Abstract
M2 macrophages are implicated in the development of pulmonary fibrosis as they generate profibrotic signals. The polarization process, at least in part, is regulated by epigenetic modulation. Because Cu,Zn–superoxide dismutase–induced H2O2 can polarize macrophages to a profibrotic M2 phenotype, we hypothesized that modulation of the redox state of the cell is involved in the epigenetic modulation of the macrophage phenotype. In this study, we show that signal transducer and activator of transcription 6 (STAT6) regulates Jumonji domain containing (Jmjd) 3, a histone H3 lysine 27 demethylase, and mutation of a redox-sensitive cysteine in STAT6 attenuates jmjd3 expression. Moreover, Jmjd3 deficiency abrogates profibrotic M2 gene expression. Treatment with leflunomide, which reduces mitochondrial reactive oxygen species production and tyrosine phosphorylation, inhibits jmjd3 expression and M2 polarization, as well as development of a fibrotic phenotype. Taken together, these observations provide evidence that the redox regulation of Jmjd3 is a unique regulatory mechanism for Cu,Zn–superoxide dismutase–mediated profibrotic M2 polarization. Furthermore, leflunomide, which reduces reactive oxygen species production and tyrosine phosphorylation, may prove to be therapeutic in the treatment of asbestos-induced pulmonary fibrosis.
Keywords: Jumonji domain containing 3; macrophage; alternative activation; Cu,Zn–superoxide dismutase; pulmonary fibrosis
Clinical Relevance
There is no effective treatment for attenuating pulmonary fibrosis development, so understanding the molecular mechanisms may lead to novel therapeutic modalities. Alveolar macrophages play a critical role in fibrosis, and the polarization of macrophages to a profibrotic phenotype promotes fibrosis. The redox regulation of epigenetic modulations may be critical for the sustained phenotype of differentiated macrophages.
Macrophage polarization is defined as the process by which macrophages differentiate into a specific phenotype important for their function (1). Macrophage polarization can be classified into two categories: the classically activated macrophages (M1 phenotype) and the alternatively activated macrophages (M2 phenotype). In the development of pulmonary fibrosis, profibrotic M2 macrophages are believed to contribute to the fibrotic pathology by increasing the generation of profibrotic immune factors, collagen synthesis substrates, such as proline, and molecules implicated in extracellular matrix dynamics (2).
Macrophages can produce high levels of reactive oxygen species (ROS), including H2O2, which is linked to the pathogenesis of pulmonary fibrosis (3, 4). Superoxide dismutase (SOD) increases H2O2 production by converting superoxide anion () to H2O2 at a rate constant 104-fold faster than the spontaneous dismutation (5). Among the three SOD enzymes, we have shown that the Cu,Zn-SOD (SOD1), which is located in the cytosol and mitochondrial intermembrane space, is increased in alveolar macrophages of patients with asbestosis, and contributes to the increased mitochondrial H2O2 production by alveolar macrophages (3). In addition, alveolar macrophages with high levels of Cu,Zn-SOD polarize to a profibrotic M2 phenotype and accelerate the development of pulmonary fibrosis (6).
In the classic Th2 cytokine–induced M2 polarization, the signal transducer and activator of transcription (STAT) 6 increases to initiate M2 gene expression (7). Studies show that Th2 cytokine–independent M2 polarization mechanisms also exist (8, 9), and we have recently shown that, independent of Th2 cytokines, Cu,Zn-SOD–mediated H2O2 production promotes M2 polarization, providing a secondary pathway for macrophage alternative activation (6). Unlike the canonical pathway, STAT6 mediates Cu,Zn-SOD–induced M2 polarization in a redox-dependent manner, in which the cysteine (Cys528) residue in the Src homology 2 (SH2) domain of STAT6 is critical for the redox activation. However, it is unknown if STAT6 is essential for Cu,Zn-SOD–mediated profibrotic M2 gene expression.
There is emerging evidence that, in addition to transient activation by cytokines, signaling pathways, and transcription factors, epigenetic modulations may be critical for the sustained phenotype of differentiated macrophages (10). Epigenetic regulation includes: (1) post-translational modifications of histones; (2) modifications of cytosine-phosphate-guanine (CpG) islands; and (3) noncoding RNA (11). The methylation status of histones is important for initiation of M2 gene transcription and suppression, especially on the histone H3 lysine 4 and 27 sites. Methylation of H3K27 suppresses transcription of multiple M2 genes, such as chitinase-like 3 (ym1), resistin-like molecule alpha (fizz1), and arginase I (arg I). In contrast, the hypermethylation of H3K4 increases M2 gene transcription (12). It is now generally accepted that the methylation status of histone H3 is dynamically regulated by site-specific demethylases and methyltransferases. The Jumonji C domain proteins are a family histone demethylases that remove methyl groups from histone H3 in a site-specific manner. Among them, the Jumonji domain containing (Jmjd) 3 specifically demethylates H3K27, which is associated with M2 gene transcriptional activation. Although it is established that Th2 cytokines, such as IL-4/-13, can increase Jmjd3, the relationship between Jmjd3 and other M2-inducing signals, such as Cu,Zn-SOD–derived H2O2, is not known.
Materials and Methods
Materials
Chrysotile asbestos was provided Dr. Peter S. Thorne (University of Iowa College of Public Health (University of Iowa College of Public Health, Iowa City, IA). p-hydroxylphenyl acetic acid, polyethylene glycol (PEG)–conjugated SOD (PEG-SOD), and horseradish peroxidase were purchased from Sigma-Aldrich (St. Louis, MO). A77 1,726, the bioactive compound of leflunomide, was purchased from EMD Millipore (Billerica, MA).
Human Subjects
We obtained human alveolar macrophages, as previously described (3), from normal subjects and patients with asbestosis under an approved protocol by the Human Subjects Review Board of the University of Iowa Carver College of Medicine (Iowa City, IA).
Mice
Wild-type (WT), Cu,Zn-SOD transgenic (Cu,Zn-SODTg), and STAT6−/− (Jackson Laboratory, Bar Harbor, ME) C57BL/6 mice were used in these studies. Experimental protocols used, as previously described (3), were approved by the University of Iowa and the University of Alabama at Birmingham (Birmingham, AL) Institutional Animal Care and Use Committee.
Cells
Human monocyte (THP-1) and murine alveolar macrophage (MH-S) cell lines were obtained from American Type Culture Collection (Manassas, VA). Cells were maintained in RPMI-1640 media with following supplements: 10% fetal bovine serum and penicillin/streptomycin. All experiments were performed with 0.5% serum supplement.
Plasmids and Transfections
Human STAT6 cDNA (NM_003153; GeneCopoeia, Rockville, MD), mutant pCDNA3.1–STAT6C528S–V5–histidine (His), and pcDNA3.1–Cu,Zn-SOD–V5–His vector have been described previously (3, 6). Plasmid vectors were transfected into cells using X-tremeGene 9 transfection reagent (Roche, Indianapolis, IN), according to the manufacturer’s instructions.
Determination of H2O2 Generation
Extracellular H2O2 production was determined fluorometrically, as previously described (13).
Adenoviral Vectors
Cells were infected with replication-deficient adenovirus type 5 with the E1 region replaced with DNA containing the cytomegalovirus (CMV) promoter region alone (Ad5.CMV) or Ad5.Cu,Zn-SOD vector (Gene Transfer Vector Core, University of Iowa Carver College of Medicine, Iowa City, IA) at a multiplicity of infection of 500 in serum-free RPMI medium.
Isolation of Nucleus
Nuclear proteins were extracted as previously described (14).
