Abstract
Please cite this paper as: Larcombe et al. (2011) Sexual dimorphism in lung function responses to acute influenza A infection. Influenza and Other Respiratory Viruses 5(5), 334–342.
Background Males are generally more susceptible to respiratory infections; however, there are few data on the physiological responses to such infections in males and females.
Objectives To determine whether sexual dimorphism exists in the physiological/inflammatory responses of weanling and adult BALB/c mice to influenza.
Methods Weanling and adult mice of both sexes were inoculated with influenza A or appropriate control solution. Respiratory mechanics, responsiveness to methacholine (MCh), viral titre and bronchoalveolar lavage (BAL) cellular inflammation/cytokines were measured 4 (acute) and 21 (resolution) days post‐inoculation.
Results Acute infection impaired lung function and induced hyperresponsiveness and cellular inflammation in both sexes at both ages. Males and females responded differently with female mice developing greater abnormalities in tissue damping and elastance and greater MCh responsiveness at both ages. BAL inflammation, cytokines and lung viral titres were similar between the sexes. At resolution, all parameters had returned to baseline levels in adults and weanling males; however, female weanlings had persisting hyperresponsiveness.
Conclusions We identified significant differences in the physiological responses of male and female mice to infection with influenza A, which occurred in the absence of variation in viral titre and cellular inflammation.
Keywords: Airway responsiveness, cellular inflammation, influenza, mice, sexual dimorphism
Introduction
Sexual dimorphism is a common feature of many human respiratory diseases including asthma, chronic obstructive pulmonary disease (COPD) and cystic fibrosis. 1 , 2 , 3 Human females generally display higher susceptibility to and increased severity of these diseases, 4 , 5 , 6 while human males are more susceptible to a wide range of bacterial, fungal and viral infections, including respiratory infections. 7 , 8 Traditionally, the dimorphism in susceptibility to infection has been associated with circulating sex steroid hormone levels 9 , 10 that influence the development and function of the immune system. 10 , 11 , 12 This results in dimorphic immune responses with androgens generally lowering, and oestrogens generally enhancing aspects of immunity. For example, adult females have superior antibody‐mediated immune responses compared with males because of higher immunoglobulin levels in their serum and pulmonary secretions. 12 , 13 Further, the associations between sex steroid hormone levels and sexual dimorphism in response to viral infections suggest that the age of infection may influence infection severity.
However, while sexual dimorphism in susceptibility to viral infection is well established, the potential influence of sex on infection severity has largely been ignored. There are virtually no data on this in humans, while animal models of lung disease 14 generally investigate only one sex. 15 , 16 The few applicable murine studies that consider both sexes suggest that the aspects of murine lung physiology do display sexual dimorphism, although they largely focus on models of allergic lung disease, rather than on respiratory viral infection. For example, female mice develop a greater immunological and inflammatory response to ovalbumin sensitisation and challenge, 17 yet male mice develop significantly greater airway hyperresponsiveness (AHR) and cellular inflammation in bronchoalveolar lavage (BAL) following intratracheal lipopolysaccharide administration. 18 The few studies of sex differences in murine models of infectious respiratory disease generally investigate bacterial or mycobacterial (not viral) infection with results that vary depending on the pathogen; female C57BL/6 mice are more susceptible to and show greater inflammatory responses to Pseudomonas aeruginosa infection than males, 3 yet male mice of various strains develop more severe lung lesions and greater mortality resulting from mycobacterial infection. 19 , 20
Thus, previous studies suggest that males may be more susceptible to infection because of their reduced ability to mount an appropriate inflammatory response compared with females. This lessened response may reduce cytokine‐/chemokine‐related damage to the airways/parenchyma in males, which may be reflected in lessened responsiveness to bronchoconstrictive agents. The present study aimed to directly investigate sexual dimorphism in the physiological and inflammatory responses of male and female BALB/c mice to influenza A infection. By investigating both adult and weanling mice, we were further able to assess the effects of age on responses to respiratory viral infection.
Materials and methods
Animals
Male and female BALB/c mice at three (weanling) and eight (adult) weeks of age were obtained from the Animal Resource Centre (Murdoch, WA, Australia) and housed in individually ventilated cages (Sealsafe, Tecniplast, Buguggiate, Italy) on non‐allergic, dust‐free bedding (Shepards Specialty Papers, Chicago, IL, USA). Food and water were provided ad libitum. All procedures were approved by the institutional animal ethics committee and conformed to National Health and Medical Research Council guidelines.
