Abstract
The majority of patients with severe or difficult-to-control asthma in the United States are obese. Epidemiological studies have clearly established that obese patients tend to have worse asthma control and increased hospitalizations and do not respond to standard controller therapy as well as lean patients with asthma. Less clear are the mechanistic underpinnings for the striking clinical differences between lean and obese patients with asthma. Because obesity is principally a disorder of metabolism and energy regulation, processes fundamental to the function of every cell and system within the body, it is not surprising that it affects the respiratory system; it is perhaps surprising that it has taken so long to appreciate how dysfunctional metabolism and energy regulation lead to severe airway disease. Although early investigations focused on identifying a common factor in obesity that could promote airway disease, an appreciation has emerged that the asthma of obesity is a manifestation of multiple anomalies related to obesity affecting all the different pathways that cause asthma, and likely also to de novo airway dysfunction. Consequently, all the phenotypes of asthma currently recognized in lean patients (which are profoundly modified by obesity), as well as those unique to one’s obesity endotype, likely contribute to obese asthma in a particular individual. This perspective reviews what we have learned from clinical studies and animal models about the phenotypes of asthma in obesity, which show how specific aspects of obesity and altered metabolism might lead to de novo airway disease and profoundly modify existing airway disease.
Keywords: asthma, obesity, phenotype, diet
Clinical Relevance
This perspective reviews what we have learned from clinical studies and animal models about the phenotypes of asthma in obesity, which show how specific aspects of obesity and altered metabolism might lead to de novo airway disease and profoundly modify existing airway disease. This leads to a number of potential research studies that are needed, and suggests changes in the way we approach this patient population.
Obese patients tend to have worse asthma control and increased hospitalizations and do not respond to standard controller therapy as well as do lean patients with asthma (1–5); in fact, the majority of patients with severe or difficult-to-control asthma in the United States are obese (6). Epidemiological studies have described two major phenotypes of obese asthma (7–9): one with later-onset asthma, lower markers of allergic inflammation, and airway reactivity that improves with weight loss; the other characterized by earlier-onset disease and higher markers of allergic inflammation. Studies in animals and humans suggest that we need to include a third phenotype: irritant/pollution-associated asthma and obesity. Compartmentalizing obese asthma into these two/three phenotypes is likely a major oversimplification. There are many phenotypes of asthma described in lean patients (10), and each of these phenotypes is likely to persist and be much complicated by obesity. We discuss our current understanding of asthma and obesity phenotypes, with the caveat that this paradigm likely oversimplifies the interactions between these two complex syndromes.
Asthma Caused by Obesity
Our work and that of others has shown that there is a group of patients with later-onset asthma, lower markers of allergic inflammation, and airway reactivity that improves with weight loss (7, 9, 11). A number of pathways pertinent to obesity may contribute to the development of this form of airway disease.
In obese humans with late-onset asthma and obesity, there is often little airway inflammation. Instead, there is increased sensitivity to airway closure and increased elastance of the peripheral airways, both of which improve with weight loss (12, 13). In lean healthy human volunteers, breathing at low lung volumes increases airway reactivity (14), and although breathing at low lung volumes likely contributes to airway disease in obesity, we have found that changes in airway reactivity with weight loss are not directly related to changes in lung volume. Our results suggest that factors other than simple changes in lung volume might contribute to the inherent airway reactivity in obesity. Modeling work suggests this augmented airway reactivity could be related either to increased compliance of the airway wall itself or to increased airway wall thickness (15). It is possible that obese individuals who develop late-onset asthma associated with minimal markers of inflammation are those patients who tend to have slightly thicker walls or more compliant airways than does the general population. This may be caused by natural biological variation at the population level (15) or by factors contributing to altered airway wall structure and function in obesity (15).
Candidate factors contributing to altered airway structure and function in obese asthma include increased oxidative stress and altered nitric oxide metabolism. Increased airway oxidative stress, measured by increased 8-isoprostanes in exhaled breath condensate, has been found in obese patients with asthma (16) and is associated with decreased airway nitric oxide, particularly in those individuals with later-onset disease (11). Nitric oxide has important bronchodilatory and homeostatic functions in the airway (17); therefore, in the context of obesity, a decrease in nitric oxide might have pathogenic consequences. One reason for the decreased levels of nitric oxide might relate to lower availability of the nitric oxide synthase substrate arginine, because of competition from increased asymmetric dimethyl arginine (11). Studies in animal models suggest that such alterations in nitric oxide metabolism can be induced by dietary changes independent of weight gain: a high-fructose diet increases asymmetric dimethyl arginine and decreases exhaled nitric oxide and is associated with increased airway resistance, even without the development of obesity (18). Changes in diet and other manifestations of the obese state could contribute to increased oxidative stress and decreased nitric oxide bioavailability in the airway, leading to the development of de novo airway disease in obesity.
Aging and the degree of obesity might be involved in the development of the late-onset asthma of obesity. Obese mice fed a high-fat diet develop increased airway reactivity only after a certain age and weight: for example, 20- to 22-week-old C57BL/6 mice (average weight, 43 g) fed a high-fat diet did not have increased airway reactivity compared with mice fed a low-fat diet, whereas 30- to 38 week-old-mice (average weight 50 g) did (19). These results suggest that an interaction between aging and weight gain may contribute to the inherent airway reactivity of obesity, and more studies on this are needed in humans. How changes in airway structure, oxidative stress, and immune function interact with aging in the setting of obesity to produce airway disease is currently a matter of speculation.
