Abstract
Disrupted brain connectivity might explain both the pathogenesis and consequences of late-life major depressive disorder (LLD). However, it remains difficult to ascertain whether and how specific circuits are affected. We reviewed literature regarding brain connectivity in LLD, and we specifically focused on the role of structural pathology. LLD is associated with greater levels of cerebrovascular disease, and greater levels of cerebrovascular disease are associated with both depression development and treatment responsiveness. Cerebrovascular disease is most often measured as white matter hyperintensity (WMH) burden, and histopathology studies suggest WMH reflect myelin damage and fluid accumulation (among other underlying pathology). WMHs appear as confluent caps around the ventricles (periventricular), as well as isolated lesions in the deep white matter. The underlying tissue damage and implications for brain connectivity may differ by WMH location or severity. WMHs are associated with lower white matter microstructural integrity (measured with diffusion tensor imaging) and altered brain function (measured with functional MRI). LLD is also associated with lower white matter microstructural integrity and grey matter loss which may also alter the network properties and function of the brain. Damage to brain structure reflected by WMH, reduced white matter microstructural integrity, and atrophy may affect brain function, and are therefore likely pathophysiological mechanisms of LLD. Additional research is needed to fully characterize the developmental course and pathology underlying these imaging markers, and to understand how structural damage explains LLD's various clinical manifestations.
Keywords: functional MRI, structural MRI, Diffusion tensor imaging, depression, connectivity, aging
Depression is a brain disease associated with altered reward processing, heightened response to emotional stimuli, and altered brain structure spanning multiple regions (1, 2). While early neuroimaging research focused on localizing these characteristics to specific brain regions, recent conceptual frameworks emphasize the need to understand mental disease as a result of complex, inter-connected networks (3). In recent years, neuroimaging methods for analyzing brain connectivity have grown in popularity (4). This research supports the idea that depression may be a disconnection syndrome; for example, recent diffusion tensor imaging research found depression was associated with reduced intra-frontal and frontal-subcortical white matter microstructural integrity (5).
Disrupted brain connectivity might explain both the pathogenesis and consequences of depression. The role of altered brain connectivity in depression is particularly important among older adults. Depression is associated with excess disability (6), and in late-life major depressive disorder (LLD), this risk may be potentiated by age-associated changes to brain structure (7-9). Indeed, LLD increases risk for dementia (10, 11) and mortality (12); among patients with LLD, the severity of brain structural pathology predicts mortality risk (13). Given these consequences, and the fact that the global population is rapidly aging (14), researchers and mental health practitioners should understand and work to advance current knowledge regarding how brain connectivity is altered in LLD.
Research conceptualizing LLD as a disconnection syndrome dates back, at least, to the vascular depression hypothesis (15, 16). The vascular depression hypothesis, which remains of central importance to understanding brain connectivity in LLD, states that “cerebrovascular disease may predispose, precipitate, or perpetuate some geriatric depressive syndromes” (15). This review provides background information on the vascular depression hypothesis then reviews current research regarding how brain connectivity is disrupted in LLD. We discuss research on structural connectivity in LLD, how variability in brain structure relates to brain function, and whether depression is characterized by local or diffuse connectivity disruption.
The vascular depression hypothesis
Early research found that white matter hyperintensities (WMH; identified on T2-weighted or T2-weighted fluid attenuated inversion recovery MRI) are more common among older adults with depression (17-20). Other early research found greater age is associated with more hyperintensities, and that hyperintensities are related to worse treatment outcomes (21). Compared with non-vascular depression, vascular depression is associated with greater levels of cognitive impairment and disability (22).
Research today continues to provide evidence for and refine the initial formulation of the vascular depression hypothesis, which included prominent roles of both WMHs and neuropsychological dysfunction. A recent systematic review confirmed that, indeed, compared with healthy controls, WMHs are more common among patients with LLD (23). Further, one large clinical study (n=217) found WMH severity and performance on several cognitive tests predicted depression severity over a course of pharmacotherapy treatment for LLD (24); this study also found that clinical vascular factors were correlated with both neuropsychological and imaging-related vascular measures. The internal validity of vascular depression is supported by research demonstrating deep white matter hyperintensities (when compared with neuropsychological and clinical measures) were best at distinguishing vascular and non-vascular sub-groups in two independent samples of LLD patients (25).
Importantly, recent longitudinal research now demonstrates that WMH progression (increases in WMH burden over time) predicts worse depression outcomes among older adults in both community (26, 27) and clinical settings (28, 29). In individuals with depression, WMH progression has been linked with the development of dementia (30). These findings clearly support the clinical relevance of the vascular depression hypothesis, and suggest cerebrovascular disease (as indicated by WMHs) is involved in the pathogenesis, persistence, and consequences of LLD. These observations provide strong motivation to further investigate the pathophysiological nature of WMHs and how they disrupt brain connectivity in depression.