Small Interfering RNA
Cells were transfected with 100 nM scrambled, STAT6 small interfering RNA (siRNA) duplex (IDT, Coralville, IA), or Jmjd3 siRNA (Dharmacon Research, Lafayette, CO), using DharmaFect reagent (Dharmacon Research), as previously described (3).
Hydroxyproline Determination
Lung tissue was digested for 24 hours at 112°C with 6 N hydrochloric acid. Hydroxyproline concentration was determined as previously described (13).
STAT6 Oxidation
Lysates were harvested under nonreducing conditions. Samples were separated on a polyacrylamide gel in the presence or absence of DTT.
STAT6-V5-His Pulldown
Cells were harvested in buffer B (10 mM Tris-HCl, pH 7.5, 0.5 M NaCl, 5 mM imidazole, and 1% Triton X-100, plus protease and phosphatase inhibitors). Lysates were briefly sonicated on ice and debris was pelleted. Talon metal (cobalt) affinity resin (Clontech, Mountain View, CA) was added to each lysate, and samples were rotated at 4°C for 2 hours and washed three times with buffer B. STAT6-V5-His proteins were eluted by adding protein sample buffer and heating at 95°C for 5 minutes.
Immunoblot Analysis
Whole-cell lysates were obtained as previously described (14) and separated by SDS-PAGE. Immunoblot analyses were performed with the designated antibodies followed by the appropriate secondary antibodies cross-linked to horseradish peroxidase.
Chromatin Immunoprecipitation Assay
The chromatin immunoprecipitation assay was conducted using the SimpleChIP enzymatic chromatin IP kit (Cell Signaling, Danvers, MA) according to the manufacturer’s instructions. Briefly, cells were fixed using 1% formaldehyde for 15 minutes at room temperature, followed by termination of fixation using excess glycine. Cells were harvested, the cell nuclei were isolated, and the resulting nuclear pellets were treated with micrococcal nuclease to digest DNA to fragments. The resulting cross-linked chromatin preparations were used for input controls (2% of total) or for immunoprecipitation using 5 μg of anti-STAT6 antibody (Cell Signaling), 10 μl histone H3 (D2B12) XP rabbit monoclonal antibody as a positive control, or 5 μg normal rabbit IgG antibody as a negative control. Protein–DNA complexes were eluted, and the chromatin was subjected to reversal of cross-links followed by DNA purification, as described in the protocol. PCR was performed using purified DNA and the following primers: 5′-CAA GCA CAT TTC ACG TAC AGA TGA-3′ and 5′-CCA CAA AGG CAG AAG GGT TTC-3′ for the murine STAT6 binding site, jmjd3 promoter region 4. RPL30 primers were used for PCR detection of the murine ribosomal protein gene locus. PCR products were resolved on a 2% agarose gel.
Quantitative Real-Time PCR
After reverse transcription of total RNA using iScript reverse transcription kit (Bio-Rad Laboratories, Hercules, CA), M1 and M2 marker gene mRNA expression was determined by quantitative real-time PCR using SYBR Green kit (Bio-Rad Laboratories, Hercules, CA). The following primer sets were used: mouse collagen Ia1 5′-GAG TTT CCG TGC CTG GCC CC-3′ and 5′-ACC TCG GGG ACC CAT CTG GC-3′; mouse Jmjd3 5′-TAC CCC CAG CAT CTA TTT GGA GAG C-3′ and 5′-TAA GTT GAG CCG AAG TGA ACC AGC C-3′; human Jmjd3 5′-GGA GGC CACACG CTG CTA C-3′ and 5′-GCC AGT ATG AAA GTT CCA GAG CTG-3′. Mouse TNF-α, Ym1, FIZZ1, β-actin, and human CCL18, and hypoxanthine-guanine phosphoribosyltransferase (HPRT) primers have been described previously (6). Data were calculated by the ∆∆CT method. The mRNA measurements were normalized to β-actin or HPRT and expressed in arbitrary units.
ELISA
Active transforming growth factor (TGF)-β, TNF-α, and Ym-1 levels in bronchoalveolar lavage (BAL) fluid and cell media were measured by ELISA (R&D, Minneapolis, MN), according to the manufacturer’s instructions.
Oxygen Consumption Assay
Oxygen consumption was measured by with an ESA BioStat multielectrode system (ESA Products, Dionex Corp., Chelmsford, MA) in conjunction with a YSI oxygen probe (5331; Yellow Springs Instruments, Yellow Springs, OH) and glass reaction chamber vials in a YSI bath assembly (5301) (Yellow Springs Instruments), all at room temperature, as previously described (15).
Jmjd3 Activity Assay
Jmjd3 activity was measured by using a Jmjd3/UTX activity assay from Epigentek (Farmingdale, NY) according to manufacturer’s manual.
Statistical Analysis
Statistical comparisons were performed using either an unpaired two-tailed t test or one-way ANOVA with a Tukey’s post hoc test. All statistical analyses are expressed with SEM and P values less than 0.05 are considered significant.
Results
STAT6 Is Required for Cu,Zn-SOD–Mediated Alternatively Activated Macrophages
We have shown that Cu,Zn-SOD overexpression induces STAT6 nuclear translocation (6), so we conducted studies to determine if STAT6 was required for Cu,Zn-SOD–mediated macrophage M2 polarization. Bone marrow–derived macrophages (BMDMs) from STAT6−/− had an M1-dominant phenotype with significantly greater tnf-α and reduced ym1 and fizz1 mRNA levels compared with WT (Figure 1A). PEG-SOD treatment, as expected, promoted M2 polarization in WT macrophages with increased ym1 and fizz1 expression. In contrast, STAT6−/− macrophages sustained an M1 phenotype in the presence or absence of PEG-SOD. These observations suggest that STAT6 is necessary for Cu,Zn-SOD–mediated M2 polarization.
Figure 1.
Signal transducer and activator of transcription (STAT) 6 is essential for Cu,Zn–superoxide dismutase (SOD)–mediated M2 polarization. (A) Wild-type (WT) and STAT6−/− bone marrow–derived macrophages (BMDMs) were cultured in the presence or absence of polyethylene glycol (PEG)–conjugated SOD (PEG-SOD) for 3 hours and then treated with chrysotile asbestos. Total RNA from macrophages was isolated, and tnf-α, chitinase-like 3 (ym1), and resistin-like molecule alpha (fizz1) gene expression were measured by quantitative RT-PCR. Results show arbitrary units normalized to β-actin mRNA (n = 3). *P < 0.05 WT versus STAT6−/−, **P < 0.05 WT versus WT + PEG-SOD, #P < 0.05 STAT6−/− + PEG-SOD versus STAT6−/−. (B) Macrophages were transfected with either scrambled or STAT6 small interfering RNA (siRNA) for 72 hours. Total mRNA was isolated and chemokine ligand (ccl-18) gene expression was measured. Results show arbitrary units normalized to hypoxanthine-guanine phosphoribosyltransferase mRNA (n = 3); *P < 0.05 STAT6 siRNA versus scrambled siRNA. (C) WT and STAT6−/− macrophages were stained with a fluorescent compound (R-PE)–labeled anti-CD23 at a 1:10 dilution. Levels of CD23 were determined by flow cytometry. Representative of three histograms of CD23-stained macrophages is shown. WT and STAT6−/− mice were exposed to chrysotile (100 μg) intratracheally. (D) Ym1 (n = 5) and (E) TNF-α (n = 3) in the bronchoalveolar lavage (BAL) fluid were measured 21 days after chrysotile exposure; *P < 0.05. (F) Total RNA from alveolar macrophages was isolated, and tnf-α, ym1, and fizz1 gene expression were measured as in A. Results show arbitrary units normalized to β-actin mRNA (n = 3); *P < 0.05 versus WT.
