Abstract
Obesity affects the incidence and severity of asthma in at least two major phenotypes: an early-onset allergic (EOA) form that is complicated by obesity and a late-onset nonallergic (LONA) form that occurs only in the setting of obesity. Both groups exhibit airway hyperresponsiveness to methacholine challenge but exhibit differential effects of weight loss. Measurements of lung function in patients with LONA obese asthma suggest that this group of individuals may simply be those unlucky enough to have airways that are more compliant than average, and that this leads to airway hyperresponsiveness at the reduced lung volumes caused by excess adipose tissue around the chest wall. In contrast, the frequent exacerbations in those with EOA obese asthma can potentially be explained by episodic inflammatory thickening of the airway wall synergizing with obesity-induced reductions in lung volume. These testable hypotheses are based on the strong likelihood that LONA and EOA obese asthma are distinct diseases. Both, however, may benefit from targeted therapeutics that impose elevations in lung volume.
Keywords: reduced lung volume, methacholine challenge, lung impedance, airway wall thickening, airway wall stiffness
Clinical Relevance
A pathophysiologic basis for the airway hyperresponsiveness of obese asthma is proposed. This provides a testable avenue for further investigation that may help lead to novel therapies.
The obesity pandemic that is sweeping the world is having a major effect on the incidence of asthma (1–3). Approximately 33% of the population in the United States is obese, but so are 50–60% of those with severe asthma (4), implicating obesity as a significant risk factor for asthma. Indeed, 250,000 cases of asthma per year in the United States are related to obesity (5). Obesity is also having a major negative impact on asthma therapy and control. Individuals with obese asthma are almost fivefold more likely than lean patients with asthma to be hospitalized for an asthma exacerbation (2). Furthermore, those with obese asthma do not respond as well as their lean counterparts to standard controller therapy with either inhaled corticosteroids or combination inhaled corticosteroid/long-acting β-agonist, and they exhibit increased use of rescue therapy (6–14). Furthermore, systemic steroid use in those with obese asthma tends to worsen their situation by making them gain even more weight, underscoring the problem of treating this patient population with therapies that were developed, for the most part, when the population was less obese than it is today.
Despite the obvious public health implications of the asthma of obesity, however, our current understanding of its pathophysiology remains very unclear. This constitutes a major impediment to the development of much-needed improvements in therapy. Nevertheless, some novel hypotheses are now being developed that potentially will explain why obese individuals are particularly susceptible to developing the symptoms of asthma. In this perspective, we focus on the phenomenon of airway hyperresponsiveness (AHR) because this is a hallmark feature of all forms of asthma and it provides a quantitative measure of disease severity. We begin with a discussion of the physiologic mechanisms that potentially explain AHR in general because these mechanisms are numerous and diverse. This provides the necessary backdrop against which to discuss how the AHR of obese asthma can potentially be linked to its pathogenesis.
Mechanistic Bases of AHR
AHR is defined as the finding of abnormal decrements in lung function after challenge with standard doses of bronchial agonist. In human patients, this invariably means abnormally large decreases in FEV1 in response to challenge with a series of escalating concentrations of methacholine aerosol, possibly with added consideration of FVC and the ratio FEV1/FVC. The precise structure–function link underlying changes in FEV1 is complex and difficult to interpret in precise physical terms, so spirometry remains an essentially empirical, albeit highly sensitive, tool (15, 16). Lung function is assessed most usefully in animal models of AHR in terms of mechanical input impedance as measured by the forced oscillation technique, because impedance is interpreted readily in terms of physiologically motivated mathematical models (17). In principle, these models allow structure to be linked directly to function (18).
In both human subjects and animal models, there is little doubt that the instigating event behind an observation of AHR is contraction of the airway smooth muscle (ASM). Accordingly, it is easy to be led to the view that AHR reflects some abnormality of the ASM itself. However, it has long been recognized that there are a number of possible abnormalities not related to the ASM that can potentially explain AHR (19–21). One important class of possibilities includes factors that alter the mechanical load against which the ASM must contract to narrow the airway lumen, and within this class are two distinct players. First, the force of ASM contraction must overcome the compressive stiffness of the airway wall. Remodeling of the airway wall might conceivably reduce this stiffness and thus allow for increased narrowing of the lumen (22), although whether this actually explains any of the AHR of asthma remains unclear (23) despite in vitro studies demonstrating proof of concept (24). The second key mechanical load opposing ASM shortening is the outward tethering force exerted on the intrapulmonary airways by the parenchyma in which they are embedded. This force of airway–parenchymal interdependence mediates the transmission of transpulmonary pressure across the airway wall and thus is tied directly to lung volume. Indeed, airways responsiveness is exquisitely sensitive to lung volume (25–27), which explains why a deep lung inflation is the most potent means of reversing established bronchoconstriction (25, 28, 29), at least in normal lungs. By the same token, reducing lung volume below normal levels is a potent means of inducing AHR (30), which has obvious implications for obesity in which mass loading of the chest wall may compress the lungs and reduce the functional residual capacity (FRC). Clearly, alterations in the stiffness and parenchymal tethering of the airway wall may occur independently of any change in the ASM itself.
