Abstract
Bronchopulmonary dysplasia (BPD), a chronic lung disease of prematurity, has been linked to endoplasmic reticulum (ER) stress. To investigate a causal role for ER stress in BPD pathogenesis, we generated conditional knockout (KO) mice (cGrp78f/f) with lung epithelial cell–specific KO of Grp78, a gene encoding the ER chaperone 78-kD glucose-regulated protein (GRP78), a master regulator of ER homeostasis and the unfolded protein response (UPR). Lung epithelial–specific Grp78 KO disrupted lung morphogenesis, causing developmental arrest, increased alveolar epithelial type II cell apoptosis, and decreased surfactant protein and type I cell marker expression in perinatal lungs. cGrp78f/f pups died immediately after birth, likely owing to respiratory distress. Importantly, Grp78 KO triggered UPR activation with marked induction of the proapoptotic transcription factor CCAAT/enhancer-binding proteins (C/EBP) homologous protein (CHOP). Increased expression of genes involved in oxidative stress and cell death and decreased expression of genes encoding antioxidant enzymes suggest a role for oxidative stress in alveolar epithelial cell (AEC) apoptosis. Increased Smad3 phosphorylation and expression of transforming growth factor-β/Smad3 targets Cdkn1a (encoding p21) and Gadd45a suggest that interactions among the apoptotic arm of the UPR, oxidative stress, and transforming growth factor-β/Smad signaling pathways contribute to Grp78 KO–induced AEC apoptosis and developmental arrest. Chemical chaperone Tauroursodeoxycholic acid reduced UPR activation and apoptosis in cGrp78f/f lungs cultured ex vivo, confirming a role for ER stress in observed AEC abnormalities. These results demonstrate a key role for GRP78 in AEC survival and gene expression during lung development through modulation of ER stress, and suggest the UPR as a potential therapeutic target in BPD.
Keywords: 78-kD glucose-regulated protein knockout, endoplasmic reticulum stress/unfolded protein response signaling, alveolar epithelial cells, apoptosis
Clinical Relevance
Bronchopulmonary dysplasia (BPD) is a major cause of mortality and morbidity in preterm neonates. Recent studies in mouse models as well as patients with BPD have suggested a role for endoplasmic reticulum (ER) stress in the pathogenesis of BPD. The chaperone 78-kD glucose-regulated protein (GRP78) is a master regulator of ER homeostasis and the unfolded protein response (UPR). In this study, analysis of mice with lung epithelial–specific knockout of Grp78 revealed developmental arrest, impaired alveolar epithelial cell (AEC) differentiation, and increased alveolar epithelial type II cell apoptosis in perinatal lungs. These results demonstrate a key role for GRP78 in AEC survival and differentiation during lung development through modulation of ER stress/UPR signaling. Pharmaceutical targeting of the ER stress/UPR pathway might be a potential treatment strategy in BPD.
Endoplasmic reticulum (ER) is the intracellular site of maturation and folding of secretory and membrane proteins. Disturbances in the ER lumenal environment, genetic mutations, viral infection, and other insults cause misfolded proteins to accumulate in the ER (1–3). ER stress results when the capacity of the ER to process misfolded proteins is exceeded. Eukaryotic cells have developed a defense mechanism, known as the unfolded protein response (UPR), which alleviates ER stress to promote cell survival, but can trigger apoptosis if ER stress is severe or prolonged. Activation of all three arms of UPR signaling via protein kinase RNA (PKR)-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6) initiates adaptive responses to increase protein folding capacity and degradation of misfolded proteins and inhibit protein translation (4). Severe or prolonged ER stress leads to apoptosis via activation of c-Jun amino terminal kinase (JNK) signaling and CCAAT/enhancer-binding proteins (C/EBP) homologous protein (CHOP) by IRE1α and PERK, respectively (4–6).
The chaperone protein 78 kD glucose-regulated protein (GRP78; also known as BiP/HSPA5) is an important modulator of ER stress/UPR signaling that binds to PERK, IRE1α, and ATF6 in nonstressed cells. In response to stress, sequestration of GRP78 by misfolded proteins leads to its release from UPR effector molecules, resulting in UPR activation (4, 7). GRP78 itself plays a crucial role in protein folding, translocation, and degradation, and its up-regulation upon UPR activation protects against apoptosis (8, 9). Loss of GRP78 leads to ER stress and UPR activation in a number of contexts. For example, GRP78 cleavage by subtilase cytotoxin in eukaryotic cells induces severe ER stress and UPR signaling (10). Homozygous deletion of Grp78 is embryonically lethal, due to ER stress and apoptosis of the inner cell mass (11). In tissue-specific Grp78 knockout (KO) animal models, resulting ER stress and activation of the UPR causes apoptosis of hematopoietic stem cells (12), while inducing differentiation of progenitor cells in intestine and esophagus (13, 14), suggesting context-dependent roles for GRP78-regulated UPR signaling/ER homeostasis in stem/progenitor cell survival versus differentiation during development and in the adult.