Given that STAT6 is instrumental for Cu,Zn-SOD–mediated M2 polarization, we hypothesized that STAT6−/− macrophages would polarize to a proinflammatory and antifibrotic M1 phenotype. Macrophages transfected with STAT6 siRNA had decreased ccl-18 expression compared with cells transfected with the scrambled siRNA (Figure 1B). In addition, flow cytometry showed that WT macrophages had significantly greater (M2a) CD23 cell surface expression compared with STAT6−/− macrophages (Figure 1C), which is consistent with differences seen in the ym1 and fizz1 gene expression.
To further test our hypothesis in vivo, WT and STAT6−/− mice were exposed to chrysotile asbestos. STAT6−/− mice showed a reduction in the M2a protein Ym1 (Figure 1D) and an increase in TNF-α (Figure 1E) in the BAL fluid compared with chrysotile-exposed WT mice. Likewise, BAL cells from STAT6−/− mice exposed to chrysotile showed reduced M2a gene expression (ym1 and fizz1), whereas M1 gene expression (tnf-α) was dramatically increased more than 25-fold compared with WT mice (Figure 1F). These data suggest that STAT6−/− macrophages have a dominant proinflammatory phenotype.
STAT6−/− Mice Are Protected from Developing Pulmonary Fibrosis
Because STAT6−/− mice have decreased profibrotic M2 markers in vivo, we postulated that STAT6−/− mice would have less fibrotic development compared with WT mice after chrysotile exposure. STAT6−/− mice had less parenchymal and peribronchial collagen deposition and essentially normal lung architecture compared with WT mice (Figure 2A). To quantitate this difference, lung homogenates from STAT6−/− mice had decreased hydroxyproline content compared with WT mice (Figure 2B). WT mice also exhibited higher levels of active TGF-β in the BAL fluid, suggesting an overall profibrotic environment in the alveolar spaces of WT mice compared with STAT6−/− mice (Figure 2C).
Figure 2.
STAT6−/− mice are protected from developing pulmonary fibrosis. WT and STAT6−/− mice were exposed to chrysotile (100 μg) intratracheally. Lungs were extracted 21 days after exposure. (A) Masson’s trichrome from WT or STAT6−/− mice. Representative micrographs of one of six animals/genotype. Magnification, ×20. (B) Hydroxyproline was measured (n = 4); *P < 0.05. (C) Active transforming growth factor (TGF)-β in the BAL fluid was measured (n = 7); *P < 0.05. (D) Lung fibroblasts were isolated from WT mice and cultured in BAL fluid from WT or STAT6−/− mice. Collagen I mRNA expression was determined by real-time PCR and normalized to β-actin (n = 3); *P < 0.05. (E) Conditioned media were obtained from fibroblasts cultured as in D, and collagen protein expression was determined by immunoblot analysis. Representative of three replicates.
Because fibroblasts are the primary cells responsible for collagen production, and because macrophages produce multiple growth factors to induce collagen synthesis from fibroblasts, WT mouse lung fibroblasts were cultured in BAL fluid from either WT mice or STAT6−/− mice. Collagen I gene expression in fibroblasts cultured with BAL fluid from STAT6−/− mice was significantly reduced compared with fibroblasts incubated with BAL fluid from WT mice (Figure 2D). Collagen I secretion from WT fibroblasts was also significantly decreased when cultured with BAL fluid from STAT6−/− mice (Figure 2E). Taken together, these data suggest that STAT6 has an important role in development of the fibrotic phenotype after chrysotile exposure.
The Cu,Zn-SOD–STAT6 Pathway Regulates Profibrotic M2 Gene Expression by Modulation of Jmjd3
In the canonical Th2 cytokine–induced M2 polarization, IL-4/IL-13 activates STAT6 and increases jmjd3 gene expression. Because Cu,Zn-SOD promotes M2 polarization using STAT6 (6), we hypothesized that Cu,Zn-SOD can increase Jmjd3 expression and activity, which results in M2 gene transcription and macrophage alternative activation.
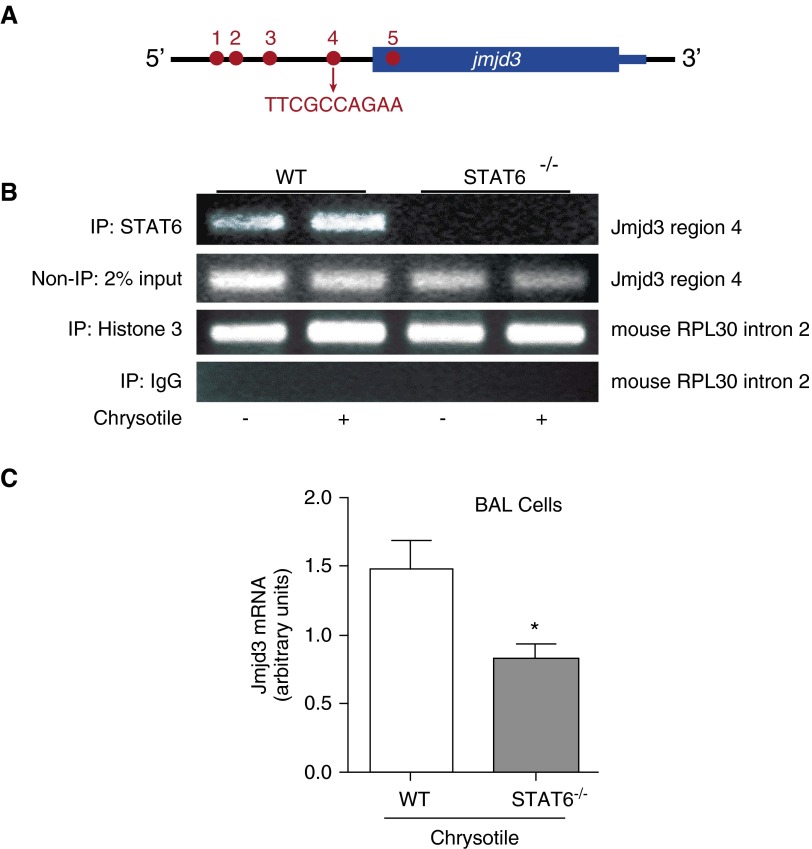
The jmjd3 promoter has five putative binding sites for STAT6 (Figure 3A), and region 4, which is the site closest to the transcription start site, has been shown to be the dominant site for STAT6 binding (12). To validate that STAT6 binds to jmjd3 promoter region 4, we performed a chromatin immunoprecipitation assay in WT and STAT6−/− BMDMs. STAT6−/− macrophages had no STAT6 binding to the jmjd3 promoter region 4 compared with WT macrophages (Figure 3B). Chrysotile increased STAT6 binding in WT macrophages. To test this observation in vivo, we isolated RNA from WT and STAT6−/− mouse alveolar macrophages after chrysotile exposure. Jmjd3 gene expression was significantly lower in cells from STAT6−/− mice (Figure 3C), which suggests that STAT6 is required for jmjd3 expression.
Figure 3.