Virus
Influenza A/Memphis/1/71 (H3N1) is a reassortant mouse‐adapted strain of influenza A of intermediate virulence that replicates to high titres and causes lung inflammation. 21 , 22 The virus stock was grown and titrated in dog kidney (MDCK) cells as described previously. 21
Infection
Mice were inoculated under light methoxyflourane anaesthesia (Medical Development Pty Ltd, Springvale, Vic., Australia) by pipetting drops of inoculum onto the nostrils. Adult mice received 104·5 plaque‐forming units (pfu) of influenza A/Mem71 diluted in 50 μl Virus Production Serum Free Medium (VP‐SFM; Gibco, Mulgrave, VIC, Australia), and weanlings received 3/5 of this dose (104·278 pfu) in a volume of 30 μl. The supernatant of uninfected MDCK cells diluted in VP‐SFM was used to inoculate control mice. Assessments were undertaken 4 (d4) and 21 (d21) days post‐inoculation; time points chosen to coincide with the peak of the viral infection and recovery. 15
Lung viral titre
Mice (10 per group) were euthanised and lung tissue collected at d4 and d21 post viral infection. Tissue was weighed and homogenised in VP‐SFM and centrifuged for 5 minutes at 250 g. Lung viral titres, normalised to lung weight, were determined by plaque assay in MDCK cells. 21
Cell counts, cytokines and lung protein analysis
Mice (18–30 per group) were lavaged by slowly washing saline in and out of the lungs three times (0·5 ml for adults and 0·3 ml for weanlings). BAL was centrifuged to separate the cell pellet and supernatant. Cells were stained with trypan blue and total cell counts obtained using a haemocytometer. Differential counts were obtained from the cytospin sample, stained with Leishman’s stain (BDH Laboratory Supplies, Poole, UK) and examined using light microscopy. The supernatant was stored for analysis of cytokines (TNF‐α, IL‐6, IFNγ, MCP‐1) by cytometric bead array (CBA) as per the manufacturers’ instructions (Mouse Inflammation kit; BD Biosciences, North Ryde, NSW, Australia) and total protein content using a colorimetric assay (Bio‐Rad, Gladesville, NSW, Australia). 23
Baseline lung function and airway hyperresponsiveness
Lung function (eight per group) was measured using a modification of the low‐frequency forced oscillation technique (LFOT) and a small‐animal ventilator (flexiVent; Scireq, Montreal, QC, Canada) as described previously. 24 , 25 Following induction of surgical anaesthesia by an i.p. injection of ketamine and xylazine (0·4 and 0·02 mg/g body weight respectively; Troy Laboratories, Glendenning, NSW, Australia), a polyethylene cannula (length = 1·0 cm, internal diameter (ID) = 0·086 cm for adults and 0·080 cm for weanlings) was inserted into the trachea and secured with silk ligature. Mice were then connected to the ventilator, ventilated at 450 breaths/minute with a tidal volume of 8 ml/kg and positive end expiratory pressure of 2 cmH2O and allowed to stabilise for 5 minutes prior to standardisation of lung volume history via three slow deep inflations to P rs = 20 cmH2O. This was sufficient to suppress spontaneous breathing during measurements without the need for paralysis.
Respiratory impedance (Z rs) was measured 26 and the Constant Phase Model 24 used to partition Z rs into airway and parenchymal components; allowing calculation of Newtonian resistance (R n), inertance (I aw), tissue damping (G) and elastance (H). In mice, R n is equivalent to airway resistance (R aw) because of the high compliance of the chest wall. Tissue hysteresivity (η) was calculated as G/H. 27 The calibration procedure in the flexiVent software was used to correct for the resistance of the tracheal cannula. I aw values were negligible and are not reported.
Once stabilised on the ventilator, LFOT measurements were taken once a minute for five minutes to establish baseline respiratory mechanics, followed by a 90‐second saline aerosol and increasing doses of methacholine (MCh) delivered by an ultrasonic nebuliser (DeVilbiss UltraNeb, Somerset, PA, USA). After each challenge, LFOT measurements were again taken once a minute for 5 minutes with the peak response used for analyses. Differences in responsiveness were assessed as the maximum responses to either a 10‐mg/ml (for weanlings) or 30‐mg/ml (for adults) MCh challenge.
Statistics
Analyses were conducted using two‐way anova with sex (i.e. male and female) and treatment (i.e. influenza or control) as factors. Data were transformed when required, P < 0·05 was considered to be significant. The Holm‐Sidak post hoc test was used to identify significant differences between groups. The Holm‐Sidak test can be used for both pairwise comparisons and comparisons versus a control group and is appropriate in this study in that it derives P values that adjust for the conduct of multiple statistical comparisons. Further, it is more powerful than the Bonferroni test. Statistical analyses were performed using sigmastat software (v3.50 SPSS Science, Chicago, IL, USA). Data are shown as mean ± SD.
