Abstract
In this article, we review the biology and physiological importance of transforming growth factor-β (TGF-β) to homeostasis in the respiratory system, its importance to innate and adaptive immune responses in the lung, and its pathophysiological role in various chronic pulmonary diseases including pulmonary arterial hypertension, chronic obstructive pulmonary disease, asthma, and pulmonary fibrosis. The TGF-β family is responsible for initiation of the intracellular signaling pathways that direct numerous cellular activities including proliferation, differentiation, extracellular matrix synthesis, and apoptosis. When TGF-β signaling is dysregulated or essential control mechanisms are unbalanced, the consequences of organ and tissue dysfunction can be profound. The complexities and myriad checkpoints built into the TGF-β signaling pathways provide attractive targets for the treatment of these disease states, many of which are currently being investigated. This review focuses on those aspects of TGF-β biology that are most relevant to pulmonary diseases and that hold promise as novel therapeutic targets.
Keywords: fibroblast, pulmonary fibrosis, COPD, asthma, pulmonary arterial hypertension
The transforming growth factor-β (TGF-β) superfamily is composed of a group of diverse polypeptides responsible for many cellular activities including proliferation, differentiation, migration, adhesion, extracellular matrix (ECM) synthesis, and cell death. There are more than 30 members of the TGF-β superfamily, including the TGF-βs themselves; bone morphogenic proteins (BMPs); activins; inhibins; nodal, myostatin, growth, and differentiation factors; and anti-Mullerian hormone (1–4). Signaling pathways initiated by TGF-β fulfill many diverse and essential functions in mammalian cells, allowing for tight regulation of varied cellular functions and the maintenance of cellular homeostasis. TGF-β is expressed by immune and nonimmune cell types and nearly all cells are responsive to the myriad effects of this critically important cytokine. TGF-β–regulated pathways can exert positive or negative effects on gene transcription, depending on the cellular context, abundance and activity of TGF-β ligands and receptors, Smad binding partners, and epigenetic changes that have wide-ranging effects on cellular functions (1). By virtue of its diverse roles in control of fundamental cellular signaling pathways, TGF-β signaling is key in both lung development and physiology, as well as in the pathogenesis of pulmonary disease states (3). For the purposes of this review, we focus on TGF-β1, 2, and 3, the TGF-β receptors I and II (TβRI and TβRII, respectively), and their roles in physiologic processes and pulmonary diseases.
TGF-β Activation
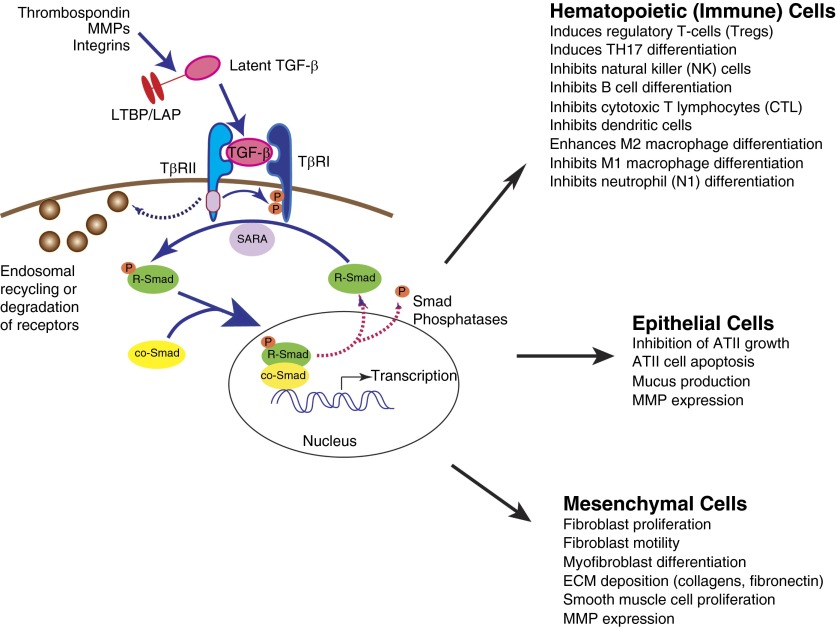
The following sections review TGF-β synthesis and activation, followed by a discussion of the signaling mechanisms and checkpoints through which TGF-β exerts its downstream effects (Figure 1). TGF-β isoforms (TGF-β1, TGF-β2, and TGF-β3) are the prototypical members of the TGF-β superfamily. TGFβ is synthesized and secreted bound noncovalently to the latency-associated peptide (LAP). Together, LAP and TGF-β form the small latent complex. The small latent complex is complexed through disulfide linkages to latent TGF-β–binding proteins in the endoplasmic reticulum, forming the large latent complex (LLC) (5, 6). Latent TGF-β cannot bind to its receptors because of steric hindrance from LAP bound to TGF-β (7). The LLC is localized primarily in the ECM, where ECM proteins are covalently crosslinked to the amino terminus of the latent TGF-β binding proteins (8). Because TGF-β can stimulate the expression of various ECM molecules, which then bind latent TGF-β, a positive feedback mechanism exists by which TGF-β may control its own accessibility and signaling (5, 9). There are multiple mechanisms by which TGF-β can be released from this complex, including acidification, extremes of temperature, oxidation, proteolytic cleavage, and traction by integrins (6). Known activating molecules include matrix metalloproteinase (MMP)-2 and -9 (10), thrombospondin-1 (11, 12), and integrins including αvβ6 (13, 14). It is noteworthy that many of these activators are up-regulated after inflammation and/or wound healing, thus allowing increased TGF-β activation in the appropriate context. MMPs and other proteinases activate latent TGF-β directly by proteolytic degradation of LAP or indirectly by digestion of ECM molecules, facilitating release of the LLC (5, 10). The integrin αvβ6, expressed by epithelial cells, is able to activate latent TGF-β when it is in direct contact with epithelial cells. Integrin αvβ6 receives injury signals from the lung epithelium via G protein–coupled receptor-mediated pathways, resulting in cytoskeletal changes in the cytoplasmic domain of the integrin, thus facilitating TGF-β activation (6, 15). Interestingly, integrin αvβ6 is up-regulated in injured epithelium, allowing for increased TGF-β activation and signaling in the setting of injury and wound healing. In addition to αvβ6, several other integrins, including αvβ8, αvβ3, and αvβ5, facilitate TGF-β activation (6). ECM stiffness may also influence TGF-β signaling and activation. In cultured cells, mechanical stress can activate latent TGF-β and substrate stiffness can modulate mesenchymal stem cell differentiation via TGF-β–mediated signaling (5, 16).
