81], [82] |
| abraxane, vandetanib | Phase I: pancreatic cancer | [29] |
| docetaxel, ganetespib | Phase II/III: second-line therapy for advanced NSCLC Phase III: Advanced NSCLC |
[76], [77], [83] |
| paclitaxel, ganetespib | Phase III: recurrent, platinium-resistant ovarian, fallopian tube or peritoneal cancer |
[84], [83] |
| curcuminoids, docetaxel | Pilot Phase II: HRPC | [85], [86] |
| anti-clusterin oligonucleotide (custirsen:TV-1011/ OGX-011), docetaxel |
Phase III: second line therapy for advanced or metastatic NSCLC | [87] |
| motesanib, carboplatin/ paclitaxel |
Phase III: nonsquamous NSCLC; Asian subgroup | [78], [88] |
| ombrabulin, docetaxel/ paclitaxel or cisplatin/ carboplatin |
Phase I: advanced solid tumors | [89], [90] |
| prednisone, cabazitaxel | Phase III: metastatic HRPC FDA approval for treatment of metastatic HRPC [14] |
[18], [91] |
| paclitaxel, MetMAb, bevacizumab |
Phase II: metastatic, triple-negative breast cancer | [92] |
| paclitaxel, trebananib | Phase II: advanced recurrent epithelial ovarian or primary peritoneal cancer |
[93], [94] |
| abraxane, capecitabine | Phase II: first-line treatment of metastatic breast cancer | [95], [96] |
| abraxane, trastuzumab, carboplatin |
Phase II: first-line therapy for advanced HER-2 positive breast cancer |
[95] |
| pertuzumab, trastuzumab, docetaxel |
Phase III: first-line treatment for HER2-positive metastatic breast cancer |
[97], [98] |
| paclitaxel, trastuzumab, MYOCET® |
Phase III: HER2-positive metastatic breast cancer | [99], [100] |
| doxorubicin and cyclophosphamide followed by abraxane |
Pilot Phase I: early-stage breast cancer | [95], [101] |
| abraxane, carboplatin, gemcitabine |
Phase II trial: locally advanced bladder cancer | [102], [103] |
| gemcitabine, abraxane | Phase II: breast cancer | [104] |
| gemcitabine, abraxane, bevacizumab |
Phase II: breast cancer | [104] |
trastuzumab, bevacizumab and pertuzumab: therapeutic monoclonal antibodies; vandetanib: VEGFR/EGFR tyrosine kinase inhibitor for thyroid cancer treatment; motesanib: angiokinase inhibitor; ombrabulin: combretastain A-4 derivative; trebananib: peptibody, angiogenesis inhibitor; curcuminoids: curcuminoid extract containing curcumin as main component and demethoxycurcumin and bisdemethoxycurcumin as minor components; AMG-386: an angiopoietin (Ang) 1 and 2 neutralizing peptibody; MetMAb: a humanized monovalent monoclonal antibody against the hepatocyte growth factor receptor (c-Met); MYOCET: a non-pegylated liposomal doxorubicin; NSCLC: non-small cell lung cancer; HRPC: hormone-refractory prostate cancer.
Figure 4.
Drugs under investigation in combination with taxanes
Abraxane is the most common drug to appear in drug combination clinical trials among the taxane-related patent applications for combination therapy filed in 2010–2014. In most cases, the toxicities were well tolerated. Double or triple combinations of drugs with abraxane is in clinical trials as first-line treatment for metastatic breast and pancreatic cancer. The combination therapy of abraxane and gemcitabine for metastatic pancreatic cancer was approved by the FDA in 2013 [34]. The FDA-approved pertuzumab injection (“Perfeta”) in 2012 [74] for use in combination with trastuzumab (“Herceptin”) and docetaxel for the treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for a metastatic disease. Ganetespib, an Hsp 90 inhibitor, combined with docetaxel for the treatment of patients with metastatic NSCLC, who have a progressed disease following one prior chemotherapy regimen, was granted a Fast Track development designation by the FDA [75] and this combination is currently undergoing Phase III trials for advanced NSCLC [76, 77]. Motesanib and carboplatin/paclitaxel combination showed improved anti-tumor activity on nonsquamous NSCLC in the Asian subgroup [78].
2.4 Drug conjugates for tumor-targeted delivery in clinical trials
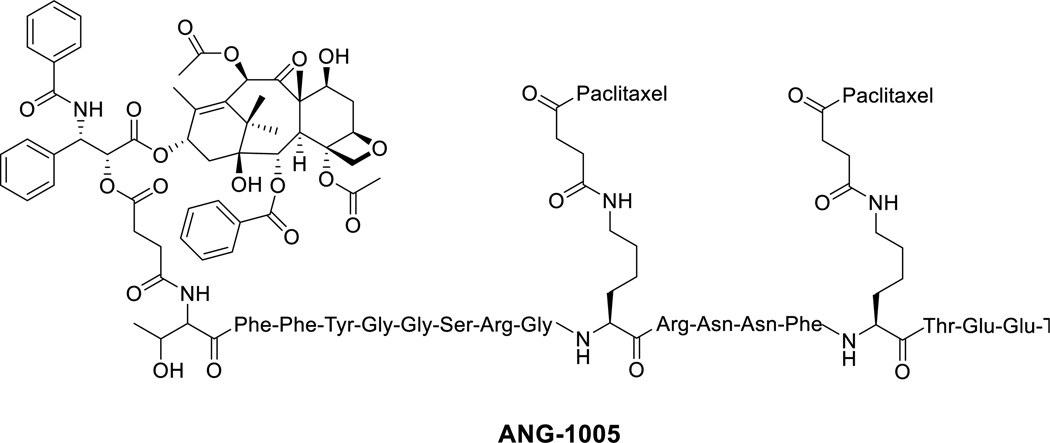
Targeted therapy represents the major hope in modern cancer treatment and a substantial step towards personalized medicine. An explosion in the development of tumor-targeted drugs and drug conjugates has been observed over the past decade, following the clinical success of trastuzumab (Herceptin®) in the treatment of metastatic human epidermal growth factor receptor 2 (HER2)-positive breast cancer [105]. Also, many drug conjugates, including many antibody-drug conjugates (ADCs) [105], as well as some small-molecule drug conjugates (SMDCs) [106], have entered clinical trials in recent years. However, the only taxane conjugate currently in clinical trials is ANG-1005 (Figure 5) [107].
Figure 5.
Chemical structure of ANG-1005
Angiopep-2 (AP-2) has been used as a peptide-based drug delivery system that provides a non-invasive and flexible platform for transporting drugs or biologically active molecules across the BBB into the central nervous system (CNS) [108, 109]. AP-2 consists of a peptide with 19 amino acid residues (TFFYGGSRGKRNNFKTEEY), derived from the Kunitz protease inhibitor (KPI) domain. This peptide binds to a specific receptor, low-density lipoprotein receptor-related protein-1 (LRP1), which is highly expressed on brain microvascular endothelial cells (BMVECs) of the BBB [108, 110]. It was found that some cancer cell lines highly express LRP1, and LRP1 overexpression is closely related to the invasivness of tumors. Therefore, the use of this peptide as a carrier to specific cancer cells is beneficial. Through clinical trials, it has been demonstrated that the drug conjugate of AP-2 with paclitaxel (ANG-1005: paclitaxel/AP-2 = 3) is efficacious in ovarian cancer and in particular, dramatically shrinks metastatic tumors within and outside the brain (e.g., in the lung) [107]. Other clinical trials on advanced brain metastatic cancer and recurrent maliganant glioma are underway [107].
3. Taxanes in preclinical development
3.1 New taxane anticancer agents and their preclinical development
Paclitaxel and docetaxel have made a significant impact on current cancer chemotherapy, but seriously suffer from the lack of tumor specificity and multi-drug resistance (MDR). Cabazitaxel exhibits improved potency against MDR-expressing cells and tumors, but its clinical application is rather limited to prostate cancer so far. Thus, the preclinical development of new taxane anticancer agents has been actively continuing (Figure 6).
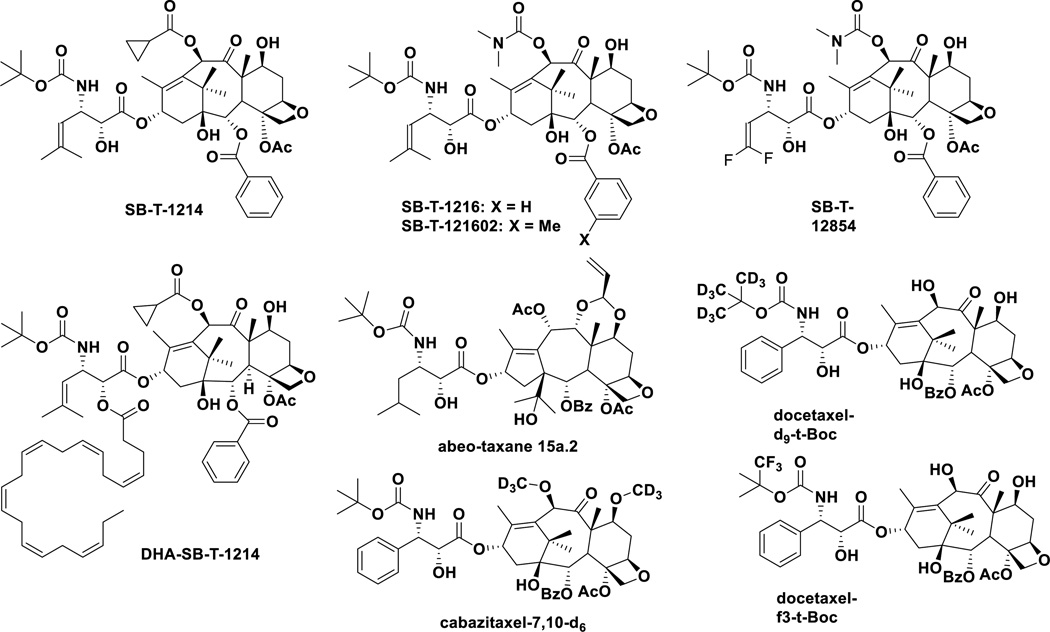
Figure 6.
