Abstract
Please cite this paper as: Tavana et al. (2011) Influenza vaccination in patients with pulmonary sarcoidosis: efficacy and safety. Influenza and Other Respiratory Viruses DOI: 10.1111/j.1750‐2659.2011.00290.x.
Background Sarcoidosis is an inflammatory, granulomatous disorder of unknown etiology. The role of cellular and humoral immune systems in this disease is unclear, whereas dysregulation of the immune system is suggested. Patients with sarcoidosis show diverse responses while exposed to various antigens. Although influenza vaccination is recommended in pulmonary sarcoidosis, its efficacy and safety has not been investigated.
Objectives To evaluate safety and immunogenicity of influenza vaccine in patients with sarcoidosis.
Patients/Methods Influenza vaccination was performed in 23 eligible patients with sarcoidosis (SP) and 26 healthy controls (HC). Antibody titers against H1N1, H3N2, and B influenza virus antigens were evaluated just before and 1 month after vaccination. Patients were followed for 6 months to assess vaccine safety.
Results Serological response and magnitude of changes in antibody titers against influenza vaccine antigens were comparable between SPs and HCs. Women showed a better serological response against B antigen (P = 0·034) than men. Twenty‐four‐hour urine calcium was associated with antibody response against H1N1 [correlation coefficient (CC) = 0·477, P = 0·003] and H3N2 (CC = 0·352, P = 0·028) antigens. Serum angiotensin‐converting enzyme correlated negatively with antibody response against B antigen (CC = −0·331, P = 0·040). Higher residual volume was associated with fewer rises in antibody titer against H3N2 antigen (CC = −0·377, P = 0·035). No major adverse events or disease flare‐up was observed during follow‐up.
Conclusions In this study, influenza vaccination did not cause any major adverse event in SPs, and their serological response was equal to HCs. Studies with larger sample size and a broader selection of subjects could help validate the results of this study.
Keywords: Influenza, sarcoidosis, vaccination
Introduction
Sarcoidosis is an inflammatory, granulomatous, multisystemic disease of unknown etiology. 1 , 2 , 3 The most frequently affected individuals are adults aged <50 years with a peak in 20–29‐year‐old females. It has been suggested that exposure of genetically susceptible individuals to particular environmental mediators can result in the development of the disease. Any organ can be involved, but lung, skin, and eye manifestations are more frequently observed. 1 , 2 , 4 Sarcoidosis is a self‐limited disorder, resolving spontaneously in up to 60% of patients. Corticosteroid is the only recommended treatment. Relapse occurs in 25–40% of treated patients 2–3 months after discontinuation of therapy and 1–8% of them die eventually from the disease. 1 , 2 , 5
The role of humoral immunity in the pathogenesis of sarcoidosis is still debated. Immunological alterations in sarcoidosis include: accumulation of T‐helper cells (ThCs) and macrophages at the sites of inflammation, reduced systemic cellular immune response (e.g., decline in delayed hypersensitivity reaction), and provoked circulating antibody response to antigens. 1 , 2 , 6 , 7 Although increased antibody titer against several antigens is observed in sarcoidosis, 8 , 9 , 10 some studies have shown impaired antibody stimulation. 11 , 12
The main pathological feature of sarcoidosis is the formation of non‐caseating granulomas at the sites of inflammation, which are composed of ThCs and other inflammatory cells. Granulomas in lungs, lymphoid system, liver, heart, and nervous system are secondary to augmented ThC‐mediated immune reactions. 2 , 5
Yet, the efficacy of vaccination in the immunocompromised and patients with autoimmune diseases is controversial. 13 , 14 Influenza vaccination is recommended for those susceptible to acute and severe influenza infection. 14 , 15 In immunological disorders, vaccination may not induce antibody production to the preventive level or, on the other hand, might overstimulate the immune system and cause disease flare‐up. 13 In a study with Mert et al., 16 standard hepatitis B virus vaccination did not provoke protective antibody titers in patients with sarcoidosis.
Influenza infection causes substantial annual morbidity and mortality among patients with chronic lung disease. Although, to our knowledge, antibody response against influenza vaccine in patients with sarcoidosis is not yet investigated, this vaccine is highly recommended in patients with chronic pulmonary diseases such as asthma, chronic obstructive pulmonary disease, and fibrosis. 17 , 18 , 19 , 20 , 21 In this case–controlled clinical trial, we aimed to evaluate the humoral response to the trivalent 2008–2009 influenza vaccine in patients with sarcoidosis and assess vaccine safety.
