Abstract
During the development of new vasoactive agents, arterial blood pressure monitoring is crucial for evaluating the efficacy of the new proposed drugs. Indeed, research focusing on the discovery of new potential therapeutic targets using genetically altered mice requires a reliable, long-term assessment of the systemic arterial pressure variation. Currently, the gold standard for obtaining long-term measurements of blood pressure in ambulatory mice uses implantable radio-transmitters, which require artery cannulation. This technique eliminates the need for tethering, restraining, or anesthetizing the animals which introduce stress and artifacts during data sampling. However, arterial blood pressure monitoring in mice via catheterization can be rather challenging due to the small size of the arteries. Here we present a step-by-step guide to illustrate the crucial key passages for a successful subcutaneous implantation of radio-transmitters and carotid artery cannulation in mice. We also include examples of long-term blood pressure activity taken from freely moving mice after a period of post-surgery recovery. Following this procedure will allow reliable direct blood pressure recordings from multiple animals simultaneously.
Keywords: Basic Protocol, Issue 111, Blood Pressure, mice, telemetry, hemodynamic, heart rate, surgery
Introduction
Hypertension is one of the major risk factors for cardiovascular diseases, arguably it is a major public health issue both in developed and developing countries1. Several animal models of experimental hypertension have been developed to mimic hypertensive responses like those observed in humans2. Among others, the ambulatory mouse represents an excellent model to study the genesis and the progression of hypertension allowing in vivo analysis of the consequences of chronic exposure to hypertension.
Blood pressure (BP) monitoring in mice has helped researchers to unravel several mechanisms involved in the physiology and pathophysiology of diseases such as hypertension and heart failure3,4. Indeed, manipulation of the mouse genome allowed generation of transgenic or gene-targeted models suitable for studying hypertension5,6. However, even gentle manipulation of conscious mice induces excitements that can potentially introduce artifacts during data acquisition, while use of sedation or tranquilizers profoundly affect blood pressure7. These aspects are particularly important and must be taken in consideration when attempting long-term BP monitoring.
There are several ways to record BP in mice, and a comparison between the most common techniques currently available has been discussed elsewhere8,9. However, the AHA recommendations for BP measurement concluded that intra-arterial measurement of BP are generally preferred because of their ability to directly measure BP over an extended period of time10. Radio-telemetry coupled with direct measurement of arterial pressure is the state-of-the-art method for monitoring physiological functions in awake and freely moving laboratory animals while minimizing stress and environmentally-associated artifacts9,11. Radio-telemetry offers the ability to automatically collect blood pressures, heart rate, body temperature and animal activity from multiple conscious animals.
Although this methodology is becoming very popular in many laboratories, radio-telemetry implantation in mice can be technically challenging. Here we show a step-by-step protocol that illustrates how to implant a pressure transducer in mice. The technique involves subcutaneous insertion of the probe in the mouse body, channel the catheter to the neck and forward near the aortic arch via the left carotid artery. Remotely captured data are shown live on the computer monitor. Data are also stored for "off-line" analysis.
Protocol
Ethics statement: All animal procedures mentioned in this video-article were reviewed and approved by the Animal Care and Use Committee (IACUC) at the University of Tennessee Health Science Center.
Note: Use sterile microsurgery instruments throughout the surgical procedure. Surgical instruments may be sterilized utilizing an infrared sterilizer at an optimum sterilizing temperature of 1,500 °F (815.6 °C). Telemeters can be reused, however, before inserting it in the animal make sure to sterilize the entire transmitter according to the manufacturer instructions and that the tip of the catheter has been refilled with a special gel provided by the manufacturer.
1. Experimental Animals
Obtain an IACUC approval for all experiments that will be performed. Consultation with institutional IACUC is highly recommended regarding requirements for post-operative analgesic use after this procedure.
Keep the animals in climate-controlled rooms having an ambient temperature of 21 °C, 60% relative humidity, and a 12-hr light-dark cycle with free access to food and water. Note: Because the mouse body has to accommodate the telemetry probe (approximatively 1 cm diameter x 2 cm long), it is preferable to use mice that weigh 20 g or more.