New taxanes in preclinical development
On the basis of extensive SAR studies of taxoids (“Taxol-like” taxanes), a series of highly potent new-generation taxoids have been developed. Most of these new-generation taxoids exhibited 2–3 orders of magnitude higher potency than those of paclitaxel and docetaxel against drug-resistant cell lines expressing MDR phenotypes [111, 112]. These new-generation taxoids possess built-in Pgp-modulating ability. The 2nd-generation taxoids have modifications at the C10 and C3’ positions, while the 3rd-generation taxoids possess modifications at the C2, C10 and C3’ positions, and either a 2-methyl-1-propenyl or 2-methylpropyl group replaces the phenyl group in paclitaxel, docetaxel and cabazitaxel at the C3’ position.
Recently, it has been shown that the ineffectiveness of standard chemotherapeutic agents could be attributed to the existence of relatively rare, highly drug resistant, quiescent or slowly proliferating tumor-initiating cells, i.e., “cancer stem cells (CSCs)” [113, 114]. These cells heighten the expression of many stem-cell related genes, exhibit similar pluripotency, and are notoriously malignant. CSCs are exclusively endowed with tumor-initiating capacity in the majority of cancer types, and are responsible for sustaining tumor growth, metastasis and relapse. Consequently, it is obviously highly important to discover and develop anticancer agents that can control or eradicate CSCs in the next-generation chemotherapy.
One of the new-generation taxoids, SB-T-1214, exhibited remarkable efficacy against highly drug-resistant (Pgp+) DLD-1 colon tumor xenograft in mice, inducing complete regression in all surviving mice with tumor growth delay >187 days, wherein paclitaxel failed to show any efficacy [111]. SB-T-1214 exhibited impressive activity against colon CSCs from HCT116, HT29 and DLD-1 cell lines using cancer spheroids in 3D cultures [115]. Most importantly, viable cells that survived this treatment regimen significantly lost the ability to form secondary spheroids. Also, it was found that the treatment of these CSCs with SB-T-1214 led to the down-regulation of a number of stem cell-related genes and significant inhibition of genes involved in retaining pluripotency [115]. In addition to SB-T-1214, SB-T-1216, SB-T-121602 and SB-12854 exhibited high potencies against HCT116 CSCs, which are orders of magnitude different from those of commonly used anticancer drugs in clinic. The IC50 values (nM) for these new-generation taxoids in comparison with known drugs are as follows: cisplatin (4,540); doxorubicin (78.0); methotrexate (32.7); paclitaxel (33.8); topotecan (451); SB-T-1214 (0.28); SB-T-1216 (0.83); SB-T-121602 (0.24) and SB-T-12854 (0.14) [116].
SB-T-12854 is one of the series of 3'-difluorovinyl-taxoids, which were designed and proven to block cytochrome C metabolic pathway, especially metabolism by CYP3A4 enzyme [112, 117]. SB-T-12854 suppressed rat lymphoma more effectively than paclitaxel [118].
Following the advance in DHA-paclitaxel (Taxoprexin) (vide supra), new-generation taxoids bearing DHA at the C2’ position have been developed and several of these DHA-taxoids exhibited excellent efficacy against (Pgp+) DLD-1 human colon as well as (Pgp-) A121 human ovarian cancer xenografts in mice, with much reduced systemic toxicity than the corresponding parent taxoids [119]. Among these DHA-taxoids, DHA-SB-T-1214 exhibited a remarkable antitumor effect on DLD-1 human colon, H460 human non-small cell lung, CFPAC-1 and PANC-1 human pancreatic cancer xenografts in mice, wherein paclitaxel and DHA-paclitaxel showed no or only marginal efficacy [57, 120].
Recently, a library of 7,10-modified paclitaxel, cabazitaxel and ortataxel analogues, using the same isoserine C13-side chain as that of SB-T-1214, were reported, and a number of these taxanes showed good to excellent potency against a panel of cancer cell lines [121].
A series of “abeo-taxanes”, bearing taxane skeletons derived from baccatin III through skeletal rearrangement, was developed with modifications at the C7- and C9-hydroxyl groups. These abeo-taxanes exhibited good potencies against various cancer cell lines that are resistant to paclitaxel, vinblastine, and doxorubicin [122]. Based on MTS assays and in vivo efficacy evaluation, abeo-taxoid 15a.2 appears most promising [122, 123]. These abeo-taxanes are also claimed to be effective against neurodegenerative disorders [122].
As a strategy to improve the metabolic stability and obtain new IPs, deuterated analogues of docetaxel and cabazitaxel were developed [124]. Introduction of a trifluoro-tert-butoxycarbonyl group was also examined [124]. These analogues should be able to mitigate the metabolism by CYP3A4 and CYP3A5. Preliminary in vitro assays against a panel of cancer cell lines look promising so far [124].
3.2 Tumor-targeted delivery of taxane anticancer agents in preclinical development
Although paclitaxel, docetaxel and cabazitaxel exhibit impressive efficacy in cancer chemotherapy, their adverse effects cause serious problems in the quality of life of patients. Accordingly, it is logical to develop efficient tumor-targeted drug delivery systems for taxane anticancer agents (Figure 7). The most common and popular approach has been the use of monoclonal antibodies, either naturally occurring [125, 126] or engineered [127, 128], following the recent successes of ADCs. However, in spite of extensive efforts along this line, very few compounds/ conjugates have reached clinical trials and have not gone further to date [129]. A hyaluronic acid – taxane conjugate [130], as well as a hyaluronidase – taxane conjugate [131] have been explored. Poly(glutamyl-glutamate)-taxane conjugates have been developed, which look promising [132, 133]. Deuterated docetaxel prodrugs conjugated to dendrimer-like “multi-arm polymers (ARM)”, 4-ARM-PEG20K-CM-Gly-d9-DOC and 4-ARM-PEG20K-BA-d9-DOC, have been developed (DOC = docetaxel) [134].
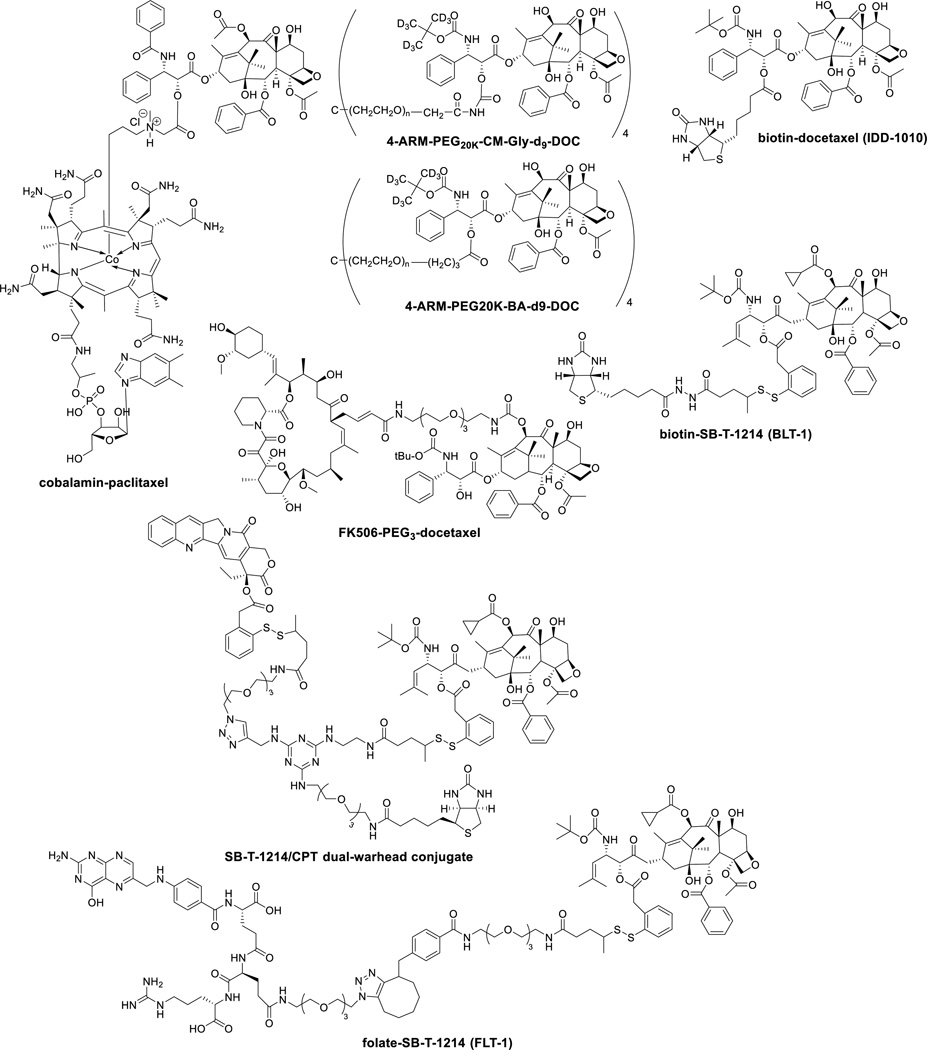
Figure 7.