In addition, changes in certain cytokine pathways described in obese mice might relate to the inherent airway reactivity in obesity. For example, this inherent airway reactivity is reduced in mice deficient in tumor necrosis factor receptor-2 (20). However, airway reactivity in obese mice is increased in mice deficient in tumor necrosis factor receptor 1 (21); therefore, global strategies to block tumor necrosis factor signaling are unlikely to be effective in this form of obese asthma (22). Another mediator that has been associated with the development of this inherent airway reactivity of obesity is IL-17A: increased IL-17A+γδ T cells and TH17 cells have been found in the lungs of mice rendered obese through a high-fat diet (23). Inherent airway reactivity in response to high-fat diet–induced obesity also appears to involve increased M1 macrophages with NLRP3 activation, IL-1β production, and innate lymphoid cells in the lung (24). Although there are increases in certain cytokines, it should be noted that this inherent airway reactivity typically occurs in the absence of overt tissue inflammation; therefore, these cytokines might affect airway smooth muscle tone or cause structural changes that contribute to altered airway function in obesity in the absence of promoting leukocyte infiltration into the airways or lung tissue.
Another factor that might contribute to altered airway function in obesity is neuronal control of airway tone. Data from animal models suggest that there are changes in the neurological control of airway smooth muscle tone through cholinergic signaling pathways in obesity (25–27). High insulin levels are present in some obese humans, and high insulin levels can increase vagal-induced bronchoconstriction both through peripheral effects on acetylcholine release (25) and through centrally mediated effects on vagal signaling (26). Leptin, an adipokine increased in obesity, can also affect the parasympathetic regulation of airway tone (27). It is difficult at this time to understand the clinical significance of these observations relating to insulin resistance and cholinergic signaling, because studies of antidiabetic medication in obese mice (28) and humans (29) have not shown efficacy in the treatment of obesity-associated asthma, and a retrospective subgroup analysis of obese patients with asthma found that body mass index (BMI) did not predict response to the anticholinergic medication tiotropium (30). Clinical studies to date have not specifically targeted the late-onset asthma of obesity. Animal studies demonstrating that cholinergic signaling affects airway tone (25–27) suggest that focused studies targeting insulin resistance and/or cholinergic pathways in the late-onset asthma of obesity associated with little overt inflammation might be worthwhile.
The development of obesity is often associated with the consumption of diets substantially different from those consumed by lean individuals. Animal models have provided insight into the contribution of pre- and perinatal nutritional composition to the lifelong risk of developing obesity and airway reactivity. Dietary factors could contribute to structural changes that affect lung function. For example, a high-fat diet alters fetal lung development: rats born to mothers fed a high-fat diet during gestation have higher levels of cytokines such as tumor necrosis factor-α in their lungs, as well as increased airway resistance and decreased respiratory system compliance (31). Furthermore, mice born to mothers fed a high-fat diet have delayed fetal lung development and reduced expression of surfactant proteins (32). Consumption of a high-fat diet during gestation could contribute to the development of asthma in offspring. A high-fat diet can also alter lung structure in adult mice: structural changes are present in the airway epithelium of adult mice fed a high-fat diet (33), and collagen content in the lungs and airways is increased (34). A high-fat diet at any time from in utero development to adulthood could lead to structural and functional changes in the airway wall that could contribute to the airway disease of obesity.
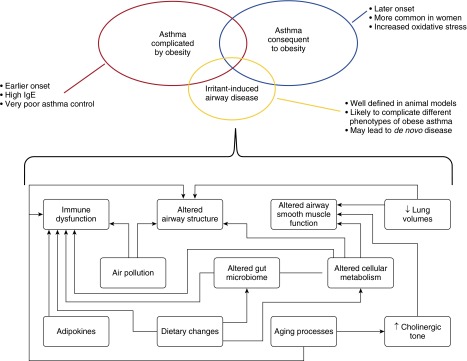
The obese state could lead to asthma in those without prior airway disease in a number of different ways (Figure 1). These include changes in lung structure and function related to diet and mechanics, oxidative signaling, cytokine derangement, and neuronal signaling pathways. These factors may also interact with aging processes in the airway and normal population variations in the airway structure, so that although not all obese individuals develop airway disease, the greater the obesity, the higher the likelihood of developing asthma.
Figure 1.
Obesity is characterized by changes in diet and aberrant metabolism that contribute to immune dysfunction, as well as by altered airway structure and function. These alterations may complicate preexisting asthma (asthma complicated by obesity), lead to de novo airway disease (asthma consequent to obesity), and increase susceptibility to airway disease related to irritants such as ozone, nitrogen dioxide, and particulate matter.
Pre-Existing Asthma Complicated by Obesity
On the basis of studies in human populations, there appears to be a phenotype of obese asthma that exhibits earlier onset of disease and higher markers of allergic inflammation (particularly serum IgE); in these patients, asthma symptoms, but not airway reactivity, improve with weight loss (7–9). These obese patients with asthma have approximately a threefold increased risk of hospital admission and a sixfold increased risk of intensive care unit admission, compared with their lean counterparts (8). Early-onset asthma in obese subjects, which is typically associated with elevated markers of Th2 inflammation, seems to be a particularly severe form of asthma associated with high health care use.