Results
Mass
Infection with Influenza A/Memphis/1/71 (H3N1) did not result in substantial weight loss in either sex or either age (Table 1) such that no mice needed to be euthanised in this study. During acute infection, infected adult mice weighed significantly less than sex‐matched controls (P < 0·001; Table 1) and adult males were significantly heavier than adult females (P < 0·001). For weanlings, males were significantly heavier than females (P < 0·001), and infection was not associated with a decrease in mass (P = 0·80). At d21, there were no significant differences in the masses of infected mice when compared to their respective controls for each age and sex (P > 0·20 in both cases), However, males were significantly heavier than females at both ages, regardless of previous infection (P < 0·001 in both cases).
Table 1.
Mass (g) of adult (8 week old) and weanling (3 week old) male and female mice, prior to infection and 4 and 21 days post‐inoculation with influenza A
| Adult male | Adult female | Weanling male | Weanling female | |
|---|---|---|---|---|
| Day 0 control | 22·10 ± 0·90 (26) | 17·71 ± 0·95 (26) | 10·84 ± 2·20 (26) | 10·96 ± 1·24 (26) |
| Day 0 flu | 22·18 ± 1·49 (30) | 17·71 ± 1·15 (30) | 11·61 ± 2·04 (30) | 10·91 ± 1·77 (30) |
| Day 4 control | 23·01 ± 1·05 (18) | 18·77 ± 1·09 (19) | 13·86 ± 1·91 (18) | 12·45 ± 0·86 (18) |
| Day 4 flu | 22·19 ± 1·26 (29)* | 17·74 ± 1·06 (30)* | 14·17 ± 1·43 (28) | 12·31 ± 1·40 (28) |
| Day 21 control | 24·05 ± 1·07 (13) | 19·57 ± 1·41 (15) | 20·83 ± 1·23 (13) | 17·16 ± 0·74 (13) |
| Day 21 flu | 24·34 ± 1·14 (18) | 20·00 ± 1·02 (20) | 20·13 ± 1·12 (18) | 17·21 ± 0·86 (18) |
*Significant effect of influenza infection compared to same sex control. Data are mean ± SD.
Viral titre
There were no significant differences in lung viral titre between males and females (adults, P = 0·46; weanlings P = 0·99; Figure 1). Weanlings had significantly higher viral titres compared with adults regardless of sex (P < 0·001). No virus was detected in any control mice, nor was any detected in mice 21 days post‐inoculation.
Figure 1.
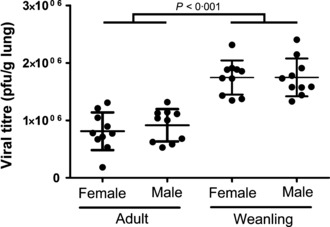
Lung viral titre (pfu/g lung) for adult (left) and weanling (right) male and female BALB/c mice 4 days post‐inoculation with influenza A. No viral titre was measured in control mice, or 21 days after infection.
Baseline lung function
During the acute phase of infection, baseline R aw, G, H and η were all significantly higher in mice infected with influenza compared to controls for both age groups (P < 0·05 in all cases; 2, 3). There was no significant effect of sex on R aw (P = 0·85), G (P = 0·86), H (P = 0·38) or η (P = 0·50) for weanling mice or on R aw (P = 0·35) or η (P = 0·33) for adult mice. Infected adult females had significantly higher baseline G and H compared with infected males at this time (P < 0·05 in both cases).
Table 2.
Baseline lung mechanics of weanling (3 weeks) and adult (8 weeks) mice during acute infection
| d4 | ||||
|---|---|---|---|---|
| R aw (cmH2O s/ml) | G (cmH2O/ml) | H (cmH2O/ml) | η | |
| Weanling male control (n = 8) | 0·45 ± 0·07 | 9·30 ± 1·56 | 48·22 ± 7·12 | 0·20 ± 0·03 |
| Weanling male flu (n = 7) | 0·57 ± 0·08* | 12·63 ± 2·67* | 57·02 ± 9·65 | 0·22 ± 0·05 |
| Weanling female control (n = 8) | 0·48 ± 0·10 | 9·20 ± 1·42 | 52·13 ± 9·08 | 0·18 ± 0·03 |
| Weanling female flu (n = 8) | 0·53 ± 0·08* | 13·29 ± 3·83* | 59·36 ± 11·98 | 0·22 ± 0·03 |
| Adult male control (n = 8) | 0·26 ± 0·03 | 5·75 ± 0·71 | 30·52 ± 2·78 | 0·19 ± 0·02 |
| Adult male flu (n = 8) | 0·28 ± 0·06* | 9·06 ± 0·92* | 34·17 ± 4·06* | 0·27 ± 0·04* |
| Adult female control (n = 8) | 0·26 ± 0·05 | 6·28 ± 0·81 | 34·11 ± 5·78 | 0·19 ± 0·01 |
| Adult female flu (n = 7) | 0·32 ± 0·07* | 13·43 ± 3·64* ** | 40·26 ± 5·85* | 0·34 ± 0·06* |
*Significant effect of influenza infection compared to same sex control.