Figure 1.
Canonical transforming growth factor-β (TGF-β) signaling cascade. TGF-β is secreted in an inactive form noncovalently bound to the LAP and covalently bound to LTBPs. TGF-β can be activated by release from this complex by a variety of mechanisms including acidification, oxidation, proteolytic cleavage, and physical force exerted through integrins such as αvβ6. Active TGF-β ligand binds TGF-β receptor (TβR) II, initiating formation of heterotetrameric complexes with TβRI. R-Smads are phosphorylated by TβRI, and with help from anchor proteins such as SARA, combine with co-Smad4 and translocate to the nucleus, where they can direct gene transcription in diverse cell types. Signal attenuation is exerted at the level of the receptor complex and by dephosphorylation of phosphorylated Smads by Smad phosphatases. AT, alveolar type; co-Smad, common-mediator SMAD; ECM, extracellular matrix; LAP, latency-associated peptide; LTBP, latent TGF-β binding protein; MMP, matrix metalloproteinase; R-Smad, receptor Smad; SARA, Smad anchor for receptor activation
TGF-β Signaling
After activation of latent TGF-β, ligand binding results in the assembly of a complex of two TβRI and two type II TGF-β receptors. These receptors both have Ser/Thr protein kinase activity and are the only known cell surface receptor Ser/Thr kinases in humans (1). Binding of TGF-β results in phosphorylation of TβRI in a Ser/Thr-rich region (glycine- and serine-rich sequence on TGF-β receptor 1 [GS region]) by TβRII; TβRI is then responsible for downstream signal propagation. Phosphorylation of TβRI results in a switch in the GS region from a site that binds the kinase-silencing FK-506–binding protein to a site that is able to bind substrate Smad proteins, enabling Smad phosphorylation and downstream canonical signaling (17). Ligand access is regulated tightly for this constitutively active kinase; many proteins function as ligand traps that bind TGF-β to prevent contact with the receptors. In addition, antagonistic ligands can oppose or inhibit TGF-β binding to tightly regulate the initiation of the signaling cascade (1).
Canonical TGF-β signaling is the essential common step in many cellular processes, and at the core of this signaling pathway are the Smad transcription factors, the phosphorylation of which is the initial event in the initiation of TGF-β signal transduction. Canonical TGF-β signaling involves Ser phosphorylation and nuclear translocation of Smad proteins and subsequent transcriptional regulation (1, 4). There are eight Smad proteins in humans and mice: the receptor, or R-Smads (1–3, 5, 8), co-Smad4 (the common partner for all R-Smads), and the Inhibitory or I-Smads (6, 7), which interfere with Smad-receptor or Smad-Smad interactions and thus serve as negative regulators of TGF-β signaling (1, 4). Smad proteins are made up of two globular domains coupled by a linker region. The C terminus of the R-Smads is conserved and contains a Ser-X-Ser motif, which can be phosphorylated by activated TβRI. The N terminus is similarly conserved among R- and co-Smads and contains an MH1 DNA-binding domain. The linker region, however, is diverse among the various Smads and contains binding sites for Smurf and ubiquitin ligases, phosphorylation sites for various classes of protein kinases, and a nuclear export signal for Smad4 (4, 18).
Multiple cytoplasmic proteins function as Smad adaptors and anchors, including the Smad anchor for receptor activation (SARA). SARA is targeted to early endosomes; when Smads are bound to SARA, they are unable to translocate to the nucleus to form transcription complexes. This anchoring also facilitates interaction with activated TβRI and TβRII, which are also internalized to early endosomes, allowing access to SARA-bound Smads (4, 19, 20). After TGF-β receptor–mediated phosphorylation of the C-terminal region of R-Smads, a binding site is created for Smad4, the common co-Smad. Smad phosphorylation by the activated receptor also decreases the affinity for SARA and increases the affinity of Smads for nucleoporins, allowing for translocation of the R-Smad/Smad4 complexes from the cytoplasm to the nucleus via the nuclear pore for participation in DNA binding (4). Smads bind DNA via the MH1 domain contained within the N-terminal domain. This action requires DNA-binding cofactors from various families of DNA-binding proteins. The variability in cofactor requirements confers a high affinity and selectivity for specific target genes, thus allowing for the remarkable diversity of transcriptional responses that can be achieved by TGF-β activation (4).
In the basal state, there is constant nucleocytoplasmic shuttling of Smads. Nuclear accumulation occurs because of both a decreased affinity of R-Smads for cytoplasmic anchors (such as SARA) and a concurrent increased affinity for nuclear factors in response to receptor-mediated phosphorylation events (4, 18, 21). Once extracellular levels of TGF-β fall, receptor inactivation occurs by internalization or degradation of the receptor, or by negative feedback mechanisms that result in a loss of Smad phosphorylation. Rapid cycles of dephosphorylation and return of R-Smads to the cytoplasm maintain the steady-state levels of phospho-Smad in the setting of sustained receptor activity (4, 22). Smad phosphatases can dephosphorylate the C-terminal Ser, thus ending DNA-binding activity (23, 24). These dephosphorylation events allow for R-Smads to return to the cytoplasm, a fundamental mechanism underlying the control of TGF-β signaling. Several R-Smad phosphatases have been identified, including PPM1A and PP2, both of which dephosphorylate residues in the tail region of Smads. Additional linker region phosphatases exist, including the small C-terminal domain Ser/Thr phosphatases (SCPs) (23). The Smad proteins can be phosphorylated and dephosphorylated many times, as long as the TGF-β receptors are active. This constant recycling of R-Smads and Smad4 allows for continuous sensing of receptor activation state and tight regulation of downstream signaling events (4).