Lung development is initiated at Embryonic Day (E) 9.5 in the mouse (15) with budding of an epithelial tube from anterior foregut endoderm. Late lung maturation (E16.5–Postnatal Day 5) is characterized by reduction in cell proliferation and emergence of more differentiated cell types, including alveolar epithelial type (AT) 1 and AT2 cells in distal lung (15). AT2 cells are cuboidal, surfactant-producing cells containing lamellar bodies that serve as progenitors for AT1 cells (which provide the major surface area for gas exchange) during development and after injury (16–18). Although signaling pathways that regulate lung morphogenesis and maturation have been extensively evaluated, effects of GRP78-modulated ER stress/UPR signaling on lung development and cellular differentiation have not been fully explored. Newborn mice expressing mutant GRP78 lacking the Lys-Asp-Glu-Leu (KDEL) retrieval sequence needed for recycling of GRP78 protein show atelectasis and respiratory failure due to impaired surfactant protein biosynthesis, although lung development before birth is normal (19). This mutant GRP78 retains sufficient chaperone function to circumvent prenatal defects, and the effect of complete GRP78 deficiency during lung maturation and distal epithelial cell differentiation has not been investigated.
Bronchopulmonary dysplasia (BPD) is a major complication of premature birth, characterized by alveolar simplification, surfactant deficiency, and respiratory distress (20). A recent study showed elevated expression of UPR components and increased apoptosis in a mouse model of hyperoxia-induced BPD and in patients with BPD, suggesting a role for ER stress/UPR signaling in BPD pathogenesis (21). In transgenic mice overexpressing a disease-linked mutation in the C-terminal peptide of surfactant protein C (SFTPC) in AT2 cells, Bridges and colleagues (22) reported that accumulation of mutant SFTPC protein (SFTPCΔexon4) perturbed lung development due to epithelial cell cytotoxicity, although epithelial cell apoptosis, as the result of ER stress/UPR activation, was not directly addressed. Although these studies are suggestive, a causal role for ER stress in disruption of lung maturation/distal lung epithelial cell differentiation has not been established.
To more directly evaluate effects of ER stress/UPR signaling on lung development and epithelial cell maturation, we generated lung epithelial cell–specific Grp78 KO mice (cGrp78f/f), with the genotype SFTPC-cre+/−;Grp78f/f, by breeding Grp78f/f with SFTPC-cre mice. cGrp78f/f mice died shortly after birth, and fetal KO lungs displayed defects in morphogenesis and alveolar epithelial cell (AEC) phenotype associated with AT2 cell apoptosis, likely resulting from ER stress/UPR-mediated cross-talk with oxidative stress and transforming growth factor (TGF)-β/Smad signaling. These results indicate that GRP78 is required for pulmonary epithelial survival/differentiation, likely through modulation of UPR signaling/ER homeostasis during lung development, and suggest that interventions to modulate UPR signaling may offer effective strategies to treat neonatal lung diseases, such as BPD.
Materials and Methods
Generation of cGrp78f/f Mice and Animal Husbandry
Heterozygous cGrp78+/f mice (genotype SFTPC-cre+/−;Grp78+/f) were generated by breeding Grp78f/f mice (11) and SFTPC-cre+/− mice (23). Heterozygotes were normal and fertile, and further crossing with Grp78f/f was performed to generate homozygous cGrp78f/f mice (genotype SFTPC-cre+/−;Grp78f/f). Genotyping details are available in the online supplement. Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Southern California.
Tissue Collection
Embryos were dissected free from the uterus and lungs were collected at E14 and E18 for RNA and protein extraction and histology. Brains and hearts were collected at E18 for RNA extraction.
Quantitative RT-PCR
cDNA was synthesized using SuperScript III reverse transcriptase (Life Technologies, Carlsbad, CA) and quantitative RT-PCR (qRT-PCR) was performed with primers shown in Table E1 in the online supplement, using Power SYBR-Green PCR Master Mix (Applied Biosystems, Foster City, CA) with the 7900-HT Fast Real-Time PCR System (Applied Biosystems). Further methods are available in the online supplement.
Western Analysis
Proteins were resolved by SDS-PAGE and electrophoretically blotted onto Immun-Blot PVDF membranes (Bio-Rad, Hercules, CA). Western blots (WBs) were performed using antibodies detailed in the online supplement.
Electron Microscopy
Lung samples were fixed in 0.5× Karnovsky’s fixative (2.5% glutaraldehyde, 2% paraformaldehyde [PFA] in 0.1 M cacodylate buffer), postfixed in 2% osmium tetroxide, stained en bloc with 1% uranyl acetate overnight, dehydrated, and embedded in Epon for transmission electron microscopy, according to standard procedures. Ultrathin sections were photographed on a JEOL JEM-2100 Electron Microscope operated at 100 kV (JEOL, Tokyo, Japan).
Immunostaining
Lung samples were fixed in 4% PFA overnight, except E18 lungs used for Pro-surfactant protein C (pro-SFTPC) staining that were fixed in Carnoy’s solution (60% ethanol, 30% chloroform, and 10% glacial acetic acid), and processed into paraffin sections using routine procedures. Antibody staining protocols are available in the online supplement.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Assay
Detection of apoptosis based on labeling of DNA strand breaks was performed using the In Situ Cell Death Detection Kit (no. 11684795910; Roche Diagnostics USA, Indianapolis, IN) according to the manufacturer’s instructions.
Microarray Analysis of Gene Expression
RNA (1 μg) from four Grp78f/f and four cGrp78f/f lungs was converted into cRNA and used for Illumina mouseRef-8 v2 whole-genome expression analysis (Illumina, San Diego, CA). Data are available in Tables E2 and E3 in the online supplement and under Gene Expression Omnibus record GSE70208 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=gvuviiqgpnollypandacc=GSE70208). Details regarding analysis of microarray data are available in the online supplement.