2. Anesthesia and Operative Preparation
Weigh the mouse using a precision scale and record its weight. Make sure to handle the animal gently and quietly.
Place the animal into the induction chamber and close it. Set oxygen flow rate at 0.5 L/min and set isoflurane concentration at 4 - 5% .
When the mouse loses consciousness, place it on a body warming plate maintained at 36 - 37 °C. Maintain the anesthesia via nosecone with isoflurane set at 2% (keep flow rate at 0.5 L/min).
Prepare the operation regions by removing the hair from the back of the neck and the ventral aspect of the neck by applying hair removal cream.
Treat the incision site with 3 applications of surgical scrub (betadine solution) alternating with 70% isopropyl alcohol.
3. Surgery
Place the mouse in a supine position. Check for reflexes by pinching the foot, and adjust anesthesia until there is no response. Make an approximately 1-cm midline incision below the neck of the mouse with a scalpel. In the left side of the cut create a subcutaneous space by carefully separating the skin from the underlying connective tissue.
Flip the mouse, and use a scalpel to make a skin incision of approximately 1.5 cm in the dorsal left side behind the scapula. Create a subcutaneous pocket along the animal's flank large enough to accommodate the device. Insert the transmitter into the pocket.
- Insert a small hemostat clamp in the back incision and maneuver it subcutaneously towards the anterior neck opening. Using the inserted-hemostat clamp, gently grasp a non-toxic polyethylene tube (4 cm length x 1 mm I.D.).
- Pull the hemostat back through the tunnel out the lateral incision in the back until the tubing protrudes from both abdominal and dorsal incision, release the tube from the hemostat. From the back, insert the catheter sensor into the tube to tunnel the tip of the pressure sensing catheter through the neck. From the neck anterior, pull and remove the polyethylene tube and close the dorsal incision using metal clips.
- Carefully separate the mandibular glands using sterile cotton tip applicators and retract the left mandibular gland using an elastic stay hook. Using fine-tipped curved forceps, locate the carotid artery along the left side of the trachea. Keep the surgical site sterile by placing and securing a sterile drape.
- Carefully isolate the vessel from the surrounding tissue and gently separate the vagus nerve (whitish in color) that is along the carotid artery away from the artery. Be careful not to cut or damage the nerve or the artery.
Pass three pieces of non-absorbable 7-0 suture underneath the isolated carotid artery section. Tie the cranial suture to close off blood flow. Pull the suture that is closest to the sternum to temporarily occlude blood flow from the aorta.
Make a loose knot using the middle suture. This will be used to secure the catheter in the vessel. Cut a small incision into the artery between the cranial and the sternal sutures using micro-scissors.
Grab the catheter with special vessel cannulation forceps, being careful not to squeeze the catheter to prevent gel loss from the probe. Gently, grab the artery with a fine tip curved tweezers forceps, retrieve the catheter, and insert it in the vessel through the small incision.
- Tighten the middle suture node around the artery and gently advance the catheter. Gently release the suture that is proximal to the sternum and continue to advance the catheter towards the transverse aorta.
- Observe the mark on the catheter that gives an approximate index of how far the catheter needs to be inserted. Once the point is reached, gently tighten both the lower and the upper suture around the catheter. The catheter is secured to the carotid artery by suture knots.
Close the skin incision with non-absorbable 5-0 suture. Once closed, seal the incision with tissue adhesive.
4. Surgical Recovery and BP Measurements
Monitor animal closely for the return of normal postures and behaviors. During the 24 hr post-surgery period administer analgesia as directed by a staff veterinarian.
Once the animals have recovered (5 - 7 days post-surgery), house them individually in a regular mouse cage placed on top of the telemetry receiver plate.
Turn the implanted transmitters "on" and "off" by using a magnetic device briefly positioned close to the animal from the outside of the cage.
Representative Results
Data can be acquired remotely by a receiver; traces are visualized on a computer screen for quality control (Figure 1a). Details such as animal ID, diastolic blood pressure and systolic blood pressure are also shown (Figure 1b). Arterial BP can be recorded continuously (24/7), or for short programmed intervals (i.e., 60 sec acquisition every hour). Data can be automatically stored in a hard disk for later analysis. Averaged BP data from a 3-day continuous recording are shown (Figure 2), it is possible to appreciate the circadian rhythm variation between dark and light cycle. Average of pressure variation and heart rate were calculated and plotted against time.