The pathophysiology and development of WMHs
Post-mortem studies are a rich source of information regarding the pathophysiology of MR identified WMHs. Early research found that WMHs indicate gliosis and demyelination (31), however the pathology of WMHs has been long recognized to vary by lesion type (32). More recently, astrogliosis and oligodendrocytes loss was observed in periventricular but not subcortical WMHs, whereas regardless of location, small vessel loss, myelin damage, and vaculoation (allowing for fluid accumulation) contributes to white matter degradation in WMHs (33); these findings suggest the pathophysiology of periventricular and subcortical WMHs may differ, and that myelin damage may occur in the absence of (or perhaps before) oligodendrocytes loss.
A recent post-mortem study of confirmed dementia cases found WMH pathology was consistent with microinfarcts, except for in juxtacortical lesion, which were enlarged perivascular spaces (34). Other research suggests ischemic, rather than small vessel disease, has a prominent role in WMH pathophysiology (35). These pathophysiological studies provide strong evidence that WMHs broadly reflect the impact of cerebrovascular disease and related processes on the brain. Note that post-mortem studies are limited by selection bias (e.g. over-representation of end-stage disease). Given that WMH pathology and etiology is likely heterogeneous, or at least multifactorial with variability by stage or severity, there is a need for in vivo studies of WMH etiology.
Several cross-sectional imaging studies have investigated the clinical correlates of WMHs. High blood pressure (36, 37) and arterial stiffness (38, 39) are among the best recognized correlates of white matter pathology. In a study of hypertensive patients with controlled blood pressure, estimated glomerular filtration rate was associated with WMH burden (40). Other factors related to vascular disease, for example inflammatory cytokines (41), may also be related to brain structural pathology. Inflammatory cytokine levels correlate with WMHs (42-44). One study among older adults with depression found peripheral markers of immune-inflammatory control, lipid metabolism, clotting process and vascular reactivity (among others) correlated with WMHs (45). Brain derived neurotrophic factor and vascular endothelial growth factor are related to future vascular brain injury among older adults (46). Elevated cortisol levels during stress may relate to white matter pathology (47). Other factors including anemia (48) and salt (49), calcium, and vitamin D (50) intake also correlate with WMHs.
Longitudinal imaging studies are needed to clarify which of these factors are involved in the etiology of WMHs, and which of these factors are markers of prevalent structural pathology. Available longitudinal evidence has mostly focused on blood pressure, and demonstrates that high blood pressure (51), and in particular high ambulatory blood pressure (52, 53) predicts WMH accumulation over time. Given the clinical importance of WMH (discussed above), there remains a need for more longitudinal research to precisely characterize the complex and likely multifactorial determinants of WMHs. Comprehensive research regarding the determinants of WMH development and progression could clarify when and how to intervene in order to prevent the consequences WMH on brain connectivity (discussed below).
Relations between WMHs and brain connectivity
Cerebrovascular disease has long been thought to disrupt brain connectivity in depression, for example an early study demonstrated that depressed patients with co-occurring clinical vascular disease had reduced auditory transmission at the pons (54). Diffusion tensor imaging (DTI), which measures the degree of anisotropic diffusion of water as a proxy of microstructural integrity, now provides in vivo confirmation of a relation between WMHs and white matter structure. This research has shown that WMHs are associated with reduced white matter microstructural integrity (55, 56). In fact, clinical atherosclerotic disease itself is associated with reduced white matter microstructural integrity (57, 58) Therefore, vascular disease and WMH are associated with white matter structure.
These structural changes have observable relations to brain function. Multi-modal imaging research has shown that “functional connectivity reflects structural connectivity” (59) (for a review, see (60)). Several recent studies have begun characterizing how WMHs affect cerebral blood flow and brain function: regions with WMH show reduced blood flow (61); periventricular and deep WMHs are associated with a decline in cerebral blood flow over a period of about 4 years (62). While these studies demonstrate a prospective association between WMH and future blood flow in the brain, it will also be important for future research to evaluate how reduced cerebral blood flow may impact white matter health over time (63) (consistent with the potential ischemic pathophysiology of WMH discussed above).
In LLD, prevalent WMHs are associated with altered functional MRI signal (64), including abnormal default-mode network connectivity (65) and more pronounced functional activation in response to an affective-reactivity task (66). White matter microstructural integrity (expressed as fractional anisotropy measured with DTI) also relates to resting state functional connectivity among older adults with depression (67). Altogether, WMHs are related to altered white matter microstructural integrity, and both WMHs and white matter microstructural integrity relate to brain function.
The relevance of WMH extent and localization
Some research has examined the spatial distribution of WMHs related to depression. WMHs are more common/extensive among older adults with depression, and increases in WMH severity may result in more of the brain being affected. For example, in older adults without depression, WMHs may be limited to bilateral parietal-temporal regions, whereas older adults with depression tend to have WMHs extending into frontal regions (68, 69). Compared with non-depressed control participants, depression is associated with greater WMHs in several tracts relevant to cognitive and affective functions (70). More extensive WMHs observed in depressed patients may reflect more severe/advanced stage cerebrovascular disease, or alternatively, an amplified impact of cerebrovascular disease on brain health due to other reasons (e.g. stress related neurotoxicity affecting cell bodies (71)).