Another class of AHR mechanism concerns the geometric arrangement of the components of the airway. For example, ASM cells are typically not aligned perfectly circumferentially around the airway but instead assume a variety of angles relative to the circumference (31). How shortening of these cells translates into airway narrowing depends on the relative stiffnesses of the airway wall in the radial and axial directions (32), both of which might conceivably be altered by airway remodeling. The most potentially important geometric factor in determining AHR, however, is the thickness of the material composing the airway wall that lies between the ASM and the lumen (19, 21). If this material becomes physically enlarged, as typically occurs when the airway is inflamed, then a perfectly normal degree of ASM shortening will cause the thickened airway to impinge on the airway lumen more than would be the case if the wall were normal. This effect is relatively mild in terms of its effect on airway resistance but it can have a large impact on lung elastance because of the increased amount of small airway closure that wall thickening can cause. In fact, this mechanism has been shown to explain almost completely the AHR seen in BALB/c mice during acute allergic inflammation (33). Furthermore, AHR can become extreme if wall thickening is combined with an increased capacity of the ASM to shorten, either as a result of increased delivery of the agonist to the ASM (34, 35) or if the ASM has an intrinsically increased capacity to shorten (36).
In addition, there are the mechanisms of AHR tied to the contractility of the ASM itself, which again come in several distinct forms. Most obvious is the possibility that asthmatic ASM is stronger than normal ASM and thus better able to constrict the airway lumen. Certainly, hypertrophy and/or hyperplasia of the ASM has been documented post mortem in patients with chronic severe asthma (37), although whether this is really a cause of asthma or simply a consequence of disease-induced overuse remains unclear. Pathologic alterations in the excitation–contraction coupling of ASM are also a distinct possibility because hypersensitivity, represented by an excessive response to an intermediate dose of agonist, is as much a feature of asthma as is an excessive response to a supramaximal dose. Indeed, the dose of methacholine eliciting a given modest decrement in lung function can be an order of magnitude of two less in an individual with severe asthma compared with a normal person (38).
Yet another class of AHR mechanism concerns the access of the agonist to the ASM. Methacholine challenge testing is founded on the tacit assumption that the administered dose of agonist is an accurate reflection of the dose that actually stimulates the ASM. This, in fact, may be a very poor assumption. Mice treated with agents known to compromise epithelial barrier function have been shown to become hyperresponsive to methacholine aerosol in a way that can only be explained by a substantially greater fraction of the administered dose reaching the ASM (35, 39). In addition, although allergically inflamed mice appear to respond to methacholine aerosol without any increase in ASM shortening, when the same animals are given intravenous injections of methacholine, they respond with greatly exaggerated ASM shortening (40). This again can be explained only on the basis of altered methacholine delivery. Specifically, inflammation is accompanied by an increase in endothelial leak, which allows an increased fraction of an intravenously administered methacholine dose to make its way into the lung tissues and thence to the ASM. In contrast, delivery of methacholine via inflamed airways is not enhanced and, if anything, would be expected to be impaired because of epithelial hypertrophy and increased mucus (33).
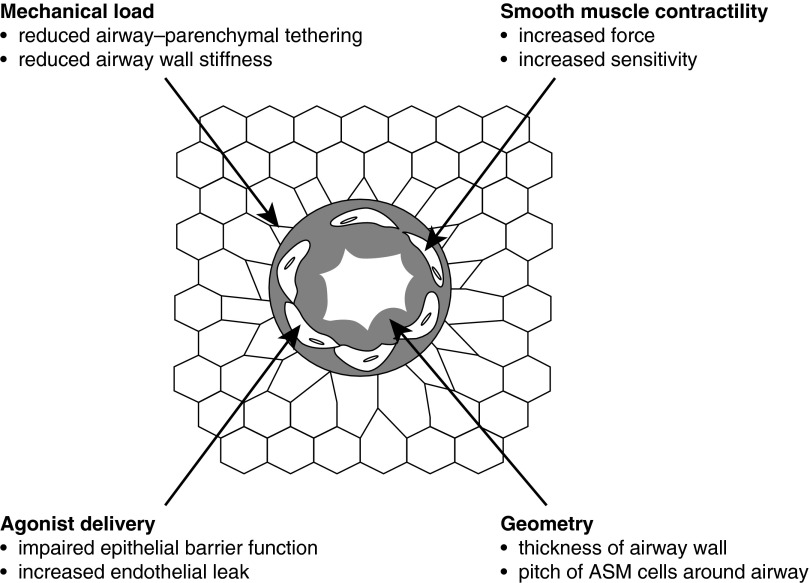
The makeup of the phenomenon of AHR can thus be multifactorial, involving mechanisms from at least four classes (Figure 1), involving (1) mechanical load, (2) geometry, (3) ASM contractility, and (4) agonist delivery. It is now well recognized that asthma presents in a variety of phenotypes (41), so it seems reasonable to suppose that different phenotypes reflect different mechanistic constellations. Accordingly, being aware of the various mechanistic possibilities is a crucial starting point for any discussion of how AHR might arise in the asthma of obesity.
