Abstract
Background
We examined how experiences with a rectal placebo gel and applicator used with receptive anal intercourse (RAI) related to young men who have sex with men’s (YMSM) likelihood of using a rectal microbicide gel and applicator in the future.
Methods
An ethnically diverse sample of 95 YMSM (aged 18 to 30 years) were asked to insert hydroxyethylcellulose (HEC) placebo gel rectally before RAI during 12 weeks and report the product’s acceptability (i.e., satisfaction with applicator and gel, respectively; perceived gel side effects; and sexual satisfaction when gel was used) and likelihood of future microbicide use. Main and interaction effects predicting future use intentions were tested using linear regression.
Results
We found a positive association between future use intentions and applicator satisfaction (b = .33; p< .001). In a subsequent interaction effects model, we found that greater gel satisfaction was associated with increased future use intentions; however, the strength of this relationship was magnified when YMSM reported greatest satisfaction with the rectal applicator.
Conclusions
Applicator satisfaction may be a salient factor in YMSM’s decision-making to use a rectal microbicide in the future. Although the importance of developing a satisfactory rectal microbicide gel for YMSM is undeniable for its future use, our results also emphasize the importance of developing strategies that increase YMSM’s comfort and skill when using a rectal applicator. Future research examining how to optimize the design, properties and characteristics of a rectal applicator as a strategy to promote greater satisfaction and use among YMSM is merited.
Keywords: acceptability, prevention, HIV/AIDS, gay, rectal microbicide, applicator satisfaction
More than 60% of all new HIV infections in the United States result from sexual contact among MSM (U.S. Centers for Disease Control and Prevention, 2012). In 2010, young men who have sex with men (YMSM) accounted for 72% of new infections among people ages 13 to 24, and 30% of all new infections among MSM (U.S. Centers for Disease Control and Prevention, 2014). Researchers have posited that YMSM’s vulnerability to HIV infection is heightened due to sexuality-related developmental milestones including serial dating and onset of anal sex (Balthasar, Jeannin, & Dubois-Arber, 2009; Bauermeister, in press; Lyons et al., 2012; Mustanski, Newcomb, Du Bois, Garcia, & Grov, 2011). Within these exchanges, consistent condom use may be compromised if MSM were never taught how to use condoms for anal sex, if they do not feel confident in their ability to negotiate condoms with their sexual partners, or if they perceive that their sexual satisfaction may be curtailed by its use (Bauermeister, Hickok, Meadowbrooke, Veinot, & Loveluck, 2014; Carballo-Diéguez et al., 2011; Kubicek et al., 2008; Kubicek, Arauz-Cuadra, & Kipke, 2015; Pingel, Thomas, Harmell, & Bauermeister, 2013). In light of these challenges, researchers and advocates have encouraged the promotion of innovative biomedical interventions for YMSM (McGowan, 2011; Underhill, Operario, Mimiaga, Skeer, & Mayer, 2010), as they may be integrated to behavioral risk reduction strategies, offer alternatives to condoms, and reduce men’s risk of HIV acquisition.
Rectal microbicides (RM) are products currently under development that, if found to be effective, could prevent or significantly reduce the risk of HIV infection (Gross, Buchbinder, Celum, Heagerty, & Seage, 1998; McGowan, 2008; McGowan, 2014). Assessing acceptability together with determining the clinical safety profile of products is increasingly recognized as a crucial step in early phases of microbicide trials, as it may indicate the need to retool the product’s formulation, its delivery system, or mode of use prior to further safety and efficacy trials (Carballo-Diéguez et al., 2008; Carballo-Diéguez et al., 2007; Carballo-Diéguez, Giguere, Dolezal, Bauermeister, Leu, Valladares, Rohan, et al., 2014; Gorbach et al., 2014; Mantell et al., 2005; Van Der Straten et al., 2012). Achieving consistent and correct use among the product’s consumers will require researchers to develop products perceived to be desirable and acceptable, both within the context of clinical trials and in the real world. Morrow and Ruiz (2008) have identified key factors associated with product acceptability, including gel-specific traits (e.g., satisfaction with gel, formulation side effects), applicator-specific characteristics (e.g., ease and comfort of use, ease carrying applicator), and contextual factors associated with its use (e.g., sexual satisfaction when RM is used in anal sex). Although prior research supports the need to examine these domains with MSM (Carballo-Diéguez et al., 2008; Carballo-Diéguez et al., 2007; Carballo-Diéguez, Giguere, Dolezal, Bauermeister, Leu, Valladares, Rohan, et al., 2014; Mantell et al., 2005), we know little of YMSM’s acceptability of rectal microbicide products.
