Abstract
Use of unauthorized synthetic drugs is a serious, forensic, regulatory and public health issue. In this scenario, consumption of drug-impregnated blotters is very frequent. For decades, blotters have been generally impregnated with the potent hallucinogen known as lysergic acid diethylamide (LSD); however, since 2013 blotter stamps with N-2 methoxybenzyl-substituted phenylethylamine hallucinogen designated as “NBOMes” have been seized in Chile. To address this issue with readily accessible laboratory equipment, we have developed and validated a new HPTLC method for the identification and quantitation of 25-C-NBOMe in seized blotters and its confirmation by GC–MS. The proposed method was validated according to SWGTOX recommendations and is suitable for routine analysis of seized blotters containing 25-C-NBOMe. With the validated method, we analyzed 15 real samples, in all cases finding 25-C-NBOMe in a wide dosage range (701.0–1943.5 µg per blotter). In this situation, we can assume that NBOMes are replacing LSD as the main hallucinogenic drug consumed in blotters in Chile.
Introduction
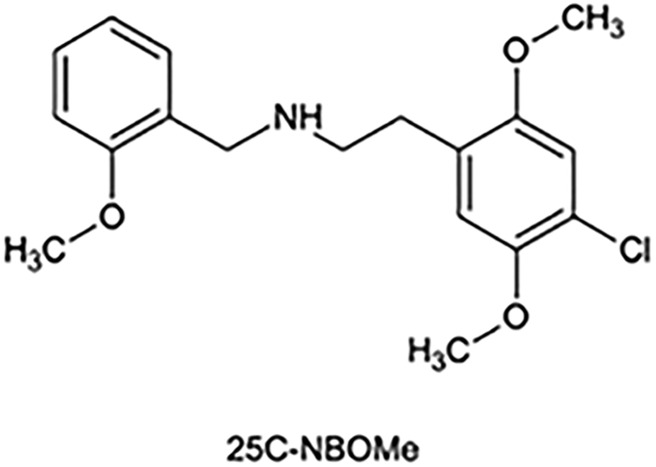
Drug abuse is a serious public health issue affecting most countries in the world. As a consequence of globalization, once a psychoactive substance is discovered or synthesized it is rapidly revealed to the entire world on the Internet. In this context, United Nations' reports indicate that new psychoactive substances (NPS) and especially those of synthetic origin have increased in prevalence and have become a legal alternative (popularly known as “legal highs”) to older scheduled drugs. Since 2010, there have been reports in numerous countries about seizures of ring-substituted phenylethylamine derivatives such as the 2,5-dimethoxy-4-halo compounds 2 CB, 2 CI and 2 CC. Quite recently, there has appeared on the market a new family of 2C drugs modified by N-substitution with a 2-methoxybenzyl group, which are commonly designated as “NBOMe” compounds (1). Since 2013, these novel substances have begun to surface in Chile. According to reports from scientific studies, these substances have high affinities for 5-HT2A receptor that in some cases could be higher than lysergic acid diethylamide (LSD) (2). In Chile, these new drugs are mainly represented by the single compound popularly known as 25-C-NBOMe (2-(4-chloro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine) (3). Figure 1 shows the chemical structure of this compound.
Figure 1.
Structure of 25-C-NBOMe.