STAT6 is required for jmjd3 gene expression. (A) Schematic of STAT6 binding sites on jmjd3 promoter. (B) Nuclear fractions were isolated from both WT and STAT6−/− BMDMs. Immunoprecipitation of STAT6 was performed from the DNA–protein complex, and PCR amplification was performed using primers to detect STAT6 binding site on jmjd3 gene region 4. Histone 3 was a positive control, and IgG was a negative control. Control DNA was amplified using primers to detect mouse recurrent pregnancy loss (RPL30) intron 2 provided by the manufacturer (Cell Signaling, Boston, MA). Representative of three replicates. (C) WT and STAT6−/− mice were exposed to chrysotile (100 μg) intratracheally. Alveolar macrophages were isolated after 21 days. Jmjd3 mRNA expression was determined by quantitative RT-PCR, and normalized to β-actin (n = 3); *P < 0.05. IP, intraperitoneal; Jmjd3, Jumonji domain containing-3.
To determine if Cu,Zn-SOD regulates M2 polarization via modulation of Jmjd3, we evaluated whether jmjd3 gene expression was increased in Cu,Zn-SODTg macrophages. Cu,Zn-SODTg BMDMs, which express high levels of Cu,Zn-SOD and exhibit a predominant M2 phenotype, had a significant increase in jmjd3 gene expression compared with WT macrophages (Figure 4A). This difference in jmjd3 expression correlated with significantly greater Jmjd3 activity in the nuclear extracts from Cu,Zn-SODTg compared with WT BMDMs (Figure 4B). Jmjd3 activity was further evaluated by determining the level of methylated histone H3 (H3K27 me3) in nuclear extracts from BMDMs. STAT6−/− macrophages showed high levels of H3K27 me3, and H3K27 me3 in WT macrophages was slightly less compared with STAT6−/− (Figure 4C). In contrast, H3K27 me3 was barely detectible in Cu,Zn-SODTg macrophages. In aggregate, these data suggest that Cu,Zn-SOD increases Jmjd3 to enhance chromatin accessibility and profibrotic M2 gene transcriptional activation.
Figure 4.
Overexpression of Cu,Zn-SOD modulates Jmjd3 expression and activity. (A) BMDMs were obtained from WT and Cu,Zn-SOD transgenic (Cu,Zn-SODTg) mice. Total RNA was isolated, and jmjd3 mRNA was measured by quantitative RT-PCR and is expressed in arbitrary units normalized to β-actin mRNA (n = 3); *P < 0.05. (B) Nuclear fractions from WT and Cu,Zn-SODTg macrophages were isolated, and Jmdj3/ubiquitously transcribed tetratricopeptide repeat, X chromosome (UTX) enzyme activity was measured (n = 4); *P < 0.05. (C) BMDMs were obtained from WT, Cu,Zn-SODTg (Tg), and STAT6−/− mice. Nuclear fractions were isolated and immunoblot analysis was performed for methylated histone H3 (H3K27 me3). Figure is representative of five experiments. (D) Macrophages were infected with a replication-deficient adenovirus vector expressing either an empty vector (cytomegalovirus [CMV]) or Cu,Zn-SOD vector (Cu,Zn-SOD) for 24 hours. After 24 hours, cells were transfected with either STAT6WT or STAT6C528S vectors for 24 hours. Total RNA from macrophages was isolated and jmjd3 gene expression was measured by quantitative RT-PCR. Results show arbitrary units normalized to β-actin mRNA. *P < 0.05 versus STAT6WT and **P < 0.05 versus all other groups; n = 4. (E) Macrophages were transfected with empty, STAT6WT, or STAT6C528S vectors. After 24 hours, cells were treated with PEG or PEG-SOD for 1 hour followed by chrysotile exposure for 3 hours. Lysates were obtained under nonreducing conditions. Samples were separated on a polyacrylamide gel in the presence or absence of dithiothreitol (DTT). Representative of three replicates. (F) Macrophages were transfected with empty, STAT6WT, or STAT6C528S in combination with scrambled or Cu,Zn-SOD siRNA. After 72 hours, cells were exposed to chrysotile for 3 hours. Lysates were obtained under nonreducing conditions. Samples were separated on a polyacrylamide gel in the presence or absence of DTT. Representative of three replicates. (G) Nuclear fractions from alveolar macrophages from normal subjects and patients with asbestosis were isolated and Jmjd3/UTX enzyme activity was measured (n = 3); *P < 0.05. (H) Total RNA was isolated from alveolar macrophages from normal subjects and patients with asbestosis. Arginase 1 gene expression was measured by quantitative RT-PCR (n = 6); *P < 0.05. (I) Macrophages were transfected with scrambled or Jmjd3 siRNA. After 72 hours, cells were exposed to chrysotile (10 μg/cm2) for 4 hours. Total RNA was isolated, and tnf-α and fizz1 gene expression was measured by quantitative RT-PCR (n = 3); *P < 0.05 versus scrambled (−), **P < 0.05 versus scrambled (+) in both tnf-α and fizz1. Inset, jmjd3 gene expression was measured (n = 3). Ns, not significant; Ox, oxidized; Red, reduced; Scr, scrambled.
Because Cu,Zn-SOD–mediated STAT6 transcriptional activation requires the redox-sensitive Cys528 residue within the SH2 domain of STAT6 (6), the effect of the redox regulation of STAT6 on jmjd3 expression was determined. Cu,Zn-SOD increased jmjd3 gene expression in cells overexpressing STAT6WT, whereas cells expressing the STAT6C528S mutant had decreased jmjd3 gene expression in the presence or absence of Cu,Zn-SOD, suggesting that jmjd3 expression is regulated in a redox-dependent manner (Figure 4D). To confirm that the Cys528 residue is required for oxidation of STAT6, macrophages were treated with PEG or PEG-SOD in the presence or absence of the reducing agent, DTT. Chrysotile increased STAT6WT oxidation under nonreducing conditions, and PEG-SOD enhanced oxidation of STAT6WT (Figure 4E). No oxidized STAT6WT was observed with the addition of DTT. In contrast, STAT6C528S was not oxidized under nonreducing conditions, including samples obtained from chrysotile-exposed cells treated with PEG-SOD.
To further validate that Cu,Zn-SOD was involved in oxidation of STAT6, we evaluated STAT6 in Cu,Zn-SOD–deficient macrophages that were generated by siRNA. Chrysotile increased STAT6WT oxidation in cells transfected with the scrambled siRNA, whereas oxidized STAT6WT was absent in Cu,Zn-SOD–deficient cells (Figure 4F). There was no evidence of STAT6 oxidation in cells transfected with STAT6C528S or with the addition of DTT. These data suggest that jmjd3 expression via STAT6 is redox regulated by Cu,Zn-SOD.
We have shown that alveolar macrophages isolated from patients with asbestosis have increased Cu,Zn-SOD activity (3), so we hypothesized that alveolar macrophages from patients with asbestosis would have increased Jmjd3 activity. Alveolar macrophages from patients with asbestosis had significantly greater Jmjd3 activity compared with the normal subjects (Figure 4G). To associate this difference of Jmjd3 activity with M2 gene expression, we determined if macrophages of patients with asbestosis had profibrotic M2 polarization. Alveolar macrophages from the patients had nearly 20-fold more arginase-1 expression than normal subjects (Figure 4H), suggesting that the alternative activation of macrophages in patients with asbestosis is modulated, in part, in an epigenetic manner.
To directly link Jmjd3 to the macrophage phenotype, Jmjd3-deficient macrophages were generated with siRNA. Chrysotile increased fizz1 expression significantly, whereas fizz1 was below control levels in Jmjd3-deficient macrophages (Figure 4I). In contrast, Jmjd3 deficiency enhanced M1 polarization, as measured by tnf-α gene expression. Taken together, these results indicate that Jmjd3 is redox regulated and is necessary for M2 polarization.
