Abstract
The hemostatic system is often subverted in patients with cancer, resulting in life-threatening venous thrombotic events. Despite the multifactorial and complex etiology of cancer-associated thrombosis, changes in the expression and activity of cancer-derived tissue factor (TF) – the principle initiator of the coagulation cascade – are considered key to malignant hypercoagulopathy and to the pathophysiology of thrombosis. However, many of the molecular and cellular mechanisms coupling the hemostatic degeneration to malignancy remain largely uncharacterized. In this review we discuss some of the tumor-intrinsic and tumor-extrinsic mechanisms that may contribute to the prothrombotic state of cancer, and we bring into focus the potential for circulating tumor cells (CTCs) in advancing our understanding of the field. We also summarize the current status of anti-coagulant therapy for the treatment of thrombosis in patients with cancer.
Keywords: Coagulation, Thrombosis, Cancer, Circulating Tumor Cells, Tissue Factor, Platelets
1. Introduction
In 1865, Armand Trousseau was the first to consider thrombotic events as reflective of malignant burden and indicative of a poor outcome for patients with cancer (1). Today, Trousseau’s syndrome is considered intrinsically related in part to cancer progression as the consequence of an aberrant thrombotic activity exploited by malignant tumors. As a result, patients diagnosed with malignancy following an initial episode of thromboembolism have a lower survival rate and higher mortality risk in comparison to those patients without an underlying thrombotic condition (2, 3). Indeed, complications from venous thromboembolism (VTE), including pulmonary embolism, are the second leading cause of death for patients with cancer, with the risk of VTE elevated from 7-fold to up to 28-fold in patients with cancer as compared to patients without cancer (3). This variability in VTE risk is correlated with the type of malignancy, patient demographics, treatment regimen, duration of follow-up period, period of study, and method of detecting and reporting VTE (4). Several mechanisms have been suggested to contribute in part and in combination to the increased thrombotic complications observed in patients with cancer: 1) the expression of tissue factor (TF) by circulating tumor cells (CTCs); 2) the shedding of procoagulant microparticles by malignant cells; 3) the interaction of cancer cells with blood platelets; 4) the generation of neutrophil extracellular traps (NETs); and 5) the secondary deleterious effects of anti-cancer therapies (3). Despite the tremendous strides made in defining the molecular and genetic drivers of tumorigenesis, much remains to be learned about the mechanisms underlying the observed increase of thrombotic events in patients with malignancy. In this review, we will summarize the complex biological processes related to cancer-associated thrombosis and we will bring to the fore some of the questions that we believe need to be addressed to better understand the prothrombotic biology of cancer.
2. The procoagulant phenotype of tumor cells and microparticles
Tissue factor is widely considered to be the major molecular driver of cancer-associated coagulopathy and thromboembolic disorders (8). TF is a membrane receptor and protein co-factor required for initiating the extrinsic coagulation cascade necessary for physiological hemostasis (4–7). TF exposure following tissue injury converts the zymogen coagulation factor VII (FVII) to activated factor VIIa (FVIIa), thus potentiating its catalytic efficiency in converting factor X to activated factor X (FXa) and factor IX to activated factor IX (FIXa). The catalytic efficiency of these reactions is increased by several orders of magnitude by the presence of calcium (Ca2+) and an anionic phospholipid surface. For instance, FIXa forms the tenase complex with its protein cofactor FVIIIa on the surface of phosphatidylserine (PS)-containing cell membranes in the presence of calcium ions to generate FXa. The protease FXa produced on phosphatidylserine (PS)-expressing cell membranes reversibly associates, in a Ca2+-dependent manner, with the cofactor factor Va (FVa), forming the prothrombinase complex (19). The latter converts pro-thrombin (FII) into the serine protease thrombin (FIIa). Thrombin is a promiscuous enzyme which cleaves fibrinogen into insoluble fibrin, activates platelets and amplifies its own production through direct activation of factor XI (FXI) and the cofactors FVIII and FV (8, 9). TF-dependent reactions are negatively regulated by the tissue factor plasma protein inhibitor (TFPI), which forms a quaternary complex with TF/FVIIa and FX to inhibit thrombin generation (20).
Membrane bound- and intravascular-TF expression and activity has been shown to be upregulated in many human cancers compared with normal tissues, often correlating with thromboembolic complications and poor prognosis (9–15). The highest levels of TF expression have been reported in cancers which are most strongly associated with a high incidence of thrombotic events such as cancers of the pancreas, brain, lung, stomach, ovaries and gliomas (10, 11, 12, 14, 16–18). In vitro, cancer cells exhibit highly procoagulant surfaces characterized by prominent expression of TF and PS which are able to generate thrombin (3–5, 11). Additionally, cultured cancer cells have been shown to endogenously synthetize FVIIa, which is capable of sustaining TF catalytic activity and thus, the amplification of the procoagulant activity of cancer cells (12). Moreover, TF-expressing microparticles (MPs) shed from growing tumors have been demonstrated to enhance thrombus propagation in a mouse model of vessel injury (15). This finding is analogous to the evidence that TF-positive MPs derived from leukocytes trigger the activation of the coagulation cascade following incorporation into thrombi. Specifically, the localization of TF-MPs into a developing thrombus has been shown to be mediated by the selective interaction of PGSL-1 on the MPs with P-selectin expressed on the membrane of activated platelets (13–15). Consequently, anti-P-selectin blocking antibodies have been shown to markedly reduce thrombotic events in mice with solid tumors (16). Furthermore, in vivo studies of carcinoma cells implanted in mice have shown that the release of TF-MPs was proportional to the size of the primary tumor and its TF-expression levels, supporting the hypothesis that primary cancer cells may be a predominant source of TF-MPs (18, 19). Subsequent experiments demonstrated that the ability of cancer cells and MPs to form a thrombus in vitro could be abrogated by TF-blocking antibodies or by annexin V, which binds to the membrane-exposed PS to inhibit activation of FX (4).
The increased expression of TF in tumor is considered to be the result of the activation of dominant-acting oncogenes or loss of recessive tumor suppressors rather than dictated by genetic aberrations of the TF gene, as different genetic loci regulate the levels of TF in cancer. For example, in colorectal cancer cells (CRC), the proto-oncogene kRAS and the tumor suppressor p53 have been shown to cooperate to cause TF regulation at a transcriptional and translational level (17). Similarly, loss of PTEN, a lipid phosphatase known to be essential for tumor suppression, has been found to be associated with profound upregulation of TF in cultured tumor cells, and promote their procoagulant activity (18). In addition, in a mouse model of tumorigenesis, the oncoprotein MET has been demonstrated to enhance the pathological procoagulant activity of cancer cells via upregulation of the hemostatic plasminogen activator inhibitor type 1 (PAI-1) and cyclooxygenase-2 (COX-2) genes (19). Furthermore, transforming growth factor β (TGFβ) has been reported to regulate TF expression through the induction of an epithelial to mesenchymal transition (EMT) in cancer cells (20).