In addition to constant nucleocytoplasmic shuttling, additional regulators of the canonical pathway exist to control TGF-β signal transduction. Among the most important of these mechanisms are the processes of phosphorylation and dephosphorylation, which can control the pool of active Smad proteins or available TGF-β receptors. Both Ser/Thr and Tyr phosphorylation can play important roles in regulating canonical and noncanonical TGF-β signaling. As noted previously, phosphorylation of the C-terminal Ser residues of the MH2 domain by activated TGF-β receptors results ultimately in downstream transcriptional regulation by R-Smads. The Smad1, 2, and 3 linker regions also have Ser/Thr sites for ERK and MAP kinases that attenuate signal accumulation. Smad3 linker region Ser/Thr residues are substrates for G1 cyclin–dependent kinases Cdk2 and Ckd4, with Cdk-mediated phosphorylation resulting in decreased Smad3 signaling activity (2, 4, 25–27). Given the individual variation in Smad linker regions, this variability can allow for selective regulation of Smad activities and tight control of signaling events after TGF-β activation. Smad phosphatases dephosphorylate Ser and Thr residues, thus facilitating Smad recycling to the cytoplasm and ending the TGF-β signal (23). Tyrosine phosphorylation has also been shown to modify downstream TGF-β signaling cascades. Recent studies have shown that phosphorylation of tyrosine residues within the cytoplasmic tail of TβRII is essential for Smad-dependent profibrotic signaling within kidney collecting duct cells, and that a tyrosine phosphatase (TCPTP) that dephosphorylates these residues inhibits profibrotic signaling via integrin-dependent mechanisms (28). We have shown that another tyrosine phosphatase, PTPα, promotes TGF-β Smad-dependent responses in vitro in fibroblasts, as well as in murine models of pulmonary fibrosis (29).
Noncanonical, or Smad-independent, pathways are also important in TGF-β signal transduction and can complement or antagonize activity via the canonical pathway. Signal transduction molecules important in the noncanonical signaling pathway include p38, ERK, JNK, PI3K, and Src family kinases. These kinases are activated in response to TGF-β stimulation and can subsequently phosphorylate other molecules that play important roles in cellular processes such as epithelial-to-mesenchymal transition and determination of cell polarity. There is extensive cross-talk between the noncanonical pathways and canonical TGF-β signaling via Smads (1). MAPKs can phosphorylate Smads in the linker regions, which can positively or negatively impact Smad signaling. For example, linker regions on Smad3 have ERK phosphorylation sites that may result in decreased responsiveness to TGF-β, whereas phosphorylation of Smad4 by ERK is necessary for maximal transcriptional responses (2). In some disease states, there may be a switch from Smad pathways to Smad-independent noncanonical pathways, with potential pathophysiological consequences (1).
TGF-β in Homeostasis and Physiological Processes
Development
Cellular homeostasis, development, and many essential physiological processes in the lungs as well as in other organ systems depend on intact and appropriate TGF-β signaling. This is perhaps demonstrated most profoundly by the lethal consequences of genetic deletion of TGF-β in mice (30, 31). Although the three isoforms of TGF-β clearly have many overlapping functions, some are unique to each isoform. Their roles in lung development have been studied extensively and each isoform is highly expressed during normal mouse lung development (32). TGF-β1 is involved in lung branching and the differentiation of alveolar and bronchiolar ducts and colocalizes with ECM proteins, such as collagen, at interfaces between epithelial and mesenchymal cells. TGF-β2 is found in endodermal bronchiolar epithelium, and TGF-β3 is expressed in the tracheal mesenchyme and the endodermal epithelial cells in bronchioles and mesodermal cells, giving rise to the visceral pleura (32, 33). TGF-β2 deletion in mice results in perinatal death from respiratory failure and structural abnormalities of the lungs. Similarly, genetic deletion of TGF-β3 is lethal in the neonatal period because of alveolar hypoplasia and decreased surfactant protein C expression (32, 34, 35). TGF-β1–deficient mice develop a diffuse systemic inflammatory syndrome and interstitial pneumonitis (30, 31). Interestingly, although Smad2-null mice die during embryogenesis, Smad3-null mice are viable and fertile, suggesting that different developmental processes are controlled by different signaling molecules within the canonical pathway. Although the absence of TGF-β is fatal, overexpression can be equally disruptive to the developmental processes. Overexpression of TGF-β1 and 2 inhibits branching morphogenesis in embryonic mouse culture in a concentration-dependent manner that is associated with suppression of n-myc. TGF-β1 overexpression also disrupts epithelial differentiation and synthesis of surfactant proteins and phospholipids (36–38).
Inflammation and Immunity
Beyond embryogenesis and development, TGF-β isoforms are necessary for the regulation of inflammation in the lungs and other organ systems, and the dysregulation of TGF-β signaling pathways may contribute to many disease states. TGF-β has long been recognized as playing a central role in inhibiting inflammation and autoimmune disease. As mentioned previously, TGF-β1 knockout mice develop severe inflammation and die shortly after birth as a result of a wasting syndrome and massive inflammatory infiltration of the heart and lungs, which are likely mediated by autoimmunity. In the immune system, TGF-β has the greatest impact on T lymphocytes, and the phenotype resulting from T-cell–specific deletion of TβRII recapitulates the phenotype resulting from global deletion of TGF-β1 (31, 39, 40). In a cellular context-dependent manner, TGF-β can direct T-cell proliferation, differentiation, activation, and survival (40). TGF-β also regulates peripheral tolerance by inhibiting proliferation and differentiation of self-reactive CD4+ and CD8+ T cells; thus, deletion of TGF-β promotes autoimmunity in mice (39, 40). Under inflammatory conditions, TGF-β, in conjunction with other inflammatory cytokines, can promote further augmentation of inflammation and autoimmunity by promoting differentiation and proliferation of T-regulatory and Th17 cells, as well as IL-9– and IL-10–producing T cells and enhancing survival of memory CD8+ T cells. Conversely, TGF-β can suppress innate immune responses. Overall, the dominant role of TGF-β in the immune system is to maintain the balance of tolerance to self and robust responses to pathogens, while containing and resolving inflammation. Disturbance of this balance may contribute to the development of pathological conditions (40).