Leflunomide Inhibits M2 Polarization
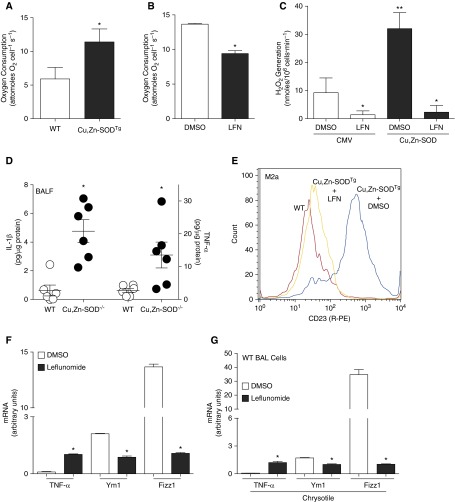
Because Cu,Zn-SOD–mediated macrophage polarization is dependent on increased H2O2 generation and STAT6 activation (6), we aimed to find a potential pharmacological agent that inhibited these pathways. Leflunomide, which has been used for rheumatoid arthritis, acts as a tyrosine kinase inhibitor, and also modulates oxidative stress by inhibiting dihydro-orotate dehydrogenase. Moreover, leflunomide has been shown to decrease oxygen consumption and the production of ROS in various cancer cell lines, and tyrosine kinase inhibitors have been used in clinical trials for the treatment of idiopathic pulmonary fibrosis (16). We hypothesized that leflunomide would abrogate the fibrotic phenotype in mice exposed to chrysotile by attenuating profibrotic M2 polarization. BMDMs from Cu,Zn-SODTg, which generate high levels of H2O2 and have a primary M2 phenotype, consumed twice as much oxygen compared with the WT macrophages (Figure 5A). WT BMDMs treated with leflunomide had a significant decrease in oxygen consumption compared with cells treated with the vehicle (Figure 5B). Because oxygen consumption is closely correlated with cellular ROS production, we further investigated whether leflunomide could inhibit Cu,Zn-SOD–mediated H2O2 generation in macrophages. Overexpression of Cu,Zn-SOD increased the generation rate of H2O2 (Figure 5C). Leflunomide treatment significantly reduced H2O2 production in cells infected with empty vector. Moreover, leflunomide decreased H2O2 generation to control levels in cells overexpressing Cu,Zn-SOD.
Figure 5.
Leflunomide (LFN) treatment decreases M2 polarization. (A) O2 consumption rate was determined in WT and Cu,Zn-SODTg BMDMs (n = 3); *P < 0.05. (B) WT BMDMs were treated with either vehicle control or LFN (200 μM). Oxygen consumption rate was determined (n = 4); *P < 0.05. (C) Macrophages were infected with adenoviral vector containing either an empty (CMV) or Cu,Zn-SOD construct. After 48 hours, cells were incubated with vehicle or LFN (200 μM). p-hydroxylphenyl acetic acid (pHPA) assay was performed, with results expressed as rate of H2O2 generation; *P < 0.05 versus dimethyl sulfoxide (DMSO) and LFN (Cu,Zn-SOD), **P < 0.05 versus all other conditions (n = 3). (D) WT and Cu,Zn-SOD−/− mice were exposed to chrysotile (100 μg intratracheally). BAL was obtained after 21 days. IL-1β and TNF-α were measured by ELISA (n = 6); *P < 0.023. (E) BMDMs from WT, Cu,Zn-SODTg + DMSO, and Cu,Zn-SODTg + LFN were stained with R-PE–labeled anti-CD23 at a 1:10 dilution. Levels of CD23 were determined by flow cytometry. Representative of three histograms of CD23-stained macrophages is shown. (F) Macrophages were treated with either vehicle (DMSO) or LFN (200 μM) overnight. Total RNA from macrophages was isolated, and tnf-α, ym1, and fizz1 gene expression were measured. Results show arbitrary units normalized to β-actin mRNA (n = 4); *P < 0.05 versus DMSO. (G) WT mice were exposed to chrysotile asbestos (100 μg intratracheally). DMSO or LFN (1.4 mg/kg) was administered intraperitoneally every other day from Day 10. Mice were killed 21 days after exposure. Total RNA from alveolar macrophages was isolated, and tnf-α, ym1, and fizz1 gene expression was measured by quantitative RT-PCR. Results show arbitrary units normalized to β-actin mRNA (n = 4); *P < 0.05 versus DMSO. BALF, bronchoalveolar lavage fluid.
The importance of Cu,Zn-SOD in modulating macrophage polarization was further demonstrated by showing that BAL fluid from chrysotile-exposed Cu,Zn-SOD−/− mice, which have decreased ROS production (3), have increased IL-1β and TNF-α compared with WT BAL fluid (Figure 5D). These observations suggest that Cu,Zn-SOD–mediated H2O2 generation is linked to profibrotic M2 polarization, and that leflunomide acts as a modulator of the cellular redox levels.
Based on these observations, we hypothesized that leflunomide would alter profibrotic M2 polarization via inhibiting ROS. Flow cytometry showed that Cu,Zn-SODTg macrophages had significantly greater M2a (CD23) cell surface expression than WT macrophages, and treatment of Cu,Zn-SODTg macrophages with leflunomide reduced CD23 expression to the level observed in WT macrophages (Figure 5E). Treatment of the murine alveolar macrophage cells with leflunomide decreased the M2a genes, ym1 and fizz1, and increased tnf-α, indicating that leflunomide promotes M1 polarization compared with cells incubated with DMSO (Figure 5F). Similar observations were recapitulated in vivo in WT mice exposed to chrysotile asbestos. The M2a genes, ym1 and fizz1, were decreased in alveolar macrophages collected from mice treated with leflunomide, whereas the M1 gene, tnf-α, was increased compared with BAL cells collected from mice treated with DMSO (Figure 5G). Taken together, these data demonstrate that macrophage polarization can be modulated pharmacologically by leflunomide.
IL-4 Does Not Use ROS to Mediate M2 Polarization
Because leflunomide also acts as a tyrosine kinase inhibitor, and phosphorylation of tyrosine (Tyr641) of STAT6 is necessary for activation, we determined if leflunomide modulated STAT6 phosphorylation in macrophages exposed to IL-4. IL-4 treatment increased STAT6 phosphorylation on Tyr641; however, leflunomide treatment decreased IL-4–induced tyrosine phosphorylation of STAT6 (Figure 6A). To determine if the reduction in STAT6 phosphorylation modulated the function of Jmjd3, we determined if there was alteration in the level of H3K27 me3. IL-4 significantly reduced H3K27 me3 in nuclear extracts, whereas leflunomide treatment increased H3K27 me3 substantially in both the absence and presence of IL-4 (Figure 6B).
Figure 6.