In this study, we examined how satisfaction with a RM gel and its applicator was associated with YMSM’s likelihood of using a RM gel in the future. Rather than proposing a hypothetical study, however, we sought to assess future use intentions from a sample of YMSM who, prior to a Phase I safety and acceptability trial of a tenofovir 1% gel, were given a rectal placebo gel and asked to use it with receptive anal intercourse (RAI) over a 3-month period. This study design sought to identify individuals who would be good candidates for the subsequent Phase I trial. We hypothesized that greater satisfaction with the rectal gel and the rectal applicator during the “run-in” period would be associated with greater likelihood of future use intentions. Above and beyond their independent association with future use intentions (i.e., main effects model), however, we hypothesized that YMSM’s future use intentions would be associated with the synergy of users’ combined satisfaction with the gel and applicator (i.e., interaction effect model).
Methods
Study data come from a larger project microbicide safety and acceptability in young men (Carballo-Diéguez, Giguere, Dolezal, Bauermeister, Leu, Valladares, Frasca, et al., 2014). The study received IRB approval of all participating institutions. After screening (Stage 1A), YMSM participated in a run-in period and were asked to apply a rectal placebo gel using a rectal-specific applicator (Stage 1B), followed by a safety trial in which participants applied tenofovir 1% gel using a vaginal applicator for rectal delivery of the gel (Stage 2). A vaginal rather than a rectal applicator was used in the safety trial (Stage 2) because the former is the only applicator for which the stability and compatibility of tenofovir 1% gel has been established. The study took place in three sites: Pittsburgh, PA, Boston, MA, and San Juan, PR. Study candidates were recruited from clinics, bars, clubs, newspaper advertisements and social networks.
Recruitment materials indicated that the investigators were looking for YMSM (ages 18–30 years) for a study about their sexual health and their feelings about inserting rectally a placebo gel resembling a microbicide gel currently under development prior to receptive anal sex.
Stage 1A – Screening
Participants underwent an eligibility pre-screening by phone or in person to determine age, same sex behavior, and presumed negative HIV status. Those who passed pre-screening were invited to the clinic for in-person screening (Visit 1). Eligibility criteria included being sexually active (operationalized as at least one RAI episode in the prior month) and engaging in some potential risk behavior. To cast a wide net for the epidemiological objective of Stage 1A (i.e., prevalence of HIV/STIs in rectum), potential risk behavior was operationalized as at least one episode of condomless RAI in the prior twelve months. After informed consent procedures, participants answered a medical history and received a physical exam including a digital rectal exam and anoscopy. Specimens were collected to test for HIV and sexually transmitted infections (STIs). YMSM had to be HIV-negative to be eligible for the study. YMSM identified as HIV-positive during Screening (Stage 1A) were excluded from the study, and referred to care. Research staff made themselves available to participants with reactive tests to provide support or additional follow-up, to the extent that the participants wanted and accepted it. HIV testing and counseling was provided during the remaining stages of the trial. In addition, participants completed a Web-based Computer-Assisted Self-Interview (CASI) that included demographic questions among other topics. HIV counseling and condoms were provided at all visits.
Stage 1B – Three-month non-clinic placebo use
Participants returned to the clinic within 28 days (Visit 2) and were informed of test results. From those who received medical clearance, we selected those fulfilling the more stringent eligibility criterion of having had condomless RAI within the prior three months. This allowed us to focus on those with more recent potential risk and invite them to enroll in Stage 1B. After undergoing a new informed consent process, receiving risk reduction counseling and provision of condoms, and updating their medical history, participants received 20 rectal applicators filled with a placebo gel and instructions to insert the entire content of one applicator rectally within 90 minutes prior to each RAI episode. We used an applicator specifically designed for the delivery of a rectal microbicide (Carballo-Diéguez et al., 2014), filled with HEC gel. HEC is also known as the “universal placebo” because of its use as placebo in most gel microbicide trials. YMSM were told that the gel did not provide any protection against HIV/STIs, and received counseling on HIV/STI risk reduction and condom use. YMSM were also given condoms. Participants were asked to call an interactive voice response system (Bauermeister, Carballo-Dieguez, Giguere, Valladares, & McGowan, 2014) at least once a week to report sexual activity and product use.