This substance is an extremely efficient selective 5-HT2 serotonin receptor (but subtype nonselective) agonist (4–6); this high affinity suggests a high potential of abuse as a hallucinogenic drug. Reports indicate that this compound is highly potent when it is inhaled or administered sublingually, but it has very low bioavailability when consumed orally. Its total duration of action varies from 3 to 8 h by inhalation and from 4 to 10 h sublingually (7). The route of administration most widely used is sublingual, using blotters applied under the tongue for a few minutes. The effects caused by sublingual application are a slight metallic taste followed by numbness of the tongue and mouth, which can last up to an hour after administering the substance; this is one of the main differences distinguishing the use of these blotters with the traditional LSD ones. Another effect of these derivatives, asides from hallucinations, seems to involve euphoric and stimulating effects that might be comparable to stimulation caused by the use of common amphetamines (2). Dozens of deaths and hospitalizations have occurred in the USA related to the consumption of these new synthetic compounds mainly resulting from traffic and other accidents, where overdosing may cause tachycardia, hypertension, hypokalemia, exacerbation of serotonin and exacerbation of serotonin sympathomimetic syndromes (8). There have been reports of hyperthermia and rhabdomyolysis with a marked increase in plasma levels of creatine kinase and transaminases, metabolic acidosis, liver and kidney failure and disseminated intravascular coagulation (9). In all cases, symptomatic treatment and maintenance of vital functions have been installed, and there is no antidote available for acute intoxication with these hallucinogens. Most cases of acute intoxication have resulted from the confusion of NBOMe blotters with blotters containing the relatively nontoxic LSD. In Chile, according to statistics from our laboratory, this substance has replaced LSD as the main hallucinogen consumed in blotters in our country (10), with thousands of units of 25-C-NBOMe seized by police (more than 2,500 in 2013). Until now, the concentration of active substance in each dose (blotter) has remained unknown. In this context, we have developed and implemented a new rapid quantitative method for testing 25-C-NBOMe in blotters by HPTLC with confirmation by GC–MS. This method was validated according recommendations of the SWGTOX (11), and its uncertainty was also evaluated (12). This robust, reliable and simple technique is suitable for blotters analysis, comparison, profiling and routine use in forensic laboratories.
Materials and methods
Reagents
Methanol, ammonia, toluene, diethylamine and cyclohexane, all of HPLC grade, were purchased from Merck (Darmstadt, Germany). Reference material of 25-C-NBOMe was generously provided by the Drug Enforcement Administration (Washington, DC).
Instrumentation
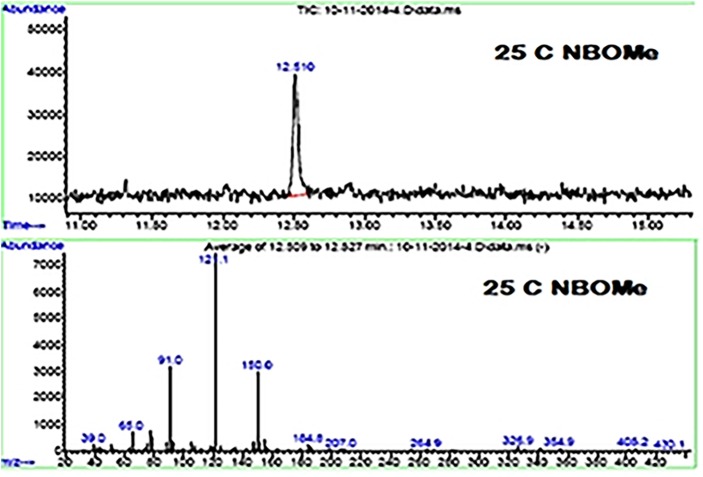
HPTLC was performed on 20 × 10 cm precoated silica gel F254 plates, (Merck, Darmstadt, Germany) previously activated at 80°C for 30 min. Standards (2 µL) and samples (1 µL) were applied in 3 mm bands with a CAMAG (Muttenz, Switzerland) ATS 4 automatic TLC sampler using a spray band technique. The first application x-axis was 15 mm, the y-axis was 8.0 mm and the distance between tracks was 5.8 mm. Plates were developed in an automatic developing chamber ADC-2 CAMAG to a distance of 70 mm with cyclohexane/toluene/diethylamine (75 + 15 + 10 v/v/v) as the mobile phase (10 mL, without saturation of the chamber). After a drying time of 5.0 min, the bands were scanned with a CAMAG TLC Scanner 4 densitometer by absorbance at 298 nm. The spectrum of each peak was recorded in the 190–400 nm range on all detected peaks mode, a scanning speed of 20 nm/s, a data resolution of 100 µm/step and reference spectrum (x = 10.0 mm, y = 5.0 mm), controlled with the Wincats Planar Chromatography Manager version 1.4.7 software. The GC–MS analysis was performed using an Agilent Technologies 6890 N GC equipped with an Agilent Technologies 5973 B MS selective detector operating in the EI mode at 70 eV using an HP-5 capillary column (30 m 0.32 × 0.25 µm). The column temperature was maintained at 75°C for 1 min, then programmed to 280°C at a rate of 25°C/min and kept constant at 280°C for 20 min. The injector temperature was 250°C, and helium was used as carrier gas at a flow rate of 1 mL min−1 with a splitless mode injection. The injection volume was 5 µL. Identification of the compound was performed by a comparison of its mass spectrum and retention time with the injected pure reference material. All chromatograms were acquired in full scan mode. Figure 2 shows the total ion chromatogram (TIC) and mass spectrum of standard of 25-C-NBOMe.