Control of Proliferation and Apoptosis
TGF-β has potent antiproliferative effects in many cell types, which contrasts with its effects on several other cell types, in which it can promote proliferation. In normal epithelial cells, including alveolar type (AT) II cells, TGF-β regulates the expression of genes promoting cell cycle arrest (41). In addition to antiproliferative effects, TGF-β can also induce apoptosis in multiple cell types, including epithelial cells, via mechanisms that depend on Smads and that correlate with its properties as a tumor suppressor (42, 43). In contrast, TGF-β can promote proliferation of mesenchymal cells, including fibroblasts and smooth muscle cells, via platelet-derived growth factor– and connective tissue growth factor–dependent and –independent mechanisms (2, 44). As noted previously, TGF-β also promotes proliferation of immune cells (40).
ECM Composition
TGF-β plays key roles in directing the composition of the ECM via control of fibroblasts. TGF-β regulates the synthesis and secretion of ECM components such as collagens, elastin, and fibronectin by fibroblasts. Indeed, TGF-β is the most potent inducer of ECM generation known (6). Collagen production requires TGF-β and Smad activity; type I collagen and connective tissue growth factor expression are decreased in fibroblasts deficient in Smad3 (2, 45). Noncanonical TGF-β signaling (via ras/MEK/ERK MAPK) also promotes collagen induction (2). TGF-β also directs secretion of MMPs and tissue inhibitors of MMP (4). Overall, TGF-β regulates ECM production and remodeling; dysregulation of these systems can have significant pathological effects on lung development and the pathogenesis of pulmonary disease, particularly pulmonary fibrosis, chronic obstructive pulmonary disease (COPD), and pulmonary vascular disease.
Wound Healing
In cutaneous injury models, TGF-β is induced rapidly, resulting in neutrophil, macrophage, and fibroblast recruitment and the subsequent release of additional TGF-β (2, 46). TGF-β can be found in the leading edge of scar tissue formation, although it is not significantly up-regulated in established lesions (2, 47). TGF-β1–deficient mice have impaired late-stage wound repair with decreased re-epithelialization and collagen deposition (2, 48). Although these findings indicate that TGF-β is essential for the wound-healing process, they also suggest that TGF-β can be involved in the initiation of the fibrotic response, which can potentially become pathological. Smad3 may be particularly important in initiation of the fibrotic response; Smad3-deficient mice have reduced inflammation after incisional wounding and resistance to cutaneous radiation-induced fibrosis. Elevated nuclear Smad3 levels are found in models of fibrosis, including bleomycin-induced pulmonary fibrosis, consistent with an essential role in fibrogenesis (2, 49).
Involvement in Pathological Processes
Many lung diseases are characterized by cycles of tissue injury followed by repair. TGF-β is induced in these circumstances and may play a role in limiting inflammation and in mediating the repair and remodeling processes. Aberrant TGF-β signaling has been shown in multiple disease states to contribute to the pathology of these lung diseases, including pulmonary fibrosis, pulmonary arterial hypertension (PAH), COPD, and asthma, among others.
Pulmonary Fibrosis
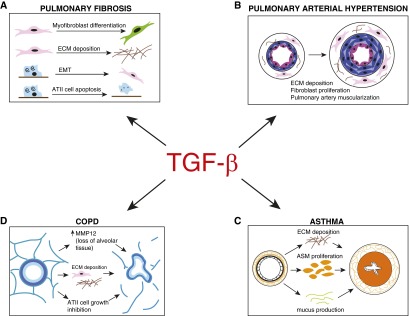
Idiopathic pulmonary fibrosis (IPF) is the most common of the idiopathic interstitial pneumonias and is characterized by a diffuse and progressive fibrotic lung disease (50, 51). IPF is believed to be triggered by injury to the alveolar epithelium, with subsequent aberrant repair mechanisms, recruitment of fibroblasts and differentiation to myofibroblasts, and unrestrained elaboration of ECM components (50, 52, 53). TGF-β is fundamental to the pathogenesis of pulmonary fibrosis (Figure 2A) (50, 53–56). In the lung, TGF-β is expressed by multiple cell types, including epithelial cells, macrophages, and fibroblasts, and levels are elevated in animal models and in patients with IPF (57–60). Pulmonary expression of TGF-β is sufficient to induce progressive fibrosis in rodents (61); conversely, blocking TGF-β signaling inhibits fibrosis in rodent models (62, 63). In vitro, TGF-β can induce myofibroblast differentiation of fibroblasts (64, 65) and mesenchymal transition of epithelial cells (66), although the importance of mesenchymal transition in pulmonary fibrosis remains controversial (67). Myofibroblasts isolated from patients with IPF exhibit a durable invasive phenotype in culture. Myofibroblasts also secrete their own TGF-β, inducing further transcriptional activation of collagens and other ECM components via Smad-dependent pathways, as well as inducing further ATII cell apoptosis, which perpetuates the aberrant wound-healing process (68–71). Targeted ATII cell deletion of TβRII provides protection from bleomycin-induced fibrosis (55). As noted previously, TGF-β activation can occur via integrins, particularly, αvβ6, and this process is of critical importance in the development of tissue fibrosis. Mice deficient in αvβ6 are protected from pulmonary fibrosis in a variety of models, including bleomycin, LPS, ventilator-induced lung injury, and radiation-induced fibrosis (6). Conversely, overexpression of αvβ6 integrin in the setting of lung injury promotes the development of fibrosis, and TGF-β itself can up-regulate the expression of this integrin, resulting in a feed-forward amplification loop (6).