Six weeks after Visit 2, participants returned for the Mid-trial Follow-up Visit (Visit 3) at which point the medical history was reviewed and updated; any reported adverse event was further explored; a physical exam including digital rectal exam and anoscopy was performed; samples were collected for STI and HIV testing if clinically indicated; used and unused applicators were collected, counted and recorded; and 20 new rectal applicators containing HEC were dispensed.
Six weeks after Visit 3, participants returned for the Final Follow-up Visit of Stage 1B (Visit 4). All procedures of Visit 3 were repeated but no rectal applicators were dispensed at this time. Additionally, participants completed a web-based CASI and semi-structured interview that included questions on gel and applicator use.
Compensation
Participants received $50 for each study visit (4 visits for Stage 1AB and 5 visits for Stage 2), an additional $50 for each visit that included a biopsy for the Stage 2 safety trial, and $50 for each video teleconference interview completed. They also received $1 per applicator returned at visits 2, 3 and 10 plus maximum incentives of $60 in Stage 1B and $40 in Stage 2 for reporting their product use consistently by phone during the trial. The maximum a participant could make by adhering to all visits and procedures from the first to the last visit of the study was $898.
Measures
Structured and semi-structured data were collected via a Web-based CASI. A descriptive summary of our variables is included in Table 1.
Table 1.
Descriptive Statistics of Stage 1B Sample (N=95)
| Variable Name | M (SD) | N (%) |
|---|---|---|
| Age | 23.19 (3.23) | |
| Race/Ethnicity | ||
| White/European American | 34 (36.0%) | |
| Black/African American | 9 (10.0%) | |
| Latino/Hispanic | 46 (48.0%) | |
| Mixed/Other | 6 (6.0%) | |
| Educational Attainment | ||
| Some high school | 1 (1.1%) | |
| High school/GED | 10 (10.5%) | |
| Some college | 44 (46.3%) | |
| College Degree | 27 (28.4%) | |
| Some Graduate School | 2 (2.1%) | |
| Graduate Degree | 11 (11.6%) | |
| Gel Satisfaction | 7.76 (1.95) | |
| Applicator Satisfaction | 7.52 (1.86) | |
| Overall satisfaction | 6.16 (2.53) | |
| Satisfaction with application process | 8.67 (1.64) | |
| Comfort with plunger | 8.70 (1.89) | |
| Side Effects Total 1 | .19 (.34) | |
| Frequency of Side Effects 1 | .18 (.16) | |
| Bothered by Side Effects 1 | .52 (.20) | |
| Sexual Satisfaction 1 | 7.96 (2.18) | |
| Comparative Satisfaction with Gel1 | ||
| Better | 34 (37.0%) | |
| No Different | 55 (59.8%) | |
| Worse | 3 (3.3%) | |
| Future Gel Use | 8.38 (1.55) | |
Notes.
3 cases had missing data for these variables (N=92)
Future Gel Use
Participants reported their likelihood of using the gel in the future on a 10-point scale (1=Not likely; 10=Extremely likely) across 6 different scenarios: use with lover, use with one-night stand, use with other types of partners, use without condoms, use if there were a 30 minute delay prior to sex, and use while under the influence of substances. We computed a mean score across these six scenarios (α=.81), where higher scores indicated greater future use intentions.
Gel Satisfaction
Participants rated their satisfaction with the gel on a 10-point scale (1=Disliked very much; 10=Liked very much). Participants answered three items specific to their experience using the product: their overall satisfaction with the gel, their immediate satisfaction with the gel, and their satisfaction with the gel after 30 minutes. We computed a mean score across these three items (α=.90), where higher scores indicated greater satisfaction with the gel.