It remains unclear why allergic asthma should be so severe in the setting of obesity, and so far, data on biomarkers of Th2 inflammation in humans have been confusing. For example, our own group reported that markers of CD4 lymphocyte function appear to be suppressed in the setting of obesity (7), and similar findings have been reported in a mouse model combining high-fat diet feeding and allergic asthma (35). Furthermore, many groups have reported decreased sputum eosinophils with increasing BMI (36, 37). On the other hand, airway wall eosinophilia may be increased in obese patients with asthma and dissociated from sputum eosinophilia (38, 39). Of note, IL-25, a cytokine produced by airway epithelium that is involved in the activation of type 2 innate lymphoid cells that can produce IL-5 and IL-13, is increased in the sputum of obese patients with asthma (40). These data in humans might be explained by altered trafficking of eosinophils in obesity and the participation of innate, rather than adaptive, immune responses in the development of airway eosinophilia in obese patients with asthma.
To try to understand the mechanisms by which obesity might alter early-onset allergic asthma, a number of studies have modeled obesity and allergic airway disease in laboratory mice (Table 1). It is apparent that the findings in the various models differ considerably: some report decreased bronchoalveolar lavage (BAL) eosinophilia, and others, increased BAL eosinophilia; some report decreased peribronchial eosinophilia, whereas others found the opposite. These studies differ in the age, strain, and sex of the mice, the type of diet, the allergen (type, dose, delivery, and duration), and the degree of obesity (e.g., the weight of the “obese” BALB/c mice in the study by Chen and colleagues (41) is similar to the weight of the “lean” control mice in some other studies). All these factors might affect immune responses as well as airway structure and function. Indeed, it is striking that any commonalities have been found at all. Nevertheless, these studies do suggest that the obese state increases allergen-induced airway reactivity and serum IgE.
Table 1.
Mouse Models of Obesity and Allergic Asthma
| Author | Year | Strain | Diet | Weight (g) | Age (wk) | Sex | Allergen | BAL | Tissue | AHR | Serum IgE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Johnston (79) | 2007 | ob/ob | 55 | M & F | OVA | ↓Eos↓Mac | ↓Inflammation | ↑ | ↑ Total | ||
| db/db | 48 | M & F | OVA | ↓Eos↔Mac | ↑ | ||||||
| Lintomen (43) | 2012 | ob/ob | 17-20 | M & F | OVA | ↓Eos | ↑Eos | ||||
| Calixto (42) | 2010 | C57BL/6 | HFD (55%) | 39 | 16 | M | OVA | ↓Eos 48hrs | ↑Eos | ||
| Ge (34) | 2013 | C57BL/6 | HFD (60%) | 40 | 18 | M | CRA | ↓Eos↑Mac | ↓Eos | ↔ | |
| Diaz (80) | 2015 | C57BL/6 | HFD (60%) | 55 | 35 | M | HDM | ↓Eos↑Mac | ↓Eos | ↑ Total | |
| Kim (44) | 2015 | C57BL/6 | HFD (60%) | 40 | 20 | F | OVA | ↔Eos↑Mac | ↔ Goblet cells | ↑ | ↑ Total |
| Dietze (81) | 2012 | AKR | HFD (40%) | 33 | 13 | F | OVA | ↑Eos | ↑OVA-specific | ||
| Chen (41) | 2015 | BALB/c | HFD (55%) | 30 | 16 | F | OVA | ↑Inflammation | ↑ | ||
| Dahm (82) | 2014 | CPEfat | 46 | 15-20 | F | OVA | ↑Eos | ↔ Inflammation | ↑ | ↑ Total (ns) |
Definition of abbreviations: AHR, airway hyperresponsiveness; BAL, bronchoalveolar lavage; CRA, cockroach antigen; Eos, eosinophils; F, female; HDM, house dust mite; HFD, high-fat diet; M, male; Mac, macrophages; ns, not significant; OVA, ovalbumin; ↑, increased; ↓, decreased; ↔, unchanged.
Some insights into the mechanisms underlying altered allergic airway disease in obesity are emerging. Increased bone marrow eosinophilia and changes in the kinetics of eosinophil trafficking to the airway were observed using both high-fat diet (42) and genetic (ob/ob) (43) models of obesity accompanied by allergen exposure. In a high-fat diet–ovalbumin model, airway reactivity was inhibited by blocking tumor necrosis factor-α and by depleting macrophages (44), suggesting a critical role for macrophage production of tumor necrosis factor-α in airway reactivity in obese allergic mice. In another study, high-fat diet–induced obesity was associated with increased collagen deposition in the lung (even before allergen challenge), whereas a cockroach allergen challenge in obesity was associated with decreased prostaglandin E2 (a bronchodilator) compared with lean mice (34). These studies show that obesity, induced through dietary or genetic means, can alter baseline airway structure, increase allergen-induced airway inflammation, and increase airway reactivity in allergic airway disease.
Many studies have focused on the potential contribution of adipokines, mediators produced by adipose tissue, to the modulation of allergic airway inflammation. For example, adiponectin, an antiinflammatory mediator that is decreased in obesity, functions in lean mice to inhibit allergic airway inflammation and airway reactivity (45, 46), an activity that appears to be related to its serum levels (47). Conversely, leptin, an adipokine that is elevated in obesity, can increase airway reactivity and serum IgE in an allergic mouse model (48). Both epidemiological data and data from mouse models suggest that adipokines might contribute to altered allergic airway inflammation.