Figure 2.
Downstream pathological effects of the TGF-β signaling cascade in selected lung diseases. (A) TGF-β influences development of pulmonary fibrosis by promoting differentiation of fibroblasts to myofibroblasts, elaboration of ECM components, epithelial to mesenchymal transition of ATII cells to fibroblasts, and ATII cell apoptosis. (B) Effects of TGF-β in the pathogenesis of pulmonary arterial hypertension include ECM deposition, fibroblast proliferation, and pulmonary artery muscularization. (C) Asthma pathogenesis is driven by TGF-β–dependent processes, including ECM deposition, airway smooth muscle cell proliferation, and mucus production. (D) TGF-β can influence the development of COPD via mechanisms including up-regulation of MMPs, leading to alveolar tissue loss, elaboration of ECM components, and ATII cell growth inhibition. COPD, chronic obstructive pulmonary disease; EMT, epithelial to mesenchymal transition.
Pulmonary Arterial Hypertension
PAH is a disease caused by the narrowing and eventual obliteration of small pulmonary arteries as a result of aberrant proliferation and dysfunction of endothelial cells and smooth muscle cells, leading to an increase in pulmonary vascular pressures (72). Mutations in the BMPRII gene, part of the larger TGF-β superfamily, have been implicated in more than 80% of patients with familial PAH and 20% of cases with sporadic idiopathic PAH (73, 74). In mouse models, overexpression of BMPRII results in pulmonary arterial muscularization and increases in pulmonary arterial pressures (75). In models of Schistosoma mansoni–induced pulmonary hypertension, pulmonary vascular remodeling and pulmonary hypertension were dependent on increased TGF-β signaling (76). This process was attenuated in Smad3 knockout mice, as well as by pharmacological blockade of the TGF-β ligand and receptor (Figure 2B) (76).
Asthma
Asthma is an inflammatory disease characterized by airway hyperresponsiveness and reversible airflow obstruction, which can result in pathological airway remodeling (6). TGF-β has been implicated in both the inflammatory and the airway remodeling components of asthma pathogenesis (Figure 2C). Studies have shown that endogenous TGF-β1 is a suppressor of asthma in murine models. TGF-β1 heterozygous mice that produce lower than baseline levels of TGF-β have exacerbated asthmatic phenotypes (77). Conversely, overexpression of TGF-β1 in ovalbumin-specific Th2 cells reduces airway hyperresponsiveness (78). Interestingly, in the airways of humans with asthma, TGF-β1 levels are elevated compared with normal control subjects, perhaps suggesting a role in the repair of injured asthmatic airways or the existence of a negative feedback loop controlling airway inflammation (40). TGF-β signaling can also promote ECM deposition, airway smooth muscle cell proliferation, and mucus production in animal models of allergic asthma, with overexpression of Smad2 resulting in airway smooth muscle cell proliferation and collagen deposition after an allergen challenge (79, 80). In asthma pathogenesis, activation of TGF-β can occur via epithelial cell, mast cell, or fibroblast activity (6).
COPD
COPD is characterized by irreversible airflow obstruction, small-airway inflammation, and destruction of alveolar architecture with airspace enlargement (3). Several studies have demonstrated impaired TGF-β1 signaling in patients with COPD (3). Investigators have identified increased TGF-β1 in the airway epithelium of smokers and those with COPD, as well as decreased expression of inhibitory Smads (81–84). Similar to the role of TGF-β in pulmonary fibrosis, in patients with COPD, TGF-β promotes fibrotic airway remodeling, which can further contribute to diminished lung function (3). Alveolar parenchymal tissue loss may be caused in part by up-regulation of MMP expression in response to TGF-β signaling, with subsequent ECM degradation (85, 86). Further injury may be potentiated by the inhibition of ATII cell growth and ATII apoptosis (Figure 2D). Some of the increases in TGF-β1 in the airway epithelium of patients with COPD may be a direct response to cigarette smoke, the most significant risk factor for the development of this disease state (3, 87, 88).
Targeting TGF-β Pathways as a Therapeutic Approach for Respiratory Diseases
Given the importance of TGF-β in disease, it stands to reason that targeting TGF-β or its downstream pathways may represent potential therapeutic options for the treatment of a myriad of pulmonary illnesses, as well as those of other organ systems. There are many ways to inhibit TGF-β, including administration of neutralizing antibodies, use of antisense nucleotides, and administration of inhibitors of the TGF-β receptor kinases. Several of these mechanisms have been attempted with variable degrees of success, and many untoward effects of interference with this complex pathway have been observed (89, 90).
Because TGF-β is considered to be a “master switch” in the fibrotic process, playing roles in parenchymal and interstitial fibrosis, as well as in airway and vascular remodeling, the TGF-β pathway is a tempting therapeutic target in diseases characterized by unregulated fibrogenesis. Many attempts have been made to ubiquitously block TGF-β. Pirfenidone, recently approved for the treatment of IPF, attenuates TGF-β production and action, although by unclear mechanisms, and has been shown to reduce decline in the vital capacity of patients with IPF (90).