**Female influenza is significantly greater than male influenza. Data are mean ± SD.
Table 3.
Baseline lung mechanics of weanling (3 weeks) and adult (8 weeks) mice 21 days post‐inoculation
| d21 | ||||
|---|---|---|---|---|
| R aw (cmH2O s/ml) | G (cmH2O/ml) | H (cmH2O/ml) | η | |
| Weanling male control (n = 8) | 0·28 ± 0·05 | 5·94 ± 0·73 | 29·65 ± 2·54 | 0·20 ± 0·02 |
| Weanling male flu (n = 7) | 0·28 ± 0·06 | 5·84 ± 0·61 | 29·90 ± 3·06 | 0·20 ± 0·02 |
| Weanling female control (n = 7) | 0·29 ± 0·06 | 6·97 ± 0·72 | 35·52 ± 2·95 | 0·20 ± 0·01 |
| Weanling female flu (n = 7) | 0·30 ± 0·07 | 6·74 ± 0·46 | 34·33 ± 1·99 | 0·20 ± 0·01 |
| Adult male control (n = 8) | 0·26 ± 0·04 | 5·34 ± 1·13 | 29·82 ± 7·18 | 0·18 ± 0·01 |
| Adult male flu (n = 8) | 0·25 ± 0·05 | 5·26 ± 1·30 | 29·74 ± 9·15 | 0·18 ± 0·01 |
| Adult female control (n = 9) | 0·26 ± 0·07 | 5·69 ± 1·15 | 30·48 ± 6·41 | 0·19 ± 0·01 |
| Adult female flu (n = 10) | 0·24 ± 0·03 | 5·47 ± 0·31 | 28·54 ± 2·64 | 0·19 ± 0·02 |
Data are mean ± SD.
At d21, lung function was similar between sexes and treatments. There were no significant differences in R aw between previously infected mice and controls or between sexes for either age (P > 0·25 in all cases). Similarly, for adult mice, there were no significant differences between previously infected mice and controls, or between sexes for G or H (P > 0·25 in both cases). Weanling females had significantly higher G (P < 0·001) and H (P < 0·001) than weanling males, but there was no effect of previous infection on these parameters (G: P = 0·47, H: P = 0·62).
Methacholine responsiveness
All respiratory parameters increased with increasing MCh for both age groups (2, 3). Female mice were more responsive than males for each lung function parameter measured for both ages. There was a strong effect of infection on responsiveness to MCh during acute infection, with maximum R aw, G, H and η all being significantly higher in infected mice than control mice regardless of sex or age (P < 0·001 in all cases). There was a significant interaction between sex and infection for H for both weanling and adult mice, whereby infected female mice showed significantly greater maximum H than infected male mice (weanlings P = 0·02, adults P < 0·01), although control males and females were not different (P > 0·05). For weanlings, there was a significant interaction between sex and treatment in G in that infected female mice were significantly more responsive than infected male mice (P < 0·01), yet male and female control mice were not significantly different (P = 0·32). For both age groups, η was higher for infected mice regardless of sex throughout the entire dose–response curve.
Figure 2.
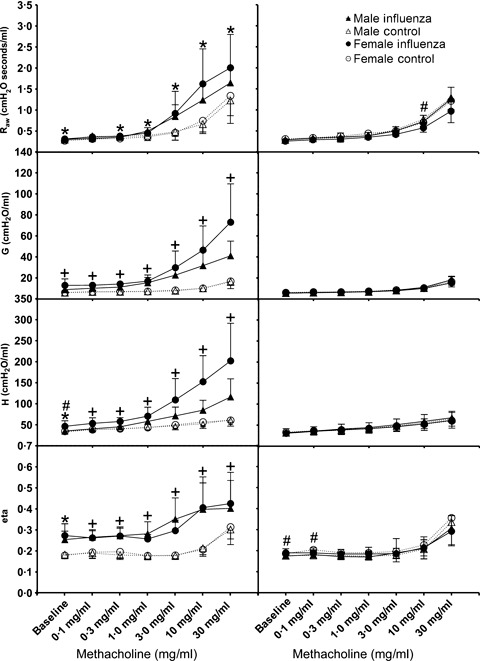
Dose–response curves for adult male (triangles) and female (circles) BALB/c mice 4 days (left panels) or 21 days (right panels) post‐inoculation with influenza A (closed symbols, solid lines) or control (open symbols, dashed lines). All data are expressed as mean ± SD (n = 7–8). Tests for significant differences were conducted at each dose using two‐way anova. * Significant effect of treatment (i.e. flu versus control), # Significant effect of sex, and + Significant interaction between treatment and sex. Significance was taken as P < 0·05 using the Holm‐Sidak post hoc test.