IL-4–mediated M2 polarization does not use oxidative stress. (A) Murine alveolar macrophages were incubated in the presence or absence of LFN (200 μM) for 2 hours and then treated with either vehicle or IL-4 (20 ng/ml). Immunoblot analysis for phosphorylated STAT6 (p-STAT6) in cell lysates was performed. Figure is representative of three experiments. (B) Macrophages were cultured in the presence or absence of IL-4 with DMSO or LFN (200 μM). Nuclear fractions were isolated, and immunoblot analysis was performed for H3K27 me3. Figure is representative of three experiments. (C) Murine alveolar macrophages were incubated in the presence or absence of LFN (200 μM) for 2 hours and then chrysotile. Immunoblot analysis for p-STAT6 in cell lysates was performed. Each lane in the figure was run on the same gel. Figure is representative of three experiments. (D) Macrophages were treated with IL-4 (20 ng/ml) or chrysotile (10 μg/cm2). Rate of H2O2 generation was measured by pHPA assay (n = 8); *P < 0.05 versus vehicle and IL-4. Macrophages were transfected with STAT6WT or STAT6C528S. After 24 hours, cells were cultured in the presence or absence of IL-4 for 4 hours. Total RNA was isolated, and (E) fizz1, (F) ym1, (G) tnf-α, and (H) il-1β gene expression was measured by quantitative RT-PCR (n = 3); *P < 0.00001 versus (−) in both groups, **P < 0.0001 versus all other groups. (I) Macrophages were transfected as described previously here. Lysates were subjected to His pulldown and immunoblot for V5 and p-STAT6 were performed. Figure is representative of three replicates. (J) Macrophages were treated with DMSO or LFN (200 μM), and then stimulated with IL-4 (20 ng/ml) for 4 hours. Total RNA was isolated, and mannose receptor and tnf-α gene expression were measured by quantitative RT-PCR (n = 5); *P < 0.05 versus DMSO (−IL-4), **P < 0.05 versus DMSO (+IL-4). Emp, empty; IB, immunoblot; PD, pull-down.
Because STAT6 oxidation is necessary for its activation (6), and leflunomide reduces ROS generation, we investigated whether leflunomide also inhibited phosphorylation of T641 in STAT6. Chrysotile exposure increased phosphorylation of STAT6, and leflunomide reduced phosphorylated STAT6 in the presence or absence of chrysotile exposure (Figure 6C). These observations suggest that chrysotile-induced modulation of STAT6 activation involves both oxidation and tyrosine phosphorylation.
Based on the two mechanisms involved in STAT6 activation after chrysotile exposure, we asked if IL-4 modulated M2 polarization via oxidative stress. The rate of generation of H2O2 in macrophages treated with IL-4 was no different than in cells treated with the vehicle, whereas chrysotile increased H2O2 generation significantly (Figure 6D). Likewise, fizz1 (Figure 6E) and ym1 expression (Figure 6F) were similar in IL-4–treated cells expressing either STAT6WT or the redox-sensitive mutant, STAT6C528S. Conversely, tnf-α (Figure 6G) and il-1β (Figure 6H) were significantly reduced with IL-4 treatment. Interestingly, cells expressing STAT6C528S had greater tnf-α and il-1β gene expression than the cells expressing STAT6WT. To further verify that STAT6WT and STAT6C528S had a similar effect on IL-4–induced M2 polarization, we confirmed that the C528S mutant did not alter Tyr641 phosphorylation of STAT6 (Figure 6I).
To determine leflunomide modulated IL-4–mediated M2 polarization, cells were stimulated with IL-4 in the presence or absence of leflunomide. IL-4 significantly increased mannose receptor expression, which was significantly decreased by leflunomide (Figure 6J). In contrast, leflunomide treatment significantly increased the M1 gene, tnf-α. These results suggest that IL-4 modulates M2 polarization by regulating the tyrosine phosphorylation (Tyr641) rather than oxidation of STAT6.
Leflunomide Attenuates Pulmonary Fibrosis
Because leflunomide treatment modulates macrophage polarization, we investigated the potential therapeutic role of leflunomide in vivo. Titanium dioxide (TiO2) exposure resulted in no collagen deposition in vehicle- (Figure 7A) or leflunomide-treated WT mice (Figure 7B). Chrysotile-exposed WT mice treated with vehicle (Figure 7C) showed widespread parenchymal collagen deposition compared with mice that received leflunomide, which had near-normal lung parenchyma (Figure 7D). The histological findings were confirmed biochemically by hydroxyproline assay (Figure 7E), suggesting that leflunomide treatment prevented the development of a fibrotic phenotype after chrysotile exposure. Because leflunomide inhibits profibrotic M2 polarization and attenuates pulmonary fibrosis development, we determined if leflunomide regulated jmjd3 gene expression in vivo. Leflunomide did not change jmjd3 gene expression in alveolar macrophages from TiO2-exposed mice (Figure 7F); however, leflunomide-treated mice had significantly less jmjd3 compared with macrophages isolated from the vehicle-treated mice after chrysotile exposure.
Figure 7.
LFN treatment attenuates pulmonary fibrosis. WT mice were exposed to titanium dioxide (TiO2) or chrysotile asbestos (100 μg intratracheally). (A and C) Vehicle (DMSO) or (B and D) LFN (1.4 mg/kg) was administered intraperitoneally every other day. Mice were killed 21 days after exposure. Lungs were extracted for Masson’s trichrome. Representative micrographs of one of five animals. (E) Hydroxyproline assay (n = 5); *P < 0.0001 versus all other groups. (F) Total RNA from alveolar macrophages was isolated, and jmjd3 gene expression was measured by quantitative RT-PCR. Results show arbitrary units normalized to β-actin mRNA (n = 4); *P < 0.05 versus DMSO, ***P < 0.0001 versus TiO2 groups. (G) BAL cells were collected. Immunoblot analysis for Cu,Zn-SOD was performed (n = 3). Cu,Zn-SODTg mice were exposed to chrysotile asbestos (100 μg intratracheally). (H) Vehicle (DMSO) or (I) LFN (1.4 mg/kg) was administered intraperitoneally every other day from Day 10. Mice were killed 21 days after exposure. Lungs were extracted for Masson’s trichrome. Representative micrographs of one of six animals.
Alveolar macrophages from patients with asbestosis have increased expression of active Cu,Zn-SOD (3), so we determined if Cu,Zn-SOD was increased in BAL cells from WT mice exposed to chrysotile asbestos. Cu,Zn-SOD expression was significantly increased in BAL cells obtained from chrysotile-exposed mice compared with mice exposed to TiO2 (Figure 7G). To determine if leflunomide treatment modulates macrophage polarization, in part by regulating Cu,Zn-SOD–mediated H2O2 generation, we investigated the potential therapeutic role of leflunomide in vivo in Cu,Zn-SODTg mice exposed to chrysotile. Cu,Zn-SODTg mice treated with vehicle (Figure 7H) showed widespread parenchymal and peribronchial collagen deposition compared with Cu,Zn-SODTg mice that received leflunomide, which had near-normal lung parenchyma (Figure 7I). In aggregate, these data underscore the role of Jmjd3 in macrophage polarization and the potential therapeutic benefits of leflunomide in attenuating the severity of the disease.
Discussion
The function of macrophages is determined by their distinct differentiation and polarization characteristics. Compared with the proinflammatory, classically activated macrophages (M1 phenotype), the alternatively activated macrophages (M2 phenotype) are associated with inflammation attenuation, repair, and, sometimes, fibrosis. We have shown that Cu,Zn-SOD–induced H2O2 can polarize macrophages to an M2 phenotype and accelerate development of pulmonary fibrosis (6); however, epigenetic regulation plays an important part in the acquisition and maintenance of macrophage phenotype. STAT6 is known to bind to the promoter region of the histone demethylase, Jmjd3, to increase jmjd3 gene expression, Jmjd3 activity, and M2 gene transcriptional activation. Based on these data, we hypothesized that profibrotic M2 polarization is regulated, in part, epigenetically. Here, we show that Cu,Zn-SOD–mediated M2 polarization is linked to the activation of STAT6. Cellular ROS levels and tyrosine phosphorylation modulate transcriptional activation of STAT6. These mechanisms rely on the redox status of Cys528 in the SH2 domain and Tyr641 in STAT6. In addition to binding to the promoter of M2 genes, STAT6 also binds to the promoter of jmjd3, a histone H3 lysine 27 demethylase. Modulating ROS production and tyrosine kinases with leflunomide decreases STAT6 activation, jmjd3 expression, and profibrotic M2 polarization, and attenuates fibrotic development (Figure 8). This alternative M2 polarization model is distinct from classical M2 polarization, which is mediated by Th2 cytokines, such as IL-4. The distinction lies in the fact that the Cu,Zn-SOD activates STAT6 in a redox- and kinase-dependent manner, whereas the Th2 cytokine, IL-4, activates STAT6 only by Tyr641 phosphorylation.