Figure 1.
The four classes of physiologic mechanism of airway hyperresponsiveness, each potentially affecting the caliber of the lumen of an airway embedded in parenchyma when its airway smooth muscle (ASM) is stimulated to contract.
Nature of Obese Asthma
The propensity for obese subjects to develop asthmatic symptoms has been recognized for some time (1–3, 42), and is supported by the observation that mouse models of obesity are hyperresponsive to bronchial challenge compared with lean control mice (43, 44). Recent studies have shown, however, that the asthma of obesity is not a single disease. In fact, there are at least two distinct major phenotypes of obese asthma (6, 41, 45–48): (1) an early-onset allergic (EOA) form that is complicated by obesity and (2) a late-onset nonallergic (LONA) form that occurs only in the setting of obesity. These two phenotypes have a common clinical manifestation in the general symptoms of asthma, but they exhibit difference responses to intervention. In particular, when individuals with LONA obese asthma lose large amounts of weight after bariatric surgery, their degree of airway closure is reduced significantly, whereas closure in those with EOA obese asthma remains unchanged (49). Furthermore, AHR measured by sensitivity to airway closure is significantly reduced after weight loss in individuals with LONA obese asthma but not in those with EOA obese asthma, even though the latter show very significant improvement in symptoms (49). In addition, those with EOA obese asthma are distinguished from their LONA counterparts by being at particular risk of asthma exacerbations (45, 46).
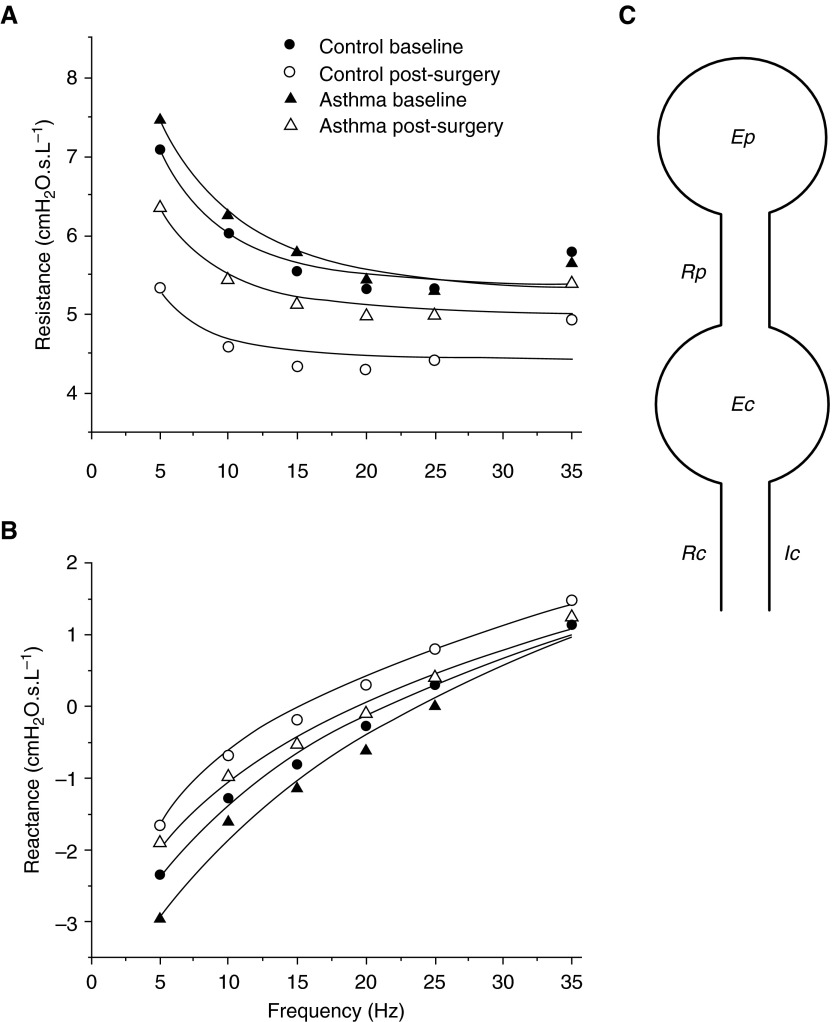
Something that both those with LONA obese asthma and those with EOA obese asthma have in common is the chronic reduction in lung volume that tends to accompany mass loading of the chest wall by adipose tissue in all individuals with obese asthma (50). Reduced lung volume is a potent mechanism for increasing AHR through the release of airway-parenchymal tethering forces, as discussed above, and thus in some way presumably represents a common pathway for all forms of obese asthma. This being the case, that which distinguishes those with LONA obese asthma and those with EOS obese asthma may be the way in which they respond to reduced lung volume. We recently uncovered a clue as to what