WMHs localized to specific tracts (e.g. those involved in social, cognitive, and affective processing) may be particularly clinically and mechanistically relevant. For example, some studies have found that periventricular WMHs predict cognitive decline over time, whereas sub-cortical WMHs do not (72, 73). In a meta-analysis, deep but not periventricular WMH correlated with depression (74). However interpreting these findings requires considering other evidence which demonstrates that periventricular, deep brain, and overall WMH are all highly correlated, and that an arbitrary, categorical distinction between periventricular and deep brain WMHs is not empirically supported (75); this study suggests WMHs extended smoothly out from the ventricles as a function of increasing overall burden. Consistent with these observations, a recent population-based study demonstrated that depression was associated with WMH accumulation around the ventricles (76), and another recent study found the ratio of WMH to non-WMH tissue differed between depressed patients and controls only in the upper cingulum (77).
These findings suggest WMHs tend to accumulate and affect tracts near ventricles. Nevertheless, the localization of WMHs may still have great clinical and mechanistic relevance. The impact of WMH localization on depression and its consequences might depend on differences in the underlying pathology that WMHs indicate (see above, e.g. whether oligodendrocyte loss occurs). Alternatively, differences in localization may simply reflect the overall the reach/severity with which WMHs affect key white matter tracts. In either case, it appears that a critical level of damage to a tract, or set of tracts, may affect brain networks and confer LLD. However, there remains a need to investigate how the spatial patterns WMH accumulation and their underlying pathology (e.g. using high-field imaging) relate to the incidence and manifestations of LLD.
Tract specific and whole-brain white matter microstructural integrity in LLD
A recent meta-analysis found, compared with healthy control participants, patients with LLD had lower fractional anisotropy (FA) across multiple regions and that the largest effects were observed in the frontal lobe, uncinate fasciculus, and cingulum (78). Voxel-based research similarly suggests LLD is associated with lower FA across multiple white matter tracts including those implicated in cognitive and affective processing (76, 79). Network based analysis of DTI data suggests LLD is associated with reduced connectivity of temporal regions (80). Altogether, these findings support a conceptualization of LLD as a disease of reduced structural brain connectivity. It does not appear variability in the microstructural integrity of a single tract is a completely accurate predictor of LLD, instead, evidence suggests LLD involves altered structural network properties and white matter damage in key regions important to cognitive and affective processing. To date, there remains a need for research to elucidate how variability in white matter structures and structural network properties impact functional connectivity patterns, and in particular, how these connectivity changes precipitate the emergence of LLD's specific clinical manifestations (e.g. altered reward processing or low mood). If specific structural changes can be linked to both alterations in functional circuitry and the specific manifestations of LLD, this might suggest that these particular circuits are generally relevant to mood and clinical depression (e.g. potentially in the absence of overt, pronounced structural pathology, as in depression among younger adults).
Grey matter structure and network organization
LLD has been associated with smaller grey matter volume across numerous brain regions (81). One study found depression was associated with lower levels of grey matter volume across multiple regions, but most significantly in the insula and anterior cingulate cortex (76). Cortical thickness may also differ between LLD patients and controls; for example, lower cortical thickness has been noted among LLD patients in right frontal, parietal, and temporal brain regions (82). However, other studies have failed to find differences in grey matter volume between depressed patients and healthy controls (79), and the regions implicated across studies are not always consistent.
A recent meta-analysis utilizing data from region of interest studies found that LLD patients (compared with healthy controls) had lower grey matter volume in the hippocampus, orbitofrontal cortex, putamen, and thalamus (83). A subsequent meta-analysis argued that a voxel-based approach provides a more comprehensive strategy to identify the grey matter correlates of depression that is not biased by the need to pre-specify regions of interest (84); this study found, compared with healthy controls, LLD patients had lower grey matter volume in the hippocampus, parahippocampus, lentiform nucleus, amygdala, medial frontal gyrus, and right subcallosal gyrus, but had significantly larger right lingual gyrus volumes. Discrepancies between the results of these studies may reflect differences in the analytic approach (e.g. region of interest vs. voxel-based approaches) or sampling characteristics (e.g. including clinical and physical health characteristics of the LLD or control group).
Nevertheless, current research generally agrees that LLD is associated with widespread loss of grey matter that may be more pronounced in regions important for cognitive and emotional processing. A recent application of network-based analysis techniques suggests LLD is associated with different regional (85) and global grey matter network properties (86). These findings suggest grey matter changes are relevant to understanding how brain connectivity is altered in LLD. However, the current multi-modal evidence-base is limited, and there remains a need to further investigate how grey matter alterations (potentially together with white matter changes) relate to structural network properties, brain function, and the incidence of depression.