Although the idea of general antagonism of TGF-β is appealing, its myriad roles in cellular homeostasis, regulation of inflammation, and tumor suppression preclude most attempts at global antagonism. Thus, more directed therapeutics targeting the TGF-β activation cascade are necessary (91). SB-431542 is a potent and selective inhibitor of TβRI and has been shown to suppress bleomycin-induced pulmonary fibrosis by attenuating R-Smad activation (92, 93). A number of other small molecules, antibodies, and ligand traps targeting TGF-β receptor kinases, as well as TGF-β ligands and TGFB1 gene promoters, are also under investigation in preclinical studies (89). One promising approach involves inhibition of integrin αvβ6, a key activator of TGF-β1. Studies have shown that administration of a blocking antibody to αvβ6 attenuated bleomycin-induced pulmonary fibrosis in mice (94). Low doses of the antibody were effective in reducing collagen expression without altering the inflammatory response (94). A humanized monoclonal antibody against αvβ6 integrin has been developed and is currently in phase II trials in patients with IPF (clinicaltrials.gov identifier NCT01371305). To our knowledge, targeting the TGF-β pathway in other respiratory diseases such as COPD and in the airway remodeling component of chronic asthma, although intuitive, has not yet been attempted. Alternative strategies may also include the targeting of molecules that cross-talk with key components of the TGF-β signaling pathway, such as the PTPα or TRPV4 channels, which could provide selective targeting of pathological TGF-β effects while avoiding the consequences and untoward effects of global TGF-β antagonism (29, 95).
Conclusions
TGF-β signaling remains a complex and incompletely understood physiological process that has significant implications for both normal physiology and the pathogenesis of disease. Further investigation into the control points that fine-tune TGF-β signaling, including phosphorylation and dephosphorylation of key components of the pathways, as well as a better understanding of the importance of these regulatory steps in disease progression or prevention, are needed. It is hoped that a more detailed knowledge of the nuances of this complex system will allow for new therapeutics to use in the treatment of disease states driven by pathological TGF-β signaling.
Footnotes
This work was supported by National Institutes of Health grant ES023932 (G.P.D.) and American Thoracic Society Foundation Unrestricted Research Grant (Y.A.).
Author Contributions: Y.A. provided the initial draft of the manuscript; G.P.D. edited the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2015-0391TR on January 21, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leask A, Abraham DJ. TGF-β signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 3.Morty RE, Königshoff M, Eickelberg O. Transforming growth factor-β signaling across ages: from distorted lung development to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:607–613. doi: 10.1513/pats.200908-087RM. [DOI] [PubMed] [Google Scholar]
- 4.Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 5.Horiguchi M, Ota M, Rifkin DB. Matrix control of transforming growth factor-β function. J Biochem. 2012;152:321–329. doi: 10.1093/jb/mvs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatler AL, Jenkins G. TGF-β activation and lung fibrosis. Proc Am Thorac Soc. 2012;9:130–136. doi: 10.1513/pats.201201-003AW. [DOI] [PubMed] [Google Scholar]
- 7.Saharinen J, Taipale J, Keski-Oja J. Association of the small latent transforming growth factor-β with an eight cysteine repeat of its binding protein LTBP-1. EMBO J. 1996;15:245–253. [PMC free article] [PubMed] [Google Scholar]
- 8.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 9.Verrecchia F, Mauviel A. Transforming growth factor-β signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 10.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-β secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 13.Miller LA, Barnett NL, Sheppard D, Hyde DM. Expression of the β6 integrin subunit is associated with sites of neutrophil influx in lung epithelium. J Histochem Cytochem. 2001;49:41–48. doi: 10.1177/002215540104900105. [DOI] [PubMed] [Google Scholar]
- 14.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin αvβ6 binds and activates latent TGF-β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin A, Jenkins G. Role of integrin-mediated TGFβ activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans. 2009;37:849–854. doi: 10.1042/BST0370849. [DOI] [PubMed] [Google Scholar]
- 16.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, Massagué J. The TGFβ receptor activation process: an inhibitor- to substrate-binding switch. Mol Cell. 2001;8:671–682. doi: 10.1016/s1097-2765(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 19.Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE domain protein that recruits Smad2 to the TGFβ receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- 20.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Massagué J. Nucleocytoplasmic shuttling of signal transducers. Nat Rev Mol Cell Biol. 2004;5:209–219. doi: 10.1038/nrm1331. [DOI] [PubMed] [Google Scholar]
- 22.Inman GJ, Nicolás FJ, Hill CS. Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-β receptor activity. Mol Cell. 2002;10:283–294. doi: 10.1016/s1097-2765(02)00585-3. [DOI] [PubMed] [Google Scholar]