this might be by measuring respiratory system impedance in individuals with LONA obese asthma and obese control subjects both before and after major weight loss achieved through bariatric surgery (51). Impedance from 5 to 35 Hz (Figures 2A and 2B) was fit with a two-compartment model featuring the compliance of both the peripheral lung and the central airways (Figure 2C). The individuals with asthma started out before weight loss with larger values of peripheral elastance compared with the control subjects, and weight loss caused peripheral elastance to decrease by 60% in the those with asthma but only by 27% in the control subjects (51). The implication of these findings is that those with LONA obese asthma are distinguished from obese control subjects by having unusually collapsible airways that cause their lung functions to become especially compromised when lung volumes are reduced by obesity.
Figure 2.
The symbols show (A) the real part and (B) the imaginary part of respiratory impedance in subjects with obese asthma and obese subjects without asthma before and after major weight loss via bariatric surgery. Also shown are the fits (lines) provided by the model shown in (C). Ec, central airway elastance; Ep, peripheral elastance; Ic, airway gas inertance; Rc, central airway resistance; Rp, peripheral resistance. Reprinted by permission from Reference 51.
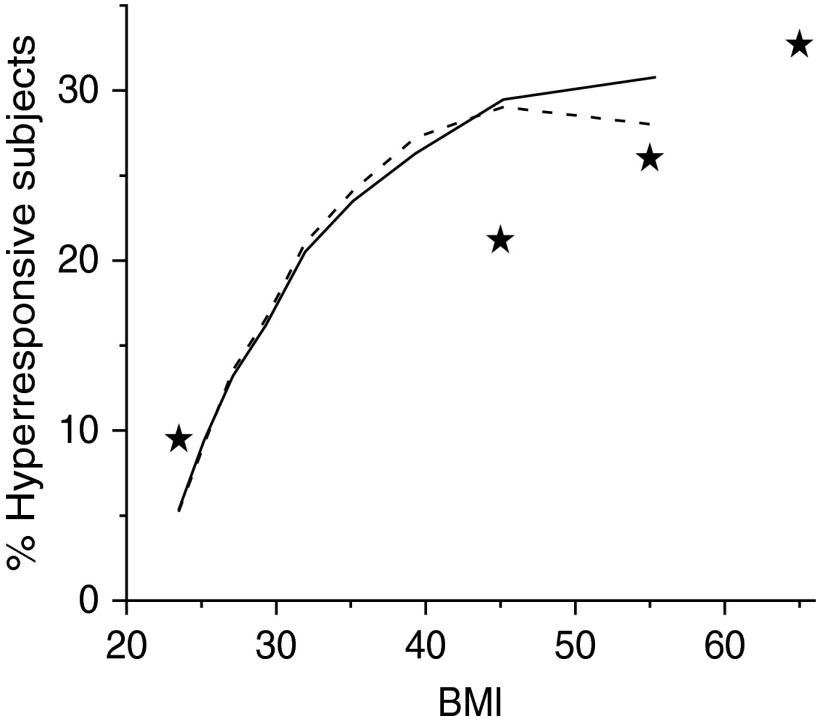
Why the airways of those with LONA obese asthma would be especially prone to collapse remains unclear, but one possibility is that these individuals simply represent a distinct subset of the population characterized by airway compliances lying toward the upper end of the naturally occurring range, perhaps as a result of genetics or inhalational exposure history. In other words, their asthma is a form of plain bad luck that manifests when they become obese. We tested this idea using a computational model of an elastic airway embedded in elastic parenchyma to simulate how airway resistance increases after bronchial challenge for a range of randomly chosen values of airway wall compliance (52). With appropriate choices for the mean and standard deviation of the parameter that controls airway wall stiffness, this model predicts a relationship between airway responsiveness and body mass index similar to that reported in obese populations (Figure 3).