Figure 2.
TIC and mass spectrum of standard of 25-C-NBOMe. This figure is available in black and white in print and in color at JCS online.
Sample preparation
Fifteen real samples of seized blotters were separately submerged in 25.0 mL of methanol (HPLC grade) and extracted for 15 min in an ultrasonic bath. An extract of each sample (2.0 mL) was added to a glass vial for chromatographic analyses, then one vial was analyzed by HPTLC and a second vial was examined by GC–MS with the aim of confirm results obtained with HPTLC.
Results and discussion
Method optimization
The extraction procedure involves a significant modification of the method previously published by Zuba et al. in 2013 (3) to obtain results in lower time and with a small use of solvents.
Method validation
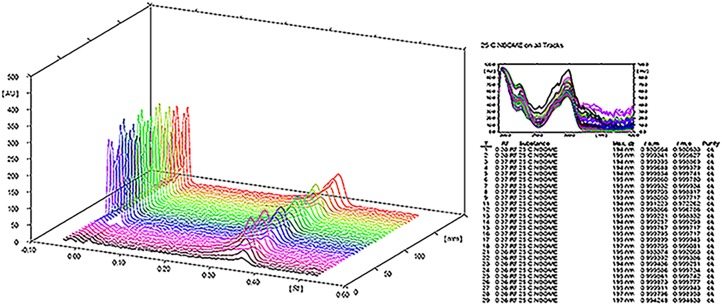
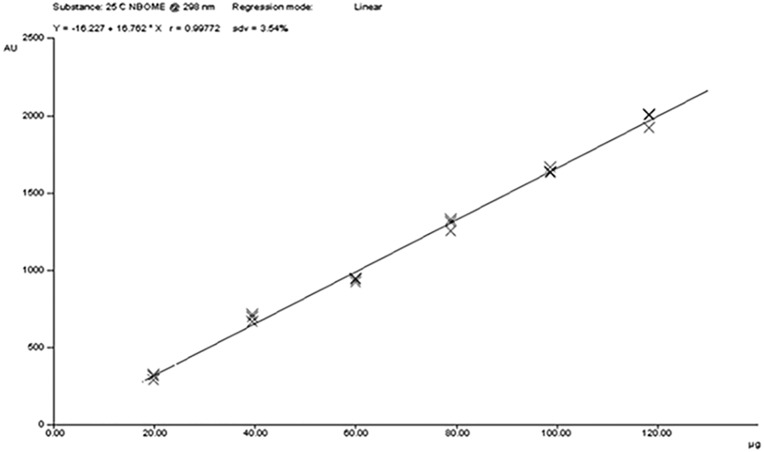
Specificity was assessed by the addition of compounds that might be found in real samples (i.e., LSD Rf 0.06 and DMT Rf 0.10). The peak purity of 25-C-NBOMe was determined by comparing the spectra recorded at three different regions of the HPTLC band [i.e., peak start (S), peak apex (M) and peak end (E)]. The results of purity and spectrum comparison of real samples and reference material of 25-C-NBOMe are shown in Figure 3. We found an acceptable correlation between the UV spectra acquired from the standard and from the real samples, and no other peaks were present at the Rf of 25-C-NBOMe (13, 14). Figure 4 shows a densitogram with 25-C-NBOMe, LSD and DMT as an example of selectivity and separation capability of the proposed method. Regarding specificity, TLC plates from another manufacturer (Macherey-Nagel, Germany) did not alter the separation process. The linearity of the method was evaluated from the calibration plot, which was constructed by analysis of six independent solutions prepared by dissolving appropriate amounts of standard of 25-C-NBOMe to give concentrations of 19.72–118.28 µg per band, and each point applied in triplicate. Data were fitted by the linear equation Y = 16.76X − 16.23; the coefficient of determination R2 was 0.99772, the slope and intercept standard deviations were 0.44 and 33.84, respectively, and the calibration plot is shown in Figure 5. The limits of detection (LOD) and quantification (LOQ) were calculated using the formulas: LOD = 3.3 × Sa/b and LOQ = 10 × Sa/b, where Sa is the standard deviation of the intercept and b is the slope of the calibration curve. Precision of the method was evaluated in terms of relative standard deviation (RSD) under conditions of repeatability and reproducibility (intermediate precision). The RSD of repeatability (intraday) was obtained by evaluating 10 independent replicates of one sample (50.2 µg/blotter) during the same day, in the same plate and by the same analyst. Intermediate precision was assessed by measuring six independent levels of concentration in three different days by the same analyst (15). Accuracy was assessed by the determination of the percentage of 25-C-NBOMe recovered by the assay at six different levels (16); chromatographic, precision, linearity and accuracy were not influenced by the time of preparation of the mobile phase, which was stored at 4°C.
Figure 3.
Results of purity and spectrum comparison of real samples and reference material of 25-C-NBOMe. This figure is available in black and white in print and in color at JCS online.
Figure 4.
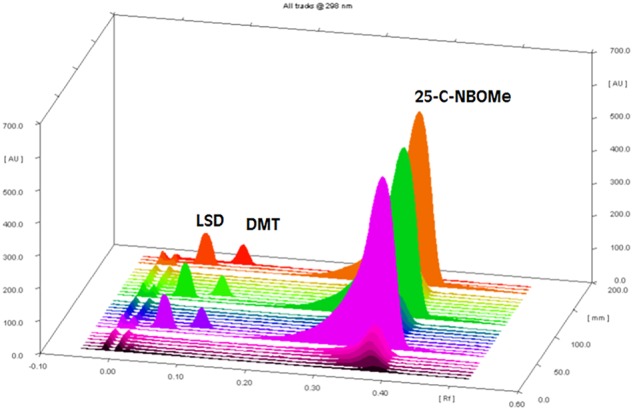
Densitogram with 25-C-NBOMe, LSD and DMT as an example of selectivity and separation capability of the proposed method. This figure is available in black and white in print and in color at JCS online.
Figure 5.
The calibration plot.
Uncertainty, in general, comprises multiple components and is calculated from the estimation of the errors associated with various steps of the analytical process (17); in this case, we applied the results from method validation, and uncertainty was calculated by using the following formula:
where CVrepeat is the RSD of repeatability, CVrecover is the RSD of accuracy assay and CVcal is the RSD from the calibration curve (18). In the case of robustness, different HPTLC plates from another manufacturer (Macherey-Nagel) did not modify the separation process.
A brief summary of validation parameters for the HPTLC method is shown in Table I. With the validated method, we analyzed 15 real samples. Quantitation was achieved by HPTLC using three points of calibration, and then the identity of all samples were confirmed by GC–MS, in all cases finding 25-C-NBOMe. Table II shows a brief summary of the results from each sample and the design or imprint of each blotter.
Table I.
Summary of Validation Parameters for Analysis of 25-C-NBOMe by HPTLC
| Parameter | Results |
|---|---|
| Rf | 0.37 |
| Linearity and range | 19.72–118.28 µg per band; y = 16.76X − 16.23, R2: 0.99772, SD: 3.64 |
| LOD and LOQ | 7.1 µg per band, and 21.63 µg per band |
| Repeatability (50.2 µg) | CV% = 5,459 |
| Intermediate precision | CV% = 6,071 |
| Amount of 25-C-NBOMe (µg/mL) | Recovery (%) |
| 18,825 | 95,635 ± 0.389 |
| 31,375 | 97,098 ± 1,166 |
| 43,925 | 102,213 ± 2,507 |
| 56,475 | 105,111 ± 1,404 |
| 69,025 | 100,971 ± 1,295 |
| 81,575 | 94,541 ± 0.430 |
| Mean: 99.26%; SD: 4.14 | |
| Relative uncertainty | 3.63% |
Table II.