Figure 3.
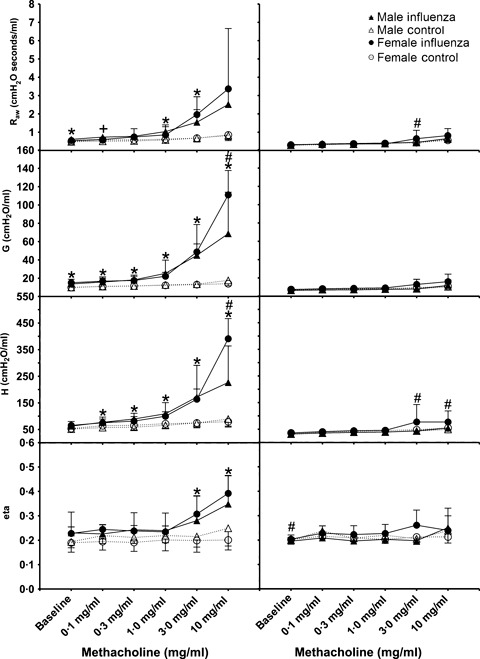
Dose–response curves for weanling male (triangles) and female (circles) BALB/c mice 4 days (left panels) or 21 days (right panels) post‐inoculation with influenza A (closed symbols, solid lines) or control (open symbols, dashed lines). All data are expressed as mean ± SD (n = 7–8). Tests for significant differences were conducted at all doses using two‐way anova. * Significant effect of treatment, # Significant effect of sex, and + Significant interaction between sex and treatment. Significance was taken as P < 0·05 using the Holm‐Sidak post hoc test.
By 21 days post infection, most parameters of respiratory function had returned to control levels for both weanlings and adults (1, 2). For adult mice, there were no residual effects of influenza infection on lung function at this time (maximum responses to MCh for previously infected mice were not different to control mice; R aw P = 0·39, G P = 0·64, H P = 0·57, ηP = 0·13), nor were there differences between sexes (R aw P = 0·06, G P = 0·19, H P = 0·24, ηP = 0·88). Previously infected female weanlings had significantly higher maximum H compared with weanling female controls (P = 0·03), yet there was no difference between previously infected male weanlings and male controls (P = 0·77).
Bronchoalveolar inflammation
At d4, total cellular inflammation was higher in infected mice compared to controls for both adults (Figure 4, P < 0·001) and weanlings (Figure 5, P < 0·001). There was no significant effect of sex on total cellular inflammation in either group. The number of macrophages, neutrophils and lymphocytes was significantly higher in both adult (P < 0·02 for all comparisons) and weanling (P < 0·009 for all comparisons) mice during acute infection; however, there was no effect of sex on numbers of any type of inflammatory cell for either age (P > 0·07 for all comparisons).
Figure 4.
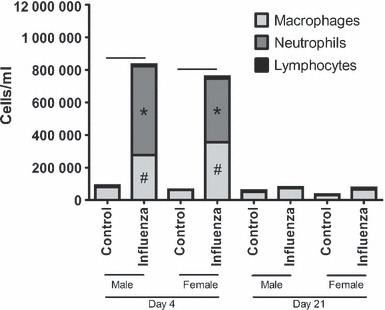
Bronchoalveolar lavage fluid differential cell counts from adult male and female BALB/c mice 4 days (left) or 21 days (right) post‐inoculation with influenza A or control. Bars indicate significant differences in total cells between groups. *,# Significant differences in the number of macrophages and neutrophils, respectively, between infected and non‐infected mice.
Figure 5.
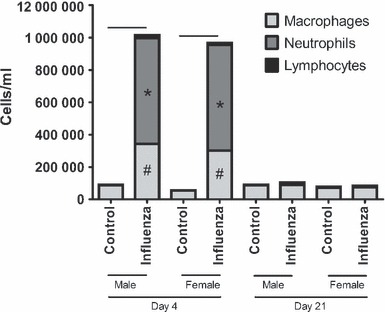
Bronchoalveolar lavage fluid differential cell counts from weanling male and female BALB/c mice 4 days (left) or 21 days (right) post‐inoculation with influenza A or control. Bars indicate significant differences in total cells between groups. *,# Significant differences in the number of macrophages and neutrophils, respectively, between infected and non‐infected mice.