Figure 3.
Percentage of the population that is hyperresponsive as a function of body mass index (BMI) predicted by computational modeling for a range of airway wall compliance (solid line) and a range of airway wall thicknesses (dashed line). Stars indicate the midregions of BMI for corresponding prevalence of asthma. Reproduced from Ref. 52 by permission of the American Physiological Society.
This modeling study, however, also showed that essentially the same degree of lung hyperresponsiveness can be predicted on the basis of thickened airway walls (52); thicker walls cause a geometric amplification of the amount of luminal narrowing that occurs when the ASM shortens, as discussed previously. Although there is no particular reason to suspect that individuals with LONA obese asthma have thicker airway walls than do obese individuals without asthma, the same cannot be said for those with EOA asthma, in whom lung inflammation is a key feature, especially during an exacerbation. One of the manifestations of inflammation is a physically thickened airway mucosa caused by engorgement of epithelial cells with mucus and a general up-regulation of innate immunity (33). Consequently, airway wall thickening would be expected to wax and wane over time within any given individual with EOA asthma. In particular, it might be relatively modest if allergic asthma is well controlled through the antiinflammatory effects of corticosteroids, but could become substantial during an acute inflammatory event precipitated by, for example, exposure to some environmental allergen. Furthermore, as pointed out above, airway wall thickness and ASM shortening have a strong synergistic effect on airways responsiveness that can even be fatal (34).
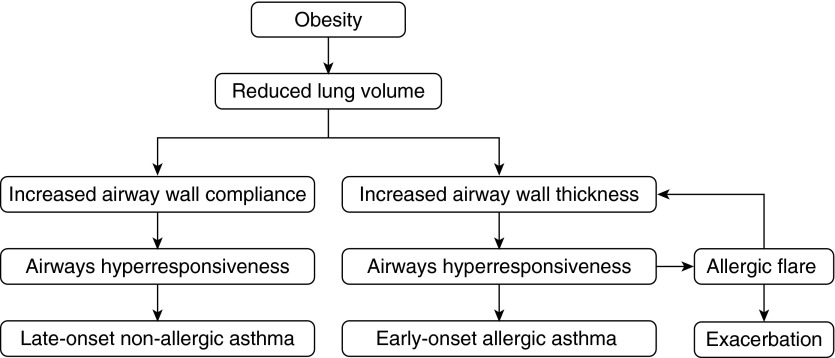
The above considerations lead to a hypothesis for the pathogenesis of obese asthma that begins with the common pathway of obesity-induced lung volume depression (Figure 4). This pathway then bifurcates to produce either LONA or EOA asthma, depending on whether the airways are more compliant or thicker, respectively, than normal. Along one arm of the bifurcation (Figure 4, right-hand side), the inflamed airway mucosa of the person with EOS asthma becomes thickened, particularly during an exacerbation, creating the risk of a severe case of status asthmaticus. Along the other arm (Figure 4, left-hand side), the unusually compliant airways of the person with LONA asthma become problematic at the decreased FRC associated with obesity.
Figure 4.
Proposed pathogenic pathways for the two principal phenotypes of obese asthma that both begin with reductions in lung volume caused by mass loading by the obese chest wall. Late-onset nonallergic obese asthma then manifests in those individuals whose primary abnormality is an airway wall that is more compliant than average (left-hand path). The early-onset allergic phenotype, in contrast, results from the synergistic interaction between inflammatory thickening of the airway wall and enhanced airway smooth muscle shortening at low lung volumes to produce the severe hyperresponsiveness characteristic of an asthma exacerbation.
Implications for Therapy
Given that reductions in lung volume are arguably a common pathway leading to both LONA and EOA obese asthma, a seemingly obvious therapeutic approach would be to impose elevations in lung volume through the application of positive pressure to the airways. The practical details of performing this during sleep are already well established because of the ubiquitous application of constant positive airway pressure for obstructive sleep apnea. Obese individuals, of course, also have a greatly increased incidence of sleep apnea. Constant positive airway pressure could thus do double duty in those individuals who also have asthma. This would offer protection against the effects of severe bronchospasm during the night and might also help mitigate or reverse any airway remodeling caused by chronic reductions in FRC, and this could prove beneficial during the day.