Analysis of 15 Real Samples of Blotters Seized in Chile
| Sample | Results by HPTLC | Confirmation by GC–MS | Amount of 25 C NBOMe by HPTLC (µg/blotter) | Design |
|---|---|---|---|---|
| 1 | 25-C-NBOMe | 25-C-NBOMe | 1,943.50 |  |
| 2 | 25-C-NBOMe | 25-C-NBOMe | 772.94 |  |
| 3 | 25-C-NBOMe | 25-C-NBOMe | 977.63 |  |
| 4 | 25-C-NBOMe | 25-C-NBOMe | 1,315.25 |  |
| 5 | 25-C-NBOMe | 25-C-NBOMe | 1,908.45 |  |
| 6 | 25-C-NBOMe | 25-C-NBOMe | 820.63 |  |
| 7 | 25-C-NBOMe | 25-C-NBOMe | 843.44 |  |
| 8 | 25-C-NBOMe | 25-C-NBOMe | 701.00 |  |
| 9 | 25-C-NBOMe | 25-C-NBOMe | 1,458.35 |  |
| 10 | 25-C-NBOMe | 25-C-NBOMe | 781.25 |  |
| 11 | 25-C-NBOMe | 25-C-NBOMe | 1,943.14 |  |
| 12 | 25-C-NBOMe | 25-C-NBOMe | 958.20 |  |
| 13 | 25-C-NBOMe | 25-C-NBOMe | 1,396.80 |  |
| 14 | 25-C-NBOMe | 25-C-NBOMe | 1,026.69 |  |
| 15 | 25-C-NBOMe | 25-C-NBOMe | 1,987.75 |  |
Sample analysis
This method is fast, with good specificity, linearity, accuracy, precision, detection and quantitation limits and low relative uncertainty. In this small number of blotters, we found from 701.0 to 1,943.5 µg per blotter. This high variability increases the potential risk of “bad trips” or acute intoxication because of high potency of this hallucinogenic drug. In all examined cases, we only found 25-C-NBOMe and no other compounds (i.e., LSD) were identified in the selected blotters.
Conclusion
An HPTLC method was developed and validated for a rapid qualitative and quantitative analysis of the content of 25-C-NBOMe in seized blotters. The main differences between this method and others previously implemented are the simple manipulation of the samples and the absence of internal standard, spraying reagents or the “scraping off” method of the plate; offering reliable results with small cost of development. In addition, a GC–MS method was used to identify 25-C-NBOMe in blotters seized in Chile in 2014. The results obtained concerning the concentration of 25-C-NBOMe are very disturbing, considering the broad variation in the amount of this compound in each blotter. In this context, we can assume that N-benzylated phenylethylamines are displacing LSD as the main hallucinogenic drug consumed in blotters in Chile. These results are alarming because the high potency and elevated toxicity of this substance, particularly, when the consumer may not be aware about what is consuming, and, in most cases, users may believe that the blotter contains LSD. To the best of our knowledge, this is the first report describing the detection of “NBOMe” derivatives in blotters by HPTLC. The proposed method can be easily implemented in forensic laboratories to establish the concentration of 25-C-NBOME in blotters seized by police officers.
Acknowledgments
The authors thank to Public Health Institute (Chile) for providing materials and reagents for this study. Furthermore, they acknowledge the Drug Enforcement Administration (DEA, USA) for the reference material for this work. The authors are also grateful to Pharmacist Lorena Delgado Rivera for her continuous support in our work. Finally, they dedicate this manuscript to the memory of Dr Q.F. Carlos Barrios Guerra, who dedicated his life to learning and teaching toxicology.