Figure 8.
Redox regulation of Jmjd3 modulates macrophage polarization and asbestosis-induced pulmonary fibrosis. Asbestos-exposed macrophages have increased Cu,Zn-SOD, which mediates macrophages to produce H2O2. Redox-sensitive STAT6 modulates jmjd3 expression, thereby regulating polarization of macrophages to a profibrotic phenotype, which contributes to asbestos-induced pulmonary fibrosis. This process can be attenuated by administration of LFN.
ROS have emerged as important regulators of macrophage polarization (17); however, most current research focuses on nitric oxide (NO) production as an indicator of M1 polarization. An increasing level of NO, which is due to the increase of inducible NOS (iNOS) during M1 polarization, leads to the accumulation of thiol nitrosylation. Intracellular targets of S-nitrosylation include multiple proinflammatory transcription factors, such as NF-κB (18) and hypoxia-inducible factor-1α (HIF-1α) (19).
Limited data are available regarding ROS and M2 polarization. The M1/2 nomenclature has been denoted as reductive or oxidative in relation to macrophages based on their intracellular glutathione levels (20). M1 macrophages have more reduced glutathione, whereas the M2 macrophages have more oxidized glutathione. This observation is consistent with our data in Cu,Zn-SODTg macrophages, which produce more H2O2, have increased oxidative stress, increased oxidized glutathione (3), and manifest a dominant M2 phenotype (6). Moreover, chrysotile exposure induces Cu,Zn-SOD localization to the mitochondria (3). Mitochondrial Cu,Zn-SOD increases oxygen consumption that likely alters mitochondrial bioenergetics to increase oxidative phosphorylation, which is characteristic of M2 macrophages (21–24).
Others and we have shown that STAT6 is required for M2 polarization in injured tissues (6, 25, 26). In the current report, we show that Cu,Zn-SOD–mediated profibrotic M2 polarization requires STAT6, and STAT6−/− mice are protected from developing pulmonary fibrosis. The protective effects of STAT6 inhibition in pulmonary fibrosis may be multifactorial, and the specificity regarding macrophage polarization warrants further investigation. Given that STAT family proteins harbor multiple cysteine residues and are potential targets for redox regulation, it is plausible that Cu,Zn-SOD–mediated H2O2 regulates macrophage phenotype by modulating other STAT family members, such as STAT1, which is required for M1 polarization, or STAT3, which is implicated in regulatory macrophage differentiation (27). Although tyrosine phosphorylation of STAT6 occurs with chrysotile exposure, the redox regulation of STAT6, Jmjd3, and M2 polarization by Cu,Zn-SOD provides a novel mechanism of macrophage alternative activation.
In contrast to the rapidly fluctuating post-translational modifications by upstream signaling proteins (kinase, phosphatase, etc.), studies indicate that epigenetic modulations have a more sustained effect (11). Recent advances in cardiovascular and cancer research suggest a potential link between ROS and epigenetic modifications. ROS can recruit DNA methyltransferases to chromatin and lead to hypomethylation in atherosclerosis (28). In addition to methylation, ROS have been shown to induce H4 acetylation and decrease histone deacetylase activity (29). ROS promotes histone acetylation by increasing recruitment of histone acetylase coactivators, such as CREB-binding protein/p300 (CBP/p300) (30); however, it is largely unknown if ROS dynamically regulates histone methylation. Macrophage differentiation is rigorously regulated epigenetically, especially through the histone modifications. Methylation of histone H3 at lysine-4, -36, and -79 is associated with activation of transcription, whereas methylation of histone H3 at lysine-9 and -27, and histone H4 at lysine-20, correlates with repression of transcription. Trimethylation of H3K27 represses transcription of M2 genes, such as arginase-1, and Jmjd3 is a specific demethylase for H3K27 me3 (12, 31–33). In fact, Jmjd3 has no effect on H3K27 me2 or methylations of H3K4 or H3K9 (33).
Leflunomide was initially approved for the treatment of rheumatoid arthritis, and it’s been associated with interstitial pneumonitis. There is some controversy if leflunomide is the culprit for interstitial lung disease in patients with rheumatoid arthritis. Studies indicate that the pneumonitis exhibits a variable incidence, and subjects with rheumatoid arthritis had interstitial pneumonitis before receiving leflunomide or had previously received methotrexate (34, 35). Alternatively activated macrophages are involved in hepatic fibrosis and contribute to the profibrotic environment. Leflunomide has been shown to suppress hepatic fibrosis in CCl4 and leptin-induced animal models (36, 37). Fibrotic markers, such as collagen Ia, hydroxyproline, and TGF-β, are decreased after systemic leflunomide treatment, but these studies did not investigate the involvement of macrophages. Despite these reports on the potential efficacy of leflunomide in treating fibrosis in vivo, little is known about the biological mechanism of how leflunomide works. Studies have shown that leflunomide treatment actively regulates matrix metalloproteinases (38–40) and promotes epithelial–mesenchymal transition (41), both of which have been linked to fibrotic development. The exact role of metalloproteinases and epithelial–mesenchymal transition in pulmonary fibrosis in vivo remains controversial. The observations in this report reveal a novel molecular mechanism by which leflunomide modulates the macrophage phenotype and attenuates the development of pulmonary fibrosis.
Acknowledgments
Acknowledgments
The authors thank Dr. Garry R. Buettner, Brett Wagner, and the Electron Spin Resonance Facility at the University of Iowa (Iowa City, IA) for assistance with oxygen consumption measurement, and Dr. Frederick Domann for the helpful discussions.
Footnotes
This work was supported by National Institutes of Health grant 2R01ES015981-07 and a Merit Review from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development 1BX001135-03.
Author Contributions: Conception and design—C.H. and A.B.C.; analysis and interpretation—C.H., J.L.L.-C., L.G., A.J.R., S.M., and A.B.C.; writing of the manuscript—C.H. and A.B.C.