To date, whilst the mechanisms describing the genetics underlying TF expression in malignancy have been largely described, is still unclear whether TF on cancer cells functions in a regulated fashion. Cell culture studies have shown that resting intravascular cells, such as monocytes, express a membrane-bound encrypted form of TF, with negligible procoagulant response, which can be subsequently decrypted to locally activate FX (21). The molecular determinants underlying the conversion of the encrypted TF into its procoagulant form (decryption) remain ill-defined, if not controversial (23). Several in vitro studies have proposed that the increase in membrane exposure of negative charged lipids, such as phosphatidylserine, is a key cellular determinant of the conversion of encrypted TF towards its active form (24–26). Moreover, mutational studies in which cysteines of the TF extracellular disulfide loop (Cys186-Cys209) were substituted with serines or alanines uncovered the importance of the disulfide isomerization for TF decryption (27, 28). In this context, the disulfide exchange to form a disulfide bond within TF has been shown to be regulated by the targeted action of the protein disulfide isomerase (PDI) (22). Adding to this complexity is the fact that tumor cells may exhibit extensive intra- and inter-procoagulant phenotypic heterogeneity, potentially deriving from stochastic events in TF protein expression and microenvironment signals (23). Along these lines, a number of studies have shown the role of the microenvironment as a key mediator of TF activation and function on endothelial cells, vascular smooth muscle cells, monocytes and macrophages (29). For instance, the vascular expression of TF as well as its procoagulant potential are known to be regulated by reactive oxygen species (ROS), inflammatory cytokines (e.g., tumor necrosis factor-α), biogenic amines (e.g., serotonin) and molecular activators (e.g., thrombin) (29, 30). However, whether tumor cells possess a cryptic form of TF and whether its activation is controlled by microenvironment-derived paracrine signals or internal cellular structural rearrangements is not known.
It is important to note that the physiological activation of the coagulation system in blood and plasma by triggers such as bacteria can only be achieved when the surface concentration of procoagulant stimuli is greater than a threshold value (44, 45). In the setting of cancer, it is unclear whether a threshold value of procoagulant activity is required to initiate the coagulation cascade and generate sufficient thrombin production to form fibrin and activate platelets. Moreover, the distinct activity of TF seems to be influenced by the physiological variance in the levels of coagulation factors, specifically coagulation factor IX (FIX) and factor X (FX) (50). These findings suggest that a diverse set of extracellular procoagulant mediators as well as exposure to different microenvironment niches may signal to influence the activity of tumor-derived TF, with potential profound prognostic implications for cancer-related thrombosis.
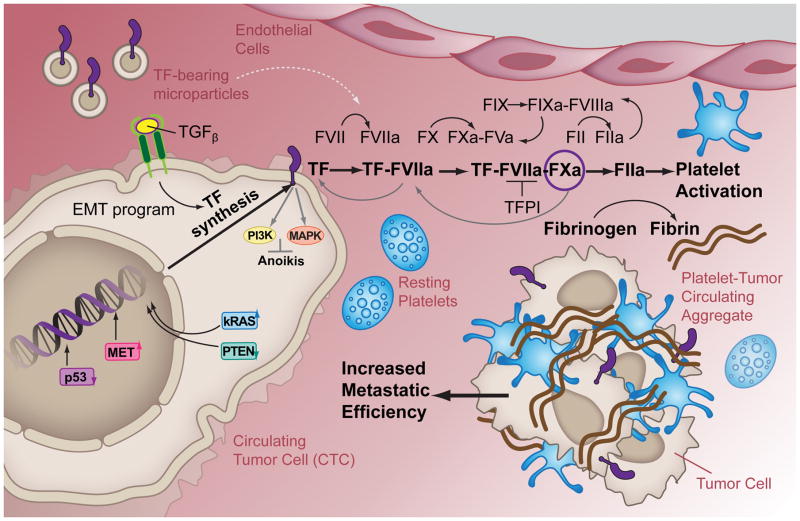
The key question remains: to what degree does the microenvironment contribute to the procoagulant phenotype of cancer cells and thus to the risk of developing thrombotic events? Indeed, the procoagulant tendency of cancer cells is considered as one of the causes responsible for the prothrombotic state of patients with cancer. It is perhaps intuitive that a contact between TF-bearing tumor cells (and/or microparticles) and the blood system would trigger the coagulation cascade in malignancy. Cancer cells shed from primary tumor and wandering in the bloodstream, referred to as circulating tumor cells (CTCs), could represent a “vector” of cancer-associated thrombosis. Figure 1 depicts the potential causes and consequences of procoagulant CTCs. It is also worth mentioning that CTCs can be found as a multicellular cluster in homotypic and/or heterotypic (associated with platelets and white blood cells) aggregates (24–26). However, the contribution of single CTCs or CTC clusters to thrombotic events remains ill-defined. Certainly, the possibility to isolate and analyze CTCs and CTC clusters may provide insights into mechanisms of thrombosis in cancer. Indeed, the biophysical, molecular and genetic profiling of CTCs from patient samples may be used to define their thrombotic potential and act as biomarkers to develop recommendations for the use of anticoagulant therapies to prevent or block the onset of vascular events in patients with cancer. Also, given the heterogeneous character of cancer, there might be subpopulations within individual tumors that possess different procoagulant phenotypes. It remains to be defined whether subpopulations of CTCs activate the coagulation cascade and platelet responses responsible for thrombosis, and whether hematogenous CTC clusters have a higher prothrombotic potential compared to single CTCs.
FIGURE 1. The procoagulant phenotype of circulating tumor cells.
In CTC, the expression of the procoagulant protein TF can be enhanced by intrinsic and extrinsic cellular routes. The intrinsic route includes the mutated activity of tumor suppressors (p53 and PTEN) and/or oncogenes (kRAS and MET) that aberrantly induce the transcription and translation of the TF gene. The extrinsic route is initiated outside the cell by TGFβ paracrine signaling, thereby dependent on the EMT program. The TF cytoplasmic domain is linked to a variety of signal transduction proteins (e.g., PI3K and MAPK) that become activated following cell intravasation to prevent anoikis. At the same time, the ectodomain of TF on the CTC surface or on shed tumor-derived microparticles (MPs) initiates the extrinsic coagulation cascade leading to thrombin generation and thus, fibrin formation and platelet activation.
The morphological abnormalities of tumor blood vessels consisting of excessive and haphazard branching, marked permeability, leakage of plasma, and clotting of extravasated fibrinogen and chronic vascular damage may exacerbate thrombotic events in patients with malignancy (28–30). Indeed, many kinds of carcinomas, including those of the breast and stomach, often succeed in stimulating extravascular coagulation in active angiogenic sites by upregulating TF expression (31, 32). However, no studies to date have explored the differential procoagulant state of CTCs when compared to tumor cells localized in a primary or metastatic mass.
3. The intricate involvement of TF-bearing tumor cells in cancer progression
Metastasis - the dissemination of cancer cells from the primary tumor mass to distant organs - occurs predominantly through the bloodstream (27) and is responsible for as much as 90% of cancer-associated mortality (28). The metastatic process is an intricate multi-step cascade during which cancer cells intravasate from the primary site into the blood vessels, circulate in the bloodstream towards distant anatomical sites, adhere to the luminal wall of micro-vessels (arterioles and capillaries) and penetrate into the surrounding tissue (extravasation) to eventually form secondary colonies (29). Perhaps TF expression confers a survival advantage to cancer cells in the circulation. Indeed, inhibition of TF, FXa or thrombin has been shown to prevent the formation of metastatic niches in SCID mice bearing human melanoma tumors (41–43). Similarly, C57Bl/6 mice injected with highly metastatic tumor cells devoid of TF were protected from metastatic disease (44). Along similar lines, metastasis was also abrogated when mice were injected with melanoma cells in presence of an anti-TF antibody which impaired TF ability to bind to FVIIa and initiate the proteolytic cascade (34).