Chemical structures of new taxane conjugates in preclinical studies
Peripheral sensory neuropathy is the most commonly reported neurotoxic side effect of paclitaxel and it limits treatment when given alone or in combination with other neurotoxic antineoplastic agents such as cisplatin. To mitigate paclitaxel neurotoxicity various neuroprotective agents have been investigated in animal and clinical studies [135]. A docetaxel conjugate with FK506, which is an immunosuppressive and neuroprotective agent, was developed. The conjugate was highly cytotoxic (IC50 1 nM) against SKOV3 ovarian, PC3 prostate, and MCF7 breast cancer cell lines, but did not affect neurite growth [136]. Cholesterol was also conjugated to taxanes [137].
Vitamins are required by all living cells for survival, but cancer cells particularly need certain vitamins and nutrients to sustain their rapid growth and proliferation, e.g., vitamin B12, folic acid (vitamin B9), biotin (vitamin B7) and riboflavin are essential for cell division. Therefore, the vitamin receptors are overexpressed on the cancer cell surface for the uptake of necessary vitamins. Accordingly, those vitamin receptors serve as highly useful targets for tumor-targeted drug delivery as well as biomarkers for identification and imaging of cancer cells. Folic acid is essential for cell division involving DNA synthesis and repair, while biotin is also essential for cell division, cell growth, fatty acid production, metabolism of fats and amino acids, and plays a role in energy production. All living cells require vitamin B12 (cobalamin) for survival, but rapidly dividing tissues have an increased demand for cobalamin.
Among those vitamin receptors, the folate receptor has been shown to be an important and relevant target [138, 139]. In fact, several folate-drug conjugates are currently in clinical trials (but not with taxanes), and notably “Vintafolide” (EC-145), a folate-vinblastine conjugate [139], has advanced to Phase III clinical trials. However, biotin receptors had not been studied until it was discovered in 2004 that those receptors were even more overexpressed than the folate and vitamin B12 receptors in a variety of cancer cells [140]. Thus, the biotin receptor has emerged as a new target for tumor-targeted drug delivery.
Biotin-taxoid (SB-T-1214) conjugates with a self-immolative disulfide linker were developed [141–144], including nano-conjugates using single-walled carbon nanotubue (SWNT) [145]. These conjugates exhibited highly efficient cancer cell – selective internalization via receptor-mediated endocytosis and high potency with much reduced toxicity in normal human cells. Also, one of these conjugates, “BLT-1” demonstrated impressive efficacy in vivo against MX-1 human breast cancer xenografts in mice, totally eradicating tumors with 20 mg/Kg/dose, once a week for 4 weeks regimen without any weight loss of mice [146]. A dual-warhead drug conjugate bearing SB-T-1214 and camptothecin, which represents a unique combination therapy through simultaneous tumor-targeted drug delivery [146]. This conjugate achieved two orders of magnitude specificity to cancer cells as compared to normal cells [146].
Another biotin-taxoid (docetaxel) conjugate through direct connection at the C2’ position of docetaxel (IDD-1010) was developed, which exhibited good in vivo efficacy against PC3 prostate cancer xenograft in mice [147]. A paclitaxel-cobalamin conjugate was examined for its efficacy in treating eye diseases, wherein its anti-angiogenic activity was confirmed [148–150]. A folate-taxoid (SB-T-1214) conjugate (FLT-1) was also developed, which exhibited remarkable cancer cell specificity (>1,000) against ID8 ovarian and MX-1 breast cancer cells through folate receptor targeting, as compared to W38 normal cells [151].
3.3 New drug combinations in preclinical development
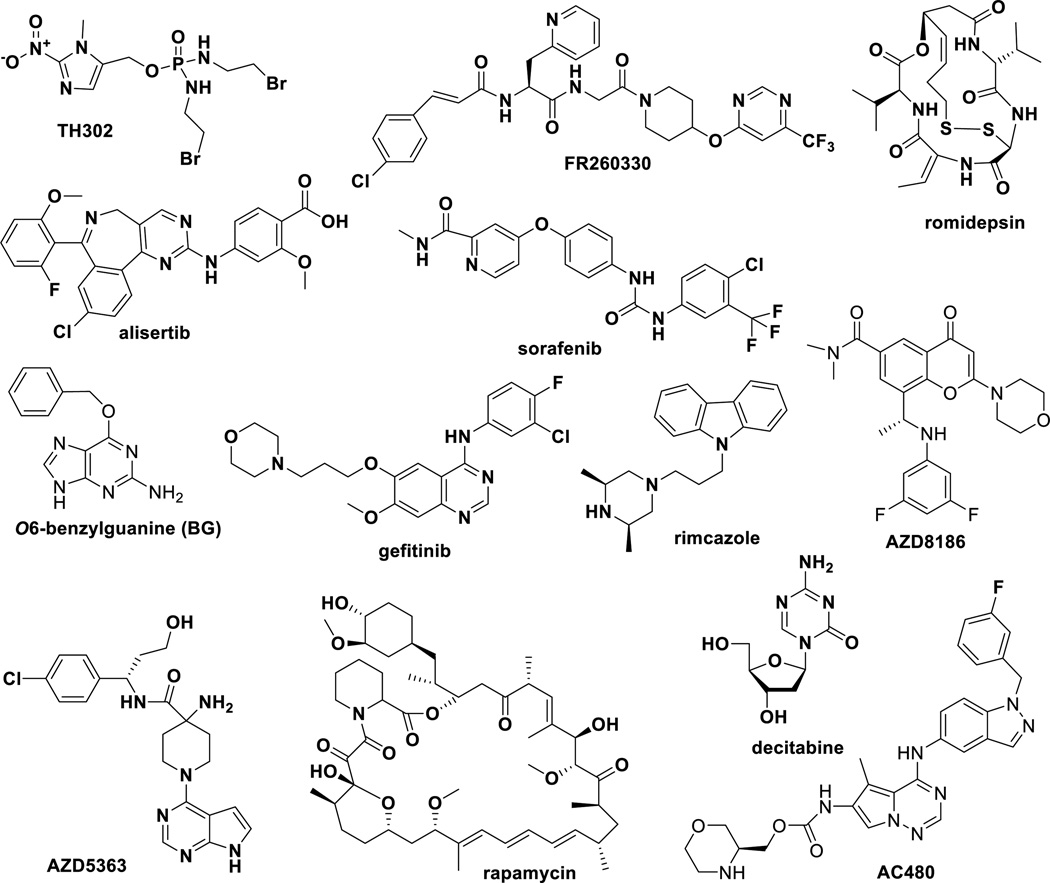
Since combination therapy of taxane anticancer agents has been successful in clinic and drug combination is one of the most translational approach for drug development, preclinical development of new drug combinations is quite active with a large number of patent applications. Selected examples are summarized in Table 2 and Figure 8. These examples have shown proof of concept in animal models. Thus, inventions with only in vitro evaluations are not included. The chemical structures of the drugs used for combination with paclitaxel, docetaxel and abraxane are shown in Figure 5. However, the structures of monoclonal antibodies are not relevant for this table and thus not included. Early indications of these drug combinations cover a variety of cancer types, including metastatic, HER2+, and inflammatory breast cancers; locally advanced or metastatic NSCLC; pancreatic, prostate, colon, ovarian, and other gastric cancers. These combinations have increased antitumor activity, especially on metastatic cancers, and proved to be well tolerated.
Table 2.
New drug combinations in preclinical development
| Drug combination | Proof of concept | References |
|---|---|---|
| paclitaxel, AC480 | xenograft in mice: MX-1 (breast) | [152] |
| abraxane, TH302, gemcitabin | xenograft in mice: Hs766t, Mia-PaCa-2, PANC-1, BxPC-3 (pancreas) |
[153] |
| paclitaxel/docetaxel, FR260330 | xenograft in mice: Calu 6 (lung), NCI-N87 (stomach), MDA-MB- 231 (breast) |
[154] |
| docetaxel, AZD8186 | xenograft in mice: PC3 (prostate), HCC70 (breast) | [155] |
| paclitaxel/docetaxel, O6- benzylguanine (BG) |
xenograft in mice: HCT116 BBR, HCT116 (colon), MDA-MB-468 (breast) |
[156] |
| paclitaxel, MPS-1 kinase inhibitor (cpd A27) |
xenograft in mice: A2780cis (ovary), NCI-H1299 (NSCLC) | [157] |
| paclitaxel, alisertib | xenograft in mice: MDA-MB-231 (breast), NCI-H82, CTG-0166 (SCLC) |
[154] |
| paclitaxel, romidepsin | xenograft in mice and in vivo imaging of inflammatory breast cancer (IBC): SUM 149, Mary-X model of IBC |
[158] |
| docetaxel, AZD5363 | xenograft in mice: BT474c, HCC-1187 (breast) | [155, 159] |
| sorafenib, docetaxel | xenograft in mice: Mia-PaCa-2 (pancreas) | [160] |
| PEGPH20, nab-paclitaxel, gemcitabine |
xenograft in mouse: BxPC-3 PDA (pancreas), MDA-MB- 468/HAS3 (breast) |
[131] |
| siRNA for B7-H3 (shB7), paclitaxel | xenograft mice: MDA-MB-231, MDA-MB-435, MDA-MB-435- shB7-H3 (breast) |
[161] |
| Hsp90 inhibitor, docetaxel | xenograft in mice: NCI-H1975, HT29 (colon), HEL92.1.7 (leukemia), HCC827 (NSCLC) |
[162] |
| decitabine/paclitaxel/ abraxane | xenograft in mice: NSCLC16325/ NSCLC16384 (SPARC- negative) |
[33] |
| abraxane, gefitinib | xenograft in mice: EGFR-expressing BT474 (breast) | [32] |
| abraxane, bevacizumab | xenograft in mice: MDA-MB-231-Luc+, MDA-MB-435-Luc+ (breast) |
[32] |
| abraxne, nab-17AAG | xenograft in mice: H358, 11358 (lung) | [32] |
| abraxane, nab-rapamycin | xenograft in mice: HT29 (colon) | [32] |
| abraxane, ABI-011 | xenograft in mice: PC3 (prostate) | [32] |
| abraxane, ABI-011, bevacizumab | xenograft in mice: HT29 (colon) | [95] |
| paclitaxel, rimcazole | xenograft in mice: MDA-MB-231 (breast) | [163] |
| paclitaxel, SIPIR-receptor antagonist |
xenograft in mice: HTB- 56 (NSCLC), MDA-MB-231 (breast) | [164] |
bevacizumab: therapeutic monoclonal antibody; AAG: 17-allylamino-geldanamycin, PEG: polyethylene glycol; ABI-011: nab-thiocolchicine dimer; SIPIR-receptor antagonist: a series of compounds were tested at the same time; Hsp90 inhibitor: a series of triazolones; NSCLC: non-small cell lung cancer; SCLC: small cell lung cancer;
Figure 8.