Conclusions and future directions
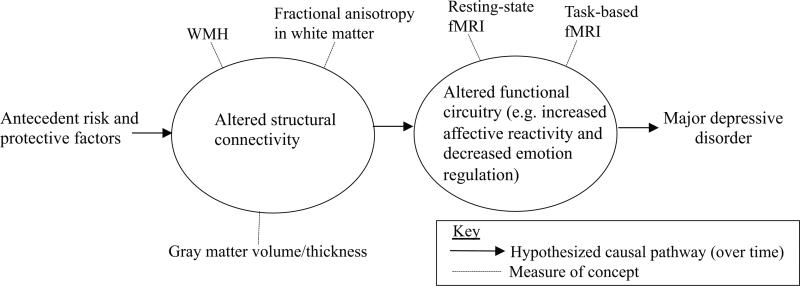
The enduring impact of the vascular depression hypothesis on the field speaks to the very powerful relationships between cerebrovascular disease, brain structure, and depression among older adults. Several studies suggest the etiology of WMHs involves vascular disease factors including blood pressure, vascular stiffness, and immunological markers (36-39, 42-45). Longitudinal evidence from a variety of settings demonstrates that WMH, and their accrual, predicts future depression (26-29). Multi-modal imaging research has confirmed that WMHs are associated with worse white matter microstructural integrity (55-57). Further, brain structural pathology is associated with altered brain function in LLD (64-66). This literature supports a conceptualization of LLD as a disconnection syndrome (Figure), wherein several physical health factors precede pathological changes to brain structural connectivity, which affects brain function, and leads to the clinical manifestations of LLD.
Figure. Conceptualization of late-life major depressive disorder as a disconnection syndrome.
Over time, risk and protective factors potentially affect structural connectivity, which affects function circuitry, leading to major depressive disorder.
Despite these exciting advancements connecting levels of analyses using multimodal imaging, substantial gaps in our understanding remain. In particular, there is a need for more longitudinal clinical neuroscience research to answer questions regarding the etiology, preventable risk factors, and course of brain connectivity changes in LLD. The model we present (Figure) is designed to summarize current knowledge and concepts that are plausible but mostly based on cross-sectional research. Longitudinal research is needed to determine which specific factors are markers of LLD rather than etiological factors, and also to clarify whether any specific factors are bi-directionally related over time (e.g. WMH and fractional anisotrophy, or reduced blood flow and WMH). Therefore, comprehensive prospective research is required to advance our understanding of how brain connectivity changes relate to the pathogenesis of LLD. In particular, there is a need to mechanistically understand how WMHs develop, what they mean pathologically, and how they relate to other structural pathology, altered brain function, and the specific clinical manifestations of LLD.
For example, current longitudinal research demonstrates high blood pressure predicts WMH accrual over time (51-53). However, the etiology of WMH development (as well grey matter and white matter microstructural integrity loss) is likely multi-factorial. Additional longitudinal research is needed to clarify whether several known correlates of WMH (e.g. cytokines and growth factors (42-45)) are in fact determinants of changes to brain connectivity. Multi-modal studies are needed to address whether/how the determinants of change to multiple aspects of brain structure differ, and how changes to different aspects of brain structure interrelate. In addition, most research examining relations between brain structure and function in LLD has focused on the role of WMHs (64-66). Future research is needed to investigate how multiple structural characteristics (including measures of grey matter, white matter microstructural integrity, and network properties) together affect functional connectivity.
More basically, future research is also needed to clarify what differences in stage or severity of WMH progression reflects about the underlying pathology. New advances in highfield imaging may help clarify the pathophysiology and course of WMHs in LLD, which would also help clarify their causes and consequences. Research is needed to determine whether WMH or fractional anisotropy changes found in depression are similar across the implicated regions in terms of their underlying pathology, consequences on brain function, and clinical relevance. Future neuroimaging studies may reveal nuisances in the level or nature of apparent structural damage across tracts (or sets of tracts) that have specific implications for the functional circuitry and clinical presentation of LLD.
Because the manifestations of depression are heterogeneous, research should test whether certain structural and functional connectivity alterations relate to specific aspects of depression's clinical presentation. The original report on the vascular depression hypothesis (15) suggested, compared with non-vascular depression, patients with vascular depression had greater executive function impairment, psychomotor retardation, lack of insight, and disability. Imaging research is now poised to clarify the pathogenic mechanisms and underlying neurobiological substrates of these clinical manifestations. For example, does damage to a particular set of white matter tracts affect brain function to cause apathy, while another set of white matter and functional alterations underlies excessive sadness? Although not reviewed in the present work, research has begun characterizing the functional connectivity of mood disturbances among older adults (87-90). Future multimodal imaging studies are needed to conclusively isolate the how brain structural changes relate to functional connectivity changes of known clinical relevance. Enhancing our knowledge of the risk cascades leading to altered brain connectivity in LLD will greatly improve our ability to identify those at risk and target novel interventions to prevent LLD and its consequences.