Normal cell survival depends on anchorage to solid substrates; thus, the detachment of cells from a matrix support results in anoikis (33). Remarkably, the functional TF-FVIIa complex is thought to orchestrate cell survival signals in TF-expressing cells through its ability to activate downstream anti-apoptotic targets such as MAP kinase and PI3 kinase, and in particular in the absence of mitogenic signaling in the context of anoikis (34, 35) (Figure 1). In addition, the survival advantage provided by the TF-FVIIa complex has been shown to be further enhanced by the presence of FXa (34). Together these observations suggest that procoagulant activity may permit cancer cells to acquire multiple alterations and survive under conditions that usually lead to death of nontransformed cells. It remains unclear whether clustering of CTC enhances the activation of the TF-FVIIa signaling cascade to increase their metastatic potential in the bloodstream as compared to single CTCs. Using mouse models of metastasis, Aceto and colleagues have demonstrated that the metastatic efficiency of CTC clusters is from 23- to 50- fold higher that single CTCs (30). Additionally, the metastatic potential of cancer cell lines has been shown to correlate with their ability to initiate the proteolytic cascade in vitro (5). As previously mentioned, genetic alterations that are responsible for tumor progression appear to confer a procoagulant phenotype and possible survival benefit. Thus, the control of the expression of the TF gene by oncogenes may create a feedback pathway that selects for procoagulant CTCs. Evidence supporting a pathological role for procoagulant activity in cancer progression is based upon a large number of epidemiological observations in which the administrations of low-molecular-weight heparins (LMWH) resulted in a delay in metastasis growth in a variety of tumors in patients (15–18). Together, these studies raise the possibility that metastasis and procoagulant activity of CTCs are intrinsically linked.
Several studies have demonstrated that TF may be directly involved in tumor growth, angiogenesis and invasion independently of its hemostatic activity (32, 89–92). TF in complex with FVIIa cleaves protease-activated receptor 2 (PAR-2), triggering a downstream transmembrane signaling cascade resulting in the phosphorylation of two serine residues on the cytoplasmic tail of TF (91, 93–95). This phosphorylation event is proposed to have at least two consequences: [1] enhanced cancer cell migration elicited via reversible release of integrin α3β1 suppression, upregulation of interleukin 8 (IL-8) and/or beta-arrestin-dependent dephosphorylation and activation of the actin filament-severing protein (cofilin) (96–98); [2] a proangiogenic switch, triggered by the upregulation of VEGF and downregulation of thrombospondin (99, 100). The potential molecular mechanisms by which TF is linked to cancer progression have been reviewed in details elsewhere (31–33).
4. The cross-talk between the hemostatic and the malignant system
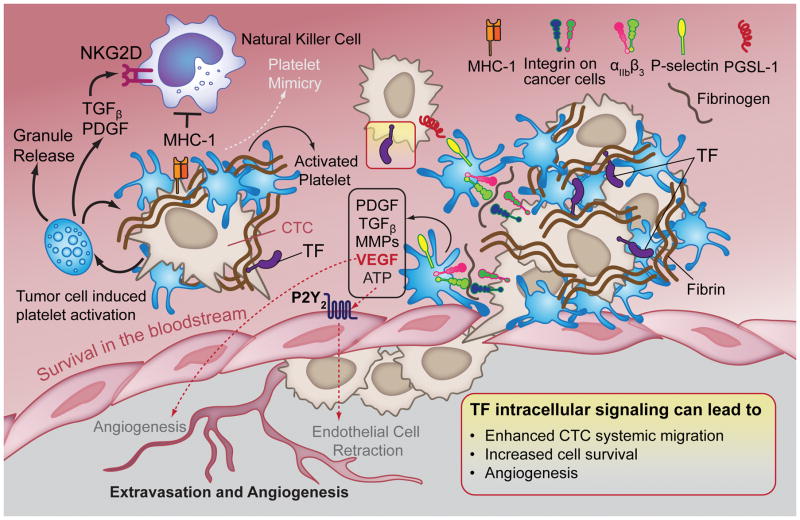
A growing body of evidence suggests that changes in the activation and regulation of the hemostatic system in patients with cancer directly contribute to cancer progression (Figure 2). During their migration through the bloodstream, cancer cells are exposed to a challenging microenvironment, including exposure to shear forces and cytotoxic natural killer (NK) cells. (34). It has been hypothesized that tumor cells may escape NK-mediated immune recognition and overcome shear forces by inducing the formation of platelet-fibrin rich microemboli (35, 36). This implicitly suggests that the role of TF in the hematogenous survival of CTC is mainly driven by thrombin generation and function. In support of this statement, platelet- or fibrinogen-depletion in mice caused a reduction in tumor colonization when compared to mice with a normal platelet count or fibrinogen levels (37–39). In addition, the ability of NK cells to lyse tumor cells in vitro has been shown to decrease with an increasing platelet count (37). Palumbo and colleagues subsequently showed that platelet-fibrin deposits form a cloak around B16-F10 melanoma cells, helping to camouflage the tumor cells to enable them to escape immune recognition and elimination (40). The mesh of fibrin, induced by TF surface expression, was found to envelop cancer cells (35, 41) preventing them from being recognized by NK cells (42, 43). In addition, the platelet-fibrin coat may enable a mechanism of molecular mimicry resulting in the acquisition of platelet-derived major histocompatibility complex (MHC-I) by cancer cells (44). Moreover, platelet-derived TGFβ and platelet-derived growth factor (PDGF) may impede NK immune surveillance by down-regulating the NK cell activating immune-receptor (NKG2D) (45) and NK cell PDGF receptor expression (46). TGFβ derived from platelet α-granules has been shown to profoundly impact tumor metastasis and survival by enhancing EMT via activation of the TGFβ/Smad and NF-KB signaling pathways in cultured tumor cells (22). Moreover, metastatic progression has been shown to be profoundly impaired in tumor-bearing mice lacking plasma transglutaminase factor XIII (FXIII), suggesting a role for FXIII-dependent platelet-fibrin coat stabilization in cancer metastasis (47).
FIGURE 2. Coagulation and platelet activation support tumor metastasis.
CTCs activate and aggregate platelets via paracrine and/or juxtacrine signals to ensure their survival in the bloodstream and to permit extravasation, proliferation and angiogenesis at the metastatic site. Aggregated platelets and fibrin surround and protect CTCs from NK-mediated lysis via physical shielding. Platelets also release TGFβ and PDGF, which function as downregulators of the NK-activating receptor NKGD. In addition, CTCs can escape the immune surveillance through molecular mimicry of platelets. Tumor cell interaction with the endothelium is thought to be mediated by platelets, specifically via P-selectin binding to PSGL-1 and integrin-fibrinogen-integrin bridges. Platelet release of growth and angiogenic factors (e.g., PDGF, TGFβ, VEGF) as well as proteases (e.g., MMPs) and adenine nucleotides (e.g., ATP) contribute to cancer cell extravasation and formation of new stable capillaries at the metastatic site. Moreover, intracellular signaling transduced via molecules downstream of TF has several benefits for tumor progression (Yellow Box).
In order to leave the circulation and develop secondary colonies, CTCs must adhere or become entrapped within the microvasculature of a target organ (48). As early as 1985, electron micrographs studies showed B16 melanoma cells trapped in an intricate network of platelets and fibrin at the lung vasculature site (49). Indeed, under in vitro flow conditions, platelets, bound to cancer cells or immobilized on a matrix surface, have been shown to facilitate tumor cell adhesion, tethering and arrest under shear flow (50–52). The formation of a platelet-tumor cell aggregate has also been implicated in the formation of a metastatic niche rich in prometastatic granulocytes in an experimental mouse model of metastasis. In this model, granulocytes were recruited by the release of selected chemokines (CXCL5 and CXCL7) from tumor cell-activated platelets. Consequently, granulocyte recruitment was prevented in platelet-depleted mice or in mice deficient in β3 integrins required for tumor cell-induced platelet aggregation (53). Importantly, several studies have shown that platelet P-selectin and αIIbβ3 act as potential receptors for tumor cells. The tethering and stable adhesion of cultured cancer cells to the subendothelium has been shown to be prevented by blockade of P-selectin and αIIbβ3 on platelet surface (50, 51, 54). Additionally, studies have provided a direct correlation between platelet concentration and the number of cancer cells tethering to human umbilical vein endothelial cells (HUVECs) in a P-selectin- and αIIbβ3-dependent manner in vitro (55).