- 23.Bruce DL, Sapkota GP. Phosphatases in SMAD regulation. FEBS Lett. 2012;586:1897–1905. doi: 10.1016/j.febslet.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, Hu M, Davis CM, Wang J, Brunicardi FC, et al. PPM1A functions as a Smad phosphatase to terminate TGFβ signaling. Cell. 2006;125:915–928. doi: 10.1016/j.cell.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGFβ/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430:226–231. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- 27.Roelen BA, Cohen OS, Raychowdhury MK, Chadee DN, Zhang Y, Kyriakis JM, Alessandrini AA, Lin HY. Phosphorylation of threonine 276 in Smad4 is involved in transforming growth factor-β-induced nuclear accumulation. Am J Physiol Cell Physiol. 2003;285:C823–C830. doi: 10.1152/ajpcell.00053.2003. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Wang H, Liao HJ, Hu W, Gewin L, Mernaugh G, Zhang S, Zhang ZY, Vega-Montoto L, Vanacore RM, et al. Integrin-mediated type II TGF-β receptor tyrosine dephosphorylation controls SMAD-dependent profibrotic signaling. J Clin Invest. 2014;124:3295–3310. doi: 10.1172/JCI71668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aschner Y, Khalifah AP, Briones N, Yamashita C, Dolgonos L, Young SK, Campbell MN, Riches DW, Redente EF, Janssen WJ, et al. Protein tyrosine phosphatase α mediates profibrotic signaling in lung fibroblasts through TGF-β responsiveness. Am J Pathol. 2014;184:1489–1502. doi: 10.1016/j.ajpath.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulkarni AB, Karlsson S. Transforming growth factor-β1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol. 1993;143:3–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Bartram U, Speer CP. The role of transforming growth factor β in lung development and disease. Chest. 2004;125:754–765. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- 33.Schmid P, Cox D, Bilbe G, Maier R, McMaster GK. Differential expression of TGFβ1, β2 and β3 genes during mouse embryogenesis. Development. 1991;111:117–130. doi: 10.1242/dev.111.1.117. [DOI] [PubMed] [Google Scholar]
- 34.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, Bu D, Lee M, Slavkin HC, Hall FL, Warburton D. Abrogation of transforming growth factor-β type II receptor stimulates embryonic mouse lung branching morphogenesis in culture. Dev Biol. 1996;180:242–257. doi: 10.1006/dbio.1996.0298. [DOI] [PubMed] [Google Scholar]
- 37.Whitsett JA, Weaver TE, Lieberman MA, Clark JC, Daugherty C. Differential effects of epidermal growth factor and transforming growth factor-β on synthesis of Mr = 35,000 surfactant-associated protein in fetal lung. J Biol Chem. 1987;262:7908–7913. [PubMed] [Google Scholar]
- 38.Zhou L, Dey CR, Wert SE, Whitsett JA. Arrested lung morphogenesis in transgenic mice bearing an SP-C-TGF-β1 chimeric gene. Dev Biol. 1996;175:227–238. doi: 10.1006/dbio.1996.0110. [DOI] [PubMed] [Google Scholar]
- 39.Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9:447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 41.Seoane J. Escaping from the TGFβ anti-proliferative control. Carcinogenesis. 2006;27:2148–2156. doi: 10.1093/carcin/bgl068. [DOI] [PubMed] [Google Scholar]
- 42.Jang CW, Chen CH, Chen CC, Chen JY, Su YH, Chen RH. TGF-β induces apoptosis through Smad-mediated expression of DAP-kinase. Nat Cell Biol. 2002;4:51–58. doi: 10.1038/ncb731. [DOI] [PubMed] [Google Scholar]
- 43.Schuster N, Krieglstein K. Mechanisms of TGF-β-mediated apoptosis. Cell Tissue Res. 2002;307:1–14. doi: 10.1007/s00441-001-0479-6. [DOI] [PubMed] [Google Scholar]
- 44.Stouffer GA, Owens GK. TGF-β promotes proliferation of cultured SMC via both PDGF-AA-dependent and PDGF-AA-independent mechanisms. J Clin Invest. 1994;93:2048–2055. doi: 10.1172/JCI117199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-β/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 46.Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB. Transforming growth factor type β induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA. 1987;84:5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Querfeld C, Eckes B, Huerkamp C, Krieg T, Sollberg S. Expression of TGF-β1, -β2 and -β3 in localized and systemic scleroderma. J Dermatol Sci. 1999;21:13–22. doi: 10.1016/s0923-1811(99)00008-0. [DOI] [PubMed] [Google Scholar]
- 48.Brown RL, Ormsby I, Doetschman TC, Greenhalgh DG. Wound healing in the transforming growth factor-β-deficient mouse. Wound Repair Regen. 1995;3:25–36. doi: 10.1046/j.1524-475X.1995.30108.x. [DOI] [PubMed] [Google Scholar]
- 49.Flanders KC, Sullivan CD, Fujii M, Sowers A, Anzano MA, Arabshahi A, Major C, Deng C, Russo A, Mitchell JB, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160:1057–1068. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 51.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 55.Li M, Krishnaveni MS, Li C, Zhou B, Xing Y, Banfalvi A, Li A, Lombardi V, Akbari O, Borok Z, et al. Epithelium-specific deletion of TGF-β receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest. 2011;121:277–287. doi: 10.1172/JCI42090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westergren-Thorsson G, Hernnäs J, Särnstrand B, Oldberg A, Heinegård D, Malmström A. Altered expression of small proteoglycans, collagen, and transforming growth factor-β1 in developing bleomycin-induced pulmonary fibrosis in rats. J Clin Invest. 1993;92:632–637. doi: 10.1172/JCI116631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergeron A, Soler P, Kambouchner M, Loiseau P, Milleron B, Valeyre D, Hance AJ, Tazi A. Cytokine profiles in idiopathic pulmonary fibrosis suggest an important role for TGF-β and IL-10. Eur Respir J. 2003;22:69–76. doi: 10.1183/09031936.03.00014703. [DOI] [PubMed] [Google Scholar]
- 58.Khalil N, O’Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, Bereznay OH, Greenberg AH. Increased production and immunohistochemical localization of transforming growth factor-β in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5:155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- 59.Khalil N, Parekh TV, O’Connor R, Antman N, Kepron W, Yehaulaeshet T, Xu YD, Gold LI. Regulation of the effects of TGF-β1 by activation of latent TGF-β1 and differential expression of TGF-β receptors (TβR-I and TβR-II) in idiopathic pulmonary fibrosis. Thorax. 2001;56:907–915. doi: 10.1136/thorax.56.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, Huang X, Sheppard D, Heller RA. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci USA. 2000;97:1778–1783. doi: 10.1073/pnas.97.4.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-β1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonniaud P, Margetts PJ, Kolb M, Schroeder JA, Kapoun AM, Damm D, Murphy A, Chakravarty S, Dugar S, Higgins L, et al. Progressive transforming growth factor β1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Respir Crit Care Med. 2005;171:889–898. doi: 10.1164/rccm.200405-612OC. [DOI] [PubMed] [Google Scholar]
- 63.Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-β and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol. 2005;175:5390–5395. doi: 10.4049/jimmunol.175.8.5390. [DOI] [PubMed] [Google Scholar]