Applicator Satisfaction
Participants also rated their satisfaction with the rectal applicator. We ascertained their satisfaction by examining their perceived ease and comfort with the application process and their satisfaction with the applicator using 9 items rated on a series of 10-point scales (1=Disliked very much/Very difficult, 10=Liked very much/Very easy). We used exploratory factor analysis (Maximum Likelihood extraction with an Oblimin rotation) to examine the domains that emerged from our applicator satisfaction data. Three factors emerged. The first factor (4 items (α=.88); 50.91% of variance) focused on participants’ overall satisfaction with the applicator (i.e., liking of the applicator, the gel application process, ease carrying applicator, and comfort using the applicator). The second factor (3 items (α=.85); 10.53% of variance) focused on their satisfaction with the application process (i.e., ease inserting the applicator, delivering the gel through the applicator, and ease following the instructions). The third factor (2 items (α=.82); 8.21% of variance) focused on participants’ comfort with the applicator’s plunger. Jointly, these three factors explained a pooled variance of 70.14%. We computed a mean score across these 9 items (α=.89), where higher scores indicated greater satisfaction with the applicator.
Perceived Gel Side Effects
Participants were presented with 10 potential side effects (e.g., leakage, bloating, soiling, gassiness, pain). For each side effect, they were asked to recall how frequently it occurred while using the gel (0=None, 1=Some, 2=A Lot), and subsequently rate on a 10-point scale how bothered they felt by each side effect (0=Not bothered at all; 9=Extremely bothered). We computed a composite score for both mean frequency of side effects and mean side effect discomfort. Recognizing that greater occasions of side effect frequency and discomfort were highly correlated (see Table 2) and would be expected to have a multiplicative effect rather than an additive effect on future gel use, we created a total perceived gel side effects score by multiplying the two mean composite scores (α=.78).
Table 2.
Correlation Matrix of Study Variables (N=92)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Applicator Satisfaction | 1 | ||||||
| 2. Gel Satisfaction | .58*** | 1 | |||||
| 3. Frequency of Side Effects | −.14 | −.18 | 1 | ||||
| 4. Bothered by Side Effects | −.25* | −.26* | .78*** | 1 | |||
| 5. Side Effects Total | −.28** | −.29** | .79*** | .95*** | 1 | ||
| 6. Sexual Satisfaction | .46*** | .80*** | −.11 | −.25* | −.25* | 1 | |
| 7. Future Gel Use | .46*** | .16 | .07 | −.02 | .06 | .20 | 1 |
p ≤ .001;
p ≤ .01;
p ≤ .05
Sexual Satisfaction
Participants were asked to rate how sexually satisfied they felt when using the gel on a 10-point scale (1=Not satisfied; 10=Very satisfied). We also asked participants to rate whether their RAI with the gel was better, worse, or no different from other occasions when the gel was not used.
Demographics
Demographic information included age, race (White, Latino, African American mixed or other) and Latino/Hispanic ethnicity, and education (1= less than 8th grade, 2= partial high school, 3= high school graduate/GED, 4= partial college, 5= college graduate, 6= partial graduate school, 7= graduate school degree).
Data Analytic Strategy
We examined our variables of interest using descriptive statistics (see Tables 1 and 2) and tested for differences across participants’ demographic characteristics (i.e., age, education, race/ethnicity). Given our limited sample size and the absence of demographic differences across variables of interest, we did not control for age, race/ethnicity, or education in our subsequent multivariate models. We used multivariate linear regression to explore the association between YMSM’s future gel use and their self-reported satisfaction with the gel and applicator, respectively, product-related side effects, and sexual satisfaction during gel use. Finally, we computed an interaction term between gel and applicator satisfaction and entered it into our regression model. Main Effects were mean centered prior to estimating the interaction term. Main and interaction terms for applicator and gel satisfaction were then z-scored and entered into our multivariate model (Aiken & West, 1991). For brevity, only statistically significant parameters (p ≤ .05) are discussed below.
RESULTS
Study participants (N=95) had a mean age of 23 years. The racial/ethnic composition of the sample was predominantly Latino (48%) and White (36.0%), followed by a fewer number of African American (10%) and Mixed/Other Race (6%) participants. Median educational attainment of participants was some college.