Explant Culture
Lung explants were dissected from E12 Grp78f/f and cGrp78f/f mice and cultured on Whatman polycarbonate membranes (13 mm in diameter and 8 μm in pore size [VWR 28158-089]; Visalia, CA) for 6 days in Dulbecco’s modified Eagles medium/F12 containing 2% FBS (no. AXE40802; Hyclone, Logan, Utah). To inhibit ER stress, Tauroursodeoxycholic acid (TUDCA; no. 580549; EMD Millipore, Temecula, CA) was added at 250 μM on the second day of culture and media changed every 48 hours. PBS was used as vehicle control. After culture, explants were photographed using a Spot FLEX digital camera (SPOT Imaging, Sterling Heights, MI) and then fixed in 4% PFA for histology.
Results
Lung Epithelial Cell-Specific KO of Grp78 Causes Early Perinatal Lethality
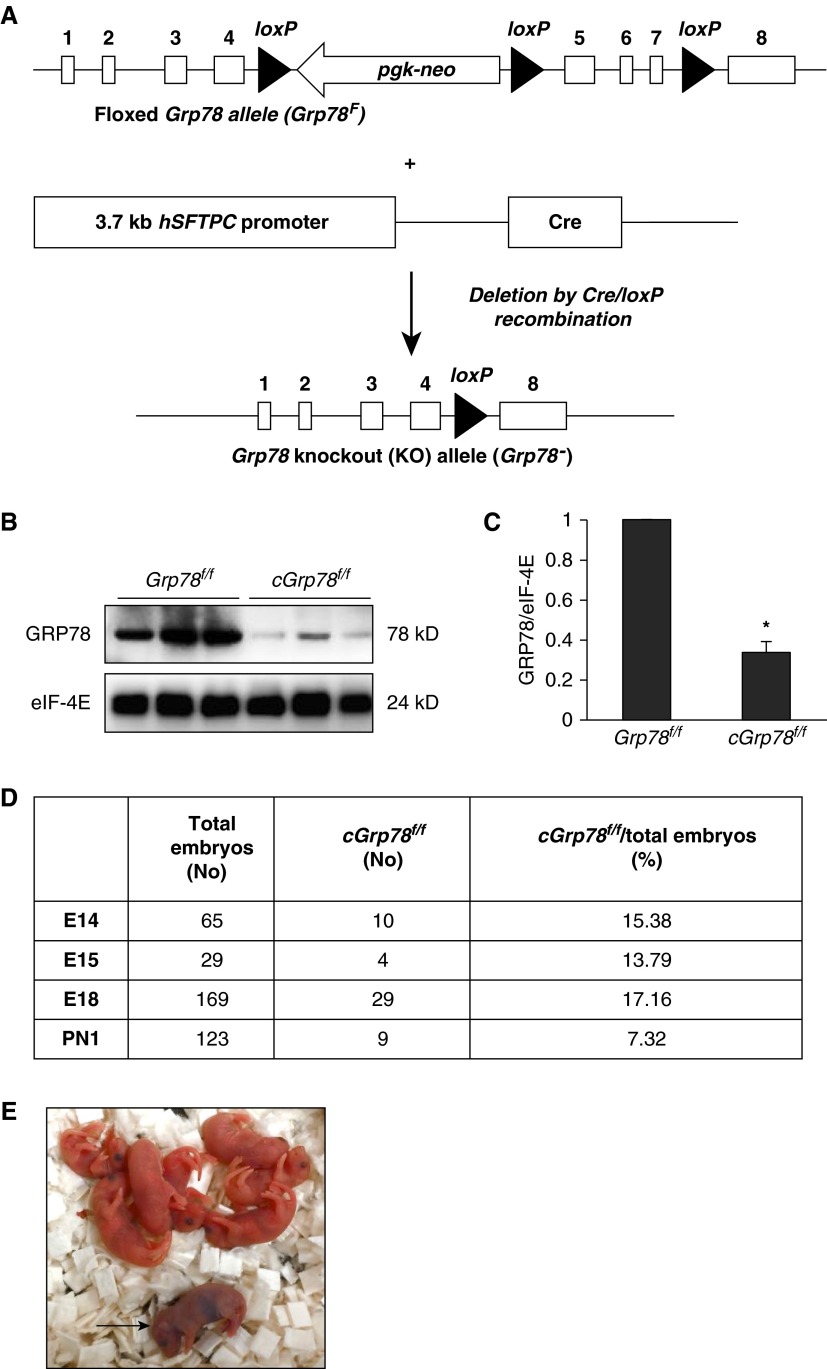
Floxed Grp78 (Grp78f/f) mice (11) were crossed to SFTPC-cre+/− mice (23) to generate mice with lung epithelial cell–specific KO of Grp78 with genotype SFTPC-cre+/−;Grp78f/f (referred to as cGrp78f/f). In these mice, Cre is under control of the human SFTPC promoter, which is active in early embryonic lung development (24) and should result in deletion of floxed exons 5–7 of Grp78 throughout the lung epithelium (Figure 1A; see also Figure E1). WB using whole-lung lysates showed an approximately 70% decrease in GRP78 expression in cGrp78f/f mice compared with controls (Grp78f/f; Figures 1B and 1C). Heterozygous mice (SFTPC-cre+/−;Grp78+/f) were alive and healthy with a normal lifespan, and did not show any abnormalities, similar to previously reported heterozygous conventional Grp78 KO mice (11). There was a slight increase in embryonic lethality in KOs, as the number of cGrp78f/f embryos was less than the expected Mendelian ratio of 25% throughout development, beginning as early as E14 (Figure 1D). Embryonic lethality is likely due to KO of Grp78 in fetal membranes (amnion and chorion) and placenta, because SFTPC expression was detected in these tissues (25). cGrp78f/f mice did not survive after birth. The very few KO pups that were born died within roughly 12 hours with respiratory distress, including cyanosis (Figure 1E), indicating that GRP78 is required for postnatal survival. Cre expression in brain and heart was detected by PCR at E18, suggesting that brain and heart defects due to Grp78 deletion may also contribute to early postnatal mortality of cGrp78f/f mice (Figure E2).
