Abstract
Objective:
The aim of this present study was to assess the impact of COX-2 over-expression on breast cancer survival.
Material and Methods:
Non-metastatic invasive breast cancer patients who received adequate loco-regional and systemic treatments were evaluated. Patients’ demographic, clinical, pathologic, and treatment-related and survival data were retrieved from their hospital files. COX-2, estrogen/progesterone receptor (ER/PR), HER-2/neu expression and Ki67 index of the tumors were determined immunohistochemically. As the primary objective, COX-2 positive and negative patients were compared in terms of overall (OS), disease-free (DFS) and breast cancer-specific survival (BCSS). Secondary objectives were to assess the independent prognostic factors for survival. In addition, the correlation of COX-2 expression with conventional prognostic and predictive factors of breast cancer was assessed.
Results:
Two hundred and seventeen patients who underwent adequate breast cancer treatment between November 2004 and December 2013 were included in the study. The median follow-up was 37 months (range: 5–107). Eighty-one (37%) patients were COX-2 positive. OS, DFS, and BCSS were similar in COX-2 positive and negative patients. Ki67 index and age were significantly correlated with COX-2 expression (r=−0.116; p=0.02; r=0.159; p=0.02). PR expression was found to be the only independent factor for predicting OS, tumor size and molecular subtype classification were found to be the only independent factors for predicting DFS, and PR expression was found to be the only independent factor for predicting BCSS.
Conclusion:
Among the independent predictive and prognostic factors of breast cancer, COX-2 over-expression was only correlated with Ki67 index and age.
Keywords: Breast cancer, survival, cyclooxygenase-2 enzyme, predictive factors, prognostic factors
INTRODUCTION
Certain prognostic and predictive factors were taken into consideration in order to predict the outcome and determine the treatment for breast cancer. Breast cancer is stratified according to tumor size, grade, axillary nodal stage, hormone (estrogen and progesterone) receptor status and HER2/neu expression as well as patient’s age and the presence of distant metastasis (1). Breast tumor molecular subtypes also have a tendency to give rise to distant metastases initially at certain body sites (2). Recently, molecular subtype classification is used to classify breast cancer for prognostication in which hormone receptors; HER2/neu and Ki67 indexes are used (2, 3).
Cyclooxygenase (COX) is the enzyme responsible for the conversion of arachidonic acid to prostaglandin H2 (4). It exists in mammals in two isoforms: COX-1 and COX-2. COX-2, a 74 kDa protein localized in the cells’ endoplasmic reticulum and nuclear membrane is expressed in response to stimuli such as inflammation and tumor promoters (5–7). COX-2 catalyzes key steps in the metabolism of arachidonic acid to prostaglandin E2 (PGE2), which in turn stimulates aromatase transcription resulting in increased estradiol concentrations.
Liu et al. (8) reported that COX-2 takes part in the initiation and progression of invasive breast cancer based on data that COX-2 expression alone could be sufficient for inducing mammary gland tumor genesis in transgenic mice. Early studies of COX-2 expression in invasive breast cancer yielded inconsistent findings, with expression reported to be between 0 and 100% of samples (9, 10). In those studies, COX-2 expression was evaluated according to mRNA levels. Immunohistochemical (IHC) studies of COX-2 antigen expression in invasive breast cancer have produced more consistent findings with moderate or strong levels of COX-2 expression, which was found in 36–56% of tumors (11–16).
Different groups have reported that COX-2 expression is a predictor of poor disease-free survival (13–15). Breast cancers expressing COX-2 are more frequently associated with higher grade, larger tumors, and poorer prognosis (17, 18). Previous studies support the consensus that COX-2 over-expression has an adverse prognostic effect in invasive breast cancer (18, 19). COX-2 over-expression is reported to be a characteristic of metastatic breast cancer (20, 21).
During the last decade, numerous groups studied the anti-cancer action mechanisms of COX-2 inhibitors. However, the underlying mechanisms are not yet completely understood. It has been demonstrated that COX-2 inhibitors induce cell cycle arrest, inhibit tumor growth, and suppress tumor neo-angiogenesis. Moreover, they potently induce apoptotic cell death in tumor cells and endothelial cells (22–26). From this point of view, it was suggested that the use of non-steroidal anti-inflammatory drug (NSAID) or acetyl salicylic acid might be associated with a survival benefit in women with breast cancer (27, 28).
This study was designed to assess the impact of the level of COX-2 expression as determined by IHC on overall survival (OS), disease-free survival (DFS) and breast cancer specific survival (BCSS) in breast cancer patients. We further analyzed the correlation between COX-2 expression and current routine prognostic and predictive factors.
MATERIAL AND METHODS
Study Design
The study was designed as a retrospective cohort study. Breast cancer patients who underwent surgical treatment at Marmara University Hospital, Istanbul were planned to be included in the study. Ethics committee approval was received for this study from the ethics committee of Marmara University, School of Medicine, Istanbul. Written informed consent was obtained from patients who participated in this study.
Inclusion and Exclusion Criteria
Patients who were clinically stage 1, 2 or 3 at diagnosis, underwent surgery for invasive breast cancer, received adequate loco-regional (surgery ± radiation treatment) and systemic treatment, had regular postoperative follow-up and had their last follow-up visit within the previous year were included in the study. Those who were clinically stage 4, had diagnosis of inflammatory breast cancer or ductal carcinoma in situ (DCIS), received no surgical treatment or inadequate loco-regional treatment, -although indicated- received inadequate or no chemotherapy, did not receive hormone treatment although their tumor was endocrine responsive (whose estrogen (ER) and/or progesterone receptor (PR) expression rates were more than 1%) or were lost to follow-up for more than 1 year were excluded from the study. Patients whose paraffin tumor blocks are missing were also excluded from outcome analysis.