- 64.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, et al. Epithelial cell α3β1 integrin links β-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-β1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 66.Willis BC, Borok Z. TGF-β-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 67.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larsson O, Diebold D, Fan D, Peterson M, Nho RS, Bitterman PB, Henke CA. Fibrotic myofibroblasts manifest genome-wide derangements of translational control. PLoS One. 2008;3:e3220. doi: 10.1371/journal.pone.0003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124:1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol. 2003;201:343–354. doi: 10.1002/path.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia H, Bodempudi V, Benyumov A, Hergert P, Tank D, Herrera J, Braziunas J, Larsson O, Parker M, Rossi D, et al. Identification of a cell-of-origin for fibroblasts comprising the fibrotic reticulum in idiopathic pulmonary fibrosis. Am J Pathol. 2014;184:1369–1383. doi: 10.1016/j.ajpath.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goumans MJ, Liu Z, ten Dijke P. TGF-β signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 73.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, III, Loyd JE, Nichols WC, Trembath RC International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 74.Machado RD, Aldred MA, James V, Harrison RE, Patel B, Schwalbe EC, Gruenig E, Janssen B, Koehler R, Seeger W, et al. Mutations of the TGF-β type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat. 2006;27:121–132. doi: 10.1002/humu.20285. [DOI] [PubMed] [Google Scholar]
- 75.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, et al. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res. 2004;94:1109–1114. doi: 10.1161/01.RES.0000126047.82846.20. [DOI] [PubMed] [Google Scholar]
- 76.Graham BB, Chabon J, Gebreab L, Poole J, Debella E, Davis L, Tanaka T, Sanders L, Dropcho N, Bandeira A, et al. Transforming growth factor-β signaling promotes pulmonary hypertension caused by Schistosoma mansoni. Circulation. 2013;128:1354–1364. doi: 10.1161/CIRCULATIONAHA.113.003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scherf W, Burdach S, Hansen G. Reduced expression of transforming growth factor β1 exacerbates pathology in an experimental asthma model. Eur J Immunol. 2005;35:198–206. doi: 10.1002/eji.200425209. [DOI] [PubMed] [Google Scholar]
- 78.Hansen G, McIntire JJ, Yeung VP, Berry G, Thorbecke GJ, Chen L, DeKruyff RH, Umetsu DT. CD4(+) T helper cells engineered to produce latent TGF-β1 reverse allergen-induced airway hyperreactivity and inflammation. J Clin Invest. 2000;105:61–70. doi: 10.1172/JCI7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-β antibody: effect on the Smad signaling pathway. J Immunol. 2005;174:5774–5780. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- 80.Gregory LG, Mathie SA, Walker SA, Pegorier S, Jones CP, Lloyd CM. Overexpression of Smad2 drives house dust mite-mediated airway remodeling and airway hyperresponsiveness via activin and IL-25. Am J Respir Crit Care Med. 2010;182:143–154. doi: 10.1164/rccm.200905-0725OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aubert JD, Dalal BI, Bai TR, Roberts CR, Hayashi S, Hogg JC. Transforming growth factor β1 gene expression in human airways. Thorax. 1994;49:225–232. doi: 10.1136/thx.49.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50s–59s. [PubMed] [Google Scholar]
- 83.Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, Okada Y, Yamasawa F, Nakahara K, Umeda A. Increased expression of transforming growth factor-β1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD) Am J Respir Crit Care Med. 2001;163:1476–1483. doi: 10.1164/ajrccm.163.6.9908135. [DOI] [PubMed] [Google Scholar]
- 84.Springer J, Scholz FR, Peiser C, Groneberg DA, Fischer A. SMAD-signaling in chronic obstructive pulmonary disease: transcriptional down-regulation of inhibitory SMAD 6 and 7 by cigarette smoke. Biol Chem. 2004;385:649–653. doi: 10.1515/BC.2004.080. [DOI] [PubMed] [Google Scholar]
- 85.Roberts AB. Medicine: smoke signals for lung disease. Nature. 2003;422:130–131. doi: 10.1038/422130a. [DOI] [PubMed] [Google Scholar]
- 86.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin α(v)β6-mediated TGF-β activation causes MMP12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 87.Marwick JA, Kirkham P, Gilmour PS, Donaldson K, MacNee W, Rahman I. Cigarette smoke-induced oxidative stress and TGF-β1 increase p21waf1/cip1 expression in alveolar epithelial cells. Ann N Y Acad Sci. 2002;973:278–283. doi: 10.1111/j.1749-6632.2002.tb04649.x. [DOI] [PubMed] [Google Scholar]
- 88.Rennard SI, Togo S, Holz O. Cigarette smoke inhibits alveolar repair: a mechanism for the development of emphysema. Proc Am Thorac Soc. 2006;3:703–708. doi: 10.1513/pats.200605-121SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9:111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 91.Rafii R, Juarez MM, Albertson TE, Chan AL. A review of current and novel therapies for idiopathic pulmonary fibrosis. J Thorac Dis. 2013;5:48–73. doi: 10.3978/j.issn.2072-1439.2012.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Higashiyama H, Yoshimoto D, Kaise T, Matsubara S, Fujiwara M, Kikkawa H, Asano S, Kinoshita M. Inhibition of activin receptor-like kinase 5 attenuates bleomycin-induced pulmonary fibrosis. Exp Mol Pathol. 2007;83:39–46. doi: 10.1016/j.yexmp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 93.Koh RY, Lim CL, Uhal BD, Abdullah M, Vidyadaran S, Ho CC, Seow HF. Inhibition of transforming growth factor-β via the activin receptor-like kinase-5 inhibitor attenuates pulmonary fibrosis. Mol Med Rep. 2015;11:3808–3813. doi: 10.3892/mmr.2015.3193. [DOI] [PubMed] [Google Scholar]
- 94.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, et al. Partial inhibition of integrin α(v)β6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 95.Rahaman SO, Grove LM, Paruchuri S, Southern BD, Abraham S, Niese KA, Scheraga RG, Ghosh S, Thodeti CK, Zhang DX, et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest. 2014;124:5225–5238. doi: 10.1172/JCI75331. [DOI] [PMC free article] [PubMed] [Google Scholar]