If the compliant airway hypothesis regarding LONA obese asthma is correct, then imposing elevations in lung volume should be equivalent to reducing body mass index to a lean value as far as lung function is concerned. The same cannot be said of those with EOA obese asthma, however, because these individuals, even when lean, still have asthma, albeit less severe, because their asthma derives from an inflammatory process driven by a response to some allergen. EOA asthma is thus still likely to require antiinflammatory therapy with its attendant side effects. Volume elevation in these individuals might conceivably aid in the inhalational distribution of antiinflammatory drugs and could offset the constrictive effects of a thickened airway wall to some degree, but is unlikely to return lung function completely to normal.
Finally, although we have deduced putative mechanistic bases for LONA and EOA obese asthma that invoke subsets of the mechanistic possibilities laid out in Figure 1, we have certainly not conclusively eliminated any of the others from contention. Our focus in this perspective has been on the biophysical mechanisms related to the balance of forces across the airway wall and the geometric features of the wall components. It must be stressed, however, that obesity may also lead to abnormalities in the ASM and/or its excitation-contraction coupling. In particular, adipose tissue has the potential to produce mediators that could conceivably act either directly or indirectly on the ASM (53) and that could have differential effects, depending on whether or not allergic inflammation is present.
Conclusions
Obesity affects the incidence and severity of both LONA and EOA asthma in different ways that suggest different pathophysiologies. The differential effects of weight loss in these two groups lead to the hypothesis that those with LONA obese asthma constitute that group of individuals unlucky enough to have airways that are more compliant than average. In contrast, the frequent exacerbations in those with EOA obese asthma potentially can be explained by inflammatory thickening of the airway wall synergizing with obesity-induced reductions in lung volume. This view of differential AHR pathogenesis provides a testable basis on which to understand LONA and EOS obese asthma as distinct diseases. It also makes one think about targeted therapeutic approaches involving imposed elevations in lung volume.
Acknowledgments
Acknowledgments
The author thanks Anne Dixon, M.D., for helpful suggestions about the manuscript.
Footnotes
This work was supported by National Institutes of Health grant R01 HL103405.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0019PS on February 24, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Quinto KB, Zuraw BL, Poon KYT, Chen W, Schatz M, Christiansen SC. The association of obesity and asthma severity and control in children. J Allergy Clin Immunol. 2011;128:964–969. doi: 10.1016/j.jaci.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Mosen DM, Schatz M, Magid DJ, Camargo CA., Jr The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol. 2008;122:507–511 e506. doi: 10.1016/j.jaci.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Vortmann M, Eisner MD. BMI and health status among adults with asthma. Obesity (Silver Spring) 2008;16:146–152. doi: 10.1038/oby.2007.7. [DOI] [PubMed] [Google Scholar]
- 4.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, Haselkorn T. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133:1549–1556. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 7.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med. 2007;101:2240–2247. doi: 10.1016/j.rmed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Camargo CA, Jr, Boulet LP, Sutherland ER, Busse WW, Yancey SW, Emmett AH, Ortega HG, Ferro TJ. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J Asthma. 2010;47:76–82. doi: 10.3109/02770900903338494. [DOI] [PubMed] [Google Scholar]
- 9.Camargo CA, Jr, Sutherland ER, Bailey W, Castro M, Yancey SW, Emmett AH, Stempel DA. Effect of increased body mass index on asthma risk, impairment and response to asthma controller therapy in African Americans. Curr Med Res Opin. 2010;26:1629–1635. doi: 10.1185/03007995.2010.483113. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178:682–687. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedón JC Childhood Asthma Management Program Research Group. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127:741–749. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh KH, Skowronski ME, Coreno AJ, Seitz RE, Villalba KD, Dickey-White H, McFadden ER. Impact of obesity on the severity and therapeutic responsiveness of acute episodes of asthma. J Asthma. 2011;48:546–552. doi: 10.3109/02770903.2011.581733. [DOI] [PubMed] [Google Scholar]