Cohorts/Outcomes/End-points
Patients were categorized into two groups according to their breast cancer COX-2 expression status as COX-2 positive and negative cancers. Patients in the two groups were compared in terms of OS (overall survival), DFS (disease free survival) and BCSS (breast cancer-specific survival).
Follow-up
In addition to the semi-annual thorough physical examination, liver function tests, chest radiography, abdominal ultra-sound (US) and bone scintigraphy were performed according to patient’s complaints/findings. Mammography and, if necessary, breast US imaging were done annually.
Objectives
The main objectives were to compare the OS, DFS and BCSS rates between COX-2 positive and negative patients. Secondary objectives were to assess the independent prognostic factors for OS, DFS, and BCSS. In addition, the correlation of COX-2 expression with conventional prognostic and predictive factors (age, tumor size, axillary lymph node involvement, tumor grade, tumor stage, ER, PR, HER2/neu and Ki67 expressions and molecular subtyping) of breast cancer was assessed as other secondary objectives.
Data
Patients’ demographic, clinical, pathologic, treatment and survival data were retrieved from their hospital files. Demographical data such as age and body mass index at the time of breast cancer diagnosis as well as gender; clinical data such as surgery date, type of operation, postoperative complication; family history, menopausal status, -if any- history of neo-adjuvant and/or adjuvant treatments, last date of follow-up and, if any, recurrences and/or death; histopathological data such as tumor histology, tumor size, regional lymphatic involvement, grade, and stage were recorded.
Tissue Sampling
For assessing COX-2 expression by IHC, tumor samples from patients that had been previously fixed in 10% buffered formalin and embedded in paraffin blocks were collected from the pathology tissue bank. Tissue samples were used for the high-throughput study. After screening the slides from each case, we selected a paraffin block that was well fixed and contained a representative section of the tumor. Tissue blocks were sectioned at a 3-µm thickness from each selected paraffin block containing breast cancer tissues with Thermo Shandon Finesse E microtome and processed for IHC staining. Following removal of paraffin with xylene, sections were re-hydrated with graded ethanol and immersed in Tris-buffered saline. The endogenous peroxidase activity was suppressed with 3% H2O2. To bring out the masked antigens, we assessed the tissue samples with pH 6.0 citrate buffer solution for 20 minutes in 400W microwave oven. The tissue samples were washed with pH 7.4 phosphate buffering solution (PBS). In order to block the non-specific immunostaining, protein blockage was performed with Super Block Solution (SensiTek HRP Anti-Polyvent Kit, ScyTek Laboratories, UK).
Immunohistochemistry for COX-2/ER/PR/HER2/neu/Ki67 Expressions
Streptavidin-biotin peroxidase technique was used to detect the expression of COX-2, Ki67 index, ER, PR and HER2/neu. IHC was performed using the COX-2 (antihuman) mouse monoclonal antibody clone, 4H12 (1:100, Novocastra, UK). One tumor tissue sample was stained with and without antibody as a positive and negative control, respectively. Immunostaining for ER, PR, HER2/neu, Ki67 index was performed using established procedures with the following antibodies for 60 minutes incubation period: a. ER (clone 6F11, mouse monoclonal, 1:40, Novocastra, UK), b. PR (clone 1A6, mouse monoclonal, 1:40, Novocastra, UK), c. HER2/neu (clone CB11, mouse monoclonal HER2/neu oncoprotein, 1:40, Novocastra, UK) and d. Ki67 index (clone MM1, mouse monoclonal, 1:50, Novocastra, UK). After the incubation period, tissue arrays were washed with PBS and reincubated with biotinylated secondary antibody and streptavidin peroxidase (SensiTek HRP Anti-Polyvalent Kit, ScyTek Laboratories, UT) for 10 minutes. They were again washed with PBS before assessment. During assessment 3.3′-diaminobenzidine tetra hydrochloride (DAB) was used. Finally, tissues were counterstained with Mayer hematoxylin and dehydrated through ethanol and closed with entellan. Assessment of slides was performed with light microscopy by a pathologist who was blinded to patients’ clinical and survival data.
Variables
Immunoreactivity for COX-2 in tumor cells was assessed using a scoring system based on staining intensity (0 = no staining, 1+ = weak staining with patchy cytoplasmic staining, 2+ = intermediate staining with mostly cytoplasmic and focally plasma membrane staining, and 3+ = strong staining with mostly plasma membrane staining). Scores 0 and 1+ were classified as “COX-2 negative”, whereas 2+ and 3+ as “COX-2 positive” (29). ER and PR status were grouped as positive and negative in which at least 1% IHC staining was regarded as positive, i.e. hormone sensitive. The tumor was regarded hormone receptor (HR) positive if either ER or PR was found to be positive, whereas it was regarded negative if both ER and PR were found to be negative. HER2/neu status of patients was graded either according to their IHC staining that revealed 0, 1+, 3+ or according to FISH (fluorescence in situ hybridization) result as positive or negative. For Her-2/neu scoring; no staining or weak incomplete membrane staining in any proportion of the tumor cells were scored as 0 or 1+, complete membrane staining either non-uniform or weak in intensity but with obvious circumferential distribution in at least 10% of cells were scored as 2+, complete intense uniform membrane staining of >30% of invasive tumor cells were scored as 3+, and were regarded as positive for HER family proteins. Those with 2+ staining were graded according to their FISH result. In the FISH technique, the green colored signals that show the centromeres (CEN-17) belonging to chromosome 17 in the nucleus of the at least 80 tumor cells in every tissue specimen that were HER2/neu 2+, and the orange colored signals that show HER2/neu gene were counted. HER2/neu and the CEN-17 ratio was evaluated and HER2/neu values ≥2 were assessed as amplified. Ki67 index staining was scored as the percentage of expression at 3 different tumor areas (×40) by counting at least 1000 tumor cells in each area. If the number of expressed cells constituted less than 10%, the Ki67 index was classified as “low”, whereas, it was regarded as “high” when the expression was found to be 10% or more. Tumors were also classified according to their molecular subtype. Tumors which were ER positive, PR positive and/or negative, HER2/neu negative were classified as “Luminal A type”; ER positive, PR positive and/or negative, HER2/neu positive were classified as “Luminal B type”; ER and PR negative, HER2/neu positive were classified as “HER2/neu type”; ER and PR and HER2/neu negative were classified as “Basal-like type”. Those with 2+ HER2/neu staining that were not verified by the FISH method were classified as “Undetermined”. Patients’ tumor size and axillary lymph node involvement were grouped according to TNM staging criteria: tumor size ≤ 2 cm as T1, 2–5 cm as T2 and ≥5 cm as T3; if no axillary lymph node involvement as N0, 1 to 3 node involvement as N1, 4 to 9 node involvement as N2, and 10 or more node involvement as N3.