Certainly, altered adipokines might be important contributors to asthma in obesity, but the lung is likely more than an innocent bystander in obesity, a disease that is first and foremost one of altered energy and metabolism. Respiration is closely linked to metabolism, and cellular metabolism obviously affects every cell type in the body. It is likely that common metabolic pathways contribute to both the development of allergic airway disease and increased visceral obesity. For example, Chitinase 3-like-1 is involved in the development of allergic responses and also in the development of visceral obesity, possibly through effects on genes involved in metabolism (49). Metabolism is a critical regulator of immune cell function; increased cellular metabolism is required to fuel the demands of immune mediator biosynthesis and cell division (50). Thus, aberrant metabolism might lead to the development of both obesity and immune dysfunction, contributing to asthma.
Aberrant metabolism could affect resident cells in the airway. Mitochondrial polymorphisms have been associated with asthma in the general population (51, 52) and changes in airway mitochondrial structure and function have been reported in airway epithelium in animal models of asthma (53, 54). Recent studies in lean animals show that markers of allergic airway inflammation (53) and transforming growth factor-β–induced collagen deposition (54) can be suppressed with mitochondrial-targeted antioxidants. These data suggest that airway epithelial mitochondrial dysfunction may contribute to allergic asthma, even in lean individuals. Mitochondrial dysfunction is a characteristic of obesity, and many studies, particularly in liver and muscle cells, suggest that obesity increases mitochondrial oxidative stress (55, 56). Interestingly, airway mitochondria are altered in animals fed a high-fat diet (33). Mitochondrial dysfunction in both allergic airway disease and obesity might thus conspire to augment allergic asthma in obesity, leading to the severe form of disease that is poorly treated by current therapies.
Diets that lead to obesity, such as those low in fiber or high in fat, may predispose to the development of allergic airway disease. Dietary fiber is fermented by colonic bacteria to generate short-chain fatty acids; in a mouse model, increasing dietary fiber consumption changed the gut microbiome (increasing the ratio of Firmicutes to Bacteroidetes), which led to increased circulating levels of the short-chain fatty acid propionate (57). Propionate reduced Th2-cell effector function and allergic airway inflammation through effects on the generation of macrophage and dendritic cell precursors (57). This suggests that a high-fiber diet might inhibit the development of allergic airway disease in later life. Another group reported suppression of regulatory T lymphocytes (Treg cells) through epigenetic modifications of the FoxP3 promoter by a high-fat diet (58); a high-fat diet in utero promoted allergic airway inflammation by suppression of Treg cell function in offspring. Dietary composition producing obesity may predispose to the development of allergic asthma.
A high-fat diet might adversely affect those with pre-existing asthma. High-fat diets can produce acute airway inflammation and changes in lung physiology. In humans, consumption of a high-fat meal increases sputum neutrophilia and Toll-like receptor 4 expression and decreases the response to the bronchodilator; this suggests that a high-fat diet alters both airway inflammation and smooth muscle function (59). The composition of fats in the diet can differentially impact inflammatory responses. Polyunsaturated and monounsaturated fatty acids, such as those found in fish oil and olive oil, respectively (e.g., Mediterranean diets), can have antiinflammatory effects, whereas saturated fatty acids, such as the palmitic acid that is abundant in red meat (e.g., Western diets), can exhibit proinflammatory effects. We recently reported that feeding subjects for 3 weeks with diets containing oleic acid: palmitic acid ratios reflecting a Western diet (1:1) versus a Mediterranean diet (12:1) caused elevated production of TNFα, IL-10, and the NLRP3-regulated cytokines IL-1β and IL-18 by LPS-stimulated peripheral blood mononuclear cells (60). Although Mediterranean versus Western diets have not shown substantial differential effects on the development of asthma in the general population (61, 62), because NLRP3 and IL-1β have been reported to be pathogenic mediators of high-fat diet–induced intrinsic methacholine hyperresponsiveness (24), a regimen with a 3:1 palmitic acid:oleic acid diet, the contribution of these dietary fats to obesity-associated asthma should be further examined with focused studies in animal models and interventional studies in obese patients with asthma.
The obese state can affect those with underlying allergic asthma in a number of ways (Figure 1). Dietary changes associated with obesity during gestation and early life might lead to the development of allergic asthma. There may be activation of innate immune pathways and changes in eosinophil trafficking in these patients. Changes in circulating adipokines may have direct effects on the airways. Changes in metabolism might affect immune function and resident cells of the airway. Dietary changes characteristic of obesity might lead to exaggerated airway inflammation. Given the myriad effects of obesity on allergic asthma, it is perhaps not surprising that these patients suffer from particularly severe disease that is refractory to current therapy.
Irritant- and Pollution-Induced Asthma in Obesity
What we eat can affect obese asthma, so too can what we breathe. Overweight and obese children are more susceptible to indoor air pollutants, especially particulate matter and nitrogen dioxide, than are lean children (63). We have reported that in nonobese mouse models, nitrogen dioxide inhalation causes allergic asthma-like disease through NLRP3 and IL-1 signaling (64) and inherent asthma-like disease through activation of the transcription factor nuclear factor-κB (NF-κB) in the airway epithelium (65). Airway epithelial NF-κB activation increases methacholine responsiveness (in the absence of allergen exposure) by increasing the abundance of airway smooth muscle (66), increases lung type 2 innate lymphoid cells, and facilitates allergic antigen sensitization (67, 68). Airway epithelial NF-κB can be activated by allergens and pollutants (69), and potentially by endogenous factors secreted by adipose tissue (70). It is possible that obese individuals might be more susceptible to air pollution through enhanced airway epithelial NF-κB activation, a hypothesis that merits further investigation.