Drugs under investigation in combination with taxanes
5. Conclusion
It has been 23 years and 19 years since paclitaxel and docetaxl, respectively, were approved by the FDA. There are still very active investigations in the clinical applications for these two drugs, especially through two or three drug combinations. The potential of cabazitaxel will be actively explored. Abraxane, albumin-bound paclitaxel nano-droplet, made a breakthrough in the formulation of this highly cytotoxic drug. This formulation substantially changed pharmacological properties of paclitaxel and has made a variety of new clinical applications possible, as represented by its use with gemcitabine for pancreatic cancer, which was unimaginable for the paclitaxel formulation. Thus, the development of new nano-formulations is a very active area. But, drug combination appears to be the most active area of clinical and translational research in this area. Nevertheless, the development of highly potent new taxanes is continuing, especially next-generation taxanes active against cancer stem cells are emerging. Highly potent new taxanes will have a substantial demand for the development of turmor-targeted drug conjugates using tumor-targeting monoclonal antibodies for ADCs, as well as small molecules such as vitamins for SMDCs.
6. Expert Opinion
First-generation taxanes, paclitaxel and docetaxel, were revolutionary anticancer agents and became block-buster drugs. Although the patents for both drugs have expired, these two drugs continue to lead cancer chemotherapy in clinic, and stimulated the generic drug markets. One of the critical reasons for the exceptional success of these two drugs is the fact that these two drugs have a unique mechanism of action, which is categorized as microtubule stabilizing agents. Although the primary mechanism of action is the cell division arrest at the G2/M stage, triggering the signaling pathway that leads to apoptosis, there seem to be other concurrently operating mechanisms to make these drugs effective. Also, because of the unique mechanism of action, these drugs are readily used for combination with other drugs, which have a different mechanism of action. These two drugs are, however, very hydrophobic and good substrates for ABC transporters, typically P-glycoprotein. Also, the molecular weights of these drugs are around 800 and thus these drugs are totally outside of the Lipinski rule for oral administration.
Although cabazitaxel, the newest FDA-approved taxane, has not appeared in the list of various clinical or preclinical studies on combination therapies yet, based on the patent literature in the last 5 years, it will surely start soon to explore the full potential of this drug. In contrast to the first-generation taxanes, cabazitaxel is a poor substrate for P-glycoprotein, which is an advantageous property.
Albumin-bound paclitaxel nano-droplet formulation, nab-paclitaxel or abraxane, is an innovative formulation for paclitaxel. This formulation not only improved solubility of highly hydrophobic paclitaxel, but also substantially reduced systemic toxicity and thus expanded its therapeutic window. This nano-formulation brought in enhanced permeability and retention (EPR) effect, characteristic to nano-scale particles, which enabled passive tumor-targeting, which can mitigate certain adverse effects. Abraxane has dramatically expanded the indications of paclitaxel as exemplified by the FDA approval of its use for pancreatic cancer treatment in combination with gemcitabine in 2013. Since paclitaxel was totally ineffective against pancreatic cancer, this was a big surprise in the field. This remarkable clinical development of abraxane has inspired researchers so that very active investigations have been ongoing in various nano-formulations, including lyposomal formulations. Thus, this line of research on the development of new nano-formulations for the three FDA-approved taxanes will continue very actively to produce promising results and eventual FDA approvals.
Currently, several taxanes are in clinical trials, including larotaxel, milataxel, BMS-184476, ortataxel and tesetaxel. Larotaxel advanced to Phase III trials in combination with cisplatin for advanced/metastatic urothelial tract or bladder cancer, but could not exceed a cisplatin/gemcitabine combination. Milataxel showed efficacy in Phase II trials for platinum-refractory NSCLC. BMS-184476 exhibited efficacy for previously treated NSCLC. Ortataxel is in Phase II trials for taxane-refractory NSCLC, metastatic breast cancer, and also recurred glioblastoma. Ortataxel is orally active and can also cross the blood brain barrier (BBB). Tesetaxel failed to demonstrate improved efficacy in Phase II trials for metastatic colorectal cancer, as compared to the standard treatment, but recently completed Phase I/II trials for solid tumors. It is difficult to judge if any of these taxanes can go through Phase III trials and reach registration at this stage.
Two taxane conjugates are also in clinical trials. DHA-paclitaxel (Taxoprexin) has advanced to Phase III trials for metastatic melanoma. A polyglutamic acid conjugate of paclitaxel (PG-paclitaxel, Opaxio) has also advanced to Phase III trials for NSCLC, ovarian cancer, and glioblastoma, which appears promising. One tumor-targeting peptide (angiopep-2) conjugate of paclitaxel, ANG-1005, is in clinical trials for metastatic ovarian cancer, advanced brain metastatic cancer and malignant glioma, showing early promise.
Numbers of second- and third-generation taxoids have been developed and continue to be developed for the pursuit of high potency taxane-based anticancer drugs. Metabolically more stable 3’-difluorovinyl-taxoids have also been developed, which can block metabolism by CYP3A4 and other cytochrome C enzymes. A notable finding is that at least several new-generation taxoids have potency against highly drug-resistant cancer stem cells (CSCs). One of these taxoids, SB-T-1214, showed promising efficacy in animal models. Also, its DHA conjugate, DHA-SB-T-1214, exhibited remarkable efficacy against DLD-1 colon, CFPAC-1 and PANC-1 pancreatic, and H460 NSCL cancer xenografts in mice.
A variety of tumor-targeting drug conjugates have been developed, using vitamins as targeting modules. Vitamin B receptors are overexpressed on various cancer cell surfaces. Thus, those conjugates undergo cancer specific vitamin receptor-mediated endocytosis to get into cancer cells with high efficiency, mitigating systemic toxicity to normal cells. Among the vitamin B receptors, the folate and biotin receptors appear to be highly promising by demonstrating very high cancer cell as well as tumor specificity in vitro and in vivo. Thus, some of those tumor-targeting conjugates will advance to clinical studies in the near future.
Highlights.
First-generation taxane anticancer drugs, paclitaxel and docetaxel, are still playing significant roles in the current cancer chemotherapy and expanding their indications.
Drug combinations are crucial to increase efficacy and mitigate adverse effects of taxanes. Thus, highly active clinical and preclinical studies are ongoing, which will lead to the development of new and efficacious cancer therapies.
Albumin-bound paclitaxel nano-droplet formulation, abraxane, brought a breakthrough in formulation, and has dramatically expanded the clinical application of paclitaxel. As such, efficacious nano-formulations, including liposomal formulations, are emerging.
Currently, several taxanes are in clinical trials, and highly potent next-generation taxanes are emerging in preclinical studies, which have efficacy against cancer stem cells.
Taxane conjugates have not been successfully developed yet so far. But, Opaxio, a paclitaxel conjugate with polyglutamic acid, is in clinical trials showing promise. Tumor-targeting drug conjugates of new-generation taxanes are emerging as potential next-generation chemotherapeutic agents with few adverse effects because of the tumor-specific drug delivery.
Acknowledgments
The research performed and inventions made in the author’s laboratory were in part supported by grants from the National Institutes of Health (NIH), U. S. A. Specifically, I Ojima is supported by NIH grant numbers CA103314 and AI 078251. B Lichtenthal, S Lee, C Wang and X Wang we supported in part by grant number CA103314.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Reference annotations
* Of interest
** Of considerable interest
- 1.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 2.Schiff PB, Fant J, Horwitz SB. Promotion of Microtubule Assembly Invitro by Taxol. Nature. 1979;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 3.Wani MC, Taylor HL, Wall ME, Coggan P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia . J. Am. Chem. Soc. 1971:932325–932327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 4.Cragg GM, Kingston DGI, Newman DJ. Anticancer agents from natural products. CRC Presss; 2011. [Google Scholar]
- 5.Bissery MC, Nohynek G, Sanderink GJ, Lavelle F. Docetaxel (Taxotere): a review of preclinical and clinical experience Part I: Preclinical experience. Anti-Cancer Drug. 1995;6(3):339–355. 363–368. doi: 10.1097/00001813-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Gueritte F. General and recent aspects of the chemistry and structure-activity relationships of taxoids. Curr Pharm Design. 2001;7(13):1229–1249. doi: 10.2174/1381612013397429. [DOI] [PubMed] [Google Scholar]
- 7.FDA. FDA approves new treatment for advanced head and neck cancer [Press Release] 2014 Retrived from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108771.htm.