Figure 1.
Embryonic and early perinatal lethality in chaperone 78-kD glucose-regulated protein (cGrp78f/f) mice. (A) Schematic diagram of floxed Grp78 (Grp78f) and knockout (KO) alleles (Grp78−). Western blotting (WB) (B) and corresponding quantitative analysis (C) show significantly decreased GRP78 expression in cGrp78f/f whole-lung lysates at Embryonic Day 18 (E18). Eukaryotic translation Initiation factor 4E (eIF-4E) was used as loading control. *P < 0.05, n = 3. (D) The number of cGrp78f/f embryos and live births at Postnatal Day 1 (PN1) was less than the expected Mendelian ratio of 25%. (E) cGrp78f/f mice (arrow) died within a few hours after birth. hSFTPC, human surfactant protein C.
GRP78 Is Necessary for Normal Distal Lung Maturation
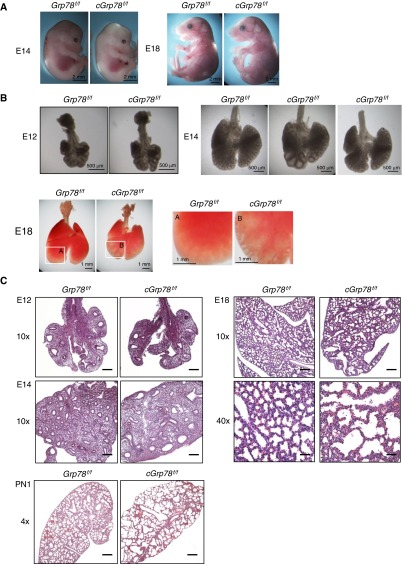
cGrp78f/f embryos appear normal at E14 and E18 (Figure 2A). At E12, no significant differences were observed between Grp78f/f and cGrp78f/f lungs. At E14, cGrp78f/f lungs appeared slightly smaller in size, with dilated distal airways compared with control lungs, although the severity of dilation varied among individual lungs. These phenotypes persisted until E18 (Figure 2B). As shown in Figure 2C, hematoxylin and eosin staining revealed that the distal airways were indeed dilated in E14 cGrp78f/f lungs. At E18, enlarged saccules with irregular size, thickened septal walls, and large amounts of cellular debris were observed in cGrp78f/f lungs. At Postnatal Day 1, KO lungs appeared simplified, with thin saccular septa (Figure 2C). Furthermore, consistent with previous findings that heterozygous conventional Grp78 KO mice are normal, morphology of lung epithelial–specific heterozygous Grp78 KO mice was normal (Figure E3).
Figure 2.
Abnormal lung morphology in cGrp78f/f mice. (A) Embryos appear macroscopically normal at both E14 and E18. (B) Lungs are morphologically normal in cGrp78f/f embryos at E12 but display dilated distal airspaces at E14 and E18. Right panels for E18 are magnified insets of the left panels A and B, showing clear dilation. Lungs of cGrp78f/f embryos also appear smaller at E18. (C) Hematoxylin and eosin staining shows normal lung morphology at E12, with dilated distal airways by E14. At E18, airspace enlargement, irregular airspace size, and cell debris in the airspaces is evident in cGrp78f/f but not in Grp78f/f mice. At PN1, lungs display alveolar hypoplasia, alveolar airspace enlargement, and thin mesenchyme. Scale bars: 200 μm for E12, E14, and E18 at 10× magnification, 50 μm at 40× magnification, and 500 μm for PN1.