Obese people have increased airway reactivity and respiratory symptoms in response to ozone (71, 72), and increases in IL-1β in induced sputum in response to ozone are directly correlated with BMI (73). Increased responses to ozone are also reported in different models of obese mice (19, 74, 75). Williams and colleagues showed that augmented airway inflammation and reactivity in obese mice in response to ozone depended on TNFα (22). Increased responses to subacute ozone exposure are also seen in adiponectin-deficient mice (adiponectin is decreased in obesity), and this appears to be related to signaling through IL-6 and IL-17A (76–78). The role of these mediators in increasing responses to ozone in obese individuals needs further exploration.
Obese individuals seem more susceptible to pollutants, and animal models support this observation, suggesting that pollutants likely contribute to the development of asthma in obesity (Figure 1). The combined effects of poor airway quality and increasing levels of obesity might synergize to produce a major increase in the development of asthma during this century, and this is an area surely deserving of additional study.
Conclusions
The clinical and biomedical research communities are beginning to appreciate that obesity is a major risk factor for the development of asthma, and that obesity contributes to far more severe asthma in those with established disease. Over the past few years, much effort has been focused on trying to understand the interrelationship between these syndromes by considering obesity and asthma as two separate entities that interact to exacerbate underlying airway disease. However, obesity is fundamentally a disorder of energy and metabolism; respiration, energy regulation, and metabolism are closely integrated systems that affect every cell in the body. We need to better understand the normal physiological interactions between the respiratory system and energy regulation–metabolism if we are to understand how dysfunction in these systems contributes to the epidemic of airway disease in obesity.
Footnotes
This work was supported by National Institutes of Health grants P30 GM103532 and R01 HL107291.
Author Contributions: A.E.D. and M.E.P. jointly conceived and drafted this manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0017PS on February 17, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vortmann M, Eisner MD. BMI and health status among adults with asthma. Obesity (Silver Spring) 2008;16:146–152. doi: 10.1038/oby.2007.7. [DOI] [PubMed] [Google Scholar]
- 2.Mosen DM, Schatz M, Magid DJ, Camargo CA., Jr The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol. 2008;122:507–11.e6. doi: 10.1016/j.jaci.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Taylor B, Mannino D, Brown C, Crocker D, Twum-Baah N, Holguin F. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008;63:14–20. doi: 10.1136/thx.2007.082784. [DOI] [PubMed] [Google Scholar]
- 4.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 5.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med. 2007;101:2240–2247. doi: 10.1016/j.rmed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 6.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, Haselkorn T. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133:1549–1556. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–15.e1, 2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E, Jarjour NN, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127:1486–93.e2. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutherland ER, Goleva E, King TS, Lehman E, Stevens AD, Jackson LP, Stream AR, Fahy JV, Leung DY Asthma Clinical Research Network. Cluster analysis of obesity and asthma phenotypes. PLoS One. 2012;7:e36631. doi: 10.1371/journal.pone.0036631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin RA, Hodgkins SR, Dixon AE, Poynter ME. Aligning mouse models of asthma to human endotypes of disease. Respirology. 2014;19:823–833. doi: 10.1111/resp.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holguin F, Comhair SA, Hazen SL, Powers RW, Khatri SS, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Fitzpatrick AM, et al. An association between L-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. Am J Respir Crit Care Med. 2013;187:153–159. doi: 10.1164/rccm.201207-1270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Alwan A, Bates JH, Chapman DG, Kaminsky DA, DeSarno MJ, Irvin CG, Dixon AE. The nonallergic asthma of obesity. A matter of distal lung compliance. Am J Respir Crit Care Med. 2014;189:1494–1502. doi: 10.1164/rccm.201401-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman DG, Irvin CG, Kaminsky DA, Forgione PM, Bates JH, Dixon AE. Influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirology. 2014;19:1170–1177. doi: 10.1111/resp.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest. 1995;96:2393–2403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bates JH, Dixon AE. Potential role of the airway wall in the asthma of obesity. J Appl Physiol (1985) 2015;118:36–41. doi: 10.1152/japplphysiol.00684.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holguin F, Fitzpatrick A. Obesity, asthma, and oxidative stress. J Appl Physiol (1985) 2010;108:754–759. doi: 10.1152/japplphysiol.00702.2009. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S, Erzurum SC. Nitric oxide metabolism in asthma pathophysiology. Biochim Biophys Acta. 2011;1810:1008–1016. doi: 10.1016/j.bbagen.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh VP, Aggarwal R, Singh S, Banik A, Ahmad T, Patnaik BR, Nappanveettil G, Singh KP, Aggarwal ML, Ghosh B, et al. Metabolic syndrome is associated with increased oxo-nitrative stress and asthma-like changes in lungs. PLoS One. 2015;10:e0129850. doi: 10.1371/journal.pone.0129850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol (1985) 2008;104:1727–1735. doi: 10.1152/japplphysiol.00075.2008. [DOI] [PubMed] [Google Scholar]