Statistical Analysis
Overall survival, disease-free survival and breast cancer specific survival estimates were calculated using Kaplan–Meier curves and tables. Survival comparisons of Kaplan-Meier curves of COX-2 positive and negative patients were made by the log-rank test. Furthermore, multivariate analyses were conducted using Cox’s proportional hazard regression model to determine independent factors affecting DFS, OS, and BCCS. Frequencies of different variables in the two patient groups were compared with Pearson’s chi-square or Fisher’s exact tests, wherever appropriate. Factors included in the multivariate analysis model on each survival were age, tumor size, axillary stage (N), pathologic stage, histologic grade, ER, PR, HER2/neu, Ki67 labeling index, COX-2 expression levels, and molecular subtype classification. The correlation test between COX-2 expression and independent factors for survival was done using Pearson correlation analysis. Correlation index was given as r-value; r-value of less than −1 was regarded as negative and +1 as positive correlation; r-value between 0–0.49 was regarded as weak, those between 0.5–0.74 as moderate, and those between 0.75–1 as strong correlation. The mean values were given with standard error (SE), p-value less than 0.05 was considered to be significant. The analysis was done by Statistical Package for the Social Sciences statistics software, version 15.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Cohort Characteristics
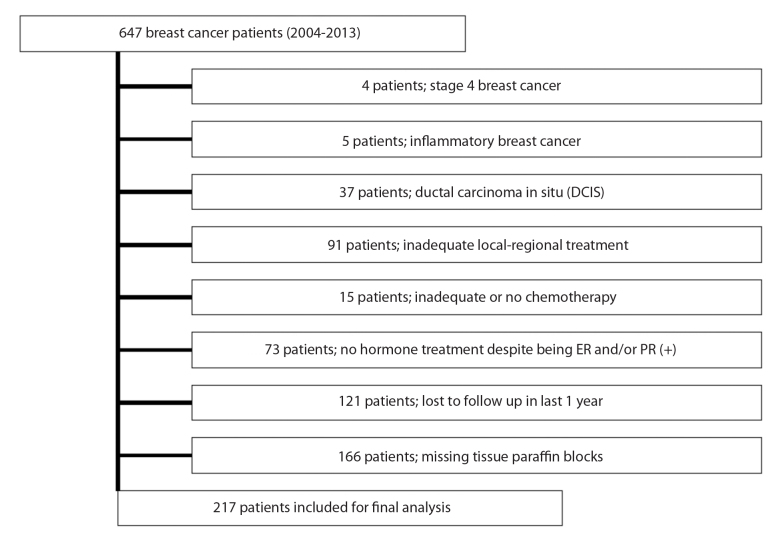
Between November 2004 and December 2013, 647 patients underwent breast cancer surgery. However, 430 patients were excluded from analysis due to the study’s exclusion criteria. Details of reasons for excluding patients were presented in Figure 1. Altogether 217 patients with non-metastatic invasive breast cancer who underwent definitive breast surgery during the study period were included in the study. Patients’ median age was 56 (27–85) years, and most of them were female (n=213). All demographic, treatment-related and histopathologic findings of the cohort were given in Table 1.
Figure 1.
Study flow-chart showing details of exclusion
Table 1.