Platelets may play a role in promoting metastasis through the selective release of key growth factors (e.g., vascular endothelium growth factor (VEGF; TGFβ; PDGF) and metalloproteases (MMPs)) at sites of CTC adhesion to the endothelium (56–58). Once released from platelet α-granules, MMPs will degrade specific components of the extracellular matrix (ECM), increasing vascular permeability and the extravasation of CTCs, as well as the release of growth factors sequestered in the ECM (59). Platelet-derived nucleotides may also promote the extravasation of CTCs. For instance, Munc13-4 deficient mice, whose platelets lack the ability to secrete dense granule components such as ADP and ATP, or mice deficient in the P2Y2-ATP receptor on endothelial cells, have been shown to exhibit a significant decrease in melanoma (B16) and breast carcinoma (LCC) cell extravasation and metastasis colony formation as compared to wild-type mice (60). Moreover, platelet-derived ADP has been shown to promote tumor cell-induced platelet aggregation (61), a potential strategy utilized by several tumor cell lines to evade the immune system and resist arterial shear stress during the hematogenous journey (36). Thus, platelet-bound adhesive molecules, platelet-derived soluble molecules and fibrin can synergistically enhance the ability of CTCs to traverse the endothelial cell barrier, penetrate the parenchyma and establish a metastatic lesion.
The assembly of a new vascular network (angiogenesis) is of central importance to the growth of solid tumors beyond 2–3 mm (62). The intimate association with the circulation is required for the tumor to acquire the necessary oxygen and nutrients, to shed metabolic waste products and to have access to proteases and cytokines which sustain further tumor growth, invasion, dissemination and extravasation (57, 63). Interestingly, tumor blood vessels display remarkable morphological abnormalities consisting of marked permeability and leakiness, and excessive and haphazard branching (59, 64). This dysregulated vessel morphology has been hypothesized to permit the access of cancer cells to the circulation, favoring contacts with platelets which, in turn, can function as a source of proangiogenic factors at the metastatic niche and contribute to tumor survival and progression (65). Indeed, platelets and fibrin have been shown to stimulate endothelial cell proliferation and augment the formation of capillary-like tubes in vitro (66, 67) and angiogenesis in vivo (68–70). Platelets are recognized as a major physiological transporter of VEGF in the blood, and have been shown to be active and selective “scavengers” of soluble tumor-derived angiogenesis regulators in patients with cancer (65, 71–77). In support of the latter, levels of proangiogenic factors were found to be more abundant in platelets isolated from mice bearing malignant tumors as compared to platelets deriving from non-tumor bearing mice (76). Along these lines, elegant studies have shown that platelets isolated from mice with cancer and activated with ADP could induce angiogenesis more efficiently than platelets obtained from cancer-free mice (78). In addition to VEGF, platelets are carriers of over 30 crucial regulators of angiogenesis (pro- and antiangiogenic factors), which can be released in a selective fashion depending on the stimuli (79–81). In this context, MCF-7 breast cancer cells have been shown to orchestrate the preferential release of proangiogenic molecules (e.g. VEGF), but not of the anti-angiogenic counterpart (e.g. endostatin), from platelet α-granules, to induce angiogenesis in vitro (79). Interestingly, treatment of human platelets with aspirin (COX-1 inhibitor) or antibodies against αIIbβ3 prior to exposure to breast cancer cells has been shown to disable the release of proangiogenic factors in vitro (79, 82, 83).
For the homeostasis of the vasculature, it is crucial to maintain a normal platelet count in the blood. Thrombocytosis is often an indicator of an advanced stage of cancer and is often associated with poor prognosis for patients with cancer (41, 84–86). Moreover, patients with metastatic cancer often display increased platelet reactivity and a concurrent high risk of developing thrombosis (87, 88). In addition to the storage and release of angiogenic regulators, key to the de novo formation of blood vessels at the metastatic foci, platelets have been shown to contribute to maintaining tumor vessel homeostasis by protecting the tumor vasculature from hemorrhage (69). This intriguing observation has been validated by inducing thrombocytopenia in tumor-bearing mice. Thrombocytopenic mice had significantly reduced levels of metastasis, compared to mice displaying a normal platelet count. The authors noted that there was a tendency for the metastatic tumors to have fragile blood vessels and evidence of hemorrhage. Transfusing these mice with fresh platelets reversed this effect and promoted angiogenesis at the metastatic site (69, 70). However, pre-activating the platelets to deplete their granule contents, before transfusion into the thrombocytopenic mice, ablated the angiogenic response without impairing platelet clotting functions. Collectively, this research suggests that the platelet secretome is of crucial importance to the maintenance of vascular homeostasis and that this ability occurs independently from thrombus formation. Along these lines, a recent study described the presence of impaired blood vessel density and maturation at the tumor niche in platelet-depleted mice (89).
5. NET(s) and cell-free DNA: enhancer of cancer-induced thrombotic events
Recent observations have shown that neutrophil extracellular traps (NETs) are involved in promoting coagulation and thrombosis in patients with cancer. NETs are networks of DNA-fibers which, in addition to their bactericidal function, can also provide a scaffold for platelet adhesion, activation and thrombus formation (90, 91). Interestingly, G-CSF, which is aberrantly expressed by diverse human tumors, including pancreatic and ovarian cancer (92, 93), have been shown to activate neutrophils and induce the release of NETs through a process called NETosis (91). A striking increase in NETosis and subsequent thrombotic degeneration was observed in murine models of metastasis following implantation of liquid or solid tumors (91). Remarkably, NETosis can be impaired by the well-known anti-platelet drug aspirin (94).
A second source of nucleic acids found in the blood, namely cell-free DNA (cfDNA), has been shown to induce thrombin generation when added to re-calcified plasma (95). The concentration of cfDNA in the blood is known to be increased in the setting of cancer and in response to treatment with chemotherapy, and thus may represent a mechanism underlying cancer-associated thrombosis, as DNA has been shown to activate the coagulation factor XII to induce thrombin generation (95, 96). Notably, plasma samples from patients with cancer normally present with higher levels of cfDNA than the samples isolated from healthy subjects, indicating a potential correlation with tumor burden (97). In addition, a direct correlation between CTC number and plasma and serum levels of cfDNA in patients with diverse types of cancer has also been demonstrated (97). Thus, cfDNA holds promise as both a biomarker for both tumor diagnosis and tracking as well as risk assessment for thrombosis.
6. Exacerbation of thrombotic events by anti-cancer therapies
In addition to the potential procoagulant phenotype of cancer cells, extensive evidence point to anti-cancer therapy as an additional cause of the enhanced risk of developing thrombosis in patients with cancer (98). Different chemotherapeutic agents, including cyclophosphamide, methotrexate, 5-fluorouracil cisplatin, gemcitabine, cyclophosphamide, doxorubicin, epirubicin, and daunorubicin, have been found to greatly potentiate the aberrant hemostatic response leading to an increased risk of VTE and associated mortality (98, 99). The underlying mechanism(s) responsible for chemotherapy–induced thrombotic events is still being resolved. Nevertheless, a range of possible mechanisms have been proposed. These include tumor cell and endothelial cell lysis and subsequent release of cytokine and procoagulant paracrine mediators, upregulated expression of TF on the surface of monocytes, platelet activation, increased endothelial cell reactivity to platelets as well as direct damage to vascular endothelium and increase in circulating levels of cfDNA (97, 99, 100). The most extensive studies regarding the causal relationship between chemotherapy and VTE have been conducted in women with early stage breast cancer, where administration of chemotherapy caused an increased risk of VTE of 2–7 folds compared with rates reported in a healthy population (101). It should be noted that in patients with breast carcinoma, chemotherapy is rarely used on its own but it is applied in combination with hormonal therapy which further augments the risk of undesired VTE of 1.5–7 folds (100–102). Chemotherapy is normally delivered by intravenous catheters, which can contribute to aberrant thrombosis in upwards of 2/3rds of cancer patients by physically traumatizing the vessel wall and thus, enhancing its thrombogenicity (98). Lastly, functional injury of endothelial cells and vascular inflammation caused by surgical interventions represents another mechanism underlying thrombotic events associated with cancer patients (98). Overall, the fact that cancer therapy and treatment may serve as a thrombotic trigger represents a major challenge for creating guidelines for treating and preventing cancer-associated thrombosis.