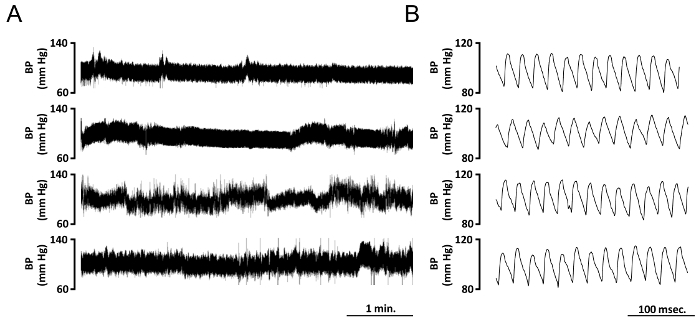 Figure 1. Typical Blood Pressure Tracings of 4 Different Mice Implanted with Telemetric Device. (A) Tracings showing real-time arterial blood pressure changes (mmHg) over a 5 min interval. Data are acquired simultaneously from four different adult mice 5 days after implantation. (B) Tracing at higher temporal resolution extracted from A, it is possible to appreciate blood pressure diastolic and systolic values, BP cycle duration, and heart rate. Please click here to view a larger version of this figure.
Figure 1. Typical Blood Pressure Tracings of 4 Different Mice Implanted with Telemetric Device. (A) Tracings showing real-time arterial blood pressure changes (mmHg) over a 5 min interval. Data are acquired simultaneously from four different adult mice 5 days after implantation. (B) Tracing at higher temporal resolution extracted from A, it is possible to appreciate blood pressure diastolic and systolic values, BP cycle duration, and heart rate. Please click here to view a larger version of this figure.
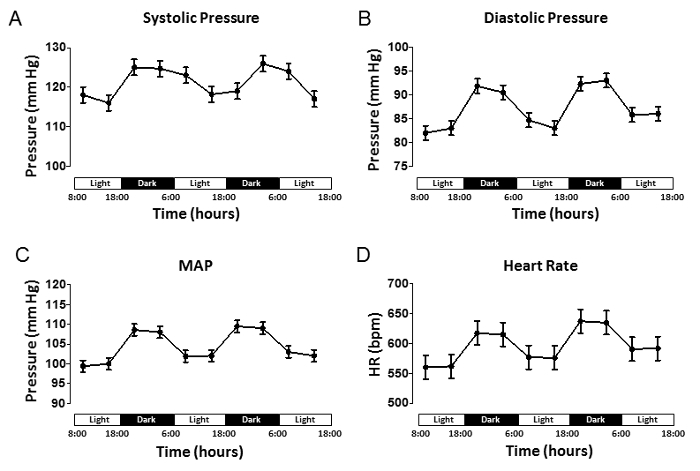 Figure 2. Long-term Arterial Blood Pressure Shows Circadian Rhythm Variation. Averaged pressure data illustrating the values for (A) systolic pressure (SP), (B) diastolic pressure (DP), (C) mean arterial pressure (MAP), and (D) heart rate expressed in beats/min. Data were extracted from continuous recordings taken during the day (light cycle, L) or night (dark cycle, D). Recording started 7 days post-surgery. All data are expressed as mean ± SEM (n = 4 mice). Please click here to view a larger version of this figure.
Figure 2. Long-term Arterial Blood Pressure Shows Circadian Rhythm Variation. Averaged pressure data illustrating the values for (A) systolic pressure (SP), (B) diastolic pressure (DP), (C) mean arterial pressure (MAP), and (D) heart rate expressed in beats/min. Data were extracted from continuous recordings taken during the day (light cycle, L) or night (dark cycle, D). Recording started 7 days post-surgery. All data are expressed as mean ± SEM (n = 4 mice). Please click here to view a larger version of this figure.