Demographic, clinical and treatment-related data of the study cohort
| Variables | COX-2 (+) n=81 | COX-2 (−) n=136 | Total n=217 | p |
|---|---|---|---|---|
| Gender; n (%) | ||||
| Female | 79 (98) | 134 (99) | 213 (98) | 0.60 |
| Male | 2 (2) | 2 (1) | 4 (2) | |
| Age; median year (SD) | 56 (11.9) | 57 (13.03) | 56 (12.6) | 0.82 |
| Operation; n (%) | ||||
| Lumpectomy | 32 (40) | 52 (38) | 84 (39) | 0.72 |
| Mastectomy | 49 (60) | 84 (62) | 133 (61) | |
| Axillary technique; n (%) | ||||
| Only sentinel node biopsy | 22 (27) | 43 (32) | 65 (30) | 0.50 |
| Axillary dissection | 59 (73) | 93 (68) | 152 (70) | |
| Radiotherapy; n (%) | ||||
| No | 37 (46) | 55 (40) | 92 (42) | 0.50 |
| Yes | 44 (54) | 81 (60) | 125 (58) | |
| Chemotherapy; n (%) | ||||
| No | 29 (36) | 55 (40) | 84 (39) | 0.50 |
| Yes | 52 (64) | 81 (60) | 133 (61) | |
| Hormonotherapy; n (%) | ||||
| No | 29 (36) | 34 (25) | 63 (29) | 0.10 |
| Yes | 52 (64) | 102 (75) | 154 (71) | |
| Trastuzumab; n (%) | ||||
| No | 69 (85) | 127 (93) | 196 (90) | 0.04 |
| Yes | 12 (15) | 9 (7) | 21 (10) | |
| Histopathologic type; n (%) | ||||
| Invasive lobular cancer | 2 (2) | 9 (7) | 11 (5) | 0.20 |
| Invasive ductal cancer | 73 (91) | 115 (84) | 188 (87) | |
| Invasive mixed cancer | 4 (5) | 11 (8) | 15 (7) | |
| Others | 2 (2) | 1 (1) | 3 (1) | |
| Tumor size; mean mm. (SD) | 25 (12.4) | 28 (17.4) | 27 (15.8) | 0.10 |
| Tumor size; n (%) | ||||
| T1 | 28 (34) | 49 (36) | 77 (35) | 0.20 |
| T2 | 50 (62) | 73 (54) | 123 (57) | |
| T3 | 3 (4) | 14 (10) | 17 (8) | |
| Histologic grade; n (%) | ||||
| I | 12 (15) | 19 (14) | 31 (14) | 0.70 |
| II | 41 (51) | 77 (57) | 118 (54) | |
| III | 28 (34) | 40 (29) | 68 (32) | |
| Axillary stage; n (%) | ||||
| N0 | 48 (59) | 63 (46) | 111 (51) | 0.13 |
| N1 | 22 (27) | 43 (32) | 65 (30) | |
| N2 | 10 (13) | 21 (15) | 31 (14) | |
| N3 | 1 (1) | 9 (7) | 10 (5) | |
| Pathological stage; n (%) | ||||
| Stage 1 | 24 (30) | 30 (22) | 54 (25) | 0.20 |
| Stage 2 | 45 (56) | 74 (54) | 119 (55) | |
| Stage 3 | 12 (14) | 32 (24) | 44 (20) | |
| ER; n (%) | ||||
| Negative | 21 (26) | 27 (20) | 48 (22) | 0.30 |
| Positive | 60 (74) | 109 (80) | 169 (78) | |
| PR; n (%) | ||||
| Negative | 32 (40) | 42 (31) | 74 (34) | 0.20 |
| Positive | 49 (60) | 94 (69) | 143 (66) | |
| HER2/neu; n (%) | ||||
| Negative | 55 (68) | 111 (82) | 166 (76) | 0.07 |
| Positive | 14 (17) | 14 (10) | 28 (13) | |
| Undetermined | 12 (15) | 11 (8) | 23 (11) | |
| Ki67 index; n (%) | ||||
| Low | 29 (36) | 71 (52) | 100 (46) | 0.02 |
| High | 52 (64) | 65 (48) | 117 (54) | |
| Molecular subtype; n (%) | ||||
| Luminal A type | 46 (57) | 94 (69) | 140 (64) | 0.24 |
| Luminal B type | 8 (10) | 7 (5) | 15 (7) | |
| HER2/neu type | 6 (7) | 7 (5) | 13 (6) | |
| Basal like type | 9 (11) | 17 (13) | 26 (12) | |
| Undetermined | 12 (15) | 11 (8) | 23 (11) |
COX-2: Cyclooxygenase-2; SD: standard deviation; ER: Estrogen receptor; PR: progesterone receptor
Cyclooxygenase-2 expression was examined by immunohistochemistry staining (Figures 2a–d) as described and was found to be positive in 81 (37%) patients. All demographic, histopathologic and treatment-related factors were similar in COX-2 positive and negative patients except trastuzumab use and Ki67 index (Table 1). Significantly more COX-2 positive patients (15%) were found to receive trastuzumab when compared to COX-2 positive patients (7%; p=0.04). Ki67 index was high in 64% of COX-2 positive patients, whereas it was high in 48% of COX-2 negative patients, the difference between groups was significant (p=0.02). COX-2 expression was observed in 57, 10, 7 and 11% of patients with Luminal A type, Luminal B type, HER2/neu type and basal-like type tumors, respectively.
Figure 2. a–d.
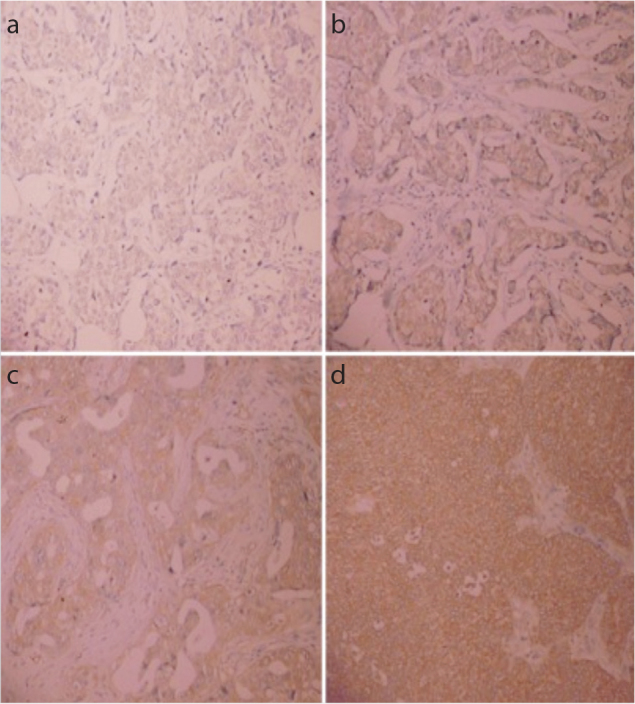
Image of Cyclooxygenase-2 (COX-2) 0 immunohistochemistry scoring (no staining) (a). Image of COX-2 1+ immunohistochemistry scoring (weak staining with patchy cytoplasmic staining) (b). Image of COX-2 2+ immunohistochemistry scoring (intermediate staining with mostly cytoplasmic and focally plasma membrane staining) (c). Image of COX-2 3+ immunohistochemistry scoring (strong staining with mostly plasma membrane staining) (d)
Survival Analysis
As of January 2014, the median follow-up period was 37 (5–107) months. 18 patients had a recurrence during the follow-up period, 14 recurrences occurred at distant sites. Four patients had a loco-regional relapse. Overall, 13 patients died during follow-up; however, only seven of them died due to breast cancer. Overall death, overall recurrence, loco-regional recurrence, distant recurrence and breast cancer-specific death rates were similar in COX-2 positive and negative patients (Table 2).