At d21, previously infected weanlings still had significantly higher total cellular inflammation compared to weanling controls (P = 0·04), made up of neutrophils and lymphocytes; with no difference between males and females (P = 0·31). There were no differences related to treatment (P = 0·10) or sex (P = 0·79) in adult mice at this time.
Cytokines and protein leak
For both weanling and adult mice during acute infection, levels of TNFα, IL‐6, IFNγ and MCP‐1 in BAL fluid (Figure 6) were all significantly higher in influenza‐infected mice compared to controls (P < 0·001 in all cases). There was no difference between weanling males and females at this time point for any cytokine (P > 0·49 in all cases); however, there were some differences between adult males and females during acute infection, with females having significantly higher IFNγ (P = 0·003) and MCP‐1 (P = 0·02) compared with males. There were no differences between adult control male and female mice. There were no differences between adult male and female mice for IL‐6 or TNFα on d4 (P > 0·24 in both cases) or in any cytokine on d21 (P > 0·15 in all cases). On d21, there was no effect of previous infection or sex (P > 0·23 in all cases) on levels of any measured cytokine for weanlings or for adult mice with respect to TNFα (P = 0·93), IL‐6 (P = 0·87) or IFNγ (P = 0·31). Previously infected adults still had significantly higher MCP‐1 than controls at this time (P = 0·04). Both adults and weanlings had significantly higher protein in their BAL on d4 (P < 0·003 in all cases; Figure 6) compared to controls. Previously infected adults still had higher BAL protein than commensurate controls on d21 (P < 0·001); however, this was not the case for weanlings (P = 0·62). There was no difference between the sexes for this parameter at any time point (P > 0·46 in all cases). There was no significant effect of influenza infection or sex on levels of IL‐10 or IL‐12p70 for either adult or weanling mice (P > 0·15 in all cases) during acute infection or 21 days post‐inoculation (data not shown).
Figure 6.
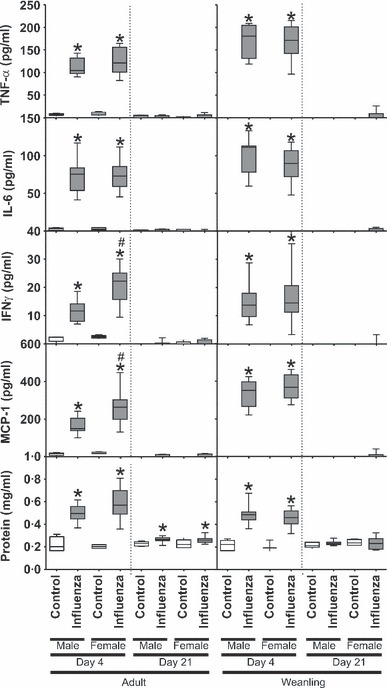
Bronchoalveolar lavage fluid levels of TNFα, IL‐6, IFNγ and MCP‐1 from adult (left) and weanling (right) male and female BALB/c mice 4 or 21 days post‐inoculation with influenza A or control. * Significant effect of influenza infection, # Significant difference between males and females.
Discussion
Data from the present study demonstrate that both weanling and adult BALB/c mice of both sexes infected with influenza A/Mem71 showed distinct physiological and inflammatory responses to infection, and hyperresponsiveness to MCh 4 days post‐inoculation. In adult mice, inflammation and hyperresponsiveness had returned to control levels 21 days post infection. Furthermore, there was sexual dimorphism in the physiological responses of BALB/c mice to influenza A, with both weanling and adult female mice showing the greatest impairments in lung function. Finally, the age of infection had profound influences on the long‐term respiratory consequences of influenza A infection, with female weanling mice alone displaying residual hyperresponsiveness 21 days after infection, when inflammation had resolved.
A number of previous studies have considered the physiological consequences of influenza infection in mice, although most use inappropriate techniques (e.g. enhanced pause: Penh), and most investigate the synergism between respiratory viral infection and allergen exposure. 16 , 28 , 29 No previous studies have considered the effects of sex or age on responses to influenza infection. Previous work by our group demonstrated that influenza infection in adult female BALB/c mice results in heightened airway responsiveness to inhaled and intravenous MCh, which resolves with clearance of the virus. 15 The adult female mouse data from the present study support our previous work.