- 8.Goldstein LJ. MDR1 gene expression in solid tumours. Eur J Cancer. 1996;32A(6):1039–1050. doi: 10.1016/0959-8049(96)00100-1. [DOI] [PubMed] [Google Scholar]
- 9.Mozzetti S, Ferlini C, Concolino P, Filippetti E, Raspaglio G, Prislei S, Gallo D, Martinelli E, Ranelletti FO, Ferrandina G, Scambia G. Class III β-Tubulin Overexpression Is a Prominent Mechanism of Paclitaxel Resistance in Ovarian Cancer Patients. Clin Cancer Res. 2005:11298–11305. [PubMed] [Google Scholar]
- 10.Darshan MSLM, Thadani-Mulero M, Levy BP, Escuin D, Zhou XK, Gjyrezi A, Chanel-Vos C, Shen R, Tagawa ST, Bander NH, Nanus DM, Giannakakou P. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011:716019–716029. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannakakou P, Plymate SR. Identifying taxane sensitivity in prostate cancer patients. WO2014047285A1. 2014 doi: 10.1158/0008-5472.CAN-13-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Leeuw RB-BL, Schiewer MJ, Ciment SJ, Den RB, Dicker AP, Kelly WK, Trabulsi EJ, Lallas CD, Gomella LG, Knudsen KE. Novel actions of next-generation taxanes benefit advanced stages of prostate cancer. Clin Cancer Res. 2015:21795–21807. doi: 10.1158/1078-0432.CCR-14-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kartner N, Riordan JR, Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- 14.FDA. FDA labeling information-Javtana (cabazitaxel) 2010 http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/201023lbl.pdf.
- 15. Abidi A. Cabazitaxel: A novel taxane for metastatic castration-resistant prostate cancer-current implications and future prospects. J Pharmacol Pharmacother. 2013;4(4):230–237. doi: 10.4103/0976-500X.119704. • This review article summarizes the characteristics of cabazitaxel, its applications to hormone-resistant prostate cancer treatment and future prospects.
- 16.Bissery MC, Bouchard H, Riou J, Vrignaud P, Combeau C, Bourzat JD. Preclinical evaluation of TXD258, a new taxoid. Proc. Am. Assoc. Cancer Res. 2000:41214. [Google Scholar]
- 17.Mita AC, Denis LJ, Rowinsky EK, Debono JS, Goetz AD, Ochoa L, Forouzesh B, Beeram M, Patnaik A, Molpus K, Semiond D, Besenval M, Tolcher AW. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15(2):723–730. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 18.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO, Investigators T. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 19.Galsky MD, Dritselis A, Kirkpatrick P, Oh WK. Cabazitaxel. Nat Rev Drug Discov. 2010;9(9):677–678. doi: 10.1038/nrd3254. [DOI] [PubMed] [Google Scholar]
- 20.Mita AC, Figlin R, Mita MM. Cabazitaxel: more than a new taxane for metastatic castrate-resistant prostate cancer? Clin Cancer Res. 24;18:6574–6579. doi: 10.1158/1078-0432.CCR-12-1584. [DOI] [PubMed] [Google Scholar]
- 21.Pivot X, Koralewski P, Hidalgo JL, Chan A, Goncalves A, Schwartsmann G, Assadourian S, Lotz JP. A multicenter phase II study of XRP6258 administered as a 1-h i.v. infusion every 3 weeks in taxane-resistant metastatic breast cancer patients. Ann Oncol. 2008;19(9):1547–1552. doi: 10.1093/annonc/mdn171. [DOI] [PubMed] [Google Scholar]
- 22.Feng L, Mumper RJ. A critical review of lipid-based nanoparticles for taxane delivery. Cancer Lett. 2013;334(2):157–175. doi: 10.1016/j.canlet.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Z, Schulz A, Wan X, Seitz J, Bludaub H, Alakhova DY, Darr DB, Perou CM, Jordan R, Ojima I, Kabanov AV, Luxenhofer R. Poly(2-oxazoline) based micelles with high capacity for 3rd generation taxoids: Preparation, in vitro and in vivo evaluation. J. Control. Release. 2015:20867–20875. doi: 10.1016/j.jconrel.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D, Noker P, Yao R, Labao E, Hawkins M, Soon-Shiong P. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. • This article describes salient features of ABI-007 (Abraxane) in the preclinical to clinical drug development in comparison to the standard formulation of paclitaxel.
- 25.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, Esmaeli B, Ring SE, Bedikian A, Hortobagyi GN, Ellerhorst JA. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–1044. [PubMed] [Google Scholar]
- 26.Stinchcombe TE. Nanoparticle albumin-bound paclitaxel: a novel Cremphor-EL-free formulation of paclitaxel. Nanomedicine. 2007;2(4):415–423. doi: 10.2217/17435889.2.4.415. [DOI] [PubMed] [Google Scholar]
- 27.Yared JA, Tkaczuk KH. Update on taxane development: new analogs and new formulations. Drug Des Devel Ther. 2012:6371–6384. doi: 10.2147/DDDT.S28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benettaib B, Elias I, Renschler M, Desai NP. Methods using pharmaceutical compositions comprising nanoparticles that comprise a taxane and an albumin for treatment of pediatric solid tumor. WO2014143613A1. 2014 [Google Scholar]
- 29.Desai NP, Soon-Shiong P. Use of nanoparticles comprising taxanes and albumin in combination with chemotherapeutic agents for treatment of pancreatic cancer. WO2011153010A1. 2011 [Google Scholar]
- 30.Yeo W, Wong N. Nanoparticles formulation comprising taxane and an albumin for treatment of hepatocellular carcinoma. WO2011119988A1. 2011 [Google Scholar]
- 31.Desai NP, Renschler M. Methods of treating melanoma comprising nanoparticles comprising taxane and a carrier protein. WO2014123612A1. 2014 [Google Scholar]
- 32.Desai NP, Trieu V. Combination therapy with thiocolchicine derivatives for treatment of proliferative disease such as cancer. WO2010105172A1. 2010 [Google Scholar]
- 33.Desai NP, Soon-Shiong P. Nanoparticle formulations comprising a taxane and an albumin in combination with other chemotherapeutic agents for the treatment of proliferative diseases such as cancer. WO2011156119A1. 2011 [Google Scholar]
- 34.FDA. 2013 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm367442.htm.
- 35.Sanofi. 2010 http://www.news.sanofi.us/index.php?s=33507&item=118530.
- 36.Parente Duena A, Garces GJ, Mis Vizcaino R. Injectable taxane pharmaceutical composition. WO2010015400A2. 2010 [Google Scholar]
- 37.Tsai CJ, Jang SH, Huang T-M, He JL. Stable pharmaceutical formulation of cabazitaxel. US20140171495A1. 2014 [Google Scholar]
- 38.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014:13813–13827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Q, Huang XE, Gao LL. A clinical study on the premedication of paclitaxel liposome in the treatment of solid tumors. Biomed. Pharmacother. 2009:63603–63607. doi: 10.1016/j.biopha.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 40. http://www.aastocks.com/en/stocks/analysis/company-fundamental/?symbol=02186.
- 41.Koudelka S, Turanek J. Liposomal paclitaxel formulations. J. Control. Release. 2012;163(3):322–334. doi: 10.1016/j.jconrel.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Crawford TC, Fetzer OS, Reiter LA, Wolfgang M. Preparation of cyclodextrin-based polymers for therapeutic delivery as antitumor agents. US20120058971A1. 2012 [Google Scholar]
- 43.Desu HR, Patel KR, Pejaver SK, Puri N. Methods and compositions for delivery of taxanes in stable oil-in-water emulsions. US20110166214A1. 2011 [Google Scholar]
- 44.Vavia PR, Patel KDK. Amino acid surfactant based nanocarrier for drug targeting. IN2010MU02958A. 2014 [Google Scholar]
- 45.Li S-D, Ernsting MJ. Cellulose-based nanoparticles for drug delivery for cancer treatment. WO2014015422A1. 2014 [Google Scholar]
- 46.Nabeta K. Non-aqueous taxane nanodispersion formulations and methods of using the same for treatment of proliferative diseases. US20140094509A1. 2014 [Google Scholar]
- 47.Zale SE, Troiano G, Ali MM, Hrkach J, Wright J. Drug loaded polymeric nanoparticles and methods of making and using same. WO2010005721A2. 2010 [Google Scholar]
- 48.Seeney CE, Klostergaard J, Yuill WA, Gibson DD. Magnetically responsive nanoparticle therapeutic constructs and methods of making and using. US20110130616A1. 2011 [Google Scholar]
- 49.Hoye TR, Wohl A, Macosko CW, Panyam J. Silicate prodrugs and nanoparticles. WO2012166949A1. 2012 [Google Scholar]
- 50.Yin R, Pan J, Zhang Y, Zhou B, Yen Y. Modified branched homopolymers forming aggregates with taxanes for nanoparticles. WO2014123791A1. 2014 [Google Scholar]
- 51.Karmali RA. Pharmaceutical compositions containing paclitaxel orotate. WO2012148494A2. 2012 [Google Scholar]
- 52.Beijnen JH, Schellens JHM, Moes J, Nuijen B. Solid pharmaceutical compositions comprising a taxane. WO2010020799A2. 2010 [Google Scholar]
- 53.Modi P. Oral formulations of chemotherapeutic agents. CA2843943A1. 2014 [Google Scholar]
- 54.Robert F, Harper K, Ackerman J, Gupta S. A phase I study of larotaxel (XRP9881) administered in combination with carboplatin in chemotherapy-naive patients with stage IIIB or stage IV non-small cell lung cancer. Cancer Chemother Pharmacol. 2010;65(2):227–234. doi: 10.1007/s00280-009-1026-5. [DOI] [PubMed] [Google Scholar]
- 55.Metzger-Filho O, Moulin C, de Azambuja E, Ahmad A. Larotaxel: broadening the road with new taxanes. Expert Opin Investig Drugs. 2009;18(8):1183–1189. doi: 10.1517/13543780903119167. [DOI] [PubMed] [Google Scholar]
- 56.Dieras V, Viens P, Veyret C, Romieu G, Awada A, Lidbrink E, Bonnefoi H, Mery-Mignard D, Dalenc F, Roche H. Larotaxel (L) in combination with trastuzumab in patients with HER2+ metastasis breast cancer (MBC): Interim analysis of an open phase II label study. J. Clin. Oncol. 2008:261070. [Google Scholar]
- 57. Ojima I, Kamath A, Seitz JD. Taxol, Taxoids and Related Taxanes. In: Hanessian S, editor. Natural Products in Medicinal Chemistry. Wiley-VCH: Weinheim; 2013. pp. 127–180. •• This book chapter summarizes the clinical and preclinical developments of new-generation taxoids and taxanes up 2011 and complements the present EOTP review.