Table 2.
Mortality and recurrence rates of COX-2 positive and negative patients
| Variables | COX-2 (+) n=81 | COX-2 (−) n=136 | Total n=217 | p |
|---|---|---|---|---|
| Survival; n (%) | 0.93 | |||
| Alive | 76 (94) | 128 (94) | 204 (94) | |
| Dead | 5 (6) | 8 (6) | 13 (6) | |
| Overall recurrence; n (%) | 0.40 | |||
| No | 76 (94) | 123 (90) | 199 (92) | |
| Yes | 5 (6) | 13 (10) | 18 (8) | |
| Loco-regional recurrence; n (%) | 0.60 | |||
| No | 80 (99) | 133 (98) | 213 (98) | |
| Yes | 1 (1) | 3 (2) | 4 (2) | |
| Distant recurrence; n (%) | 0.50 | |||
| No | 77 (95) | 126 (93) | 203 (94) | |
| Yes | 4 (5) | 10 (7) | 14 (6) | |
| Breast cancer specific survival; n (%) | ||||
| Alive | 76 (94) | 128 (94) | 204 (94) | 0.20 |
| Death from breast cancer | 1 (1) | 6 (4) | 7 (3) | |
| Death from other reasons | 4 (5) | 2 (2) | 6 (3) |
COX-2: Cyclooxygenase-2
The median OS was 32 months in COX-2 positive patients and 40 months in COX-2 negative patients. Median DFS was 31.5 months in COX-2 positive patients and 39 months in COX-2 negative patients. Median BCSS was 32 months in COX-2 positive patients and 40 months in COX-2 negative patients. 5-year OS, DFS and BCSS were similar in COX-2 positive (91.9%, 87.4%, 100%, respectively) and negative (92%, 87.3%, 93.9%, respectively) patients (p=0.82, 0.28 and 0.12, respectively) (Table 3, Figures 3 a–c).
Table 3.
Survival estimates of COX-2 positive and negative patients
| COX-2 (+) | COX-2 (−) | p | |
|---|---|---|---|
| 5 year-OS | 91.9% | 92% | 0.82 |
| 5 year-DFS | 87.4% | 87.3% | 0.28 |
| 5 year-BCSS | 100% | 93.9% | 0.12 |
OS: overall survival; DFS: disease-free survival; BCSS: breast cancer-specific survival; COX-2: Cyclooxygenase-2
Figure 3. a–c.

Overall survival (OS) curves of COX-2 positive and negative patients (a). Disease free survival (DFS) curves of COX-2 positive and negative patients (b). Breast cancer specific survival (BCSS) curves of COX-2 positive and negative patients (c)
Independent Factors for Survival
Progesterone receptor expression was found to be an independent factor for predicting OS. PR positive patients had better OS. Tumor size and molecular subtype classification were found to be independent factors for predicting DFS. It was shown that the DFS was longer in tumors with a smaller size. Patients having Luminal A and HER2/neu type cancers had better DFS. In addition, PR expression was found to be an independent factor for predicting BCSS. PR positive patients had better BCSS. Other independent factors were not associated with OS, DFS or BCSS.
Correlation with COX-2 Expression
There was a significant weak negative correlation between COX-2 expression and patient age (r=−0.116; p=0.02). Also, there was a significant weak positive correlation between COX-2 expression and Ki67 index score (r=0.159; p=0.02). On the other hand, the weak negative correlation between COX-2 expression and ER, PR expressions, tumor size, axillary (N) as well as pathologic stage were not found to be significant (r=−0.071; p=0.30, r=−0.088; p=0.20, r=−0.042; p=0.54, r=−0.125; p=0.07 and r=−0.117; p=0.08, respectively). Also, the weak positive correlation between COX-2 expression and HER2/neu expression, histologic grade and molecular sub-type classification were not found to be significant (r=0.124; p=0.85, r=0.032; p=0.64 and r=0.046; p=0.52, respectively).
DISCUSSION
In this study, the aim was to assess the level of COX-2 expression as determined by IHC on OS, DFS, and BCSS in Turkish breast cancer patients. We found that 5-year OS, DFS, and BCSS were similar in COX-2 positive and negative patients. PR expression was shown to be an independent factor for both OS and BCSS. On the other hand, tumor size and molecular subtype classification were independent predictors for DFS. Further evaluation of the independent predictive factors revealed that only Ki67 index and patient age had a correlation with COX-2 expression.