Expression of AEC Differentiation Markers Is Reduced in Lungs of cGrp78f/f Mice
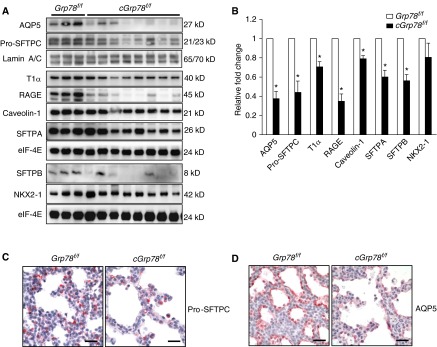
To examine if GRP78 has a function in AEC differentiation, WB was performed to evaluate expression of AT2 and AT1 cell markers in cGrp78f/f mice at E18 using whole-lung lysates. Although AT2 cell marker NK2 Homeobox 1 (NKX2-1), expression was unchanged, expression of other AT2 cell markers, surfactant protein A (SFTPA), surfactant protein B (SFTPB), and surfactant protein C (SFTPC), and AT1 cell markers, Aquaporin5 (AQP5), T1α, receptor for advanced glycation endproducts (RAGE), and caveolin-1, was significantly reduced in cGrp78f/f mice (Figures 3A and 3B). Consistent with these findings, immunostaining demonstrated reduced expression of pro-SFTPC and AQP5 in lungs of cGrp78f/f mice at E18 (Figures 3C and 3D). Expression levels of the transcripts encoding SFTPC, SFTPA, NKX2-1, T1α, and RAGE (Sftpc, Sftpa, Nkx2-1, Pdoplanin (Pdpn) and Advanced Glycosylation End Product-Specific Receptor [Ager]) were also significantly reduced in cGrp78f/f mice (Figure E4).
Figure 3.
KO of Grp78 reduces expression of alveolar epithelial type (AT) 2 and AT1 cell markers. Representative WB using whole-lung lysates (A) and corresponding quantitative analysis (B) show decreased expression of AT2 and AT1 cell markers in cGrp78f/f mice at E18 (n ≥ 3, *P < 0.05). Immunohistochemistry confirmed decreased expression of pro-SFTPC (red) (C) and AQP5 (red) (D) in cGrp78f/f mice at E18. Scale bars: 50 μm. AQP5, Aquaporin5; NKX2-1, NK2 homeobox 1; RAGE, receptor for advanced glycation endproducts; SFTPA, surfactant protein A; SFTPB, surfactant protein B.
Activation of ER Stress/UPR Signaling in Lungs of cGrp78f/f Mice
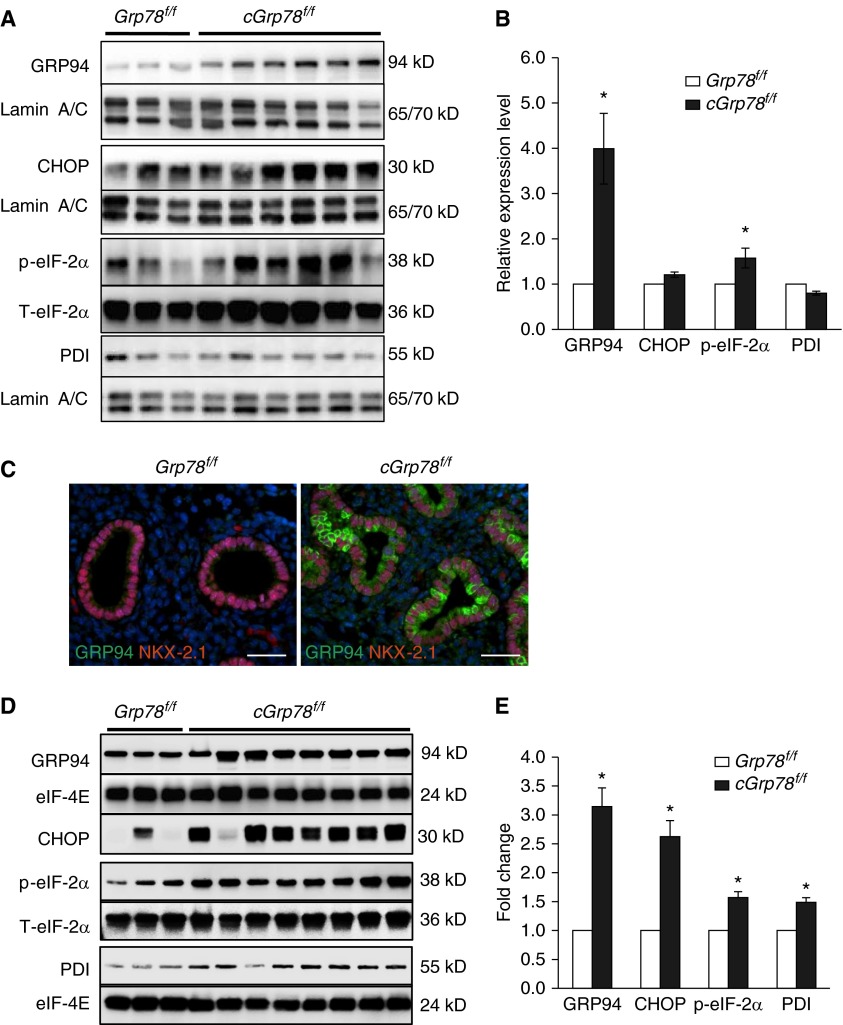
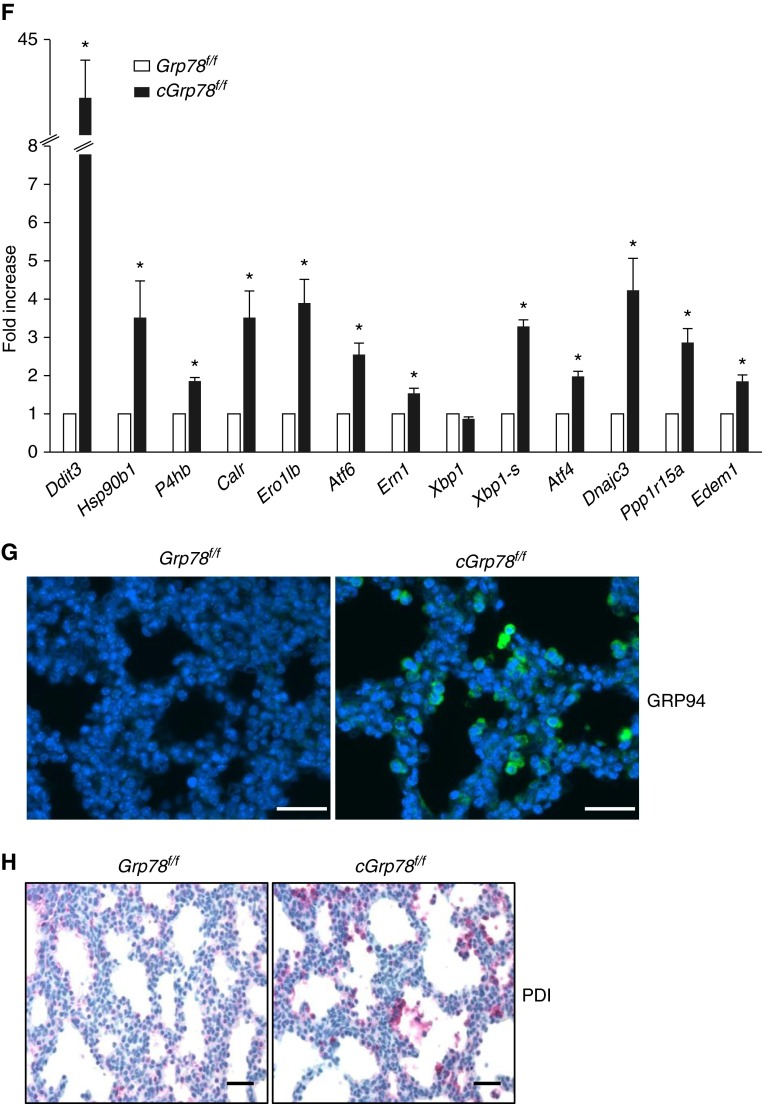