Originally Published in Press as DOI: 10.1165/rcmb.2015-0183OC on December 23, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, Ieronymaki E, Androulidaki A, Venihaki M, Margioris AN, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci USA. 2012;109:9517–9522. doi: 10.1073/pnas.1119038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 3.He C, Murthy S, McCormick ML, Spitz DR, Ryan AJ, Carter AB. Mitochondrial Cu,Zn–superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J Biol Chem. 2011;286:15597–15607. doi: 10.1074/jbc.M110.187377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murthy S, Ryan A, He C, Mallampalli RK, Carter AB. Rac1-mediated mitochondrial H2O2 generation regulates MMP-9 gene expression in macrophages via inhibition of SP-1 and AP-1. J Biol Chem. 2010;285:25062–25073. doi: 10.1074/jbc.M109.099655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fridovich I. Superoxide anion radical (), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 6.He C, Ryan AJ, Murthy S, Carter AB. Accelerated development of pulmonary fibrosis via Cu,Zn–superoxide dismutase–induced alternative activation of macrophages. J Biol Chem. 2013;288:20745–20757. doi: 10.1074/jbc.M112.410720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heller NM, Qi X, Junttila IS, Shirey KA, Vogel SN, Paul WE, Keegan AD. Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Sci Signal. 2008;1:ra17. doi: 10.1126/scisignal.1164795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron MJ, Arreaza GA, Zucker P, Chensue SW, Strieter RM, Chakrabarti S, Delovitch TL. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in nonobese diabetic mice by potentiation of regulatory T helper-2 cell function. J Immunol. 1997;159:4686–4692. [PubMed] [Google Scholar]
- 9.Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34:216–223. doi: 10.1016/j.it.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang IV, Schwartz DA. Epigenetic control of gene expression in the lung. Am J Respir Crit Care Med. 2011;183:1295–1301. doi: 10.1164/rccm.201010-1579PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, Carson WF, IV, Cavassani KA, Li X, Lukacs NW, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114:3244–3254. doi: 10.1182/blood-2009-04-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murthy S, Adamcakova-Dodd A, Perry SS, Tephly LA, Keller RM, Metwali N, Meyerholz DK, Wang Y, Glogauer M, Thorne PS, et al. Modulation of reactive oxygen species by Rac1 or catalase prevents asbestos-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;297:L846–L855. doi: 10.1152/ajplung.90590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter AB, Hunninghake GW. A constitutive active MEK → ERK pathway negatively regulates NF-κB-dependent gene expression by modulating TATA-binding protein phosphorylation. J Biol Chem. 2000;275:27858–27864. doi: 10.1074/jbc.M003599200. [DOI] [PubMed] [Google Scholar]
- 15.Wagner BA, Venkataraman S, Buettner GR. The rate of oxygen utilization by cells. Free Radic Biol Med. 2011;51:700–712. doi: 10.1016/j.freeradbiomed.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 17.Brüne B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T, von Knethen A, Weigert A. Redox control of inflammation in macrophages. Antioxid Redox Signal. 2013;19:595–637. doi: 10.1089/ars.2012.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess DT, Stamler JS. Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem. 2012;287:4411–4418. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brüne B, Zhou J. Nitric oxide and superoxide: interference with hypoxic signaling. Cardiovasc Res. 2007;75:275–282. doi: 10.1016/j.cardiores.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Murata Y, Shimamura T, Hamuro J. The polarization of T(h)1/T(h)2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int Immunol. 2002;14:201–212. doi: 10.1093/intimm/14.2.201. [DOI] [PubMed] [Google Scholar]
- 21.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tavakoli S, Zamora D, Ullevig S, Asmis R. Bioenergetic profiles diverge during macrophage polarization: implications for the interpretation of 18F-FDG PET imaging of atherosclerosis. J Nucl Med. 2013;54:1661–1667. doi: 10.2967/jnumed.112.119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Chi F, Guo T, Punj V, Lee WN, French SW, Tsukamoto H. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J Clin Invest. 2015;125:1579–1590. doi: 10.1172/JCI76468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan MH, Whitfield JR, Boros DL, Grusby MJ. Th2 cells are required for the Schistosoma mansoni egg–induced granulomatous response. J Immunol. 1998;160:1850–1856. [PubMed] [Google Scholar]
- 27.Joshi AD, Raymond T, Coelho AL, Kunkel SL, Hogaboam CM. A systemic granulomatous response to Schistosoma mansoni eggs alters responsiveness of bone-marrow–derived macrophages to Toll-like receptor agonists. J Leukoc Biol. 2008;83:314–324. doi: 10.1189/jlb.1007689. [DOI] [PubMed] [Google Scholar]
- 28.Hiltunen MO, Turunen MP, Häkkinen TP, Rutanen J, Hedman M, Mäkinen K, Turunen AM, Aalto-Setälä K, Ylä-Herttuala S. DNA hypomethylation and methyltransferase expression in atherosclerotic lesions. Vasc Med. 2002;7:5–11. doi: 10.1191/1358863x02vm418oa. [DOI] [PubMed] [Google Scholar]
- 29.Rahman I. Oxidative stress, transcription factors and chromatin remodelling in lung inflammation. Biochem Pharmacol. 2002;64:935–942. doi: 10.1016/s0006-2952(02)01153-x. [DOI] [PubMed] [Google Scholar]
- 30.Escobar J, Pereda J, López-Rodas G, Sastre J. Redox signaling and histone acetylation in acute pancreatitis. Free Radic Biol Med. 2012;52:819–837. doi: 10.1016/j.freeradbiomed.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 31.De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Kruidenier L, Chung CW, Cheng Z, Liddle J, Che K, Joberty G, Bantscheff M, Bountra C, Bridges A, Diallo H, et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488:404–408. doi: 10.1038/nature11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang Y, Zhu Z, Han G, Lin H, Xu L, Chen CD. JMJD3 is a histone H3K27 demethylase. Cell Res. 2007;17:850–857. doi: 10.1038/cr.2007.83. [DOI] [PubMed] [Google Scholar]
- 34.Kamata Y, Nara H, Kamimura T, Haneda K, Iwamoto M, Masuyama J, Okazaki H, Minota S. Rheumatoid arthritis complicated with acute interstitial pneumonia induced by leflunomide as an adverse reaction. Intern Med. 2004;43:1201–1204. doi: 10.2169/internalmedicine.43.1201. [DOI] [PubMed] [Google Scholar]
- 35.Suissa S, Hudson M, Ernst P. Leflunomide use and the risk of interstitial lung disease in rheumatoid arthritis. Arthritis Rheum. 2006;54:1435–1439. doi: 10.1002/art.21806. [DOI] [PubMed] [Google Scholar]
- 36.Si HF, Li J, Lü XW, Jin Y. Suppressive effects of leflunomide on leptin-induced collagen I production involved in hepatic stellate cell proliferation. Exp Biol Med (Maywood) 2007;232:427–436. [PubMed] [Google Scholar]
- 37.Yao HW, Li J, Chen JQ, Xu SY. Inhibitory effect of leflunomide on hepatic fibrosis induced by CCl4 in rats. Acta Pharmacol Sin. 2004;25:915–920. [PubMed] [Google Scholar]
- 38.Huang JL, Wu SY, Xie XJ, Wang MX, Zhu S, Gu JR. Inhibiting effects of leflunomide metabolite on overexpression of CD147, MMP-2 and MMP-9 in PMA differentiated THP-1 cells. Eur J Pharmacol. 2011;670:304–310. doi: 10.1016/j.ejphar.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 39.Ohta Y, Hayashi M, Kanemaru T, Abe K, Ito Y, Oike M. Dual modulation of airway smooth muscle contraction by Th2 cytokines via matrix metalloproteinase-1 production. J Immunol. 2008;180:4191–4199. doi: 10.4049/jimmunol.180.6.4191. [DOI] [PubMed] [Google Scholar]
- 40.Tchetverikov I, Kraan MC, van El B, Hanemaaijer R, DeGroot J, Huizinga TW. Leflunomide and methotrexate reduce levels of activated matrix metalloproteinases in complexes with α2 macroglobulin in serum of rheumatoid arthritis patients. Ann Rheum Dis. 2008;67:128–130. doi: 10.1136/ard.2006.067827. [DOI] [PubMed] [Google Scholar]
- 41.Namba T, Tanaka KI, Ito Y, Hoshino T, Matoyama M, Yamakawa N, Isohama Y, Azuma A, Mizushima T. Induction of EMT-like phenotypes by an active metabolite of leflunomide and its contribution to pulmonary fibrosis. Cell Death Differ. 2010;17:1882–1895. doi: 10.1038/cdd.2010.64. [DOI] [PubMed] [Google Scholar]