Acknowledgements
SFS is supported by T32MH019986. This work was also supported by the National Institute of Mental Health through a research grant (R01MH076079) and the Advanced Center in Intervention and Services Research in Late-life Depression Prevention (P30MH09033), and by the National Institute on Aging through the University of Pittsburgh Alzheimer's Disease Research Center (P50AG005133) and the Pittsburgh Pepper Center (P30AG024827).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annual review of psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 2.Treadway MT, Pizzagalli DA. Imaging the pathophysiology of major depressive disorder - from localist models to circuit-based analysis. Biology of mood & anxiety disorders. 2014;4:5. doi: 10.1186/2045-5380-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sporns O. The human connectome: a complex network. Annals of the New York Academy of Sciences. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- 4.Friston KJ. Functional and effective connectivity: a review. Brain connectivity. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- 5.Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. Journal of psychiatry & neuroscience : JPN. 2013;38:49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 7.Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR American journal of neuroradiology. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- 8.Awad IA, Spetzler RF, Hodak JA, Awad CA, Carey R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke; a journal of cerebral circulation. 1986;17:1084–1089. doi: 10.1161/01.str.17.6.1084. [DOI] [PubMed] [Google Scholar]
- 9.Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Kawashima R, et al. Correlations among Brain Gray Matter Volumes, Age, Gender, and Hemisphere in Healthy Individuals. PloS one. 2011;6:e22734. doi: 10.1371/journal.pone.0022734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., 3rd Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. The British journal of psychiatry : the journal of mental science. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y, Huang C, Zhao K, Ma L, Qiu X, Zhang L, et al. Depression as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. International journal of geriatric psychiatry. 2013;28:441–449. doi: 10.1002/gps.3845. [DOI] [PubMed] [Google Scholar]
- 12.Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. Journal of affective disorders. 2002;72:227–236. doi: 10.1016/s0165-0327(01)00413-x. [DOI] [PubMed] [Google Scholar]
- 13.Levy RM, Steffens DC, McQuoid DR, Provenzale JM, MacFall JR, Krishnan KR. MRI lesion severity and mortality in geriatric depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2003;11:678–682. doi: 10.1176/appi.ajgp.11.6.678. [DOI] [PubMed] [Google Scholar]
- 14.Cauley J. The Demography of Aging. In: Newman AC J, editor. The Epidemiology of Aging. Springer; 2013. pp. 3–14. [Google Scholar]
- 15.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Archives of general psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 16.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Molecular psychiatry. 2013 doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan KR, Goli V, Ellinwood EH, France RD, Blazer DG, Nemeroff CB. Leukoencephalopathy in patients diagnosed as major depressive. Biological psychiatry. 1988;23:519–522. doi: 10.1016/0006-3223(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 18.Coffey CE, Figiel GS, Djang WT, Cress M, Saunders WB, Weiner RD. Leukoencephalopathy in elderly depressed patients referred for ECT. Biological psychiatry. 1988;24:143–161. doi: 10.1016/0006-3223(88)90270-3. [DOI] [PubMed] [Google Scholar]
- 19.Coffey CE, Figiel GS, Djang WT, Saunders WB, Weiner RD. White matter hyperintensity on magnetic resonance imaging: clinical and neuroanatomic correlates in the depressed elderly. The Journal of neuropsychiatry and clinical neurosciences. 1989;1:135–144. doi: 10.1176/jnp.1.2.135. [DOI] [PubMed] [Google Scholar]
- 20.Dolan RJ, Poynton AM, Bridges PK, Trimble MR. Altered magnetic resonance white-matter T1 values in patients with affective disorder. The British journal of psychiatry: the journal of mental science. 1990;157:107–110. doi: 10.1192/bjp.157.1.107. [DOI] [PubMed] [Google Scholar]
- 21.Hickie I, Scott E, Mitchell P, Wilhelm K, Austin MP, Bennett B. Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression. Biological psychiatry. 1995;37:151–160. doi: 10.1016/0006-3223(94)00174-2. [DOI] [PubMed] [Google Scholar]
- 22.Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Silbersweig D, Charlson M. Clinically defined vascular depression. American Journal of Psychiatry. 1997;154:562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- 23.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. Journal of neurology, neurosurgery, and psychiatry. 2008;79:619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 24.Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Archives of general psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sneed JR, Rindskopf D, Steffens DC, Krishnan KR, Roose SP. The vascular depression subtype: evidence of internal validity. Biological psychiatry. 2008;64:491–497. doi: 10.1016/j.biopsych.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Firbank MJ, Teodorczuk A, van der Flier WM, Gouw AA, Wallin A, Erkinjuntti T, et al. Relationship between progression of brain white matter changes and late-life depression: 3-year results from the LADIS study. The British Journal of Psychiatry. 2012;201:40–45. doi: 10.1192/bjp.bp.111.098897. [DOI] [PubMed] [Google Scholar]