- 20.Williams AS, Chen L, Kasahara DI, Si H, Wurmbrand AP, Shore SA. Obesity and airway responsiveness: role of TNFR2. Pulm Pharmacol Ther. 2013;26:444–454. doi: 10.1016/j.pupt.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu M, Williams AS, Chen L, Wurmbrand AP, Williams ES, Shore SA. Role of TNFR1 in the innate airway hyperresponsiveness of obese mice. J Appl Physiol (1985) 2012;113:1476–1485. doi: 10.1152/japplphysiol.00588.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams AS, Mathews JA, Kasahara DI, Wurmbrand AP, Chen L, Shore SA. Innate and ozone-induced airway hyperresponsiveness in obese mice: role of TNF-α. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1168–L1177. doi: 10.1152/ajplung.00393.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews JA, Wurmbrand AP, Ribeiro L, Neto FL, Shore SA. Induction of IL-17A precedes development of airway hyperresponsiveness during diet-induced obesity and correlates with complement factor D. Front Immunol. 2014;5:440. doi: 10.3389/fimmu.2014.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, Iwakura Y, Israel E, Bolger K, Faul J, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nie Z, Jacoby DB, Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol. 2014;51:251–261. doi: 10.1165/rcmb.2013-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leiria LO, Arantes-Costa FM, Calixto MC, Alexandre EC, Moura RF, Folli F, Prado CM, Prado MA, Prado VF, Velloso LA, et al. Increased airway reactivity and hyperinsulinemia in obese mice are linked by ERK signaling in brain stem cholinergic neurons. Cell Reports. 2015;11:934–943. doi: 10.1016/j.celrep.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Arteaga-Solis E, Zee T, Emala CW, Vinson C, Wess J, Karsenty G. Inhibition of leptin regulation of parasympathetic signaling as a cause of extreme body weight-associated asthma. Cell Metab. 2013;17:35–48. doi: 10.1016/j.cmet.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shore SA, Williams ES, Zhu M. No effect of metformin on the innate airway hyperresponsiveness and increased responses to ozone observed in obese mice. J Appl Physiol (1985) 2008;105:1127–1133. doi: 10.1152/japplphysiol.00117.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon AE, Subramanian M, DeSarno M, Black K, Lane L, Holguin F. A pilot randomized controlled trial of pioglitazone for the treatment of poorly controlled asthma in obesity. Respir Res. 2015;16:143. doi: 10.1186/s12931-015-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters SP, Bleecker ER, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, Boushey HA, Calhoun WJ, Castro M, et al. National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Predictors of response to tiotropium versus salmeterol in asthmatic adults. J Allergy Clin Immunol. 2013;132:1068–1074.e1. doi: 10.1016/j.jaci.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffiths PS, Walton C, Samsell L, Perez MK, Piedimonte G. Maternal high-fat hypercaloric diet during pregnancy results in persistent metabolic and respiratory abnormalities in offspring. Pediatr Res. doi: 10.1038/pr.2015.226. [online ahead of print] 23 Dec 2015; DOI: 10.1038/pr.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayor RS, Finch KE, Zehr J, Morselli E, Neinast MD, Frank AP, Hahner LD, Wang J, Rakheja D, Palmer BF, et al. Maternal high-fat diet is associated with impaired fetal lung development. Am J Physiol Lung Cell Mol Physiol. 2015;309:L360–L368. doi: 10.1152/ajplung.00105.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leishangthem GD, Mabalirajan U, Singh VP, Agrawal A, Ghosh B, Dinda AK. Ultrastructural changes of airway in murine models of allergy and diet-induced metabolic syndrome. ISRN Allergy. 2013;2013:261297. doi: 10.1155/2013/261297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ge XN, Greenberg Y, Hosseinkhani MR, Long EK, Bahaie NS, Rao A, Ha SG, Rao SP, Bernlohr DA, Sriramarao P. High-fat diet promotes lung fibrosis and attenuates airway eosinophilia after exposure to cockroach allergen in mice. Exp Lung Res. 2013;39:365–378. doi: 10.3109/01902148.2013.829537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Vries A, Hazlewood L, Fitch PM, Seckl JR, Foster P, Howie SE. High-fat feeding redirects cytokine responses and decreases allergic airway eosinophilia. Clin Exp Allergy. 2009;39:731–739. doi: 10.1111/j.1365-2222.2008.03179.x. [DOI] [PubMed] [Google Scholar]
- 36.van Veen IH, Ten Brinke A, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63:570–574. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 37.Lessard A, Turcotte H, Cormier Y, Boulet LP. Obesity and asthma: a specific phenotype? Chest. 2008;134:317–323. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 38.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, Bafadhel M, Singapuri A, Siddiqui S, Woods J, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Respir Crit Care Med. 2013;188:657–663. doi: 10.1164/rccm.201208-1470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Wiel E, Ten Hacken NH, van den Berge M, Timens W, Reddel HK, Postma DS. Eosinophilic inflammation in subjects with mild-to-moderate asthma with and without obesity: disparity between sputum and biopsies. Am J Respir Crit Care Med. 2014;189:1281–1284. doi: 10.1164/rccm.201310-1841LE. [DOI] [PubMed] [Google Scholar]