- 58.Sternberg CN, Skoneczna IA, Castellano D, Theodore C, Blais N, Voog E, Bellmunt J, Peters F, Le-Guennec S, Cerbone L, Risse ML, Machiels JP. Larotaxel with Cisplatin in the first-line treatment of locally advanced/metastatic urothelial tract or bladder cancer: a randomized, active-controlled, phase III trial (CILAB) Oncology. 2013;85(4):208–215. doi: 10.1159/000354085. [DOI] [PubMed] [Google Scholar]
- 59.Beer M, Lenaz L, Amadori D. Phase II study of ortataxel in taxane-resistant breast cancer. J. Clin. Oncol. 2008:261066. [Google Scholar]
- 60.ClinicalTrials.gov. An Efficacy Study Of Ortataxel In Recurrent Glioblastoma. 2013 https://clinicaltrials.gov/ct2/show/NCT01989884.
- 61.Moore M, Jones C, Harker G, Lee F, Ardalan B, Saif M, Hoff P, Coomes J, Rollins C, Felt K. Phase II trial of DJ-927, an oral tubulin depolymerization inhibitor, in the treatment of metastatic colorectal cancer. J. Clin. Oncol. 2006:243591. [Google Scholar]
- 62.Roche M, Kyriakou H, Seiden M. Drug evaluation: tesetaxel--an oral semisynthetic taxane derivative. Curr Opin Investig Drugs. 2006;7(12):1092–1099. [PubMed] [Google Scholar]
- 63.Beeram M, Papadopoulos K, Pantnaik A, Qureshi A, Tolcher A. Phase I dose-ranging, pharmacokinetic (PK) study of tesetaxel, a novel orally active tubulin-binding agent. J. Clin. Oncol. 2010:2813075. [Google Scholar]
- 64.Ramanathan RK, Picus J, Raftopoulos H, Bernard S, Lockhart AC, Frenette G, Macdonald J, Melin S, Berg D, Brescia F, Hochster H, Cohn A. A phase II study of milataxel: a novel taxane analogue in previously treated patients with advanced colorectal cancer. Cancer Chemother Pharmacol. 2008;61(3):453–458. doi: 10.1007/s00280-007-0489-5. [DOI] [PubMed] [Google Scholar]
- 65.Camps C, Felip E, Sanchez JM, Massuti B, Artal A, Paz-Ares L, Carrato A, Alberola V, Blasco A, Baselga J, Astier L, Voi M, Rosell R. Spanish Lung Cancer, G. Phase II trial of the novel taxane BMS-184476 as second-line in non-small-cell lung cancer. Ann Oncol. 2005;16(4):597–601. doi: 10.1093/annonc/mdi120. [DOI] [PubMed] [Google Scholar]
- 66.Bradley MO, Webb NL, Anthony FH, Devanesan P, Witman PA, Hemamalini S, Chander MC, Baker SD, He L, Horwitz SB, Swindell CS. Tumor targeting by covalent conjugation of a natural fatty acid to paclitaxel. Clin. Cancer. Res. 2001;7(10):3229–3238. [PubMed] [Google Scholar]
- 67.Bedikian AY, DeConti RC, Conry R, Agarwala S, Papadopoulos N, Kim KB, Ernstoff M. Phase 3 study of docosahexaenoic acid-paclitaxel versus dacarbazine in patients with metastatic malignant melanoma. Ann Oncol. 2011;22(4):787–793. doi: 10.1093/annonc/mdq438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bradley MO, Swindell CS, Anthony FH, Witman PA, Devanesan P, Webb NL, Baker SD, Wolff AC, Donehower RC. Tumor targeting by conjugation of DHA to paclitaxel. J. Control. Release. 2001;74(1–3):233–236. doi: 10.1016/s0168-3659(01)00321-2. [DOI] [PubMed] [Google Scholar]
- 69.Paz-Ares L, Ross H, O'Brien M, Riviere A, Gatzemeier U, Von Pawel J, Kaukel E, Freitag L, Digel W, Bischoff H, Garcia-Campelo R, Iannotti N, Reiterer P, Bover I, Prendiville J, Eisenfeld AJ, Oldham FB, Bandstra B, Singer JW, Bonomi P. Phase III trial comparing paclitaxel poliglumex vs docetaxel in the second-line treatment of non-small-cell lung cancer. Br J Cancer. 2008;98(10):1608–1613. doi: 10.1038/sj.bjc.6604372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeyapalan SAMGJD. A phase II study of paclitaxel poliglumex (PPX), temozolamide (TMZ), and radiation (RT) for newly diagnosed high-grade gliomas. J Clin Oncol. 2011;29(suppl):2036. [Google Scholar]
- 71.Dipetrillo T, Suntharalingam M, Ng T, Fontaine J, Horiba N, Oldenburg N, Perez K, Birnbaum A, Battafarano R, Burrows W, Safran H. Neoadjuvant paclitaxel poliglumex, cisplatin, and radiation for esophageal cancer: a phase 2 trial. Am J Clin Oncol. 2012;35(1):64–67. doi: 10.1097/COC.0b013e318201a126. [DOI] [PubMed] [Google Scholar]
- 72.Markman M. Management of toxicities associated with the administration of taxanes. Expert Opin Drug Saf. 2003;2(2):141–146. doi: 10.1517/14740338.2.2.141. [DOI] [PubMed] [Google Scholar]
- 73.Hagiwara H, Sunada Y. Mechanism of taxane neurotoxicity. Breast Cancer. 2004;11(1):82–85. doi: 10.1007/BF02968008. [DOI] [PubMed] [Google Scholar]
- 74.FDA. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm307549.htm.
- 75.MPR. http://www.empr.com/drugs-in-the-pipeline/fda-fast-tracks-ganetespib-for-non-small-cell-lung-adenocarcinoma/article/311445/.
- 76.Ramalingam SS, Goss GD, Andric ZG, Bondarenko I, Zaric B, Ceric T, Poddubskaya EV, Ciuleanu T-E, Spicer JF, Felip E, Hirsh V, Manegold C, Rosell R, Khuri FR, Vukovic VM, Teofilovici F, El-Hariry I, Guo W, Bahcall SR, Fennell D. A randomized study of ganetespib, a heat shock protein 90 inhibitor, in combination with docetaxel versus docetaxel alone for second-line therapy of lung adenocarcinoma (GALAXY-1) J. Clin. Oncol. 2013;31(18):CRA8007. [Google Scholar]
- 77.NIH. https://www.clinicaltrials.gov/ct2/show/NCT01798485.
- 78.Hei Y-J, Yao B. Combination of motesanib, a taxane and a platinum-containing anti-cancer drug for use in the treatment of non-small cell lung cancer in a population subset of Asian patients. WO2012142325A1. 2012 [Google Scholar]
- 79.Conlin AK, Seidman AD, Bach A, Lake D, Dickler M, D'Andrea G, Traina T, Danso M, Brufsky AM, Saleh M, Clawson A, Hudis CA. Phase II trial of weekly nanoparticle albumin-bound paclitaxel with carboplatin and trastuzumab as first-line therapy for women with HER2-overexpressing metastatic breast cancer. Clin. Breast Cancer. 2010;10(4):281–287. doi: 10.3816/CBC.2010.n.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Desai NP, Tao C, De T, Ci SX, Trieu V. Nanoparticle formulations comprising taxane derivatives for cancer treatment. WO2010118365A1. 2010 [Google Scholar]
- 81. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. • This article describes unanticipated high efficacy of nab-paclitaxel (Abraxane) in pancreatic cancer in combitation with gemcitabine.
- 82.Desai NP, Soon-Shiong P. Nanoparticle formulations comprising a taxane and an albumin in combination with other chemotherapeutic agents for treatment of cancer. WO2011123393A1. 2011 [Google Scholar]
- 83.Vukovic V. Specific cancer treatment regimens with ganetespib. WO2014189937A1. 2014 [Google Scholar]
- 84.NIH. https://www.clinicaltrials.gov/ct2/show/study/NCT01962948?term=paclitaxel+and+ganetespib&rank=1.