To investigate effects of Grp78 deficiency on UPR signaling in AECs, lungs were harvested from embryos at E14 and E18. WB showed increased GRP94 and p-eIF2α expression at E14 in cGrp78f/f lungs (Figures 4A and 4B). Immunostaining and colocalization with NKX2-1 at E14 showed increased GRP94 specifically in respiratory epithelial cells (Figure 4C). At E18, GRP94, p-eIF2α, CHOP, and protein disulfide isomerase (PDI) were all significantly increased (Figures 4D and 4E). Furthermore, qRT-PCR analysis showed increased expression of transcripts encoding GRP94 (Hsp90b1), CHOP (Ddit3), PDI (P4 hb), and other major UPR components in cGrp78f/f mice (Figure 4F). Immunostaining showed that cells strongly expressing GRP94 and PDI are epithelial cells lining the saccules (Figures 4G and 4H) in distal lung. We evaluated whether Grp78 KO also induces ER stress in bronchiolar epithelial cells at E18. Although ER stress markers, GRP94 and PDI, were increased in AECs, they were not induced in bronchiolar epithelial cells at E18 (Figures E5A and E5B). Moreover, club cell marker CCSP (SCGB1A1) immunostaining appeared similar in cGrp78f/f and Grp78f/f mice (Figure E5C), together indicating selective effects of Grp78 KO on AT2 cells.
Figure 4.
Unfolded protein response activation in lungs of cGrp78f/f mice. Representative WB using whole-lung lysates (A) and corresponding quantitative analysis (B) show significant increases of GRP94 and p-eiF2α protein in E14 lungs of cGrp78f/f mice (n ≥ 3, *P < 0.05). (C) Immunostaining demonstrates increased GRP94 expression in NKX2-1+ epithelial cells of Grp78 KO cGrp78f/f mice at E14. Scale bars: 50 μm. Representative WB (D) and corresponding quantitative analysis (E) using whole-lung lysates shows significant increases of GRP94, CCAAT/enhancer-binding proteins (C/EBP) homologous protein (CHOP), p-eiF2α, and protein disulfide isomerase (PDI) in E18 lung of cGrp78f/f mice (n ≥ 3, *P < 0.05). (F) Quantitative RT-PCR (qRT-PCR) shows increased mRNA expression of Hsp90b1, Ddit3, and P4 hb (encoding GRP94, CHOP, and PDI), and other endoplasmic reticulum (ER) stress markers in E18 lungs of cGrp78f/f mice (n = 3, *P < 0.05). Immunostaining demonstrates increased GRP94 (green) (G) and PDI (red) (H) expression in epithelial cells lining the airspaces in cGrp78f/f mice at E18. Scale bars: 50 μm.