- 27.Teodorczuk A, O'Brien JT, Firbank MJ, Pantoni L, Poggesi A, Erkinjuntti T, et al. White matter changes and late-life depressive symptoms: longitudinal study. The British journal of psychiatry : the journal of mental science. 2007;191:212–217. doi: 10.1192/bjp.bp.107.036756. [DOI] [PubMed] [Google Scholar]
- 28.Taylor WD, Steffens DC, MacFall JR, McQuoid DR, Payne ME, Provenzale JM, et al. White matter hyperintensity progression and late-life depression outcomes. Archives of general psychiatry. 2003;60:1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- 29.Khalaf A, Edelman K, Tudorascu D, Andreescu C, Reynolds CF, Aizenstein H. White Matter Hyperintensity Accumulation During Treatment of Late-Life Depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steffens DC, Potter GG, McQuoid DR, MacFall JR, Payne ME, Burke JR, et al. Longitudinal magnetic resonance imaging vascular changes, apolipoprotein E genotype, and development of dementia in the neurocognitive outcomes of depression in the elderly study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2007;15:839–849. doi: 10.1097/JGP.0b013e318048a1a0. [DOI] [PubMed] [Google Scholar]
- 31.Braffman BH, Zimmerman RA, Trojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer WW. Brain MR: pathologic correlation with gross and histopathology. 2. Hyperintense white-matter foci in the elderly. AJR American journal of roentgenology. 1988;151:559–566. doi: 10.2214/ajr.151.3.559. [DOI] [PubMed] [Google Scholar]
- 32.Chimowitz MI, Estes ML, Furlan AJ, Awad IA. Further observations on the pathology of subcortical lesions identified on magnetic resonance imaging. Archives of neurology. 1992;49:747–752. doi: 10.1001/archneur.1992.00530310095018. [DOI] [PubMed] [Google Scholar]
- 33.Murray ME, Vemuri P, Preboske GM, Murphy MC, Schweitzer KJ, Parisi JE, et al. A Quantitative Postmortem MRI Design Sensitive to White Matter Hyperintensity Differences and their Relationship with Underlying Pathology. Journal of neuropathology and experimental neurology. 2012;71:1113–1122. doi: 10.1097/NEN.0b013e318277387e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Veluw SJ, Zwanenburg JJ, Rozemuller AJ, Luijten PR, Spliet WG, Biessels GJ. The spectrum of MR detectable cortical microinfarcts: a classification study with 7-tesla postmortem MRI and histopathology. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35:676–683. doi: 10.1038/jcbfm.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas AJ, Perry R, Barber R, Kalaria RN, O'Brien JT. Pathologies and pathological mechanisms for white matter hyperintensities in depression. Annals of the New York Academy of Sciences. 2002;977:333–339. doi: 10.1111/j.1749-6632.2002.tb04835.x. [DOI] [PubMed] [Google Scholar]
- 36.Basile AM, Pantoni L, Pracucci G, Asplund K, Chabriat H, Erkinjuntti T, et al. Age, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes. The LADIS (Leukoaraiosis and Disability in the Elderly) Study. Cerebrovascular diseases (Basel, Switzerland) 2006;21:315–322. doi: 10.1159/000091536. [DOI] [PubMed] [Google Scholar]
- 37.Longstreth WT, Jr., Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke; a journal of cerebral circulation. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 38.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, DeCarli C, Au R, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–991. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarumi T, Ayaz Khan M, Liu J, Tseng BM, Parker R, Riley J, et al. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. Journal of Cerebral Blood Flow & Metabolism. 2014;34:971–978. doi: 10.1038/jcbfm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takami T, Yamano S, Okada S, Sakuma M, Morimoto T, Hashimoto H, et al. Major risk factors for the appearance of white-matter lesions on MRI in hypertensive patients with controlled blood pressure. Vascular Health and Risk Management. 2012;8:169–176. doi: 10.2147/VHRM.S30507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. International journal of geriatric psychiatry. 2011 doi: 10.1002/gps.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, et al. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology. 2015;84:825–832. doi: 10.1212/WNL.0000000000001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fornage M, Chiang YA, O'Meara ES, Psaty BM, Reiner AP, Siscovick DS, et al. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: the Cardiovascular Health Study. Stroke; a journal of cerebral circulation. 2008;39:1952–1959. doi: 10.1161/STROKEAHA.107.508135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 2012;78:720–727. doi: 10.1212/WNL.0b013e318248e50f. [DOI] [PubMed] [Google Scholar]
- 45.Diniz BS, Sibille E, Ding Y, Tseng G, Aizenstein HJ, Lotrich F, et al. Plasma biosignature and brain pathology related to persistent cognitive impairment in late-life depression. Mol Psychiatry. 2015;20:594–601. doi: 10.1038/mp.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pikula A, Beiser AS, Chen TC, Preis SR, Vorgias D, DeCarli C, et al. Serum brain-derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury: Framingham Study. Stroke; a journal of cerebral circulation. 2013;44:2768–2775. doi: 10.1161/STROKEAHA.113.001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox SR, Bastin ME, Ferguson KJ, Maniega SM, MacPherson SE, Deary IJ, et al. Brain white matter integrity and cortisol in older men: the Lothian Birth Cohort 1936. Neurobiology of aging. 2015;36:257–264. doi: 10.1016/j.neurobiolaging.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inzitari M, Studenski S, Rosano C, Zakai NA, Longstreth WT, Cushman M, et al. Anemia is associated with the progression of white matter disease in older adults with high blood pressure: the Cardiovascular Health Study. Journal of the American Geriatrics Society. 