- 85.Mahammedi H, Planchat H, Cure H, Barthomeuf C, Bayet-Robert M, Mouret-Reynier M, Abrial C, Thivat E, Atger M, Savareux L, Guy L, Goyard J, Chollet PJM, Nabholtz J, Durando X, Eymard J. Pilot phase II study with docetaxel in combination with curcuminoids in patients with hormone-resistant prostate cancer (HRPC) J. Clin. Oncol. 2011:29. [Google Scholar]
- 86.Barthomeuf C, Chollet P, Cure H, Planchat E. Methods and pharmaceutical compositions for the treatment of hormone-refractory prostate cancers. WO2012146706A1. 2012 [Google Scholar]
- 87.Duksin C, Tessler S. Methods for treating non-small cell lung cancer using combination of taxanes, an anti-clusterin oligonucleotide and platinum-based chemotherapeutic agents. WO2012156817A2. 2012 [Google Scholar]
- 88.Kubota K, Ichinose Y, Scagliotti G, Spigel D, Kim JH, Shinkai T, Takeda K, Kim SW, Hsia TC, Li RK, Tiangco BJ, Yau S, Lim WT, Yao B, Hei YJ, Park K. Phase III study (MONET1) of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous nonsmall-cell lung cancer (NSCLC): Asian subgroup analysis. Ann Oncol. 2014;25(2):529–536. doi: 10.1093/annonc/mdt552. [DOI] [PubMed] [Google Scholar]
- 89.Bahleda R, Sessa C, Del Conte G, Gianni L, Capri G, Varga A, Oprea C, Daglish B, Hospitel M, Soria JC. Phase I clinical and pharmacokinetic study of ombrabulin (AVE8062) combined with cisplatin/docetaxel or carboplatin/paclitaxel in patients with advanced solid tumors. Invest. New Drugs. 2014;32(6):1188–1196. doi: 10.1007/s10637-014-0119-0. [DOI] [PubMed] [Google Scholar]
- 90.Cohen P, Oprea IC. An antitumoral combination comprising ombrabulin, a taxane derivative and a platinum derivative. WO2011158206A1. 2011 [Google Scholar]
- 91.Gupta S. Novel antitumoral use of cabazitaxel in metastatic prostate cancer. WO2011051894A1. 2011 [Google Scholar]
- 92.Patel PH, Peterson AC. Anti-c-Met antibodies and taxanes combined with anti-VEGR antibodies for treatment of ER-/PR-/HER- metastatic breast cancer. US20110287003A1. 2011 [Google Scholar]
- 93.Karlan BY, Oza AM, Richardson GE, Provencher DM, Hansen VL, Buck M, Chambers SK, Ghatage P, Pippitt CH, Jr, Brown JV, 3rd, Covens A, Nagarkar RV, Davy M, Leath CA, 3rd, Nguyen H, Stepan DE, Weinreich DM, Tassoudji M, Sun YN, Vergote IB. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J Clin Oncol. 2012;30(4):362–371. doi: 10.1200/JCO.2010.34.3178. [DOI] [PubMed] [Google Scholar]
- 94.Weinreich DM. Treatment of ovarian cancer using a specific binding agent of human angiopoietin-2 in combination with a taxane. WO2011038139A1. 2011 [Google Scholar]
- 95.Desai NP, Soon-Shiong P. Nanoparticles of paclitaxel and albumin in combination with bevacizumab against cancer. US20100112077A1. 2010 [Google Scholar]
- 96.Somer BG, Schwartzberg LS, Arena FP, Epperson A, Fu D, Fortner BV. Phase II trial of nab-paclitaxel (nanoparticle albumin-bound paclitaxel (ABX)) + capecitabine (XEL) in first-line treatment of metastatic breast cancer (MBC) J. Clin. Oncol. 2007;25(18) [Google Scholar]
- 97.Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A, Clark E, Ross G, Benyunes MC, Baselga J. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCormack PL. Pertuzumab: a review of its use for first-line combination treatment of HER2-positive metastatic breast cancer. Drugs. 2013;73(13):1491–1502. doi: 10.1007/s40265-013-0109-0. [DOI] [PubMed] [Google Scholar]
- 99.Baselga J, Manikhas A, Cortes J, Llombart A, Roman L, Semiglazov VF, Byakhov M, Lokanatha D, Forenza S, Goldfarb RH, Matera J, Azarnia N, Hudis CA, Rozencweig M. Phase III trial of nonpegylated liposomal doxorubicin in combination with trastuzumab and paclitaxel in HER2-positive metastatic breast cancer. Ann Oncol. 2014;25(3):592–598. doi: 10.1093/annonc/mdt543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rozencweig M, Goldfarb RH, Forenza S. Non-pegylated liposomal doxorubicin triple combination therapy. US8026267B2. 2011 [Google Scholar]
- 101.Robert N, Krekow L, Stokoe C, Clawson A, Iglesias J, O'Shaughnessy J. Adjuvant dose-dense doxorubicin plus cyclophosphamide followed by dose-dense nab-paclitaxel is safe in women with early-stage breast cancer: a pilot study. Breast Cancer Res. Treat. 2011;125(1):115–120. doi: 10.1007/s10549-010-1187-2. [DOI] [PubMed] [Google Scholar]
- 102.Desai NP, Soon-Shiong P. Use of nanoparticles comprising taxanes and albumin in combination with chemotherapeutic agents for the treatment of bladder cancer. WO2011153009A1. 2011 [Google Scholar]
- 103.Smith DC, Grivas P, Daignault S, Hafez K, Wood DP, Lee CT, Montie JE, Weizer AZ, Montgomery J, Hussain M. A phase II trial of neoadjuvant ABI-007, carboplatin, and gemcitabine (ACG) in patients with locally advanced carcinoma of the bladder. J. Clin. Oncol. 2011:29. doi: 10.1016/j.urology.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 104.Chirgwin J, Chua SL. Management of breast cancer with nanoparticle albumin-bound (nab)-paclitaxel combination regimens: a clinical review. Breast J. 2011;20(5) doi: 10.1016/j.breast.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 105.Lianos GD, Vlachos K, Zoras O, Katsios C, Cho WC, Roukos DH. Potential of antibody-drug conjugates and novel therapeutics in breast cancer management. Onco Targets Ther. 2014:7491–7500. doi: 10.2147/OTT.S34235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Srinivasarao M, Galliford CV, Low PS. Principles in the design of ligandtargeted cancer therapeutics and imaging agents. Nat. Rev. Drug Discov. 2015:14203–14219. doi: 10.1038/nrd4519. [DOI] [PubMed] [Google Scholar]
- 107.Castaigne J-P, Demeule M, Lawrence B. Treatment of ovarian cancer using an anticancer agent conjugated to an angiopep-2 analog. WO2010121379A1. 2010 [Google Scholar]
- 108.Demeule M, Regina A, Che C, Poirier J, Nguyen T, Gabathuler R, Castaigne JP, Beliveau R. Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther. 2008;324(3):1064–1072. doi: 10.1124/jpet.107.131318. [DOI] [PubMed] [Google Scholar]
- 109.Bertrand Y, Currie JC, Demeule M, Regina A, Che C, Abulrob A, Fatehi D, Sartelet H, Gabathuler R, Castaigne JP, Stanimirovic D, Beliveau R. Transport characteristics of a novel peptide platform for CNS therapeutics. J Cell Mol Med. 2010;14(12):2827–2839. doi: 10.1111/j.1582-4934.2009.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bowen BC, Bradley WG. Amyotrophic lateral sclerosis: the search for a spectroscopic marker of upper motoneuron involvement. Arch Neurol. 2001;58(5):714–716. doi: 10.1001/archneur.58.5.714. [DOI] [PubMed] [Google Scholar]
- 111.Ojima I, Chen J, Sun L, Borella CP, Wang T, Miller ML, Lin S, Geng X, Kuznetsova L, Qu C, Gallager D, Zhao X, Zanardi I, Xia S, Horwitz SB, Mallen-St Clair J, Guerriero JL, Bar-Sagi D, Veith JM, Pera P, Bernacki RJ. Design, synthesis, and biological evaluation of new-generation taxoids. J Med Chem. 2008;51(11):3203–3221. doi: 10.1021/jm800086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ojima I, Das M. Recent advances in the chemistry and biology of new generation taxoids. J Nat Prod. 2009;72(3):554–565. doi: 10.1021/np8006556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kamb A, Wee S, Lengauer C. Why is cancer drug discovery so difficult? Nature Rev. Drug Discov. 2007:6115–6120. doi: 10.1038/nrd2155. [DOI] [PubMed] [Google Scholar]
- 114.Dalerba P, Cho RW, Clarke MF. Cancer Stem Cells: Models and Concepts. Annual Rev. Med. 2007:58267–58284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 115. Botchkina GI, Zuniga ES, Das M, Wang Y, Wang H, Zhu S, Savitt AG, Rowehl RA, Leyfman Y, Ju J, Shroyer K, Ojima I. New-generation taxoid SB-T-1214 inhibits stem cell-related gene expression in 3D cancer spheriods induced by purified colon tumor-initiating cells. Mol. Cancer. 2010:9192–9204. doi: 10.1186/1476-4598-9-192. • This article revealed the activity of a 2nd-generation taxoid against colon cancer stem cells for the first time.