Altered ER Structure in Lungs of cGrp78f/f Mice
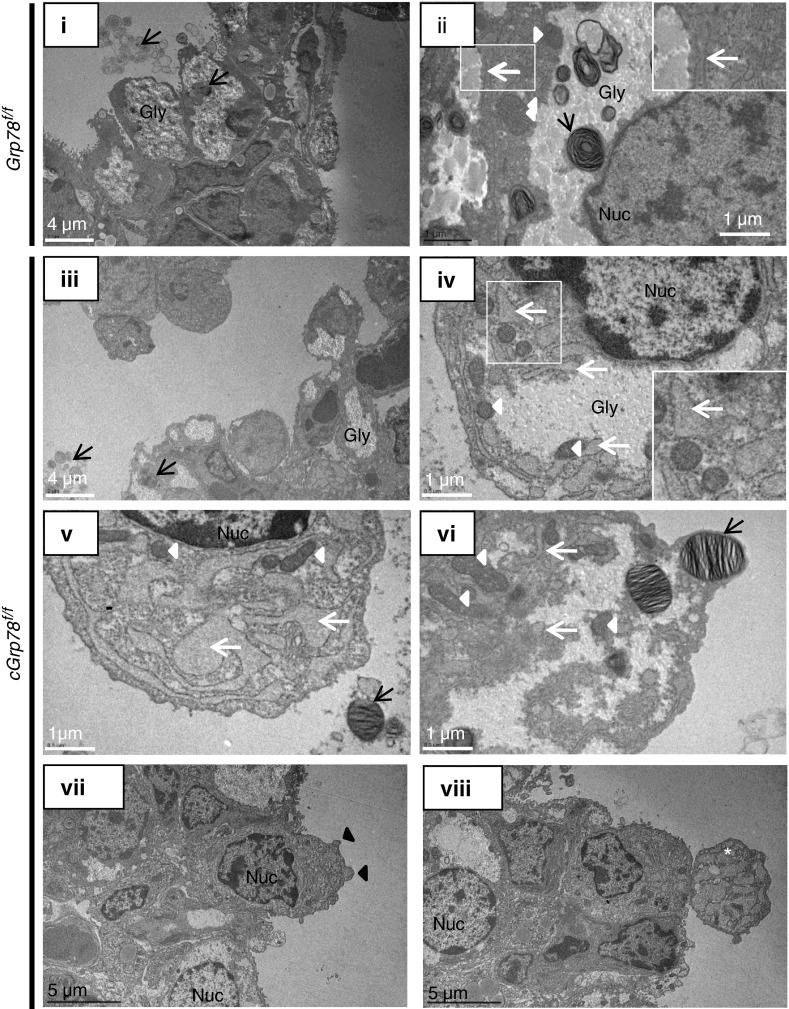
ER expansion has been reported in cells undergoing ER stress (26, 27), leading us to examine ER structure using electron microscopy. As shown in Figure 5, epithelial cells are tightly packed in Grp78f/f mice (Figure 5, i), but are more loosely arranged and appear to be detaching from the epithelial layer in cGrp78f/f mice (Figure 5, iii) at E18. Abnormal ER expansion (white arrows) was observed in cGrp78f/f mice (Figures 5, iv–vi) when compared with Grp78f/f mice (Figure 5, ii) at E18. Although secreted lamellar bodies and normally developed intracellular lamellar bodies were observed in cGrp78f/f mice (black arrows), lamellar bodies were seldom observed in cells with greater ER expansion (Figures 5, iv, v, vii, and viii). In addition, whereas there was no increase in the number of autophagosomes, more mitochondria (Figures 5, arrowheads, iv and vi), and features of apoptosis (28) (e.g., condensed chromatin at the nuclear membrane [Figures 5, iv, v, vii, and viii], cell surface blebbing [Figure 5, black arrowheads, vii], and possible apoptotic bodies [Figure 5, viii]) were observed in cGrp78f/f mice.
Figure 5.
Grp78 KO alters ER structure. Representative electron micrographs of lung at E18 show that epithelial cells are not tightly connected, and some are detaching from the epithelium (iii) and ER (white arrows) is abnormally expanded (iv, v, and vi) in cGrp78f/f compared with control Grp78f/f mice (i and ii). Lamellar bodies (black arrows) are observed within cells and in airspaces in cGrp78f/f mice (iii and vi) and Grp78f/f mice, but they are seldom observed in cells with greater ER expansion (iv and v). More mitochondria (white arrowheads) and no increase in autophagosomes are observed in cGrp78f/f mice. Marginalization and condensation of chromatin in the nucleus (iv, vii, and viii), cell surface blebbing (black arrowheads; vii) and possible apoptotic bodies (white star; viii) are observed in cGrp78f/f mice. Gly, Glycogen; Nuc, nucleus.
Increased Apoptosis in Lung Epithelial Cells in cGrp78f/f Mice
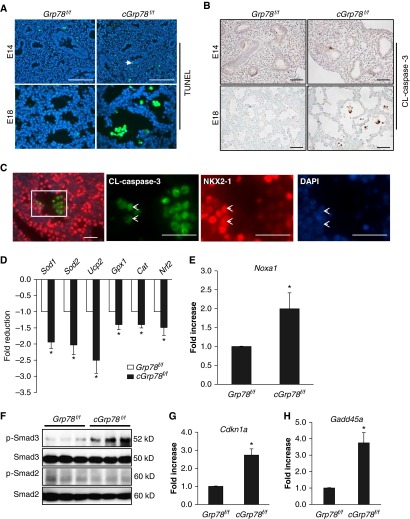
To further investigate effects of UPR activation on AEC apoptosis in cGrp78f/f mice, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays were performed in E14 and E18 lungs. Very few apoptotic epithelial cells (green, arrow) were detected in cGrp78f/f lungs at E14 (Figure 6A), whereas increased numbers of apoptotic cells located within saccules were detected in E18 lungs. Detection of cleaved (CL)-caspase 3 by immunohistochemistry confirmed that cells located in airspaces, but not bronchiolar epithelial cells (Figure E6), were undergoing apoptosis (Figure 6B). Double staining for CL-caspase 3 and NKX2-1 showed some NKX2-1–positive epithelial cells expressing CL-caspase 3 (Figure 6C, arrow), indicating that AECs undergo apoptosis in cGrp78f/f mice. Ki67 staining showed no apparent differences in cell proliferation between cGrp78f/f and Grp78f/f mice (Figure E7). Together with up-regulation of CHOP (Figure 4), these data suggest that ER stress due to Grp78 KO induces AEC apoptosis at E18. We also determined if Grp78 KO affects pulmonary vascular development by staining lung sections for endothelial marker CD31 (platelet/endothelial cell adhesion molecule 1 [PECAM-1]). No obvious differences in CD31 expression were found between Grp78f/f and cGrp78f/f embryos (Figure E8).