- 40.Marijsse GS, Seys SF, Schelpe AS, Dilissen E, Goeminne P, Dupont LJ, Ceuppens JL, Bullens DM. Obese individuals with asthma preferentially have a high IL-5/IL-17A/IL-25 sputum inflammatory pattern. Am J Respir Crit Care Med. 2014;189:1284–1285. doi: 10.1164/rccm.201311-2011LE. [DOI] [PubMed] [Google Scholar]
- 41.Chen YP, Zhang JH, Li CQ, Sun QX, Jiang XH. Obesity enhances Th2 inflammatory response via natural killer T cells in a murine model of allergic asthma. Int J Clin Exp Med. 2015;8:15403–15412. [PMC free article] [PubMed] [Google Scholar]
- 42.Calixto MC, Lintomen L, Schenka A, Saad MJ, Zanesco A, Antunes E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol. 2010;159:617–625. doi: 10.1111/j.1476-5381.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lintomen L, Calixto MC, Schenka A, Antunes E. Allergen-induced bone marrow eosinophilopoiesis and airways eosinophilic inflammation in leptin-deficient ob/ob mice. Obesity (Silver Spring) 2012;20:1959–1965. doi: 10.1038/oby.2012.93. [DOI] [PubMed] [Google Scholar]
- 44.Kim JY, Sohn JH, Lee JH, Park JW. Obesity increases airway hyperresponsiveness via the TNF-α pathway and treating obesity induces recovery. PLoS One. 2015;10:e0116540. doi: 10.1371/journal.pone.0116540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118:389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 46.Verbout NG, Benedito L, Williams AS, Kasahara DI, Wurmbrand AP, Si H, Halayko AJ, Hug C, Shore SA. Impact of adiponectin overexpression on allergic airways responses in mice. J Allergy (Cairo) 2013;2013:349520. doi: 10.1155/2013/349520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams AS, Kasahara DI, Verbout NG, Fedulov AV, Zhu M, Si H, Wurmbrand AP, Hug C, Ranscht B, Shore SA. Role of the adiponectin binding protein, T-cadherin (Cdh13), in allergic airways responses in mice. PLoS One. 2012;7:e41088. doi: 10.1371/journal.pone.0041088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Ahangari F, Sood A, Ma B, Takyar S, Schuyler M, Qualls C, Dela Cruz CS, Chupp GL, Lee CG, Elias JA. Chitinase 3-like-1 regulates both visceral fat accumulation and asthma-like Th2 inflammation. Am J Respir Crit Care Med. 2015;191:746–757. doi: 10.1164/rccm.201405-0796OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loftus RM, Finlay DK. Immunometabolism: cellular metabolism turns immune regulator. J Biol Chem. 2016;291:1–10. doi: 10.1074/jbc.R115.693903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flaquer A, Heinzmann A, Rospleszcz S, Mailaparambil B, Dietrich H, Strauch K, Grychtol R. Association study of mitochondrial genetic polymorphisms in asthmatic children. Mitochondrion. 2014;14:49–53. doi: 10.1016/j.mito.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Zifa E, Daniil Z, Skoumi E, Stavrou M, Papadimitriou K, Terzenidou M, Kostikas K, Bagiatis V, Gourgoulianis KI, Mamuris Z. Mitochondrial genetic background plays a role in increasing risk to asthma. Mol Biol Rep. 2012;39:4697–4708. doi: 10.1007/s11033-011-1262-8. [DOI] [PubMed] [Google Scholar]
- 53.Aguilera-Aguirre L, Bacsi A, Saavedra-Molina A, Kurosky A, Sur S, Boldogh I. Mitochondrial dysfunction increases allergic airway inflammation. J Immunol. 2009;183:5379–5387. doi: 10.4049/jimmunol.0900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaffer OA, Carter AB, Sanders PN, Dibbern ME, Winters CJ, Murthy S, Ryan AJ, Rokita AG, Prasad AM, Zabner J, et al. Mitochondrial-targeted antioxidant therapy decreases transforming growth factor-β-mediated collagen production in a murine asthma model. Am J Respir Cell Mol Biol. 2015;52:106–115. doi: 10.1165/rcmb.2013-0519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arruda AP, Pers BM, Parlakgül G, Güney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20:1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taddeo EP, Laker RC, Breen DS, Akhtar YN, Kenwood BM, Liao JA, Zhang M, Fazakerley DJ, Tomsig JL, Harris TE, et al. Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Mol Metab. 2014;3:124–134. doi: 10.1016/j.molmet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 58.Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, Roberts LK, Wong CH, Shim R, Robert R, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015;6:7320. doi: 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 59.Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. 2011;127:1133–1140. doi: 10.1016/j.jaci.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 60.Kien CL, Bunn JY, Fukagawa NK, Anathy V, Matthews DE, Crain KI, Ebenstein DB, Tarleton EK, Pratley RE, Poynter ME. Lipidomic evidence that lowering the typical dietary palmitate to oleate ratio in humans decreases the leukocyte production of proinflammatory cytokines and muscle expression of redox-sensitive genes. J Nutr Biochem. 2015;26:1599–1606. doi: 10.1016/j.jnutbio.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lv N, Xiao L, Ma J. Dietary pattern and asthma: a systematic review and meta-analysis. J Asthma Allergy. 2014;7:105–121. doi: 10.2147/JAA.S49960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brigham EP, Kolahdooz F, Hansel N, Breysse PN, Davis M, Sharma S, Matsui EC, Diette G, McCormack MC. Association between Western diet pattern and adult asthma: a focused review. Ann Allergy Asthma Immunol. 2015;114:273–280. doi: 10.1016/j.anai.