2008;56:1867–1872. doi: 10.1111/j.1532-5415.2008.01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heye AK, Thrippleton MJ, Chappell FM, Valdes Hernandez MdC, Armitage PA, Makin SD, et al. Blood pressure and sodium: association with MRI markers in cerebral small vessel disease. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 doi: 10.1038/jcbfm.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payne ME, Anderson JJB, Steffens DC. Calcium and vitamin D intakes may be positively associated with brain lesions in depressed and non-depressed elders. Nutrition research (New York, NY) 2008;28:285–292. doi: 10.1016/j.nutres.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allan CL, Zsoldos E, Filippini N, Sexton CE, Topiwala A, Valkanova V, et al. Lifetime hypertension as a predictor of brain structure in older adults: cohort study with a 28-year follow-up. The British journal of psychiatry : the journal of mental science. 2015;206:308–315. doi: 10.1192/bjp.bp.114.153536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolfson L, Wakefield DB, Moscufo N, Kaplan RF, Hall CB, Schmidt JA, et al. Rapid Buildup of Brain White Matter Hyperintensities Over 4 Years Linked to Ambulatory Blood Pressure, Mobility, Cognition, and Depression in Old Persons. The journals of gerontology Series A, Biological sciences and medical sciences. 2013 doi: 10.1093/gerona/glt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White WB, Wolfson L, Wakefield DB, Hall CB, Campbell P, Moscufo N, et al. Average Daily Blood Pressure, not Office Blood Pressure, is Associated with Progression of Cerebrovascular Disease and Cognitive Decline in Older People. Circulation. 2011;124:2312–2319. doi: 10.1161/CIRCULATIONAHA.111.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalayam B, Alexopoulos GS, Musiek FE, Kakuma T, Toro A, Silbersweig D, et al. Brainstem evoked response abnormalities in late-life depression with vascular disease. The American journal of psychiatry. 1997;154:970–975. doi: 10.1176/ajp.154.7.970. [DOI] [PubMed] [Google Scholar]
- 55.Taylor WD, Bae JN, MacFall JR, Payne ME, Provenzale JM, Steffens DC, et al. Widespread effects of hyperintense lesions on cerebral white matter structure. AJR American journal of roentgenology. 2007;188:1695–1704. doi: 10.2214/AJR.06.1163. [DOI] [PubMed] [Google Scholar]
- 56.Taylor WD, Payne ME, Krishnan KR, Wagner HR, Provenzale JM, Steffens DC, et al. Evidence of white matter tract disruption in MRI hyperintensities. Biological psychiatry. 2001;50:179–183. doi: 10.1016/s0006-3223(01)01160-x. [DOI] [PubMed] [Google Scholar]
- 57.Rowe Bijanki K, Arndt S, Magnotta VA, Nopoulos P, Paradiso S, Matsui JT, et al. Characterizing white matter health and organization in atherosclerotic vascular disease: a diffusion tensor imaging study. Psychiatry research. 2013;214:389–394. doi: 10.1016/j.pscychresns.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoptman MJ, Gunning-Dixon FM, Murphy CF, Ardekani BA, Hrabe J, Lim KO, et al. Blood pressure and white matter integrity in geriatric depression. Journal of affective disorders. 2009;115:171. doi: 10.1016/j.jad.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cerebral Cortex (New York, NY) 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain structure & function. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- 61.Brickman AM, Zahra A, Muraskin J, Steffener J, Holland CM, Habeck C, et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry research. 2009;172:117–120. doi: 10.1016/j.pscychresns.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Veen PH, Muller M, Vincken KL, Hendrikse J, Mali WP, van der Graaf Y, et al. Longitudinal relationship between cerebral small-vessel disease and cerebral blood flow: the second manifestations of arterial disease-magnetic resonance study. Stroke; a journal of cerebral circulation. 2015;46:1233–1238. doi: 10.1161/STROKEAHA.114.008030. [DOI] [PubMed] [Google Scholar]
- 63.Chen JJ, Rosas HD, Salat DH. The Relationship between Cortical Blood Flow and Sub-Cortical White-Matter Health across the Adult Age Span. PloS one. 2013;8:e56733. doi: 10.1371/journal.pone.0056733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patel MJ, Boada FE, Price JC, Sheu LK, Tudorascu DL, Reynolds CF, et al. Association of small vessel ischemic white matter changes with BOLD fMRI imaging in the elderly. Psychiatry research. 2012;204:117–122. doi: 10.1016/j.pscychresns.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF, 3rd, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry research. 2011;194:39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aizenstein HJ, Andreescu C, Edelman KL, Cochran JL, Price J, Butters MA, et al. fMRI correlates of white matter hyperintensities in late-life depression. The American journal of psychiatry. 2011;168:1075–1082. doi: 10.1176/appi.ajp.2011.10060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tadayonnejad R, Yang S, Kumar A, Ajilore O. Multimodal Brain Connectivity Analysis in Unmedicated Late-Life Depression. PloS one. 2014;9:e96033. doi: 10.1371/journal.pone.0096033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor WD, MacFall JR, Steffens DC, Payne ME, Provenzale JM, Krishnan KRR. Localization of age-associated white matter hyperintensities in late-life depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:539–544. doi: 10.1016/S0278-5846(02)00358-5. [DOI] [PubMed] [Google Scholar]
- 69.Greenwald BS, Kramer-Ginsberg E, Krishnan KR, Ashtari M, Auerbach C, Patel M. Neuroanatomic localization of magnetic resonance imaging signal hyperintensities in geriatric depression. Stroke; a journal of cerebral circulation. 1998;29:613–617. doi: 10.1161/01.str.29.3.613. [DOI] [PubMed] [Google Scholar]