An important finding of the present study was that we measured no differences in lung viral titre of age‐matched male and female mice, suggesting that the sexual dimorphism in lung function responsiveness (and some cytokines) was not because of higher viral replication. This infers that the ability of male and female mice to clear primary influenza infection is similar. Although there appears to be no literature comparing viral titres in male and female mice infected with influenza, one previous study found no significant difference in mean viral titre in the lungs of male and female mice infected with respiratory syncytial virus. 26
Cellular inflammation was not significantly different between males and females at either age in the present study, yet infected adult females had higher levels of IFNγ and MCP‐1 compared with infected adult males. IFNγ has many potential modes of action, including direct inhibition of viral replication and pro‐inflammatory activities. Female mice have previously been shown to produce higher levels of IFNγ in response to Mycobacterium tuberculosis, 30 , 31 possibly related to increased levels of estradiol. Similarly, amongst other functions, MCP‐1 recruits monocytes, memory T cells and dendritic cells to sites of infection. 32 , 33 MCP‐1 is expressed by monocytes, epithelial cells and smooth muscle cells and is capable of directly inactivating some respiratory viruses. 34 Of importance to this study is the knowledge that higher levels of MCP‐1 have been associated with greater mortality and severity of illness in humans infected with virulent influenza. 35 We speculate that the macrophage chemo‐attractant function of MCP‐1 may play an important role in mediating the inflammatory response by recruitment of circulating leucocytes into the lung. Despite the fact that we found no significant differences between male and female mice in terms of pulmonary inflammation, this influx explains the significant macrophage infiltrate observed in the lungs of mice infected with influenza. We also found that infected mice had increased protein leak into the alveolar compartment compared to controls. This agrees with the previous findings from our group 15 and suggests an increased permeability of the epithelium following respiratory viral infection, 23 which we have previously speculated as a mechanism for viral‐induced hyperresponsiveness. We found that there was no significant difference in total protein in the BAL between the sexes at either age, although there was a trend towards more in infected adult females compared with infected adult males. We expected that the greater physiological responses of female mice would be associated with a greater amount of total protein in the BAL, in association with increased permeability of the alveolar‐capillary barrier.
In summary, we have identified significant differences in the physiological responses of male and female mice to infection with influenza A. These differences occurred in the absence of variation in viral titre and BAL cellular inflammation. In all measured parameters where there was a difference, females showed the greatest response. Further, the physiological sexual dimorphism identified in adult mice was largely replicated in weanling mice. Of particular importance is the fact that adult mice of both sexes and weanling male mice showed complete recovery 21 days post infection; however, female weanling mice had measureable influenza‐induced hyperresponsiveness at this time. This shows that both sex and age of infection can have important effects on the physiological and inflammatory responses of mice to respiratory viral infection. Future studies investigating potential sexual dimorphism in response to viral infection should take into account the potential effects of sex hormones, which are likely to play a significant role in any differences found. This could be achieved by assessing sex hormone levels for each subject and using these data as covariates in statistical analyses. Additionally, studies employing oestrogen‐receptor knockout or gonadectomised mice could assist in identifying the mechanisms underlying sexual dimorphism in response to respiratory viral infection.
Acknowledgements
This project was funded by NH&MRC grants #458561, #458513, #458562 and #454518.
References
- 1. Caracta CF. Gender differences in pulmonary disease. Mt Sinai J Med 2003; 70:215. [PubMed] [Google Scholar]
- 2. FitzSimmons S. The changing epidemiology of cystic fibrosis. J Pediatr 1993; 123:1–9. [DOI] [PubMed] [Google Scholar]
- 3. Guilbault C, Stotland P, Lachance C et al. Influence of gender and interleukin‐10 deficiency on the inflammatory response during lung infection with Pseudomonas aeruginosa in mice. Immunology 2002; 107:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agusti A, MacNee W, Donaldson K, Cosio M. Hypothesis: does COPD have an autoimmune component? Thorax 2003; 58:832–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corteling R, Trifilieff A. Gender comparison in a murine model of allergen‐driven airway inflammation and the response to budesonide treatment. BMC Pharmacol 2004; 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Da Silva JAP. Sex hormones and glucocorticoids: interactions with the immune system. Ann N Y Acad Sci 1999; 876:102. [DOI] [PubMed] [Google Scholar]