7. Clinical considerations for the procoagulant phenotype of cancer cells
Anticoagulant prophylaxis in patients with cancer has been shown to be effective in reducing rates of venous thromboembolism (VTE) with no significant increased risk of serious bleeding or effect on mortality (103, 104). However, guidelines indicate that patients with solid tumors being treated in an ambulatory setting should not be routinely prophylaxed for venous thromboembolism primarily due to low incidence of thrombosis in these studies (<5%) (105). Therefore, risk stratification methods for thrombosis in patients with cancer are needed for future trials so that only patient subgroups with relatively high thrombotic risk are included in studies. In line with this, clinical parameters have been used to identify patients with cancer who are at highest risk to develop thrombosis. Primary disease site is one of the most important clinical parameters associated with thrombosis (106). Randomized controlled clinical trials evaluating thromboprophylaxis for patients with cancer based on primary disease site have shown mixed results for the efficacy of VTE prophylaxis. For example, TOPIC-1 and TOPIC-2 evaluated thromboprophylaxis with certoparin, low molecular weight heparin, versus placebo in patients with metastatic breast cancer and stage III or IV non-small cell lung cancer (NSCLC), respectively. TOPIC-1 showed no difference in VTE rates (4%) for metastatic breast cancer patients given VTE prophylaxis, while TOPIC-2 showed a reduction in VTE from 8.3% in the placebo arm to 4.5% in the prophylaxis arm with no increased risk of bleeding (103). Rates of VTE for patients with pancreatic cancer have been shown to decrease from 28% to 12% in the FRAGEM trial for patients receiving VTE thromboprophylaxis (104). Patients with newly diagnosed grade III/IV glioma given VTE prophylaxis saw VTE rates decrease from 15% to 9% in the PRODIGE trial, although a trend toward increased intracranial bleeding was observed (107). In addition to primary disease site, the Khorana score was developed and independently validated and includes four other clinical parameters by which to stratify risk of VTE for patients with cancer and includes disease site, pretreatment platelet count, hemoglobin level or use of erythropoietic agents, body mass index, and leukocyte count (108, 109). The Khorana score has been individually validated, with six month cumulative incidence of VTE increasing with score (score 0 = 1.5%, score 1 = 3.8%, score 2 = 9.4%, score 3 or greater = 17.7%). ASCO guidelines recommend utilization of the risk score to assess patient risk to develop thrombosis, and future studies to evaluate thromboprophylaxis in patients with cancer would likely benefit from utilizing clinical scores in order to prescreen patient populations and include only patients with relatively high thrombosis incidence. Despite the success of the Khorana index, it excludes several disease specific and patient specific characteristics that have been independently associated with risk to develop thrombosis. A multitude of biomarkers have been associated with venous thrombosis in patients with cancer (110). Theoretically, improved specificity in stratifying patients by risk to develop thrombosis might be achieved with the inclusion of individual patient biomarkers. The Vienna Cancer and Thrombosis study (VCATS) identified D-dimer and soluble P-selectin levels as independent predictors of VTE and the initial results of a risk score that includes these values are promising for identifying patients at highest risk to develop cancer-associated thrombosis (109). The extensive evidence to support a procoagulant phenotype for cancer cells would suggest that CTCs may be intimately involved with aberrant activation of coagulation and may even be prognostic for thrombosis in patients with cancer. As such, CTCs make for an intriguing potential biomarker for thrombosis in patients with cancer. The first study to assess the correlation between circulating tumor cells and development was performed in 2009, retrospectively analyzing the development of thrombosis as a function of pre-treatment CTC count (111). This study revealed that detectable levels of CTCs at baseline correlated with a hazard ratio for venous thrombosis of 5.3. Moreover, baseline CTC counts greater than five had double the risk to develop thrombosis than baseline CTC counts below five; however these results were not statistically significant. Similarly, the presence of CTCs correlated with levels of D-dimer, a marker of coagulation activation (112) Therefore, the hypothesis that CTCs might be a potential biomarker for thrombosis is supported by the studies published to date. More studies, and in particular, studies on patients with cancers of known high thrombotic risk are needed to further explore the association of CTCs with thrombosis.
In vitro, cancer cells exhibit significant heterogeneity in their morphology, viability and metastatic potential. Efforts to distinguish critical features of CTCs, such as stem cell markers, have demonstrated that cancer stem cells overexpress TF (113). As CTC stem cells are a mere subpopulation of the total CTCs population, this finding suggests heterogeneity in the procoagulant phenotype of CTCs. In support of this, in vitro studies have shown heterogeneity in cancer cell surface expression of TF and PS, both of which are critical to determining the procoagulant phenotype of cancer cells. As such, the utility of implementing CTC analysis as a means to rationally base thrombosis prophylaxis may hinge upon determining critical features of CTCs in addition to CTC counts as a marker for risk to develop thrombosis (114).
Finally, the host response to procoagulant tumor cells in the peripheral blood may be different depending on a number of factors to include platelet count, levels of coagulation factors and number of CTCs or CTC clusters, all of which may determine an individual’s propensity to develop thrombosis over the course of their disease. In fact, debate exists over the utility of circulating TF as a predictor of thrombosis, as it has a demonstrated association with thrombosis in pancreatic cancer, but not all patients with high levels of circulating TF develop thrombosis (115). Further, circulating TF is not associated with thrombosis in patients with colorectal cancer or brain glioma (116). Therefore, the development of thrombosis may not rely on of the mere initiation of coagulation by circulating TF, but rather the magnitude of the host’s coagulation response to that trigger. Our studies have shown that physiological variance of coagulation factors IX and X influence in vitro coagulation kinetics to equivalent procoagulant TF triggers, and evaluation of individual patient coagulation factor levels may hold promise in predicting who is at greater risk to develop thrombosis in the presence of procoagulant TF or CTCs (117).
8. Conclusive remarks and perspectives
Tumor cells may serve as a prothrombotic trigger through the aberrant regulation of coagulation activity and enhanced platelet activation. However, like the metastatic process itself, mechanisms by which the coagulation cascade is activated in patients with cancer is difficult to dissect directly. Importantly, differently from other cancer phenotypes, the mechanisms contributing to coagulation may be strictly influenced by the blood microenvironment. For instance, there are no procoagulant genes that undergo mutation to trigger thrombotic behaviors, yet there are potential paracrine or juxtacrine cross-talk mechanisms that might drive a genotypic and phenotypic conversion towards a procoagulant behavior. The prediction of a thrombotic event in patients with cancer is also complicated by the fact that we do not fully understand when and how the procoagulant power of tumors overwhelms the endogenous anticoagulant pathways present in the vasculature. Although the exact molecular triggers behind the initiation of thrombosis in patient with cancer remain ill-defined, one exciting advance is the identification and possible isolation of CTCs and CTC clusters and detection of cfDNA in patient blood samples. We propose that novel studies of CTC and cfDNA biophysics and transport may help bridge the gap in our understanding of molecular and cellular mechanism of thrombosis in cancer. The study of cfDNA and identification and isolation of CTCs from patients with cancer might help us in deciphering changes in the procoagulant phenotype of tumors in real time and establish whether specific patient subpopulations are associated with a greater risk of developing thrombotic events. Together with this, experimental and computer modelling approaches are also likely to be predictive of the prothrombotic risk of certain tumors. Future research will identify the mechanisms underlying the activation of the coagulation cascade by cfDNA and CTCs in the bloodstream, and the potential role of the blood microenvironment in reprogramming the procoagulant phenotype. The possibility that a tumor cell may escape the immune response in the bloodstream and be successful in its progression may be regulated by its prothrombotic activity. Several questions remain: Are CTC clusters more procoagulant and thus, more aggressive than single CTCs? What is the contribution of CTCs to the pool of cfDNA, and what role does cfDNA play in initiating the coagulation cascade? Does activation of the coagulation cascade and platelets help or hinder metastasis? Is the coagulation pathway activated only during primary dissemination or also during metastatic relapse? Answering these questions may help provide a rationale for the use of cfDNA and CTCs for cancer diagnosis, therapy and prognosis.