Materials and methods
Study design
Subjects
This trial was performed in Shahid Modarres Medical Center, Tehran, Iran from September 2008 to March 2009. Twenty‐six patients from the outpatient clinic who met the inclusion criteria (20–60‐year‐old patients with pulmonary sarcoidosis with relevant clinical and radiological features confirmed with compatible histological characteristics) were included in the study. Transbronchial Lung Biopsy was the most commonly used method of tissue diagnosis (74% versus 22% by mediastinoscopy and 4% by video‐assisted thoracoscopic surgery). Stages of sarcoidosis were defined as follows: stage I, hilar adenopathy alone; stage II, combination of adenopathy plus infiltrates; stage III, infiltrates alone; and stage IV, fibrosis. Most cases (91·3%) were in stage I. Exclusion criteria were organ (renal, heart, or hepatic) failure, collagen vascular diseases, diabetes mellitus, any acute disease, treatment with high‐dose (>60 mg/day) steroid, and immunosuppression (e.g., organ transplantation, AIDS). Twenty‐six age‐ and sex‐matched healthy hospital staff volunteers were enrolled as controls. Three cases failed to complete the study because of loss to follow‐up.
Contraindications for vaccine inoculation (e.g., egg allergy), any psychological disease that interfered with follow‐up, and influenza vaccination within the past 5 years were exclusion criteria in both groups. Demographic and lung variables were recorded to assess their relationship with antibody response (1, 2). The Ethics Committee of Shahid Beheshti Medical University approved the study protocol, and all the study subjects gave written informed consent according to the Declaration of Helsinki. The guidelines of the European Committee for Proprietary Medicinal Products for the evaluation of influenza vaccine immunogenicity were considered in all stages of the study. 22 The protocol of this trial was registered in ClinicalTrials.gov (NCT00828828).
Table 1.
Demographic features and smoking history
| Sarcoidosis group, n = 23 | Control group, n = 26 | |
|---|---|---|
| Baseline characteristics | ||
| Age, mean (SD) | 45·83 (7·09) | 42·23 (8·08) |
| Male/female, n | 8/15 | 9/17 |
| BMI, mean (SD) | 28·21 (5·56) | 25·84 (3·92) |
| Smoking habit, n (%) | 2 (8·7) | 0 (0) |
SD, standard deviation; BMI, body mass index.
Table 2.
Pulmonary clinical and para‐clinical features in the sarcoidosis group
| Stage of sarcoidosis | |
| Stage I, n (%) | 21 (91·30) |
| Stage II, n (%) | 0 (0) |
| Stage III, n (%) | 2 (8·70) |
| Stage IV, n (%) | 0 (0) |
| Clinical and para‐clinical characteristics | |
| DLCO, mean (SD) | 94·38 (14·99) |
| TLC, mean (SD) | 97·89 (18·10) |
| RV, mean (SD) | 120·37 (35·43) |
| RV/TLC, mean (SD) | 41·89 (12·81) |
| FEV1, mean (SD) | 87·50 (17·69) |
| FVC, mean (SD) | 90·16 (15·70) |
| FEV1/FVC, mean (SD) | 83·17 (9·13) |
| MMFR, mean (SD) | 70·52 (26·53) |
| ESR, mean (SD) | 21·33 (13·91) |
| Serum ACE level, mean (SD) | 78·78 (35·04) |
| 24‐hour urine calcium, mean (SD) | 179·16 (58·09) |
| Eye involvement, n (%) | 3 (13·0) |
| Low‐dose corticosteroid therapy*, n (%) | 1 (4·3) |
DLCO, diffusing capacity of the lung for carbon monoxide; TLC, total lung capacity; RV, residual volume; FEV1, forced expiratory volume in 1 second; FVC, functional vital capacity; MMFR, maximal midexpiratory flow rate 25–75%; ESR, erythrocyte sedimentation rate; ACE, angiotensin‐converting enzyme (measured with ELISA method).
*<15 mg/day.