Discussion
Implantable radio-telemetry has improved significantly over the last decade; smaller probe size makes the implant less traumatic for the animal, prolonged battery life helps to reduce the costs, and independent telemeter frequencies eliminate crosstalk between receivers. Telemetry is considered the state-of-the-art method for collecting a wide variety of physiological parameters from freely moving animals without the artifacts associated with the use of restraint, human interaction, or anesthesia that are required by other techniques8,9 .
However, some of the disadvantages of his technique are associated with the initial costs of the instrument and software. The transmitters are fragile and susceptible to damage, and the battery eventually does run out of power and need to be replaced. Although the company offers to refurbish those damaged probes at a lower cost, the cost can be still prohibitive for some laboratories. Furthermore, this procedure can be technically challenging and practice is required to achieve reliable BP measurement. Finally, there is some post-surgery associated mortality, which in our hands is about 5%. This can dramatically increase if a specific treatment has to be tested or if a genetic manipulation compromises the mouse health.
The following critical factors are essential for the successful execution of the procedure: maintain tissue hydration with sterile saline throughout the entire procedure. Always handle the transmitter with great care; avoid holding it by the pressure sensor as this may cause the gel to leak out or damage the transmitter. When inserting the catheter, tie the central suture with a double knot, as failure to do so will result in the catheter exiting the vessel. The required length of the catheter that has to be inserted in the carotid can change according to the mouse strain and weight used in that particular study. Therefore, it is highly recommended to run some preliminary experiment to determine how deep the catheter needs to be inserted to avoid occlusion of the aorta. Finally, monitor the animal daily and make sure that the skin above the transmitter is not stretched or necrotic. If extended necrosis or infection occurs, euthanize the animals according to institutional policy.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the National Institutes of Health (NIH/NHLBI) [Grant no. HL114869] and the support from UTHSC to SM.
References
- Danaei G, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornas WC, Silva ME. Animal models for the study of arterial hypertension. J Biosci. 2011;36(4):731–737. doi: 10.1007/s12038-011-9097-y. [DOI] [PubMed] [Google Scholar]
- Henze M, et al. Persistent alterations in heart rate variability, baroreflex sensitivity, and anxiety-like behaviors during development of heart failure in the rat. Am J Physiol Heart Circ Physiol. 2008;295(1):H29–H38. doi: 10.1152/ajpheart.01373.2007. [DOI] [PubMed] [Google Scholar]
- Hoffmann DS, et al. Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension. 2008;51(4):1058–1065. doi: 10.1161/HYPERTENSIONAHA.107.107219. [DOI] [PubMed] [Google Scholar]
- Lerman LO, Chade AR, Sica V, Napoli C. Animal models of hypertension: an overview. J Lab Clin Med. 2005;146(3):160–173. doi: 10.1016/j.lab.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Johns C, Gavras I, Handy DE, Salomao A, Gavras H. Models of experimental hypertension in mice. Hypertension. 1996;28(6):1064–1069. doi: 10.1161/01.hyp.28.6.1064. [DOI] [PubMed] [Google Scholar]
- Vatner SF, Braunwald E. Cardiovascular control mechanisms in the conscious state. N Engl J Med. 1975;293(19):970–976. doi: 10.1056/NEJM197511062931906. [DOI] [PubMed] [Google Scholar]
- Zhao X, et al. Arterial Pressure Monitoring in Mice. Curr Protoc Mouse Biol. 2011;1:105–122. doi: 10.1002/9780470942390.mo100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesall SE, Hoff JB, Vollmer AP, D'Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol. 2004;286(6):H2408–H2415. doi: 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]
- Kurtz TW, et al. Recommendations for blood pressure measurement in humans and experimental animals. Part 2: Blood pressure measurement in experimental animals: a statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Hypertension. 2005;45(2):299–310. doi: 10.1161/01.HYP.0000150857.39919.cb. [DOI] [PubMed] [Google Scholar]
- Kurtz TW, et al. Recommendations for blood pressure measurement in animals: summary of an AHA scientific statement from the Council on High Blood Pressure Research, Professional and Public Education Subcommittee. Arterioscler Thromb Vasc Biol. 2005;25(3):478–479. doi: 10.1161/01.ATV.0000153088.15433.8f. [DOI] [PubMed] [Google Scholar]