Participants reported high satisfaction for both the gel and applicator (see Table 1), with few participants reporting any side effects as a result of using the gel. When asked about their sexual satisfaction while using the product, participants reported high sexual satisfaction, with the majority noting that sexual intercourse with the gel was better (37%) or no different (59.8%) than sex without the gel. In bivariate analyses, we found that gel satisfaction was higher for Latinos (M=8.31, SD=2.04) than Whites (M=7.05, SD=1.57; F(3,88)=2.99; p≤.05). Sexual satisfaction was also higher for Latinos (M=8.32, SD=2.04) than Whites (M=7.16; SD=2.70; F(3,88)=3.00; p≤.05). We observed no other mean differences across our variables of interest by race/ethnicity, age, or educational attainment.
Future gel use was associated with greater applicator satisfaction (β=.42; p ≤ .001) when entered into a multivariate linear regression analysis (Table 3; F(4,87)=4.39; p ≤ .01). We found no association between future gel use and gel satisfaction, side effects, or sexual satisfaction in our main effects model. This main effects model had an adjusted R2 of 13.0%.
Table 3.
Main Effects Regression Model of Future Gel Use among YMSM (N=92)
| b | SE | Beta (β) | t statistic | p value | |
|---|---|---|---|---|---|
| (Constant) | 5.90 | .71 | 8.28 | .001 | |
| Applicator Satisfaction | .33 | .10 | .42 | 3.47 | .001 |
| Gel Satisfaction | −.15 | .13 | −.21 | −1.18 | .24 |
| Side Effects | .70 | .41 | .18 | 1.70 | .09 |
| Sexual Satisfaction | .14 | .10 | .22 | 1.34 | .18 |
Notes. Adjusted R-Squared is 13% for this main effects model; F(4,87)=4.39; p≤.01.
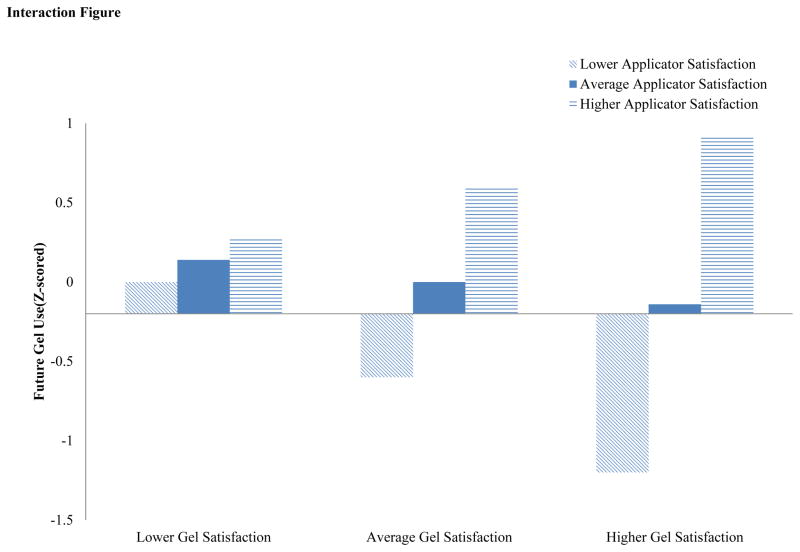
We then examined whether future gel use was influenced by the concurrent evaluation of YMSM’s satisfaction with the gel and the applicator. Using an interaction effects regression model (Table 4; F(5,86)=6.06; p ≤ .001), we found support for this interaction (β=.34; p ≤ .001). In Figure 1, we note a cross-over interaction effect between gel and applicator satisfaction. Future gel use scores were highest when YMSM felt highly satisfied by both gel and applicator; however, future gel use scores decreased if YMSM reported greater dissatisfaction with the applicator, even if they reported satisfaction with the gel. This interaction term model explained a larger percentage of the variance (R2=21.8%) than the main effects model.
Table 4.