- 7. Moller AP, Sorci G, Erritz J. Sexual dimorphism in immune defense. Am Nat 1998; 152:605–619. [DOI] [PubMed] [Google Scholar]
- 8. Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg 1999; 134:935. [DOI] [PubMed] [Google Scholar]
- 9. Klein S. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev 2000; 24:627–638. [DOI] [PubMed] [Google Scholar]
- 10. Zuk M, McKean KA. Sex differences in parasite infections: patterns and processes. Int J Parasitol 1996; 26:1009–1024. [PubMed] [Google Scholar]
- 11. Grossman C. Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. J Steroid Biochem Mol Biol 1989; 34:241–251. [DOI] [PubMed] [Google Scholar]
- 12. Verthelyi D. Sex hormones as immunomodulators in health and disease. Int Immunopharmacol 2001; 1:983–993. [DOI] [PubMed] [Google Scholar]
- 13. Miller L, Hunt J. Sex steroid hormones and macrophage function. Life Sci 1996; 59:1–14. [DOI] [PubMed] [Google Scholar]
- 14. Card JW, Voltz JW, Ferguson CD et al. Male sex hormones promote vagally mediated reflex airway responsiveness to cholinergic stimulation. Am J Physiol Lung Cell Mol Physiol 2006; 292:L908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bozanich EM, Gualano RC, Zosky GR et al. Acute influenza A infection induces bronchial hyper‐responsiveness in mice. Respir Physiol Neurobiol 2008; 162:190–196. [DOI] [PubMed] [Google Scholar]
- 16. Suzuki S, Suzuki Y, Yamamoto N, Matsumoto Y, Shirai A, Okubo T. Influenza A virus infection increases IgE production and airway responsiveness in aerosolized antigen‐exposed mice. J Allergy Clin Immunol 1998; 102:732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Melgert BN, Postma DS, Kuipers I et al. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy 2005; 35:1496. [DOI] [PubMed] [Google Scholar]
- 18. Card JW. Gender differences in murine airway responsiveness and lipopolysaccharide‐induced inflammation. J Immunol 2006; 177:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamamoto Y. Sex differences in host resistance to Mycobacterium marinum infection in mice. Infect Immun 1991; 59:4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamamoto Y, Tomioka H, Sato K, Saito H, Yamada Y, Setogawa T. Sex differences in the susceptibility of mice to infection induced by Mycobacterium intracellulare . Am Rev Respir Dis 1990; 142:430–433. [DOI] [PubMed] [Google Scholar]
- 21. Gualano R, Hansen M, Vlahos R et al. Cigarette smoke worsens lung inflammation and impairs resolution of influenza infection in mice. Respir Res 2008; 9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reading PC, Morey LS, Crouch EC, Anders EM. Collectin‐mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol 1997; 71:8204–8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol 2004; 286:L231–L246. [DOI] [PubMed] [Google Scholar]
- 24. Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 1992; 72:168–178. [DOI] [PubMed] [Google Scholar]
- 25. Sly PD, Hayden MJ, Petak F, Hantos Z. Measurement of low‐frequency respiratory impedance in infants. Am J Respir Crit Care Med 1996; 154:161–166. [DOI] [PubMed] [Google Scholar]
- 26. Collins R, Gualano R, Zosky G et al. Hyperresponsiveness to inhaled but not intravenous methacholine during acute respiratory syncytial virus infection in mice. Respir Res 2005; 6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fredberg JJ, Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol 1989; 67:2408–2419. [DOI] [PubMed] [Google Scholar]
- 28. Tsitoura DC, Kim S, Dabbagh K, Berry G, Lewis DB, Umetsu DT. Respiratory infection with influenza A virus interferes with the induction of tolerance to aeroallergens. J Immunol 2000; 165:3484–3491. [DOI] [PubMed] [Google Scholar]
- 29. Wohlleben G, Muller J, Tatsch U et al. Influenza A virus infection inhibits the efficient recruitment of Th2 cells into the airways and the development of airway eosinophilia. J Immunol 2003; 170:4601–4611. [DOI] [PubMed] [Google Scholar]
- 30. Cua D, Hinton D, Stohlman S. Self‐antigen‐induced Th2 responses in experimental allergic encephalomyelitis (EAE)‐resistant mice. Th2‐mediated suppression of autoimmune disease. J Immunol 1995; 155:4052–4059. [PubMed] [Google Scholar]
- 31. Huygen K, Palfliet K. Strain variation in interferon [gamma] production of BCG‐sensitized mice challenged with PPD: II. Importance of one major autosomal locus and additional sexual influences. Cell Immunol 1984; 85:75–81. [DOI] [PubMed] [Google Scholar]
- 32. Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T‐lymphocyte chemoattractant. Proc Natl Acad Sci USA 1994; 91:3652–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu L, Warren M, Rose W, Gong W, Wang J. Human recombinant monocyte chemotactic protein and other C‐C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol 1996; 60:365–371. [DOI] [PubMed] [Google Scholar]
- 34. Lehrer RI, Daher K, Ganz T, Selsted ME. Direct inactivation of viruses by MCP‐1 and MCP‐2, natural peptide antibiotics from rabbit leukocytes. J Virol 1985; 54:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Jong MD, Simmons CP, Thanh TT et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12:1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]