Outcome measures
Outcome measures included frequency of protective antibody titers before vaccination, magnitude of change in antibody titer after vaccination, and serological response [i.e., ≥4‐fold rise in hemagglutination inhibition (HI) titers] to each of the three antigens of the trivalent influenza vaccine 1 month after vaccination. Occurrence of local (erythema, pain, induration, or ecchymosis at injection site) or systemic (malaise, fever, shivering, myalgia, headache, Guillain–Barré Syndrome) adverse reaction to influenza vaccine was also recorded. All patients were followed for 6 months (months 1, 2, 4, and 6) after vaccination for signs and symptoms of sarcoidosis flare‐up (shortness of breath, dry cough, wheezing, enlarged lymph nodes, skin, or eye symptoms), number of influenza episodes, and adverse reaction to the vaccine, either local or systemic. All assessments were made by the same pulmonologist.
Vaccination and antibody titration
All study participants received 0·5 ml of the trivalent influenza vaccine (influvac; Solvay Pharma, Weesp, Netherlands) by subcutaneous injection into the upper arm. The injection was given into the opposite arm from which blood was drawn. The vaccine contained 15 μg hemagglutinin of each of the three strains, namely Brisbane/59/2007 (H1N1), Brisbane/10/2007 (H3N2), and Florida/4/2006 (B), according to the World Health Organization guidelines for the influenza vaccination campaign of 2008–2009.
A 10 ml blood sample was drawn just before vaccination and 1 month thereafter. Serum samples were separated and stored frozen (−20°C) immediately after collection. Hemagglutination inhibition test was employed for antibody titration using the hemagglutinin antigens that were representative of the virus strains contained in the vaccine. The test was performed using twofold dilutions of the serum from 1:10 to 1:1280. Titers of <1:10 were calculated as 1:5.
Analysis
Continuous variables were presented by mean (SD) or median (interquartile range, IQR). The paired between‐group comparisons of the continuous variables were performed using the non‐parametric Mann–Whitney U test. Significance of differences between each pair of dichotomous variables was evaluated by Fisher’s exact test.
To identify the association of independent factors, including demographic and pulmonary variables (listed in 1, 2), with the magnitude of increase in antibody titers against the vaccine, Kendall’s τ‐b and Spearman’s ρ tests were used.
P < 0·05 was considered to indicate statistical significance in all comparisons.
Results
Baseline characteristics
Baseline characteristics for both groups are summarized in Table 1. There was no significant difference in age, sex, body mass index, and smoking habit between groups. Table 2 demonstrates clinical and para‐clinical variables of patients with sarcoidosis.
Response measures among study groups
Serological response to influenza vaccine antigens tended to be more frequent in patients with sarcoidosis than in healthy controls (H1N1: 74% versus 50%; H3N2: 83% versus 62%; B: 65% versus 50%), although the differences did not reach statistical significance. Magnitude of change in antibody titer against all three antigens was similar in sarcoidosis and control groups (Table 3, Figure 1). Protection rate against the antigens before vaccination did not differ between groups, yet the sarcoidosis group showed significantly higher protection rate against B antigen after vaccination (P = 0·026).
Table 3.
Response measures to 2008–2009 influenza vaccine antigens
| Sarcoidosis group, n = 23 | Control group, n = 26 | |
|---|---|---|
| Brisbane/59/2007 (HIN1) | ||
| Protective (≥1:40) HI titer before vaccination, n (%) | 7 (30·4) | 7 (26·9) |
| Protective (≥1:40) HI titer after vaccination, n (%) | 19 (82·6) | 17 (65·4) |
| Magnitude of change, ×fold, median (IQR) | 8 (15) | 3 (15) |
| Serologic response (≥4 fold HI titer rise), n (%) | 17 (73·9) | 13 (50·0) |
| Mean antibody titer changes after vaccination, n, M/F | 7·75/15·47 | 8·89/13·24 |
| Brisbane/10/2007 (H3N2) | ||
| Protective (≥1:40) HI titer before vaccination, n (%) | 16 (69·6) | 11 (42·3) |
| Protective (≥1:40) HI titer after vaccination, n (%) | 22 (95·7) | 21 (80·8) |
| Magnitude of change, ×fold, median (IQR) | 8 (12) | 4 (9) |
| Serologic response (≥4 fold HI titer rise), n (%) | 19 (82·6) | 16 (61·5) |
| Mean antibody titer changes after vaccination, n, M/F | 14·25/19·33 | 6·78/16·82 |
| Florida/4/2006 (B) | ||
| Protective (≥1:40) HI titer before vaccination, n (%) | 13 (56·5) | 12 (46·2) |
| Protective (≥1:40) HI titer after vaccination, n (%) | 22 (95·7) | 18 (69·2) |
| Magnitude of change, ×fold, median (IQR) | 4 (6) | 3 (7) |
| Serologic response (≥4 fold HI iter rise), n (%) | 15 (65·2) | 13 (50·0) |
| Mean antibody titer changes after vaccination, n, M/F | 2·63/28·80 | 2·22/9·53 |
HI, hemagglutination inhibition; IQR, interquartile range; M, male; F, female.