Interaction Effects Regression Model of Future Gel Use among YMSM (N=92)
| b | SE | Beta (β) | t statistic | p value | |
|---|---|---|---|---|---|
| (Constant) | 6.99 | .80 | 8.77 | .001 | |
| Applicator Satisfaction | .60 | .17 | .41 | 3.58 | .001 |
| Gel Satisfaction | −.14 | .24 | −.10 | −.58 | .56 |
| Applicator x Gel Satisfaction Interaction | .46 | .14 | .34 | 3.28 | .001 |
| Side Effects | .69 | .39 | .17 | 1.76 | .08 |
| Sexual Satisfaction | .17 | .10 | .27 | 1.70 | .09 |
Notes. Adjusted R-Squared is 21.8% for this interaction effects model; F(5,86)=6.06; p≤.001. Main Effects were mean centered prior to estimating the interaction term. Main and interaction terms for applicator and gel satisfaction are reported as standardized (z-score) terms.
Figure 1.
DISCUSSION
The effectiveness of rectal microbicides will be dependent on their clinical safety, efficacy, and individuals’ consistent and correct product use. Informed by Morrow and Ruiz’s framework (2008), we examined whether YMSM’s anticipated future gel use was associated with gel-specific traits (e.g., satisfaction with gel, formulation side effects), applicator-specific characteristics (e.g., applicator satisfaction), and contextual factors associated with its use (e.g., sexual satisfaction when using the product), after they had participated in a product acceptability trial using a HEC placebo gel with RAI.
Satisfaction with the rectal applicator delivery device in microbicide trials, above and beyond YMSM’s satisfaction with the gel as part of their 12-week acceptability trial, was the only covariate associated with future gel use among YMSM within our main effects regression model. The absence of an association between gel satisfaction and future use intentions in our analyses may be due to participants’ limited contact with the gel as a stand-alone agent (i.e., unlike a rectal lubricant, participants would have had limited contact with the gel as it was discharged into the rectum through the use of the applicator). Once we examined whether YMSM’s likelihood of future gel use was influenced by users’ concurrent satisfaction of the applicator and rectal gel, however, we found support for an interactions effect model. Specifically, future gel use was highest when YMSM felt highly satisfied by both gel and applicator; however, future gel use scores were more likely to decrease if YMSM reported greater dissatisfaction with the applicator process, even if they reported satisfaction with the gel. These findings support Morrow and Ruiz’s framework (2008) and underscore the importance of considering how the concurrent acceptability of different product-related traits (e.g., product, applicator, and context) may influence users’ acceptability of a rectal microbicide (Carballo-Diéguez, Giguere, Dolezal, Bauermeister, Leu, Valladares, Rohan, et al., 2014; Gross et al., 1998; McGowan, 2014). Future research focused on how product adherence varies based on a rectal applicator’s appeal, comfort, and ease of use may be warranted. Furthermore, behavioral strategies focused on building YMSM’s self-efficacy and behavioral capability to use a rectal applicator should be included in all future rectal microbicide trials. Alternatively, applicator-related difficulties could be circumvented with the development of a rectal microbicide that is applied similar to a lubricant. Although speculative at present, the delivery of a rectal microbicide without an applicator may increase acceptability and adherence further.
Several factors limit the generalizability of these study results. Participants were not randomly selected and are not representative of YMSM in the cities where the research was conducted. Age of the participants may make results not generalizable to older populations. Participants volunteered for a rectal microbicide study and received significant financial remuneration if they participated in all stages of the trial; therefore, YMSM may have been particularly interested in this kind of product and have been subject to social desirability. By eligibility criteria, all participants acknowledged having URAI in the prior three months, and although URAI is not per se a risk behavior unless partners are serodiscordant and, as of late, not on oral pre-exposure prophylaxis, lack of consistent condom use may have heightened participants’ risk perception and willingness to try and adhere to rectal microbicide use. Furthermore, it is possible that use of a gel with an active microbicide component may have resulted in different levels of adherence. Finally, we used a newly designed rectal applicator as part of Stage 1B. Therefore, it is possible that the specific characteristics of this applicator (Carballo-Diéguez, Giguere, Dolezal, Bauermeister, Leu, Valladares, Rohan, et al., 2014), as compared to the vaginal applicators used in prior RM studies, influenced the evaluation that participants made of the applicator and how it was used. Future research examining how to optimize the design, appeal and satisfaction with microbicide-delivered applicators may be warranted.