The strength of the current study is that we included patients with strict criteria. Setting the inclusion and exclusion criteria when designing the study enabled us to assess a relatively homogenous population. Patients with stage 4 breast cancer, inflammatory breast cancer, DCIS, those who had inadequate loco-regional treatment, who were lost-to-follow-up and those with missing paraffin blocks were excluded from the study. Furthermore, the pathologist was blinded to the prognostic data and the cohort of the patients. In earlier series, COX-2 over-expression was found to be associated with lower OS and DFS (13, 14). However, there is no evidence showing a relationship between COX-2 over-expression and BCSS, yet. Therefore, our study is unique due to one of its objectives, which is to assess the impact of COX-2 over-expression on BCSS.
However, there are also some drawbacks of the present study. Due to its retrospective design, it was open to bias to some extent. Although the number of patients who were in the scope of the study was 647 patients at the beginning, most of the patients were excluded due to our exclusion criteria shown in Figure 1. Therefore, only 217 patients remained for final analysis. Furthermore, because they did not have further FISH/SISH or CISH assessment, 23 cases with HER2/neu 2+ staining were grouped as “undetermined” for their sub-typing. In addition, the history of selective COX-2 inhibitor drug and NSAID use of patients were not complete, which we believe might have affected the survival outcome of this study. Lastly, the median follow-up period was relatively short for non-metastatic breast cancer patients in whom the life expectancy is longer when compared to other cancer patients.
The proportion of immunohistochemically identified COX-2 positive tumors varies widely among studies (ranging between 4.5 and 85%) (30). Variations in COX-2 expression are partly attributable to the different scoring systems and cut-off values used for COX-2 immunoreactivity. Kelly et al. (31) scored even weak COX-2 staining as positive immunoreactivity while Boland et al. (32) considered COX-2 staining as positive only in case of moderate staining in the specimens. For clinically significant classification of COX-2 expression, we applied semi-quantitative scoring, which reflects staining intensity. In our cohort, COX-2 over-expression was found in 81 (37%) patients, which is concordant with other previously reported series. This also facilitated us to compose two cohorts with a satisfactory number of subjects.
In earlier series, COX-2 over-expression was reported to be associated with lower OS and DFS (13, 14). However, in this study, we found that COX-2 over-expression has no impact on OS, DFS, and BCSS. Both COX-2 positive and negative patients had similar survival rates. The reason for this finding might be our short follow-up period when compared to similar studies as in one study with a median follow-up of 19 years (8). Therefore, we do not exclude a type II error in our findings due to our limited sample size.
Our secondary objective was to assess the independent prognostic factors for OS, DFS, and BCSS. In earlier studies, negative ER and PR status, HER2/neu positivity, increased tumor size, lymph node involvement, high histologic grade and molecular subtyping were reported as poor prognostic factors for OS (1). Although there are studies supporting that Ki67 index is one of the determining factors for molecular subtyping in breast cancer (17), we did not use Ki67 index over-expression as a criterion for this purpose in our study. We have chosen the molecular subtype classification without including Ki67 index as in most of the studies (18) since this classification is easier to perform and to evaluate the data. In our study, PR expression was found to be the only independent factor for predicting OS and BCSS. Furthermore, tumor size and molecular subtype classifications were found to be independent factors for predicting DFS.
Currently, the mechanism by which COX-2 is up-regulated in breast cancer is unknown. One possibility is that cancer cells become intrinsically more active in expressing COX-2 than non-neoplastic cells (25). To this end, both inactivation of tumor suppressor genes, such as p53, and activation of oncogenes, such as HER2/neu, have been implicated in the induction of COX-2. However, we observed no significant association between COX-2 and HER2/neu expression patterns. The relationship between HER2/neu gene amplification and COX-2 over-expression has been a subject of controversy (19, 21, 33–36). The correlation of COX-2 expression with conventional prognostic and predictive factors for breast cancer including HER2/neu expression was assessed as our secondary objective. In previous studies, COX-2 over-expression was found to be associated with large tumor size, high histologic grade, negative ER/PR status, high proliferation rate, high p53 expression, and HER2/neu amplification along with axillary node involvement and ductal type histology (13, 33). The results consistently support a correlation of COX-2 over-expression with high histologic grade, negative ER/PR status and high Ki67 index (13, 37). In patients with hormone receptor-positive tumors, the COX-2 expression had a negative influence on outcome. One study showed that COX-2 played a role in hormonal pathways and could explain the results found in previously published studies (8). In our study, we only showed a positive weak correlation between the COX-2 over-expression and Ki67 index, and a negative weak correlation between COX-2 expression and patient age. We found COX-2 over-expression and high Ki67 index in the specimen to have a weak significant positive correlation. Similarly, we determined that the young patient age and high COX-2 expression had a weak significant negative correlation. Other variables seemed to have no correlation with COX-2 over-expression. Data from similar studies showed that COX-2 over-expression is significantly associated with advanced stage of breast cancer, luminal B and basal-like type tumors (29). Bos et al. (20) reported that the brain metastasis of breast cancer is mediated by increased expression of a set of genes, including COX-2, and is seen less after combination treatment with cetuximab. These findings might support the clinical application of combination treatments with COX-2 inhibitors for patients with luminal-B or basal-like type breast cancers. Previous reports have shown a correlation between COX-2 expression and response to hormone therapy in early breast cancer, whereby about one-third of the patient group was treated with adjuvant endocrine or chemotherapy. Witton et al. (38) also showed that COX-2 expression is associated with poor outcome in ER-negative, but not in ER-positive breast cancer. In contrast, Haffty et al. (34) reported that the significance of COX-2 expression is limited to HR-positive tumors in breast cancer patients treated with partial mastectomy and radiotherapy.