Acknowledgments
The authors would like to acknowledge Katya X. for design of the artwork. This work was supported by the National Institutes of Health (R01HL101972 and U54CA143906). O.J.T.M. is an American Heart Association Established Investigator (13EIA12630000).
Footnotes
DISCLOSURES
P.K. has ownership in Epic Sciences, which has licensed the HD-CTC technology. No conflicts of interest, financial or otherwise, are declared by the other authors.
Contributor Information
Annachiara Mitrugno, Email: mitrugno@ohsu.edu.
Garth W. Tormoen, Email: tormoeng@ohsu.edu.
Peter Kuhn, Email: pkuhn@usc.edu.
Owen J.T. McCarty, Email: mccartyo@ohsu.edu.
References
- 1.Trousseau A. Phlegmasia alba dolens. Lectures on clinical medicine Delivered at the Hotel-Dieu. 1865:281–332. [Google Scholar]
- 2.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–50. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 3.Levitan N, Dowlati A, Remick SC, Tahsildar HI, Sivinski LD, Beyth R, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine. 1999;78(5):285–91. doi: 10.1097/00005792-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Williams JC, Mackman N. Tissue factor in health and disease. Front Biosci. 2012;4:358–72. doi: 10.2741/383. [DOI] [PubMed] [Google Scholar]
- 5.Kasthuri RS, Glover SL, Boles J, Mackman N. Tissue factor and tissue factor pathway inhibitor as key regulators of global hemostasis: measurement of their levels in coagulation assays. Semin Thromb Hemost. 2010;36(7):764–71. doi: 10.1055/s-0030-1265293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg. 2009;108(5):1447–52. doi: 10.1213/ane.0b013e31819bceb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackman N, Taubman M. Tissue factor: past, present, and future. Arterioscler Thromb Vasc Biol. 2009;29(12):1986–8. doi: 10.1161/ATVBAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruf W, Mueller BM. Tissue factor signaling. Thromb Haemost. 1999;82(2):175–82. [PubMed] [Google Scholar]
- 9.Mann KG, Butenas S, Brummel K. The dynamics of thrombin formation. Arterioscler Thromb Vasc Biol. 2003;23(1):17–25. doi: 10.1161/01.atv.0000046238.23903.fc. [DOI] [PubMed] [Google Scholar]
- 10.Metcalf RL, Fry DJ, Swindell R, McGurk A, Clamp AR, Jayson GC, et al. Thrombosis in ovarian cancer: a case control study. Br J Cancer. 2014;110(5):1118–24. doi: 10.1038/bjc.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callander NS, Varki N, Rao LV. Immunohistochemical identification of tissue factor in solid tumors. Cancer. 1992;70(5):1194–201. doi: 10.1002/1097-0142(19920901)70:5<1194::aid-cncr2820700528>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Koizume S, Jin MS, Miyagi E, Hirahara F, Nakamura Y, Piao JH, et al. Activation of cancer cell migration and invasion by ectopic synthesis of coagulation factor VII. Cancer Res. 2006;66(19):9453–60. doi: 10.1158/0008-5472.CAN-06-1803. [DOI] [PubMed] [Google Scholar]
- 13.Muller I, Klocke A, Alex M, Kotzsch M, Luther T, Morgenstern E, et al. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB journal. 2003;17(3):476–8. doi: 10.1096/fj.02-0574fje. [DOI] [PubMed] [Google Scholar]
- 14.Furie B, Furie BC. Role of platelet P-selectin and microparticle PSGL-1 in thrombus formation. Trends Mol Med. 2004;10(4):171–8. doi: 10.1016/j.molmed.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. JEM. 2003;197(11):1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. JEM. 2009;206(9):1913–27. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105(4):1734–41. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 18.Rong Y, Post DE, Pieper RO, Durden DL, Van Meir EG, Brat DJ. PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res. 2005;65(4):1406–13. doi: 10.1158/0008-5472.CAN-04-3376. [DOI] [PubMed] [Google Scholar]
- 19.Boccaccio C, Sabatino G, Medico E, Girolami F, Follenzi A, Reato G, et al. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature. 2005;434(7031):396–400. doi: 10.1038/nature03357. [DOI] [PubMed] [Google Scholar]
- 20.Vrana JA, Stang MT, Grande JP, Getz MJ. Expression of tissue factor in tumor stroma correlates with progression to invasive human breast cancer: paracrine regulation by carcinoma cell-derived members of the transforming growth factor beta family. Cancer Res. 1996;56(21):5063–70. [PubMed] [Google Scholar]
- 21.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26(3):456–61. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 22.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103(38):13932–7. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milsom C, Yu J, May L, Meehan B, Magnus N, Al-Nedawi K, et al. The role of tumor-and host-related tissue factor pools in oncogene-driven tumor progression. Thromb Res. 2007;120(Suppl 2):S82–91. doi: 10.1016/S0049-3848(07)70135-4. [DOI] [PubMed] [Google Scholar]
- 24.Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, Baehring F, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol. 2012;9(1):016003. doi: 10.1088/1478-3975/9/1/016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips KG, Lee AM, Tormoen GW, Rigg RA, Kolatkar A, Luttgen M, et al. The thrombotic potential of circulating tumor microemboli: computational modeling of circulating tumor cell-induced coagulation. American journal of physiology Cell physiology. 2015;308(3):C229–36. doi: 10.1152/ajpcell.00315.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King MR, Phillips KG, Mitrugno A, Lee TR, de Guillebon AM, Chandrasekaran S, et al. A physical sciences network characterization of circulating tumor cell aggregate transport. Am J Physiol Cell Physiol. 2015 May 15;308(10):C792–802. doi: 10.1152/ajpcell.00346.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 28.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 29.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 30.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–22. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milsom C, Rak J. Tissue factor and cancer. Pathophysiol Haemos Thromb. 2008;36(3–4):160–76. doi: 10.1159/000175154. [DOI] [PubMed] [Google Scholar]
- 32.Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J Clin Oncol. 2009;27(29):4834–8. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruf W, Yokota N, Schaffner F. Tissue factor in cancer progression and angiogenesis. Thromb Res. 2010;125(Suppl 2):S36–8. doi: 10.1016/S0049-3848(10)70010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2(12):1091–9. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–34. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egan K, Cooke N, Kenny D. Living in shear: platelets protect cancer cells from shear induced damage. Clin Exp Metastasis. 2014 Aug;31(6):697–704. doi: 10.1007/s10585-014-9660-7. [DOI] [PubMed] [Google Scholar]
- 37.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–300. [PubMed] [Google Scholar]
- 38.Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002;62(23):6966–72. [PubMed] [Google Scholar]
- 39.Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104(2):397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- 40.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–85. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 41.van Es N, Sturk A, Middeldorp S, Nieuwland R. Effects of cancer on platelets. Semin Oncol. 2014;41(3):311–8. doi: 10.1053/j.seminoncol.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Lipinski B, Egyud LG. Resistance of cancer cells to immune recognition and killing. Med Hypotheses. 2000;54(3):456–60. doi: 10.1054/mehy.1999.0876. [DOI] [PubMed] [Google Scholar]