Figure 1.
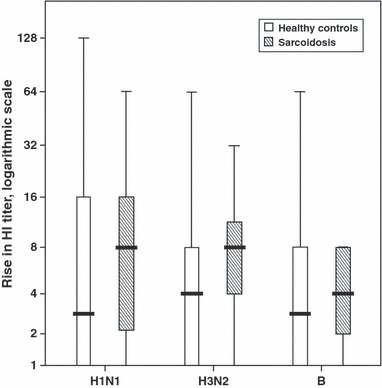
Boxplot; distribution of the magnitude of HI antibody rise against each of the three antigens for both study groups. HI: hemagglutinin antibody.
Factors predictive of antibody response
In regard to gender, female subjects in both groups showed a greater increase in antibodies against all antigens, still only significant for B antigen (P = 0·034 among patients with sarcoidosis; P = 0·008 including all subjects).
The 24‐hour urinary calcium (UCa) was independently associated with the magnitude of antibody increase against H1N1 [correlation coefficient (CC) = 0·477, P = 0·003] and H3N2 (CC = 0·352, P = 0·028) antigens (Table 4). Blood level of angiotensin‐converting enzyme (ACE) was independently associated with lower antibody response against B antigen (CC = −0·331, P = 0·040, Table 4). Higher respiratory residual volume (RV) was independently associated with fewer rises in the magnitude of antibody titer against H3N2 antigen (CC = −0·377, P = 0·035, Table 4).
Table 4.
Factors associated with magnitude of antibody increment against antigens of 2008–2009 influenza vaccine in patients with sarcoidosis
| Correlation coefficient | P value | |
|---|---|---|
| Brisbane/59/2007 (HIN1) | ||
| 24‐hour urine calcium | 0·477 | 0·003 |
| Residual volume | −0·236 | NS |
| Serum ACE level | −0·241 | NS |
| Female sex* | – | NS |
| Brisbane/10/2007 (H3N2) | ||
| 24‐hour urine calcium | 0·352 | 0·028 |
| Residual volume | −0·377 | 0·035 |
| Serum ACE level | −0·198 | NS |
| Female sex* | – | NS |
| Florida/4/2006 (B) | ||
| 24‐hour urine calcium | 0·101 | NS |
| Residual volume | −0·026 | NS |
| Serum ACE level | −0·331 | 0·040 |
| Female sex* | – | 0·034 |
NS, not significant; ACE, Angiotensin‐converting enzyme.
*Analyzed with chi‐squared test
Adverse events and vaccine safety
No major systemic or local adverse event was noted, but minor pain and erythema at the injection site were seen in 17·4% of the patients with sarcoidosis and 11·5% of the controls. After 6 months of follow‐up, no sign of disease flare‐up or major adverse events were observed in the patients. No episode of influenza like symptoms was recorded in the course of follow‐up.
Discussion
This study found patients with sarcoidosis and healthy controls to have similar serological response to influenza vaccine. High antibody titers against several antigens (Epstein–Barr, herpes simplex, rubella, measles and parainfluenza viruses, and mismatched blood) have been detected in patients with sarcoidosis. 5 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 In contrast, in the study of Mert et al., 16 none of 16 patients with sarcoidosis developed antibody response to hepatitis B vaccine. In another study, peripheral blood B cells obtained from patients with sarcoidosis produced less immunoglobulin against pokeweed‐mitogen compared to normal B cells. 12
The role of cellular and humoral immunity in sarcoidosis is not yet clear. Dominance of ThC type 1 cytokines (e.g., IFN‐γ and IL‐2) in the lung and accumulation of ThCs in the granulomatous lesions that leads to cutaneous anergy (e.g., to Mantoux test) are seen in sarcoidosis. A weaker immune response in the periphery in contrary to accumulation of immune T cells in the injured sites is referred to as ‘immune paradox’. 1 , 23 The humoral immune response is largely provoked by cellular immunity. ThCs have an important role in stimulating B cells to produce antibodies; consequently, their depletion in the peripheral blood can reduce ThC‐mediated B‐cell antibody response (as observed against hepatitis B vaccine). 16 , 23 Cytotoxic T cells, in addition to ThCs, play a role in stimulating B‐cell antibody production against influenza infection. 24 Cytotoxic T‐cell distribution is generally not affected in sarcoidosis, 1 , 23 and this could explain the profound response of patients to influenza vaccine in our study.