- 116.Zuniga ES. Ph.D. Thesis. Stony Brook University; 2012. Design, synthesis and biological evaluation of new-generation taxoid-based tumor-targeting drug conjugates. [Google Scholar]
- 117.Kuznetsova L, Sun L, Chen J, Zhao X, Seitz J, Das M, Li Y, Veith JM, Pera P, Bernacki RJ, Xia S, Horwitz SB, Ojima I. Synthesis and biological evaluation of novel 3'-difluorovinyl taxoids. J. Fluorine Chem. 2012:143177–143188. doi: 10.1016/j.jfluchem.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Otova B, Ojima I, Vaclavikova R, Hrdy J, Ehrlichova M, Soucek P, Voborilova J, Nemcova V, Zanardi I, Horsky S, Kovar J, Gut I. Second-generation taxanes effectively suppress subcutaneous rat lymphoma: role of disposition, transport, metabolism, in vitro potency and expression of angiogenesis genes. Invest. New Drugs. 2012;30(3):991–1002. doi: 10.1007/s10637-011-9654-0. [DOI] [PubMed] [Google Scholar]
- 119.Ojima I. Taxoid-Fatty Acid Conjugates and Pharmaceutical Compositions Thereof. US7820839B2. 2010 [Google Scholar]
- 120.Seitz J, Ojima I. Drug Conjugates with Polyunsaturated Fatty Acids. In: Kratz F, Senter P, Steinhagen H, editors. Drug Delivery in Oncology – From Research Concepts to Cancer Therapy. Vol. 3. Wiley-VCH: Weinheim; 2011. pp. 1323–1360. [Google Scholar]
- 121.Jing Y-r, Zhou W, Li W-l, Zhao L-x, Wang Y-f. The synthesis of novel taxoids for oral administration. Bioorg. Med. Chem. 2014:22194–22203. doi: 10.1016/j.bmc.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 122.McChesney JD, Venkataraman S, Henri JT. Preparation of taxane analogues for the treatment of cancer and neurodegenerative disorders. WO2011028571A1. 2011 [Google Scholar]
- 123.McChesney JD, Henri JT, Venkataraman SK, Gundluru MK. Taxane and abeo-taxane analogs as Antitumor agents. WO2013106029A1. 2013 [Google Scholar]
- 124.Kozlowski A, Riley TA, McManus SP. Preparation of deuterated and/or fluorinated taxane derivatives. WO2012088433A1. 2012 [Google Scholar]
- 125.Chari RVJ. Potent cell-binding agent drug conjugates. WO2009134870A1. 2009 [Google Scholar]
- 126.Chari RVJ. Potent cell-binding agent drug conjugates. US20100092495A1. 2010 [Google Scholar]
- 127.Liu J, King D. Site-specific attachment of drugs or other agents to engineered antibodies with C-terminal cysteine-containing extensions. WO2009026274A1. 2009 [Google Scholar]
- 128.Hamada H, Mikuni K, Nakanishi K, Mandai T. Antitumor compositions containing taxoid derivatives covalently linked to tumor-specific antibody. US20070025995A1. 2007 [Google Scholar]
- 129.Gastaldi D, Zonari D, Dosio F. Targeted taxane delivery systems: recent advances. Drug Deliv. Lett. 2011;1(2):105–117. [Google Scholar]
- 130.Klostergaard J, Farquhar D, Ghosh SC, Price RE, Kundra V, Friedman RS. Anticancer agent-hyaluronic acid conjugate compositions and methods of use. WO2008134528A1. 2008 [Google Scholar]
- 131.Maneval DC, Shepard HM, Thompson CB. Protein sequences of human hyaluronidase and combination therapy with an anti-hyaluronan agent and a tumor-targeted taxane. WO2013151774A1. 2013 [Google Scholar]
- 132.Fazioni S, Hovda K, Livi V, McKennon M, Siviero L, Spoonemore H. Method for determining the amount of conjugated taxane in poly(glutamic acid)-taxane conjugates. WO2008107174A1. 2008 [Google Scholar]
- 133.Bai H, Tsang KY, Jin Y, Yu L. Large scale process for preparing poly(glutamyl-glutamate) conjugates for anticancer drug delivery. WO2014175898A1. 2014 [Google Scholar]
- 134.Kozlowski A, Riley TA, McManus SP. Multi-arm polymeric prodrug conjugates of taxane-based compounds as a drug delivery systems. WO2012088422A1. 2012 [Google Scholar]
- 135.Scripture CD, Figg WD, Sparreboom A. Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol. 2006;4(2):165–172. doi: 10.2174/157015906776359568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mutz MW, Webb R, III, Gestwicki JE. Therapeutic agents having reduced toxicity. WO2011130317A2. 2011 [Google Scholar]
- 137.Sengupta S, Kulkarni A, Rao P, Roy B. Compositions of a chemotherapeutic agent-cholesterol conjugate for treating cancer and methods for making the same. WO2013188763A1. 2013 [Google Scholar]
- 138.Leamon CP, Reddy JA. Folate-targeted chemotherapy. Adv. Drug Deliv. Rev. 2004:561127–561141. doi: 10.1016/j.addr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 139.Vlahov IR, Leamon CP. Engineering Folate–Drug Conjugates to Target Cancer: From Chemistry to Clinic. Bioconjugate Chem. 2012:231357–231369. doi: 10.1021/bc2005522. [DOI] [PubMed] [Google Scholar]
- 140.Russell-Jones G, McTavish K, McEwan J, Rice J, Nowotnik D. Vitamin-mediated targeting as a potential mechanism to increase drug uptake by tumours. J. Inorg. Biochem. 2004:981625–981633. doi: 10.1016/j.jinorgbio.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 141.Chen SY, Zhao XR, Chen JY, Chen J, Kuznetsova L, Wong SS, Ojima I. Mechanism-Based Tumor-Targeting Drug Delivery System Validation of Efficient Vitamin Receptor-Mediated Endocytosis and Drug Release. Bioconjugate Chem. 2010;21(5):979–987. doi: 10.1021/bc9005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ojima I, Zuniga ES, Berger WT, Seitz JD. Tumor-targeting drug delivery of new generation taxoids. Future Med. Chem. 2012:433–450. doi: 10.4155/fmc.11.167. • This review article summarizes various approaches to the tumor-targeted drug delivery of new-generation taxoids.
- 143.Ojima I. Drug Conjugate. US. 7 282,590 B2 2007. [Google Scholar]
- 144.Ojima I. Drug Conjugates with heterobifunctional linkers. US 7,847,119 B2 2010. [Google Scholar]
- 145.Chen J, Chen S, Zhao X, Kuznetsova LV, Wong SS, Ojima I. Functionalized Single-walled Carbon Nanotubes as Rationally Designed Vehicles for Tumor-Targeted Drug Delivery. J. Am. Chem. Soc. 2008:13016778–13016785. doi: 10.1021/ja805570f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vineberg JG. Ph.D. Thesis. Stony Brook University; 2014. Design, synthesis, and biological evaluation of novel taxoid-based drug conjugates and theranostic imaging agents towards tumor-targeted chemotherapy. [Google Scholar]
- 147.Rayan A. Conjugate of a taxane and biotin and uses thereof. WO2014191989A1. 2014 [Google Scholar]
- 148.Gebhard JR, Vollmer D, Patel D, Daugherty C. Cobalamin taxane bioconjugates useful as oral anti-cancer or anti-angiogenic drugs. WO2008115805A2. 2008 [Google Scholar]
- 149.Gebhard JR, Patel D. Cobalamin taxane bioconjugates for treating eye disease. WO2010088287A1. 2010 [Google Scholar]
- 150.Gebhard JR, Patel D. Taxane compounds for treating eye disease. US20100016256A1. 2010 [Google Scholar]
- 151.Seitz JD, Vineberg JG, Herlihy E, Park B, Melief E, Ojima I. Design, synthesis and biological evaluation of a highly-potent and cancer cell selective folate-taxoid conjugate. Bioorg. Med Chem. 2015;23(9):2187–2194. doi: 10.1016/j.bmc.2015.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Armstrong RC, Wierenga WD. Combination of an indazolylaminopyrrolotriazine and taxane for cancer treatment. WO2010105016A1. 2010 [Google Scholar]
- 153.Hart CP, Sun JD, Elenbaas BA, Gualberto A. Treatment of pancreatic cancer with a combination of a hypoxia-activated prodrug and a taxane. WO2015013448A1. 2015 [Google Scholar]
- 154.Chakravarty A, Ecsedy JA, Kleinfield RW, Le KN, Shyu WC, Venkatakrishnan K. Methods of treating cancer using aurora kinase inhibitors. WO2013142491A1. 2013 [Google Scholar]
- 155.Hancox UJ, Cosulich S, Davies BR. Combination treatment of cancer comprising 8-[(1R)-1-(3,5-difluorophenylamino)ethyl]-N,N-dimethyl-2-morpholino-4-oxo-4H–chromene-6-carboxamide and a taxane. WO2014135851A1. 2014 doi: 10.1021/jm501629p. [DOI] [PubMed] [Google Scholar]
- 156.Liu L, Gerson S. O6-methylguanine methyltransferase (MGMT) inhibitor in combination with other chemotherapeutic agents for the treatment of neoplastic disorders. US8791081B2. 2014 [Google Scholar]
- 157.Wengner AM, Siemeister G. Combination of a imidazopyridazine derivative and a mitotic agent for the treatment of cancer. WO2014198776A1. 2014 [Google Scholar]
- 158.Robertson FM. Combination therapy comprising paclitaxel and romidepsin for treating an inflammatory breast cancer. WO2013085902A1. 2013 [Google Scholar]
- 159.Davies BR. Pharmaceutical combination comprising AZD5363 and taxanes for treatment of cancer. WO2012131399A1. 2012 [Google Scholar]
- 160.Ulivi P, Arienti C, Zoli W, Scarsella M, Carloni S, Fabbri F, Tesei A, Chiadini E, Orlandi A, Passeri D, Zupi G, Milandri C, Silvestrini R, Amadori D, Leonetti C. In Vitro and In Vivo Antitumor Efficacy of Docetaxel and Sorafenib Combination in Human Pancreatic Cancer Cells. Curr Cancer Drug Targets. 2010:10600–10610. doi: 10.2174/156800910791859489. [DOI] [PubMed] [Google Scholar]
- 161.Fodstad O. B7-H3 antagonists and taxanes for treating cancer. WO2012004410A1. 2012 [Google Scholar]
- 162.Blackman RK, Foley KP, Proia D. Combination therapy using with taxanes and triazolone HSP90 inhibitory compounds for treatment of cancer. WO2011049946A1. 2011 [Google Scholar]
- 163.Patel L, Williams S, Wilding I, Read M, Spruce BA, Watt S. Combination therapy comprising a taxane and a sigma receptor ligand such as rimcazole. WO2010128309A1. 2010 [Google Scholar]
- 164.Shankar G, Bentzien F, Lamb P, Matthews D. Synergistic effects between sphingosine-1-phosphate receptor antagonists and antimicrotubule agents for the treatment of cancer. WO2010045601A2. 2010 [Google Scholar]