Within these limitations, our study makes important contributions for the design and implementation of future rectal microbicide trials. Our evidence suggests that applicator satisfaction may be a salient factor in YMSM’s decision-making to use a RM in the future. Although the importance of developing a satisfactory RM gel for YMSM is undeniable for its future use, our results also emphasize the importance of developing strategies that increase YMSM’s comfort and skill when using a rectal applicator. Future research examining how to optimize the design, properties and characteristics of a rectal applicator as a strategy to promote greater satisfaction and use among YMSM is merited.
Acknowledgments
We greatly appreciate the hard work of the study staff at the sites, and are indebted to the study participants for volunteering their time. This research was sponsored by the US National Institutes of Health (NIH), including NICHD and NIMH, under R01 HD59533 (Carballo-Diéguez and McGowan, Co-PIs) and co-sponsored by CONRAD. Additional support came from the National Institute of Mental Health to the HIV Center for Clinical and Behavioral Studies at NY State Psychiatric Institute and Columbia University (P30-MH43520; Principal Investigator: Robert Remien). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
References
- Aiken LS, West Stephen G. Multiple Regression: Testing and Interpreting Interactions. Thousand Oak, CA: Sage Publications, Inc; 1991. [Google Scholar]
- Balthasar H, Jeannin A, Dubois-Arber F. First anal intercourse and condom use among men who have sex with men in Switzerland. Archives of Sexual Behavior. 2009 doi: 10.1007/s10508-008-9382-5. [DOI] [PubMed] [Google Scholar]
- Bauermeister JA. Sexual Partner Typologies among Single Young Men who Have Sex with Men. AIDS and Behavior. doi: 10.1007/s10461-014-0932-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauermeister JA, Carballo-Dieguez A, Giguere R, Valladares J, McGowan I. Interactive Voice Response System (IVRS): Data Quality Considerations and Lessons Learned During a Microbicide Placebo Adherence Trial With Young Men Who Have Sex With Men. Journal of Adolescent Health. 2014;54(2):S57–S58. [Google Scholar]
- Bauermeister JA, Hickok AM, Meadowbrooke C, Veinot T, Loveluck J. Self-efficacy among young men who have sex with men: An exploratory analysis of hiv/aids risk behaviors across partner types. AIDS and Behavior. 2014;18(1):69–77. doi: 10.1007/s10461-013-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo-Diéguez A, Dolezal C, Bauermeister JA, O’Brien W, Ventuneac A, Mayer K. Preference for gel over suppository as delivery vehicle for a rectal microbicide: results of a randomised, crossover acceptability trial among men who have sex with men. Sexually Transmitted Infections. 2008;84(6):483–487. doi: 10.1136/sti.2008.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo-Diéguez A, Exner T, Dolezal C, Pickard R, Lin P, Mayer KH. Rectal microbicide acceptability: results of a volume escalation trial. Sexually Transmitted Diseases. 2007;34(4):224–229. doi: 10.1097/01.olq.0000233715.59239.83. [DOI] [PubMed] [Google Scholar]
- Carballo-Diéguez A, Giguere R, Dolezal C, Bauermeister J, Leu CS, Valladares J, … McGowan I. Adherence to Rectal Gel Use Among Mainly Ethnic Minority Young Men Who have Sex with Men During A 3-Month Placebo Gel Trial: Implications for Microbicide Research. AIDS and Behavior. 2014;18(9):1726–1733. doi: 10.1007/s10461-014-0768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo-Diéguez A, Giguere R, Dolezal C, Bauermeister J, Leu CS, Valladares J, … McGowan I. Rectal-Specific Microbicide Applicator: Evaluation and Comparison with a Vaginal Applicator Used Rectally. AIDS and Behavior. 2014;18(9):1734–1745. doi: 10.1007/s10461-014-0793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo-Diéguez A, Ventuneac A, Dowsett GW, Balan I, Bauermeister J, Remien RH, … Mabragaña M. Sexual pleasure and intimacy among men who engage in “bareback sex”. AIDS and Behavior. 