A higher serological response in women (2–11‐fold) is not an unexpected event because they have higher cellular and humoral immune activity. 25
As granulomas secrete ACE and calcitriol, serum ACE level and UCa are used to monitor sarcoidosis activity. 1 , 23 Our study found serum ACE level and UCa to be associated with lower and higher antibody response, respectively. Greater immune activity at the inflammation sites (i.e., granulomatous lesions) and higher UCa (as a result of higher cellular immune system activation at the inflammation sites) can be associated with greater antibody titer, as observed in our study. The negative association of ACE level with antibody response could be questioned because serum ACE level is under the influence of several factors. 26 , 27 , 28
We found an association between RV and antibody increment; nevertheless, the lack of association between other spirometry parameters and serological response decreases the merit of this finding. However, higher RV can be observed in patients with sarcoidosis compared with normal controls, which is explained by obstruction of the airways as a result of endobronchial involvement. 1 , 4 , 29 Most patients with sarcoidosis (91%) were in stage I, and because of the low number of patients in other stages, it is not possible to make an estimate about the effect of staging on their humoral response. Only one of the patients was on low‐dose steroid treatment; therefore, steroid is unlikely to be an interfering factor with antibody response in this study.
Influenza vaccine is found to be effective and safe in other autoimmune disease (e.g., lupus erythematosus and rheumatoid arthritis), and no disease flare‐up has been observed. 30 In our study, no major adverse event or disease flare‐up was noted after 6 months of clinical follow‐up.
Our study population does mostly include patients with stage I disease (93·1%); hence, further studies with greater power and samples representative of all disease stages would add to and help validate the results of this study.
Only one patient was on low‐dose corticosteroid therapy, which renders corticosteroid an improbable confounding variable. No episode of flu‐like symptom was recorded during 6 months after vaccination in patients with sarcoidosis, thus natural exposure to circulating influenza antigens is unlikely to play a role in our patients antibody response to the vaccine.
Conclusions
Patients with sarcoidosis did not show any major adverse reaction or disease flare‐up following influenza vaccination, and their serological response was comparable with healthy individuals. No episode of influenza infection was observed during the 6‐month follow‐up. Findings on relations of ACE, RV, and UCa with antibody response lack adequate power because of the small sample size and the fact that this study was not primarily intended to evaluate such relations. As our patient population is essentially representative of patients with stage I sarcoidosis, studies with larger sample size, including patients in all disease stages, would help to generalize the results of this study.
References
- 1. Dempsey OJ, Paterson EW, Kerr KM, Denison AR. Sarcoidosis. BMJ 2009; 339:620–625. [DOI] [PubMed] [Google Scholar]
- 2. Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J 1999; 14:735–737. [DOI] [PubMed] [Google Scholar]
- 3. Marcoval J, Mañá J. Specific (granulomatous) oral lesions of sarcoidosis: report of two cases. Med Oral Patol Oral Cir Bucal 2010; 15:e456–e458. [PubMed] [Google Scholar]
- 4. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997; 336:1224–1234. [DOI] [PubMed] [Google Scholar]
- 5. Belfer MH, Stevens RW. Sarcoidosis: a primary care review. Am Fam Physician 1998; 58:2041–2050, 2055–6. [PubMed] [Google Scholar]
- 6. Müller‐Quernheim J. Sarcoidosis: immunopathogenetic concepts and their clinical application. Eur Respir J 1998; 12:716–738. [DOI] [PubMed] [Google Scholar]
- 7. Zissel G, Prasse A, Müller‐Quernheim J. Immunologic response of sarcoidosis. Semin Respir Crit Care Med 2010; 31:390–403. [DOI] [PubMed] [Google Scholar]
- 8. James DG, Neville E, Walker A. Immunology of sarcoidosis. Am J Med 1975; 59:388–394. [DOI] [PubMed] [Google Scholar]