- 43.Egyud LG, Lipinski B. Significance of fibrin formation and dissolution in the pathogenesis and treatment of cancer. Med Hypotheses. 1991;36(4):336–40. doi: 10.1016/0306-9877(91)90006-k. [DOI] [PubMed] [Google Scholar]
- 44.Placke T, Kopp HG, Salih HR. Modulation of natural killer cell anti-tumor reactivity by platelets. J Innate Immun. 2011;3(4):374–82. doi: 10.1159/000323936. [DOI] [PubMed] [Google Scholar]
- 45.Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69(19):7775–83. doi: 10.1158/0008-5472.CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- 46.Gersuk GM, Westermark B, Mohabeer AJ, Challita PM, Pattamakom S, Pattengale PK. Inhibition of human natural killer cell activity by platelet-derived growth factor (PDGF). III. Membrane binding studies and differential biological effect of recombinant PDGF isoforms. Scand J Immunol. 1991;33(5):521–32. doi: 10.1111/j.1365-3083.1991.tb02522.x. [DOI] [PubMed] [Google Scholar]
- 47.Palumbo JS, Barney KA, Blevins EA, Shaw MA, Mishra A, Flick MJ, et al. Factor XIII transglutaminase supports hematogenous tumor cell metastasis through a mechanism dependent on natural killer cell function. Journal of thrombosis and haemostasis: JTH. 2008;6(5):812–9. doi: 10.1111/j.1538-7836.2008.02938.x. [DOI] [PubMed] [Google Scholar]
- 48.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 49.Crissman JD, Hatfield J, Schaldenbrand M, Sloane BF, Honn KV. Arrest and extravasation of B16 amelanotic melanoma in murine lungs. A light and electron microscopic study. Lab Invest. 1985;53(4):470–8. [PubMed] [Google Scholar]
- 50.McCarty OJ, Mousa SA, Bray PF, Konstantopoulos K. Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood. 2000;96(5):1789–97. [PubMed] [Google Scholar]
- 51.Dardik R, Kaufmann Y, Savion N, Rosenberg N, Shenkman B, Varon D. Platelets mediate tumor cell adhesion to the subendothelium under flow conditions: involvement of platelet GPIIb-IIIa and tumor cell alpha(v) integrins. Int J Cancer. 1997;70(2):201–7. doi: 10.1002/(sici)1097-0215(19970117)70:2<201::aid-ijc11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 52.McCarty OJ, Jadhav S, Burdick MM, Bell WR, Konstantopoulos K. Fluid shear regulates the kinetics and molecular mechanisms of activation-dependent platelet binding to colon carcinoma cells. Biophys J. 2002;83(2):836–48. doi: 10.1016/S0006-3495(02)75212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A. 2014;111(30):E3053–61. doi: 10.1073/pnas.1411082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Felding-Habermann B, Habermann R, Saldivar E, Ruggeri ZM. Role of beta3 integrins in melanoma cell adhesion to activated platelets under flow. J Biol Chem. 1996;271(10):5892–900. doi: 10.1074/jbc.271.10.5892. [DOI] [PubMed] [Google Scholar]
- 55.Burdick MM, Konstantopoulos K. Platelet-induced enhancement of LS174T colon carcinoma and THP-1 monocytoid cell adhesion to vascular endothelium under flow. Am J Physiol Cell Physiol. 2004;287(2):C539–47. doi: 10.1152/ajpcell.00450.2003. [DOI] [PubMed] [Google Scholar]
- 56.Poggi A, Stella M, Donati MB. The importance of blood cell-vessel wall interactions in tumour metastasis. Baillieres Clin Haematol. 1993;6(3):731–52. doi: 10.1016/s0950-3536(05)80196-9. [DOI] [PubMed] [Google Scholar]
- 57.Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol. 2002;3(7):425–30. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- 58.Lowe KL, Navarro-Nunez L, Watson SP. Platelet CLEC-2 and podoplanin in cancer metastasis. Thromb Res. 2012;129(Suppl 1):S30–7. doi: 10.1016/S0049-3848(12)70013-0. [DOI] [PubMed] [Google Scholar]
- 59.Weinberg RA. The biology of cancer. Garland Science; Taylor & Francis Group; New York: 2012. [Google Scholar]
- 60.Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-Derived Nucleotides Promote Tumor-Cell Transendothelial Migration and Metastasis via P2Y2 Receptor. Cancer Cell. 2013;24(1):130–7. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Mitrugno A, Williams D, Kerrigan SW, Moran N. A novel and essential role for FcgammaRIIa in cancer cell-induced platelet activation. Blood. 2014;123(2):249–60. doi: 10.1182/blood-2013-03-492447. [DOI] [PubMed] [Google Scholar]
- 62.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 63.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pinedo HM, Verheul HM, D’Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352(9142):1775–7. doi: 10.1016/s0140-6736(98)05095-8. [DOI] [PubMed] [Google Scholar]
- 66.Pipili-Synetos E, Papadimitriou E, Maragoudakis ME. Evidence that platelets promote tube formation by endothelial cells on matrigel. Br J Pharmacol. 1998;125(6):1252–7. doi: 10.1038/sj.bjp.0702191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Contrino J, Goralnick S, Qi J, Hair G, Rickles FR, Kreutzer DL. Fibrin induction of tissue factor expression in human vascular endothelial cells. Circulation. 1997;96(2):605–13. [PubMed] [Google Scholar]
- 68.Iba O, Matsubara H, Nozawa Y, Fujiyama S, Amano K, Mori Y, et al. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106(15):2019–25. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 69.Ho-Tin-Noe B, Goerge T, Cifuni SM, Duerschmied D, Wagner DD. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res. 2008;68(16):6851–8. doi: 10.1158/0008-5472.CAN-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kisucka J, Butterfield CE, Duda DG, Eichenberger SC, Saffaripour S, Ware J, et al. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc Natl Acad Sci U S A. 2006;103(4):855–60. doi: 10.1073/pnas.0510412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelets effects on tumor growth. Semin Oncol. 2014;41(3):359–69. doi: 10.1053/j.seminoncol.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 72.Peterson JE, Zurakowski D, Italiano JE, Jr, Michel LV, Fox L, Klement GL, et al. Normal ranges of angiogenesis regulatory proteins in human platelets. Am J Hematol. 2010;85(7):487–93. doi: 10.1002/ajh.21732. [DOI] [PubMed] [Google Scholar]
- 73.Verheul HM, Hoekman K, Luykx-de Bakker S, Eekman CA, Folman CC, Broxterman HJ, et al. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3(12 Pt 1):2187–90. [PubMed] [Google Scholar]
- 74.Gisterek I, Matkowski R, Lacko A, Sedlaczek P, Szewczyk K, Biecek P, et al. Serum vascular endothelial growth factors a, C and d in human breast tumors. Pathol Oncol Res. 2010;16(3):337–44. doi: 10.1007/s12253-009-9211-8. [DOI] [PubMed] [Google Scholar]
- 75.Peterson JE, Zurakowski D, Italiano JE, Jr, Michel LV, Connors S, Oenick M, et al. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis. 2012;15(2):265–73. doi: 10.1007/s10456-012-9259-z. [DOI] [PubMed] [Google Scholar]