- 70.Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. The American journal of psychiatry. 2008;165:524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conrad CD. Chronic Stress-Induced Hippocampal Vulnerability: The Glucocorticoid Vulnerability Hypothesis. Reviews in the neurosciences. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Groot JC, De Leeuw FE, Oudkerk M, Van Gijn J, Hofman A, Jolles J, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Annals of neurology. 2002;52:335–341. doi: 10.1002/ana.10294. [DOI] [PubMed] [Google Scholar]
- 73.van den Heuvel DM, ten Dam VH, de Craen AJ, Admiraal-Behloul F, Olofsen H, Bollen EL, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. Journal of neurology, neurosurgery, and psychiatry. 2006;77:149–153. doi: 10.1136/jnnp.2005.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L, Leonards CO, Sterzer P, Ebinger M. White matter lesions and depression: a systematic review and meta-analysis. Journal of psychiatric research. 2014;56:56–64. doi: 10.1016/j.jpsychires.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 75.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical Mapping of White Matter Hyperintensities (WMH): Exploring the Relationships Between Periventricular WMH, Deep WMH, and Total WMH Burden. Stroke; a journal of cerebral circulation. 2005;36 doi: 10.1161/01.STR.0000150668.58689.f2. 10.1161/1101.STR.0000150668.0000158689.f0000150662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tudorascu DL, Rosano C, Venkatraman VK, MacCloud RL, Harris T, Yaffe K, et al. Multimodal MRI markers support a model of small vessel ischemia for depressive symptoms in very old adults. Psychiatry research. 2014;224:73–80. doi: 10.1016/j.pscychresns.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taylor WD, Zhao Z, Ashley-Koch A, Payne ME, Steffens DC, Krishnan RR, et al. Fiber tract-specific white matter lesion severity Findings in late-life depression and by AGTR1 A1166C genotype. Human brain mapping. 2013;34:295–303. doi: 10.1002/hbm.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wen MC, Steffens DC, Chen MK, Zainal NH. Diffusion tensor imaging studies in late-life depression: systematic review and meta-analysis. International journal of geriatric psychiatry. 2014;29:1173–1184. doi: 10.1002/gps.4129. [DOI] [PubMed] [Google Scholar]
- 79.Sexton CE, Allan CL, Le Masurier M, McDermott LM, Kalu UG, Herrmann LL, et al. Magnetic resonance imaging in late-life depression: multimodal examination of network disruption. Archives of general psychiatry. 2012;69:680–689. doi: 10.1001/archgenpsychiatry.2011.1862. [DOI] [PubMed] [Google Scholar]
- 80.Charlton RA, Leow A, GadElkarim J, Zhang A, Ajilore O, Yang S, et al. Brain connectivity in late-life depression and aging revealed by network analysis. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2015;23:642–650. doi: 10.1016/j.jagp.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andreescu C, Butters MA, Begley A, Rajji T, Wu M, Meltzer CC, et al. Gray matter changes in late life depression--a structural MRI analysis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2566–2572. doi: 10.1038/sj.npp.1301655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mackin RS, Tosun D, Mueller SG, Lee JY, Insel P, Schuff N, et al. Patterns of reduced cortical thickness in late-life depression and relationship to psychotherapeutic response. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2013;21:794–802. doi: 10.1016/j.jagp.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sexton CE, Mackay CE, Ebmeier KP. A Systematic Review and Meta-Analysis of Magnetic Resonance Imaging Studies in Late-Life Depression. The American Journal of Geriatric Psychiatry. 2013;21:184195. doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 84.Du M, Liu J, Chen Z, Huang X, Li J, Kuang W, et al. Brain grey matter volume alterations in late-life depression. Journal of psychiatry & neuroscience : JPN. 2014;39:397–406. doi: 10.1503/jpn.130275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lim HK, Jung WS, Aizenstein HJ. Aberrant topographical organization in gray matter structural network in late life depression: a graph theoretical analysis. International psychogeriatrics / IPA. 2013;25:1929–1940. doi: 10.1017/S104161021300149X. [DOI] [PubMed] [Google Scholar]
- 86.Ajilore O, Lamar M, Leow A, Zhang A, Yang S, Kumar A. Graph theory analysis of cortical-subcortical networks in late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2014;22:195–206. doi: 10.1016/j.jagp.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kenny ER, O'Brien JT, Cousins DA, Richardson J, Thomas AJ, Firbank MJ, et al. Functional Connectivity in Late-Life Depression Using Resting-State Functional Magnetic Resonance Imaging. The American Journal of Geriatric Psychiatry. 2010;18:643–651. doi: 10.1097/JGP.0b013e3181cabd0e. [DOI] [PubMed] [Google Scholar]
- 88.Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup JK, Lim KO, et al. Functional connectivity in apathy of late-life depression: a preliminary study. Journal of affective disorders. 2013;149:398–405. doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bohr IJ, Kenny E, Blamire A, O'Brien JT, Thomas AJ, Richardson J, et al. Resting-state functional connectivity in late-life depression: higher global connectivity and more long distance connections. Frontiers in psychiatry. 2012;3:116. doi: 10.3389/fpsyt.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andreescu C, Tudorascu DL, Butters MA, Tamburo E, Patel M, Price J, et al. Resting state functional connectivity and treatment response in late-life depression. Psychiatry research. 2013;214 doi: 10.1016/j.pscychresns.2013.08.007. 10.1016/j.pscychresns.2013.1008.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]