- 9. Pasquali J, Godin D, Urlacher A, Pelletier A, Pauli G, Storck D. Abnormalities of in vitro responses to polyclonal activation of peripheral blood lymphocytes in patients with active sarcoidosis. Eur J Clin Invest 1985; 15:82–88. [DOI] [PubMed] [Google Scholar]
- 10. Byrne EB, Evans AS, Fouts DW, Israel HL. A seroepidemiological study of Epstein–Barr virus and other viral antigens in sarcoidosis. Am J Epidemiol 1973; 97:355–363. [DOI] [PubMed] [Google Scholar]
- 11. Majer RV, Green PJ. The immune response in sarcoidosis. Lancet 1987; 2:195–196. [PubMed] [Google Scholar]
- 12. Katz P, Fauci A. Inhibition of polyclonal B cell activation by suppressor monocytes in patients with sarcoidosis. Clin Exp Immunol 1978; 32:554–562. [PMC free article] [PubMed] [Google Scholar]
- 13. Nosal GJ. Vaccination and autoimmunity. J Autoimmun 2000; 14:15–22. [DOI] [PubMed] [Google Scholar]
- 14. Aron‐Maor A, Shoenfeld Y. Vaccination and autoimmunity; in Shoenfeld Y, Rose NR. (eds): Infection and autoimmunity. Amsterdam: Elsevier BV, 2004; 105–116. [Google Scholar]
- 15. Herron A, Dettleff G, Hixon B et al. Influenza vaccination in patients with rheumatic diseases: safety and efficacy. JAMA 1979; 242:53–56. [PubMed] [Google Scholar]
- 16. Mert A, Bilir M, Ozaras R, Tabak F, Karayel T, Senturk H. Results of Hepatitis B Vaccination in sarcoidosis. Respiration 2000; 67:543–545. [DOI] [PubMed] [Google Scholar]
- 17. Kmiecik T, Arnoux S, Kobryn A, Gorski P. Influenza vaccination in adults with asthma: safety of an inactivated trivalent influenza vaccine. J Asthma 2007; 44:817–822. [DOI] [PubMed] [Google Scholar]
- 18. Bansal AS, Bruce J, Hogan PG, Allen RK. An assessment of peripheral immunity in patients with sarcoidosis using measurements of serum vitamin D3, cytokines and soluble CD23. Clin Exp Immunol 1997; 110:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wat D, Gelder C, Hibbitts S et al. Is there a role for influenza vaccination in cystic fibrosis? J Cyst Fibros 2008; 7:85–88. [DOI] [PubMed] [Google Scholar]
- 20. Brundage JF. Cases and deaths during influenza pandemics in the United States. Am J Prev Med 2006; 31:252–256. [DOI] [PubMed] [Google Scholar]
- 21. Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Influenza vaccination health impact and cost effectiveness among adults aged 50–64 and 65 and older. Am J Prev Med 2006; 31:72–79. [DOI] [PubMed] [Google Scholar]
- 22. Note for guidance on harmonisation of requirements for influenza vaccines. Committee for Proprietary Medicinal Products (CPMP); 1997. [http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf].
- 23. Lazarus A. Sarcoidosis: epidemiology, etiology, pathogenesis, and genetics. Dis Mon 2009; 55:649–660. [DOI] [PubMed] [Google Scholar]
- 24. La Gruta NL, Turner SJ, Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus‐specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J Immunol 2004; 172:5553–5560. [DOI] [PubMed] [Google Scholar]
- 25. Nalbandian G, Kovats S. Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen‐presenting cells. Immunol Res 2005; 31:91–106. [DOI] [PubMed] [Google Scholar]
- 26. Eyries M, Michaud A, Deinum J et al. Increased shedding of angiotensin‐converting enzyme by a mutation identified in the stalk region. J Biol Chem 2001; 276:5525–5532. [DOI] [PubMed] [Google Scholar]
- 27. Kramers C, Danilov SM, Deinum J et al. Point mutation in the stalk of angiotensin‐converting enzyme causes a dramatic increase in serum angiotensin‐converting enzyme but no cardiovascular disease. Circulation 2001; 104:1236–1240. [DOI] [PubMed] [Google Scholar]
- 28. Nesterovitch AB, Hogarth KD, Adarichev VA et al. Angiotensin I‐converting enzyme mutation (Trp1197Stop) causes a dramatic increase in blood ACE. PLoS ONE 2009; 4:e8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baydur A, Alsalek M, Louie SG, Sharma OP. Respiratory muscle strength, lung function, and dyspnea in patients with sarcoidosis. Chest 2001; 120:102–108. [DOI] [PubMed] [Google Scholar]
- 30. Stojanovich L. Influenza vaccination of patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Clin Dev Immunol 2006; 13:373–375. [DOI] [PMC free article] [PubMed] [Google Scholar]