CONCLUSION
Survival rates were similar in both COX-2 positive and negative non-metastatic breast cancer patients. COX-2 over-expression seemed to be correlated with higher Ki67 index and young patient age. However, these correlations were not strong. The only independent factor for OS and BCSS was PR expression status while tumor size and molecular subtype were independent factors for DFS. Results of studies with larger sample size and longer follow-up should be evaluated to reach solid conclusions.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Marmara University School of Medicine.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – S.A.G., M.Ü.U., B.M.G.; Design - S.A.G., M.Ü.U., B.M.G.; Supervision – H.K., B.M.G.; Resources – S.A.G., M.Ü.U., H.K., B.M.G.; Materials – H.K.; Data Collection and/or Processing – S.A.G., M.Ü.U., S.Ş., Y.N.; Analysis and/or Interpretation – S.A.G., M.Ü.U., B.M.G.; Literature Search – S.A.G., M.Ü.U.; Writing Manuscript– M.Ü.U., S.A.G.; Critical Review – B.M.G., H.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Decker T, Hungermann D, Bocker W. Prognostic and predictive factors of invasive breast cancer. Pathologe. 2009;30:49–55. doi: 10.1007/s00292-008-1105-0. http://dx.doi.org/10.1007/s00292-008-1105-0. [DOI] [PubMed] [Google Scholar]
- 2.Gabos Z, Thoms J, Ghosh S, Hanson J, Dechenes J, Sabri S, et al. The association between biological subtype and locoregional recurrence in newly diagnosed breast cancer. Breast Cancer Res Treat. 2010;124:187–194. doi: 10.1007/s10549-010-1135-1. http://dx.doi.org/10.1007/s10549-010-1135-1. [DOI] [PubMed] [Google Scholar]
- 3.Hortobagyi GN. Toward individualized breast cancer therapy: translating biological concepts to the bedside. Oncologist. 2012;17:577–584. doi: 10.1634/theoncologist.2012-0032. http://dx.doi.org/10.1634/theoncologist.2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. http://dx.doi.org/10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 5.Méric JB, Rottey S, Olaussen K, Soria JC, Khayat D, Rixe O. Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol. 2006;59:51–64. doi: 10.1016/j.critrevonc.2006.01.003. http://dx.doi.org/10.1016/j.critrevonc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 7.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. http://dx.doi.org/10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 8.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. http://dx.doi.org/10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 9.Parrett M, Harris R, Joarder F, Ross MS, Clausen KP, Robertson FM. Cyclooxygenase-2 gene expression in human breast cancer. Int J Oncol. 1997;10:503–507. doi: 10.3892/ijo.10.3.503. http://dx.doi.org/10.3892/ijo.10.3.503. [DOI] [PubMed] [Google Scholar]
- 10.Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455–460. doi: 10.1093/jnci/90.6.455. http://dx.doi.org/10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- 11.Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. http://dx.doi.org/10.1002/1097-0142(20001215)89:12<2637::AID-CNCR17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 12.Half E, Tang XM, Gwyn K, Sahin A, Wathen K, Sinicrope FA. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62:1676–1681. [PubMed] [Google Scholar]
- 13.Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 14.Denkert C, Winzer KJ, Muller BM, Weichert W, Pest S, Kobel M, et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer. 2003;97:2978–2987. doi: 10.1002/cncr.11437. http://dx.doi.org/10.1002/cncr.11437. [DOI] [PubMed] [Google Scholar]
- 15.Spizzo G, Gastl G, Wolf D, Gunsilius E, Steurer M, Fong D. Correlation of COX-2 and Ep-CAM overexpression in human invasive breast cancer and its impact on survival. Br J Cancer. 2003;88:574–578. doi: 10.1038/sj.bjc.6600741. http://dx.doi.org/10.1038/sj.bjc.6600741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe O, Shimizu T, Imamura H, Utada Y, Okabe T, Kimura K. Expression of cyclooxygenase-2 in malignant and benign breast tumors. Anticancer Res. 2003;23:3215–3221. [PubMed] [Google Scholar]
- 17.Costa C, Soares R, Reis-Filho JS, Leitao D, Amendoeira I, Schmitt FC. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol. 2002;55:429–434. doi: 10.1136/jcp.55.6.429. http://dx.doi.org/10.1136/jcp.55.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor JK, Avent J, Lee RJ, Fischbach J, Gaffney DK. Cyclooxygenase-2 expression correlates with diminished survival in invasive breast cancer treated with mastectomy and radiotherapy. Int J Radiat Oncol Biol Phys. 2004;58:1034–1040. doi: 10.1016/j.ijrobp.2003.08.032. http://dx.doi.org/10.1016/j.ijrobp.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Park K, Han S, Shin E, Kim HJ, Kim JY. Cox-2 expression on tissue micro-array of breast cancer. Eur J Surg Oncol. 2006;32:1093–1096. doi: 10.1016/j.ejso.2006.05.010. http://dx.doi.org/10.1016/j.ejso.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. http://dx.doi.org/10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh B, Berry JA, Shoher A, Ayers GD, Wei C, Lucci A. COX-2 involvement in breast cancer metastasis to bone. Oncogene. 2007;26:3789–3796. doi: 10.1038/sj.onc.1210154. http://dx.doi.org/10.1038/sj.onc.1210154. [DOI] [PubMed] [Google Scholar]