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu KD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, Williams DL, Peng RD, McCormack MC, Matsui EC. Being overweight increases susceptibility to indoor pollutants among urban children with asthma. J Allergy Clin Immunol. 2013;131:1017–1023, 1023.e1–1023.e3. doi: 10.1016/j.jaci.2012.12.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin RA, Ather JL, Lundblad LK, Suratt BT, Boyson JE, Budd RC, Alcorn JF, Flavell RA, Eisenbarth SC, Poynter ME. Interleukin-1 receptor and caspase-1 are required for the Th17 response in nitrogen dioxide-promoted allergic airway disease. Am J Respir Cell Mol Biol. 2013;48:655–664. doi: 10.1165/rcmb.2012-0423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bevelander M, Mayette J, Whittaker LA, Paveglio SA, Jones CC, Robbins J, Hemenway D, Akira S, Uematsu S, Poynter ME. Nitrogen dioxide promotes allergic sensitization to inhaled antigen. J Immunol. 2007;179:3680–3688. doi: 10.4049/jimmunol.179.6.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pantano C, Ather JL, Alcorn JF, Poynter ME, Brown AL, Guala AS, Beuschel SL, Allen GB, Whittaker LA, Bevelander M, et al. Nuclear factor-κB activation in airway epithelium induces inflammation and hyperresponsiveness. Am J Respir Crit Care Med. 2008;177:959–969. doi: 10.1164/rccm.200707-1096OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ather JL, Hodgkins SR, Janssen-Heininger YM, Poynter ME. Airway epithelial NF-κB activation promotes allergic sensitization to an innocuous inhaled antigen. Am J Respir Cell Mol Biol. 2011;44:631–638. doi: 10.1165/rcmb.2010-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ather JL, Foley KL, Suratt BT, Boyson JE, Poynter ME. Airway epithelial NF-κB activation promotes the ability to overcome inhalational antigen tolerance. Clin Exp Allergy. 2015;45:1245–1258. doi: 10.1111/cea.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ckless K, Hodgkins SR, Ather JL, Martin R, Poynter ME. Epithelial, dendritic, and CD4(+) T cell regulation of and by reactive oxygen and nitrogen species in allergic sensitization. Biochim Biophys Acta. 2011;1810:1025–1034. doi: 10.1016/j.bbagen.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 71.Alexeeff SE, Litonjua AA, Suh H, Sparrow D, Vokonas PS, Schwartz J. Ozone exposure and lung function: effect modified by obesity and airways hyperresponsiveness in the VA normative aging study. Chest. 2007;132:1890–1897. doi: 10.1378/chest.07-1126. [DOI] [PubMed] [Google Scholar]
- 72.Dong GH, Qian Z, Liu MM, Wang D, Ren WH, Fu Q, Wang J, Simckes M, Ferguson TF, Trevathan E. Obesity enhanced respiratory health effects of ambient air pollution in Chinese children: the Seven Northeastern Cities study. Int J Obes. 2013;37:94–100. doi: 10.1038/ijo.2012.125. [DOI] [PubMed] [Google Scholar]
- 73.Todoric K, Zhou H, Zhang H, Mills K, Peden DB, Hernandez ML. Body mass index correlates with pollutant-induced interleukin-1β in sputum and blood. Ann Allergy Asthma Immunol. 2015;114:251–253. doi: 10.1016/j.anai.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L856–L865. doi: 10.1152/ajplung.00386.2005. [DOI] [PubMed] [Google Scholar]
- 75.Rivera-Sanchez YM, Johnston RA, Schwartzman IN, Valone J, Silverman ES, Fredberg JJ, Shore SA. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol (1985) 2004;96:2200–2206. doi: 10.1152/japplphysiol.00960.2003. [DOI] [PubMed] [Google Scholar]
- 76.Kasahara DI, Kim HY, Williams AS, Verbout NG, Tran J, Si H, Wurmbrand AP, Jastrab J, Hug C, Umetsu DT, et al. Pulmonary inflammation induced by subacute ozone is augmented in adiponectin-deficient mice: role of IL-17A. J Immunol. 2012;188:4558–4567. doi: 10.4049/jimmunol.1102363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kasahara DI, Williams AS, Benedito LA, Ranscht B, Kobzik L, Hug C, Shore SA. Role of the adiponectin binding protein, T-cadherin (cdh13), in pulmonary responses to subacute ozone. PLoS One. 2013;8:e65829. doi: 10.1371/journal.pone.0065829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kasahara DI, Kim HY, Mathews JA, Verbout NG, Williams AS, Wurmbrand AP, Ninin FM, Neto FL, Benedito LA, Hug C, et al. Pivotal role of IL-6 in the hyperinflammatory responses to subacute ozone in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2014;306:L508–L520. doi: 10.1152/ajplung.00235.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, Shore SA. Allergic airway responses in obese mice. Am J Respir Crit Care Med. 2007;176:650–658. doi: 10.1164/rccm.200702-323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diaz J, Warren L, Helfner L, Xue X, Chatterjee PK, Gupta M, Solanki MH, Esposito M, Bonagura V, Metz CN. Obesity shifts house dust mite-induced airway cellular infiltration from eosinophils to macrophages: effects of glucocorticoid treatment. Immunol Res. 2015;63:197–208. doi: 10.1007/s12026-015-8717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dietze J, Bocking C, Heverhagen JT, Voelker MN, Renz H. Obesity lowers the threshold of allergic sensitization and augments airway eosinophilia in a mouse model of asthma. Allergy. 2012;67:1519–1529. doi: 10.1111/all.12031. [DOI] [PubMed] [Google Scholar]
- 82.Dahm PH, Richards JB, Karmouty-Quintana H, Cromar KR, Sur S, Price RE, Malik F, Spencer CY, Barreno RX, Hashmi SS, et al. Effect of antigen sensitization and challenge on oscillatory mechanics of the lung and pulmonary inflammation in obese carboxypeptidase E-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2014;307:R621–R633. doi: 10.1152/ajpregu.00205.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]