- 13.Anderson WJ, Lipworth BJ. Does body mass index influence responsiveness to inhaled corticosteroids in persistent asthma? Ann Allergy Asthma Immunol. 2012;108:237–242. doi: 10.1016/j.anai.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Telenga ED, Tideman SW, Kerstjens HA, Hacken NH, Timens W, Postma DS, van den Berge M. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy. 2012;67:1060–1068. doi: 10.1111/j.1398-9995.2012.02855.x. [DOI] [PubMed] [Google Scholar]
- 15.Mead J. Problems in interpreting common tests of pulmonary mechanical function. In: Macklem PT, Permutt S, editors. The lung in transition between health and disease. New York, NY: Marcell Dekker; 1979. pp. 43–51. [Google Scholar]
- 16.Irvin CG, Bates JH. Physiologic dysfunction of the asthmatic lung: what’s going on down there, anyway? Proc Am Thorac Soc. 2009;6:306–311. doi: 10.1513/pats.200808-091RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bates JH, Rincon M, Irvin CG. Animal models of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;297:L401–L410. doi: 10.1152/ajplung.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bates JHT. Lung mechanics. An inverse modeling approach. Cambridge, NY: Cambridge University Press; 2009. [Google Scholar]
- 19.Moreno RH, Hogg JC, Paré PD. Mechanics of airway narrowing. Am Rev Respir Dis. 1986;133:1171–1180. doi: 10.1164/arrd.1986.133.6.1171. [DOI] [PubMed] [Google Scholar]
- 20.Brusasco V, Pellegrino R. Complexity of factors modulating airway narrowing in vivo: relevance to assessment of airway hyperresponsiveness. J Appl Physiol (1985) 2003;95:1305–1313. doi: 10.1152/japplphysiol.00001.2003. [DOI] [PubMed] [Google Scholar]
- 21.Bates JH, Maksym GN. Mechanical determinants of airways hyperresponsiveness. Crit Rev Biomed Eng. 2011;39:281–296. doi: 10.1615/critrevbiomedeng.v39.i4.30. [DOI] [PubMed] [Google Scholar]
- 22.Paré PD, McParland BE, Seow CY. Structural basis for exaggerated airway narrowing. Can J Physiol Pharmacol. 2007;85:653–658. doi: 10.1139/Y07-051. [DOI] [PubMed] [Google Scholar]
- 23.McParland BE, Macklem PT, Pare PD. Airway wall remodeling: friend or foe? J Appl Physiol (1985) 2003;95:426–434. doi: 10.1152/japplphysiol.00159.2003. [DOI] [PubMed] [Google Scholar]
- 24.Khan MA, Ellis R, Inman MD, Bates JH, Sanderson MJ, Janssen LJ. Influence of airway wall stiffness and parenchymal tethering on the dynamics of bronchoconstriction. Am J Physiol Lung Cell Mol Physiol. 2010;299:L98–L108. doi: 10.1152/ajplung.00011.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bates JH, Cojocaru A, Lundblad LK. Bronchodilatory effect of deep inspiration on the dynamics of bronchoconstriction in mice. J Appl Physiol (1985) 2007;103:1696–1705. doi: 10.1152/japplphysiol.00698.2007. [DOI] [PubMed] [Google Scholar]
- 26.Bates JH, Lauzon AM. Parenchymal tethering, airway wall stiffness, and the dynamics of bronchoconstriction. J Appl Physiol (1985) 2007;102:1912–1920. doi: 10.1152/japplphysiol.00980.2006. [DOI] [PubMed] [Google Scholar]
- 27.Bates JH, Lauzon AM, Dechman GS, Maksym GN, Schuessler TF. Temporal dynamics of pulmonary response to intravenous histamine in dogs: effects of dose and lung volume. J Appl Physiol (1985) 1994;76:616–626. doi: 10.1152/jappl.1994.76.2.616. [DOI] [PubMed] [Google Scholar]
- 28.Black LD, Henderson AC, Atileh H, Israel E, Ingenito EP, Lutchen KR. Relating maximum airway dilation and subsequent reconstriction to reactivity in human lungs. J Appl Physiol (1985) 2004;96:1808–1814. doi: 10.1152/japplphysiol.01170.2003. [DOI] [PubMed] [Google Scholar]
- 29.Jensen A, Atileh H, Suki B, Ingenito EP, Lutchen KR. Selected contribution: airway caliber in healthy and asthmatic subjects: effects of bronchial challenge and deep inspirations. J Appl Physiol (1985) 2001;91:506–515, discussion 504–505. doi: 10.1152/jappl.2001.91.1.506. [DOI] [PubMed] [Google Scholar]
- 30.Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol (1985) 1987;62:1324–1330. doi: 10.1152/jappl.1987.62.3.1324. [DOI] [PubMed] [Google Scholar]
- 31.Lei M, Ghezzo H, Chen MF, Eidelman DH. Airway smooth muscle orientation in intraparenchymal airways. J Appl Physiol (1985) 1997;82:70–77. doi: 10.1152/jappl.1997.82.1.70. [DOI] [PubMed] [Google Scholar]