- 22.Jendrossek V, Handrick R, Belka C. Celecoxib activates a novel mitochondrial apoptosis signaling pathway. FASEB J. 2003;17:1547–1549. doi: 10.1096/fj.02-0947fje. http://dx.doi.org/10.1096/fj.02-0947fje. [DOI] [PubMed] [Google Scholar]
- 23.Song X, Lin HP, Johnson AJ, Tseng PH, Yang YT, Kulp SK, et al. Cyclooxygenase-2, player or spectator in cyclooxygenase-2 inhibitor-induced apoptosis in prostate cancer cells. J Natl Cancer Inst. 2002;94:585–591. doi: 10.1093/jnci/94.8.585. http://dx.doi.org/10.1093/jnci/94.8.585. [DOI] [PubMed] [Google Scholar]
- 24.Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 25.Leahy KM, Ornberg RL, Wang Y, Zweifel BS, Koki AT, Masferrer JL. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625–631. [PubMed] [Google Scholar]
- 26.Lin HP, Kulp SK, Tseng PH, Yang YT, Yang CC, Chen CS. Growth inhibitory effects of celecoxib in human umbilical vein endothelial cells are mediated through G1 arrest via multiple signaling mechanisms. Mol Cancer Ther. 2004;3:1671–1680. [PubMed] [Google Scholar]
- 27.Blair CK, Sweeney C, Anderson KE, Folsom AR. NSAID use and survival after breast cancer diagnosis in post-menopausal women. Breast Cancer Res Treat. 2007;101:191–197. doi: 10.1007/s10549-006-9277-x. http://dx.doi.org/10.1007/s10549-006-9277-x. [DOI] [PubMed] [Google Scholar]
- 28.Hyun CL, Lee HE, Kim KS, Kim SW, Kim JH, Choe G, et al. The effect of chromosome 17 polysomy on HER-2/neu status in breast cancer. J Clin Pathol. 2008;61:317–321. doi: 10.1136/jcp.2007.050336. http://dx.doi.org/10.1136/jcp.2007.050336. [DOI] [PubMed] [Google Scholar]
- 29.Kostopoulos I, Arapantoni-Dadioti P, Gogas H, Papadopoulos S, Malamou-Mitsi V, Scopa CD, et al. Evaluation of the prognostic value of HER-2 and VEGF in breast cancer patients participating in a randomized study with dose-dense sequential adjuvant chemotherapy. Breast Cancer Res Treat. 2006;96:251–261. doi: 10.1007/s10549-005-9062-2. http://dx.doi.org/10.1007/s10549-005-9062-2. [DOI] [PubMed] [Google Scholar]
- 30.Singh-Ranger G, Salhab M, Mokbel K. The role of cyclooxygenase-2 in breast cancer: review. Breast Cancer Res Treat. 2008;109:189–198. doi: 10.1007/s10549-007-9641-5. http://dx.doi.org/10.1007/s10549-007-9641-5. [DOI] [PubMed] [Google Scholar]
- 31.Kelly LM, Hill AD, Kennedy S, Connolly EM, Ramanath R, Teh S, et al. Lack of prognostic effect of Cox-2 expression in primary breast cancer on short-term follow-up. Eur J Surg Oncol. 2003;29:707–710. doi: 10.1016/j.ejso.2003.07.001. http://dx.doi.org/10.1016/j.ejso.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer. 2004;90:423–429. doi: 10.1038/sj.bjc.6601534. http://dx.doi.org/10.1038/sj.bjc.6601534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucci A, Krishnamurthy S, Singh B, Bedrosian I, Meric-Bernstam F, Reuben J, et al. Cyclooxygenase-2 expression in primary breast cancers predicts dissemination of cancer cells to the bone marrow. Breast Cancer Res Treat. 2009;117:61–68. doi: 10.1007/s10549-008-0135-x. http://dx.doi.org/10.1007/s10549-008-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haffty BG, Yang Q, Moran MS, Tan A, Reiss M. Estrogen-dependent prognostic significance of cyclooxygenase-2 expression in early-stage invasive breast cancers treated with breast-conserving surgery and radiation. Int J Radiat Oncol Biol Phys. 2008;71:1006–1013. doi: 10.1016/j.ijrobp.2007.11.063. http://dx.doi.org/10.1016/j.ijrobp.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 35.Subbaramaiah K, Howe LR, Port ER, Brogi E, Fishman J, Liu CH, et al. HER-2/neu status is a determinant of mammary aromatase activity in vivo: evidence for a cyclooxygenase-2-dependent mechanism. Cancer Res. 2006;66:5504–5511. doi: 10.1158/0008-5472.CAN-05-4076. http://dx.doi.org/10.1158/0008-5472.CAN-05-4076. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz KJ, Callies R, Wohlschlaeger J, Kimmig R, Otterbach F, Bohr J, et al. Overexpression of cyclo-oxygenase-2 is an independent predictor of unfavourable outcome in node-negative breast cancer, but is not associated with protein kinase B (Akt) and mitogen-activated protein kinase (ERK1/2, p38) activation or with Her-2/neu signalling pathways. J Clin Pathol. 2006;59:685–691. doi: 10.1136/jcp.2005.030650. http://dx.doi.org/10.1136/jcp.2005.030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerlikowske K, Molinaro AM, Gauthier ML, Berman HK, Waldman F, Bennington J, et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst. 2010;102:627–637. doi: 10.1093/jnci/djq101. http://dx.doi.org/10.1093/jnci/djq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witton CJ, Hawe SJ, Cooke TG, Bartlett JM. Cyclooxygenase 2 (COX2) expression is associated with poor outcome in ER-negative, but not ER-positive, breast cancer. Histopathology. 2004;45:47–54. doi: 10.1111/j.1365-2559.2004.01898.x. http://dx.doi.org/10.1111/j.1365-2559.2004.01898.x. [DOI] [PubMed] [Google Scholar]