2011;15(Suppl 1):S57–65. doi: 10.1007/s10461-011-9900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach PM, Kelly CW, Borgerding Ja, Ramjee G, Tembo T, Kumwenda N, … Maslankowski L. Effects of partnership change on microbicide gel adherence in a clinical trial (HPTN 035) AIDS and Behavior. 2014;18(5):855–861. doi: 10.1007/s10461-013-0651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M, Buchbinder SP, Celum C, Heagerty P, Seage GR. Rectal microbicides for U.S. gay men. Are clinical trials needed? Are they feasible? HIVNET Vaccine Preparedness Study Protocol Team. Sexually Transmitted Diseases. 1998;25(6):296–302. doi: 10.1097/00007435-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Kubicek K, Arauz-Cuadra C, Kipke MD. Attitudes and Perceptions of Biomedical HIV Prevention Methods: Voices from Young Men Who Have Sex with Men. Archives of Sexual Behavior. 2015;44(2):487–497. doi: 10.1007/s10508-014-0398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek K, Carpineto J, McDavitt B, Weiss G, Iverson EF, Au CW, … Kipke MD. Integrating professional and folk models of hiv risk: Ymsm’s perceptions of high-risk sex. AIDS Education and Prevention. 2008;20(3):220–238. doi: 10.1521/aeap.2008.20.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Pitts M, Grierson J, Smith A, McNally S, Couch M. Age at first anal sex and HIV/STI vulnerability among gay men in Australia. Sexually Transmitted Infections. 2012;88(4):252–257. doi: 10.1136/sextrans-2011-050253. [DOI] [PubMed] [Google Scholar]
- Mantell JE, Myer L, Carballo-Diéguez A, Stein Z, Ramjee G, Morar NS, Harrison PF. Microbicide acceptability research: Current approaches and future directions. Social Science and Medicine. 2005;60(2):319–330. doi: 10.1016/j.socscimed.2004.05.011. [DOI] [PubMed] [Google Scholar]
- McGowan I. Rectal microbicides: a new focus for HIV prevention. Sexually Transmitted Infections. 2008;84(6):413–417. doi: 10.1136/sti.2008.031328. [DOI] [PubMed] [Google Scholar]
- McGowan I. Rectal Microbicides: Can We Make Them and Will People Use Them? AIDS and Behavior. 2011;15(Suppl 1):66–71. doi: 10.1007/s10461-011-9899-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan I. The development of rectal microbicides for HIV prevention. Expert Opinion on Drug Delivery. 2014;11:69–82. doi: 10.1517/17425247.2013.860132. [DOI] [PubMed] [Google Scholar]
- Morrow KM, Ruiz MS. Assessing Microbicide Acceptability: A Comprehensive and Integrated Approach. AIDS Behavior. 2008;12(2):272–283. doi: 10.1007/s10461-007-9266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustanski BS, Newcomb ME, Du Bois SN, Garcia SC, Grov C. HIV in young men who have sex with men: a review of epidemiology, risk and protective factors, and interventions. Journal of Sex Research. 2011;48(2–3):218–53. doi: 10.1080/00224499.2011.558645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingel ES, Thomas L, Harmell C, Bauermeister JA. Creating comprehensive, youth centered, culturally appropriate sex education: What do young gay, bisexual, and questioning men want? Sexuality Research and Social Policy. 2013;10(4):293–301. doi: 10.1007/s13178-013-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Centers for Disease Control and Prevention. Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report. 2012;17(4) Retrieved from http://www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental. [Google Scholar]
- Underhill K, Operario D, Mimiaga MJ, Skeer MR, Mayer KH. Implementation science of pre-exposure prophylaxis: preparing for public use. Current HIV/AIDS Reports. 2010;7(4):210–9. doi: 10.1007/s11904-010-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Straten a, Montgomery ET, Cheng H, Wegner L, Masenga G, Von Mollendorf C, Woodsong C. High acceptability of a vaginal ring intended as a microbicide delivery method for HIV prevention in African women. AIDS and Behavior. 2012;16(7):1775–1786. doi: 10.1007/s10461-012-0215-0. [DOI] [PubMed] [Google Scholar]