- 32.Bates JH, Martin JG. A theoretical study of the effect of airway smooth muscle orientation on bronchoconstriction. J Appl Physiol (1985) 1990;69:995–1001. doi: 10.1152/jappl.1990.69.3.995. [DOI] [PubMed] [Google Scholar]
- 33.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol (1985) 2004;96:2019–2027. doi: 10.1152/japplphysiol.00924.2003. [DOI] [PubMed] [Google Scholar]
- 34.Bates JH, Cojocaru A, Haverkamp HC, Rinaldi LM, Irvin CG. The synergistic interactions of allergic lung inflammation and intratracheal cationic protein. Am J Respir Crit Care Med. 2008;177:261–268. doi: 10.1164/rccm.200706-832OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen GB, Leclair TR, von Reyn J, Larrabee YC, Cloutier ME, Irvin CG, Bates JH. Acid aspiration-induced airways hyperresponsiveness in mice. J Appl Physiol (1985) 2009;107:1763–1770. doi: 10.1152/japplphysiol.00572.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Affonce DA, Lutchen KR. New perspectives on the mechanical basis for airway hyperreactivity and airway hypersensitivity in asthma. J Appl Physiol (1985) 2006;101:1710–1719. doi: 10.1152/japplphysiol.00344.2006. [DOI] [PubMed] [Google Scholar]
- 37.James AL, Elliot JG, Jones RL, Carroll ML, Mauad T, Bai TR, Abramson MJ, McKay KO, Green FH. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med. 2012;185:1058–1064. doi: 10.1164/rccm.201110-1849OC. [DOI] [PubMed] [Google Scholar]
- 38.Brannan JD, Lougheed MD. Airway hyperresponsiveness in asthma: mechanisms, clinical significance, and treatment. Front Physiol. 2012;3:460. doi: 10.3389/fphys.2012.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bates JH, Wagers SS, Norton RJ, Rinaldi LM, Irvin CG. Exaggerated airway narrowing in mice treated with intratracheal cationic protein. J Appl Physiol (1985) 2006;100:500–506. doi: 10.1152/japplphysiol.01013.2005. [DOI] [PubMed] [Google Scholar]
- 40.Cojocaru A, Irvin CG, Haverkamp HC, Bates JH. Computational assessment of airway wall stiffness in vivo in allergically inflamed mouse models of asthma. J Appl Physiol (1985) 2008;104:1601–1610. doi: 10.1152/japplphysiol.01207.2007. [DOI] [PubMed] [Google Scholar]
- 41.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 42.Juel CT, Ali Z, Nilas L, Ulrik CS. Asthma and obesity: does weight loss improve asthma control? a systematic review. J Asthma Allergy. 2012;5:21–26. doi: 10.2147/JAA.S32232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shore SA. Obesity and asthma: lessons from animal models. J Appl Physiol (1985) 2007;102:516–528. doi: 10.1152/japplphysiol.00847.2006. [DOI] [PubMed] [Google Scholar]
- 44.Shore SA, Lang JE, Kasahara DI, Lu FL, Verbout NG, Si H, Williams ES, Terry RD, Lee A, Johnston RA. Pulmonary responses to subacute ozone exposure in obese vs. lean mice. J Appl Physiol (1985) 2009;107:1445–1452. doi: 10.1152/japplphysiol.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E, Jarjour NN, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127:1486–1493 e1482. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutherland ER, Goleva E, King TS, Lehman E, Stevens AD, Jackson LP, Stream AR, Fahy JV, Leung DY Asthma Clinical Research Network. Cluster analysis of obesity and asthma phenotypes. PLoS One. 2012;7:e36631. doi: 10.1371/journal.pone.0036631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pradeepan S, Garrison G, Dixon AE. Obesity in asthma: approaches to treatment. Curr Allergy Asthma Rep. 2013;13:434–442. doi: 10.1007/s11882-013-0354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515 e501-502. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chapman DG, Irvin CG, Kaminsky DA, Forgione PM, Bates JH, Dixon AE. Influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirology. 2014;19:1170–1177. doi: 10.1111/resp.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salome CM, Munoz PA, Berend N, Thorpe CW, Schachter LM, King GG. Effect of obesity on breathlessness and airway responsiveness to methacholine in non-asthmatic subjects. Int J Obes. 2008;32:502–509. doi: 10.1038/sj.ijo.0803752. [DOI] [PubMed] [Google Scholar]
- 51.Al-Alwan A, Bates JH, Chapman DG, Kaminsky DA, DeSarno MJ, Irvin CG, Dixon AE. The nonallergic asthma of obesity. A matter of distal lung compliance. Am J Respir Crit Care Med. 2014;189:1494–1502. doi: 10.1164/rccm.201401-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bates JH, Dixon AE. Potential role of the airway wall in the asthma of obesity. J Appl Physiol (1985) 2015;118:36–41. doi: 10.1152/japplphysiol.00684.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]