- 76.Klement GL, Yip TT, Cassiola F, Kikuchi L, Cervi D, Podust V, et al. Platelets actively sequester angiogenesis regulators. Blood. 2009;113(12):2835–42. doi: 10.1182/blood-2008-06-159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kerr BA, Miocinovic R, Smith AK, Klein EA, Byzova TV. Comparison of tumor and microenvironment secretomes in plasma and in platelets during prostate cancer growth in a xenograft model. Neoplasia. 2010;12(5):388–96. doi: 10.1593/neo.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuznetsov HS, Marsh T, Markens BA, Castano Z, Greene-Colozzi A, Hay SA, et al. Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow-derived cells. Cancer Discov. 2012;2(12):1150–65. doi: 10.1158/2159-8290.CD-12-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Battinelli EM, Markens BA, Italiano JE., Jr Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118(5):1359–69. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237–49. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 81.Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111(3):1227–33. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Battinelli EM, Markens BA, Kulenthirarajan RA, Machlus KR, Flaumenhaft R, Italiano JE., Jr Anticoagulation inhibits tumor cell-mediated release of platelet angiogenic proteins and diminishes platelet angiogenic response. Blood. 2014;123(1):101–12. doi: 10.1182/blood-2013-02-485011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amirkhosravi A, Amaya M, Siddiqui F, Biggerstaff JP, Meyer TV, Francis JL. Blockade of GpIIb/IIIa inhibits the release of vascular endothelial growth factor (VEGF) from tumor cell-activated platelets and experimental metastasis. Platelets. 1999;10(5):285–92. doi: 10.1080/09537109975915. [DOI] [PubMed] [Google Scholar]
- 84.Erdemir F, Kilciler M, Bedir S, Ozgok Y, Coban H, Erten K. Clinical significance of platelet count in patients with renal cell carcinoma. Urol Int. 2007;79(2):111–6. doi: 10.1159/000106322. [DOI] [PubMed] [Google Scholar]
- 85.Levin J, Conley CL. Thrombocytosis Associated with Malignant Disease. Arch Intern Med. 1964;114:497–500. doi: 10.1001/archinte.1964.03860100079008. [DOI] [PubMed] [Google Scholar]
- 86.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–8. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cooke NM, Egan K, McFadden S, Grogan L, Breathnach OS, O’Leary J, et al. Increased platelet reactivity in patients with late-stage metastatic cancer. Cancer Med. 2013;2(4):564–70. doi: 10.1002/cam4.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buller HR, van Doormaal FF, van Sluis GL, Kamphuisen PW. Cancer and thrombosis: from molecular mechanisms to clinical presentations. JTH. 2007;5(Suppl 1):246–54. doi: 10.1111/j.1538-7836.2007.02497.x. [DOI] [PubMed] [Google Scholar]
- 89.Li R, Ren M, Chen N, Luo M, Deng X, Xia J, et al. Presence of intratumoral platelets is associated with tumor vessel structure and metastasis. BMC Cancer. 2014;14:167. doi: 10.1186/1471-2407-14-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107(36):15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A. 2012;109(32):13076–81. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Joshita S, Nakazawa K, Sugiyama Y, Kamijo A, Matsubayashi K, Miyabayashi H, et al. Granulocyte-colony stimulating factor-producing pancreatic adenosquamous carcinoma showing aggressive clinical course. Intern Med. 2009;48(9):687–91. doi: 10.2169/internalmedicine.48.1900. [DOI] [PubMed] [Google Scholar]
- 93.Savarese TM, Mitchell K, McQuain C, Campbell CL, Guardiani R, Wuu J, et al. Coexpression of granulocyte colony stimulating factor and its receptor in primary ovarian carcinomas. Cancer Lett. 2001;162(1):105–15. doi: 10.1016/s0304-3835(00)00623-6. [DOI] [PubMed] [Google Scholar]
- 94.Lapponi MJ, Carestia A, Landoni VI, Rivadeneyra L, Etulain J, Negrotto S, et al. Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J Pharmacol Exp Ther. 2013;345(3):430–7. doi: 10.1124/jpet.112.202879. [DOI] [PubMed] [Google Scholar]
- 95.Swystun LL, Mukherjee S, Liaw PC. Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. Journal of thrombosis and haemostasis: JTH. 2011;9(11):2313–21. doi: 10.1111/j.1538-7836.2011.04465.x. [DOI] [PubMed] [Google Scholar]
- 96.Geddings JE, Mackman N. New players in haemostasis and thrombosis. Thromb Haemost. 2014;111(4):570–4. doi: 10.1160/TH13-10-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11(6):426–37. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 98.Falanga A, Marchetti M. Anticancer treatment and thrombosis. Thromb Res. 2012;129(3):353–9. doi: 10.1016/j.thromres.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 99.De Cicco M. The prothrombotic state in cancer: pathogenic mechanisms. Crit Rev Oncol Hematol. 2004;50(3):187–96. doi: 10.1016/j.critrevonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 100.Haddad TC, Greeno EW. Chemotherapy-induced thrombosis. Thromb Res. 2006;118(5):555–68. doi: 10.1016/j.thromres.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 101.Connolly GC, Khorana AA. Risk stratification for cancer-associated venous thromboembolism. Best Pract Res Clin Haemat. 2009;22(1):35–47. doi: 10.1016/j.beha.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 102.Deitcher SR, Gomes MP. The risk of venous thromboembolic disease associated with adjuvant hormone therapy for breast carcinoma: a systematic review. Cancer. 2004;101(3):439–49. doi: 10.1002/cncr.20347. [DOI] [PubMed] [Google Scholar]
- 103.Haas SK, Freund M, Heigener D, Heilmann L, Kemkes-Matthes B, von Tempelhoff GF, et al. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin Appl Thromb Hemost. 2012;18(2):159–65. doi: 10.1177/1076029611433769. [DOI] [PubMed] [Google Scholar]
- 104.Maraveyas A, Waters J, Roy R, Fyfe D, Propper D, Lofts F, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer. 2012;48(9):1283–92. doi: 10.1016/j.ejca.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 105.Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(17):2189–204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 106.Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(29):4839–47. doi: 10.1200/JCO.2009.22.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perry JR, Julian JA, Laperriere NJ, Geerts W, Agnelli G, Rogers LR, et al. PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. Journal of thrombosis and haemostasis: JTH. 2010;8(9):1959–65. doi: 10.1111/j.1538-7836.2010.03973.x. [DOI] [PubMed] [Google Scholar]
- 108.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–7. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377–82. doi: 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 110.Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122(12):2011–8. doi: 10.1182/blood-2013-04-460147. [DOI] [PubMed] [Google Scholar]
- 111.Mego M, De Giorgi U, Broglio K, Dawood S, Valero V, Andreopoulou E, et al. Circulating tumour cells are associated with increased risk of venous thromboembolism in metastatic breast cancer patients. Br J Cancer. 2009;101(11):1813–6. doi: 10.1038/sj.bjc.6605413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mego M, Karaba M, Minarik G, Benca J, Sedlackova T, Tothova L, et al. Relationship between Circulating Tumor Cells, Blood Coagulation, and Urokinase-Plasminogen-Activator System in Early Breast Cancer Patients. Breast J. 2015;21(2):155–60. doi: 10.1111/tbj.12388. [DOI] [PubMed] [Google Scholar]
- 113.Milsom C, Anderson GM, Weitz JI, Rak J. Elevated tissue factor procoagulant activity in CD133-positive cancer cells. Journal of thrombosis and haemostasis: JTH. 2007;5(12):2550–2. doi: 10.1111/j.1538-7836.2007.02766.x. [DOI] [PubMed] [Google Scholar]
- 114.Tormoen GW, Recht O, Gruber A, Levine RL, McCarty OJ. Phosphatidylserine index as a marker of the procoagulant phenotype of acute myelogenous leukemia cells. Phys Biol. 2013;10(5):056010. doi: 10.1088/1478-3975/10/5/056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15(22):6830–40. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. Journal of thrombosis and haemostasis: JTH. 2012;10(7):1363–70. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- 117.Tormoen GW, Cianchetti FA, Bock PE, McCarty OJ. Development of coagulation factor probes for the identification of procoagulant circulating tumor cells. Front Oncol. 2012;2:110. doi: 10.3389/fonc.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]