References
- 1.Casale J.F., Hays P.A.; Characterization of eleven 2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine (NBOMe) derivatives and differentiation from their 3- and 4-methoxybenzyl analogues—part I; Microgram Journal, (2012); 9: 84–109. [Google Scholar]
- 2.Bersani F.S., Corazza O., Albano G., Valeriani G., Santacroce R., Bolzan F. et al. ; 25C-NBOMe: preliminary data on pharmacology, psychoactive effects, and toxicity of a new potent and dangerous hallucinogenic drug; BioMed Research International, (2014); 2014: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuba D., Sekuła K., Buczek A.; 25C-NBOMe—new potent hallucinogenic substance identified on the drug market; Forensic Science International, (2013); 227: 7–14. [DOI] [PubMed] [Google Scholar]
- 4.Lawn W., Barratt M., Williams M., Horne A., Winstock A.; The NBOMe hallucinogenic drug series: patterns of use, characteristics of users and self-reported effects in a large international sample; Journal of Psychopharmacology, (2014); 28: 780–788. http://www.ncbi.nlm.nih.gov/pubmed/24569095?dopt=Abstract. [DOI] [PubMed] [Google Scholar]
- 5.Braden M.R., Parrish J.C., Naylor J.C., Nichols D.E.; Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists; Molecular Pharmacology, (2006); 70: 1956–1964. [DOI] [PubMed] [Google Scholar]
- 6.Hansen M., Phonekeo K., Paine J.S., Leth-Petersen S., Begtrup M., Bräuner-Osborne H. et al. ; Synthesis and structure−activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists; ACS Chemical Neuroscience, (2014); 5: 243––2249.. http://pubs.acs.org/doi/abs/10.1021/cn400216u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halberstadt A.L., Geyer M.A.; Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response; Neuropharmacology, (2014); 77: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose S.R., Poklis J.L., Poklis A.; A case of 25I-NBOMe (25-I) intoxication: a new potent 5-HT2A agonist designer drug; Clinical Toxicology (Philadelphia, PA), (2013); 51: 174–177. http://www.ncbi.nlm.nih.gov/pubmed/23473462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill S., Doris T., Gurung S.; Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: case series; Clinical Toxicology, (2013); 51: 487–492. http://informahealthcare.com/doi/abs/10.3109/15563650.2013.802795. [DOI] [PubMed] [Google Scholar]
- 10.Duffau B., Rojas S., Jofré S., Kogan M., Triviño I., Fuentes P.; Thin-layer chromatography method for the detection of N,N-dimethyltryptamine in seized street samples; Journal of Planar Chromatography—Modern TLC, (2014); 27: 477–479. [Google Scholar]
- 11.SWGTOX; Scientific Working Group for Forensic Toxicology (SWGTOX) Standard Practices for Method Validation in Forensic Toxicology; Journal of Analytical Toxicology, (2013); 37: 452–454. [DOI] [PubMed] [Google Scholar]
- 12.González A.G., Herrador M.Á.; A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles; TrAC, Trends in Analytical Chemistry, (2007); 26: 227–238. [Google Scholar]
- 13.Srivastava M.; High-performance thin-layer chromatography (HPTLC). Springer, Berlin/Heidelberg, (2011). [Google Scholar]
- 14.Spangenberg B., Poole C.F., Weins C.; Quantitative thin-layer chromatography: a practical survey. Springer, Berlin/Heidelberg, (2011). [Google Scholar]
- 15.Renger B., Végh Z., Ferenczi-Fodor K.; Validation of thin layer and high performance thin layer chromatographic methods; Journal of Chromatography A, (2011); 1218: 2712–2721. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann C., Smeyers-Verbeke J., Massart D.L., McDowall R.D.; Validation of bioanalytical chromatographic methods; Journal of Pharmaceutical and Biomedical Analysis, (1998); 17: 193–218. [DOI] [PubMed] [Google Scholar]
- 17.Kristiansen J.; The guide to expression of uncertainty in measurement approach for estimating uncertainty: an appraisal; Clinical Chemistry, (2003); 49: 1822–1829. http://www.clinchem.org/cgi/content/abstract/49/11/1822. [DOI] [PubMed] [Google Scholar]
- 18.Müller I.B., Windberg C.N.; Validation of an HPLC method for quantitation of MDMA in tablets; Journal of Chromatographic Science, (2005); 43: 434–437. [DOI] [PubMed] [Google Scholar]