Figure 6.
Grp78 KO results in apoptosis, activation of oxidative stress and transforming growth factor (TGF)-β signaling in alveolar epithelial cell (AEC). (A) Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and (B) immunohistochemistry for cleaved (CL)-caspase 3 demonstrate increased numbers of TUNEL+ cells (green) and CL-caspase 3+ cells (brown) located in the airspaces in E18, but not E14, cGrp78f/f embryos. 4′,6-diamidino-2-phenylindole (DAPI) or methyl green is the nuclear counterstain. Scale bars: 50 μm. (C) Double immunofluorescence staining shows colocalization of CL-caspase 3 and NKX2-1 in cells detaching from the epithelial layer in cGrp78f/f lung. The three panels to the right are individual channels showing higher magnification of the rectangular area in the left image. Arrowheads shows CL-caspase 3+/NKX2-1+ cells. Scale bars: 50 μm. qRT-PCR using whole-lung RNA harvested from cGrp78f/f and Grp78f/f mice at E18 shows that Grp78 KO reduces expression of genes encoding antioxidant enzymes (D) and increases expression of Noxa1, a gene involved in generation of reactive oxidant species (E) (n = 3, *P < 0.05). (F) WB demonstrates that Grp78 KO increases p-Smad3, but not p-Smad2, expression. qRT-PCR shows increased expression of Cdkn1a (encoding p21) (G) and Gadd45a (H) in cGrp78f/f mice (n = 3–4, *P < 0.05).
Activation of Oxidative Stress and TGF-β Signaling in cGrp78f/f Mice
To determine if ER stress caused by absence of GRP78 activates oxidative stress in cGrp78f/f mice, qRT-PCR using whole-lung RNA harvested from embryos at E18 was performed. Loss of Grp78 reduced expression of genes encoding antioxidant enzymes, Sod1, Sod2, Usp2, Gpx1, Cat (Catalase), and Nrf2 (Figure 6D), whereas expression of Noxa1, P4 hb (PDI), Ppp1r15a (growth arrest and DNA damage-inducible protein [GADD34]), and Ero1lb (endoplasmic reticulum oxidoreductase beta [ERO1β]), genes involved in generation of reactive oxygen species (ROS), was increased (Figures 4F and 6E). In addition, p-Smad3 (but not p-Smad2) (Figure 6F), and known targets of TGF-β–mediated apoptosis, Cdkn1a (encoding p21; Figure 6G) (29) and Gadd45a (Figure 6H) (30), were increased in cGrp78f/f lungs at E18. We also examined if ER stress due to loss of GRP78 stimulated autophagy, as the autophagy pathway has been reported to be activated in response to ER stress (31). WB for microtubule-associated protein 1A/1B–light chain (LC) 3 showed no change in conversion of LC3-I (cytoplasmic form) to LC3-II (autophagosome membrane–bound form) in cGrp78f/f lungs at E18 (Figure E9).
Microarray Analysis of Messenger RNA from cGrp78f/f and Grp78f/f Lung
Microarray analysis using whole-lung RNA from E18 cGrp78f/f and Grp78f/f embryos (Figures E10A and E10B) revealed 83 up-regulated genes with greater than twofold change, with CHOP (Ddit3) at the top of the list, and 7 down-regulated genes (Figure E10B and Tables E2 and E3). Genes labeled with an asterisk (*) in Tables E2 and E3 were confirmed to be up-regulated by qRT-PCR (Figure 4). Ingenuity pathway analysis revealed that the most significantly altered pathway in cGrp78f/f lungs was ER stress/UPR signaling (Figure E10C). Analysis of gene networks and pathways affected in cGrp78f/f lungs demonstrated UPR activation, as evidenced by up-regulation of several genes involved in ER stress–mediated apoptosis and death (e.g., Ddit3, Trib3, Atf3, Lcn2, and Gadd45a), ER-associated protein degradation (e.g., Ayvn1, Herpud1, and Sel1l), and protein folding and trafficking (e.g., Mdfd2, Creld2, and Srm) (Figure E10D). Ingenuity pathway analysis also showed that genes involved in calcium, cytoskeletal, and GADD45A signaling, which are highly associated with ER homeostasis and apoptosis, were affected in cGrp78f/f E18 embryos (Figure E10C). Genes involved in protein synthesis, which lead to increased ROS generation, ATP depletion, and apoptosis in cells with ER stress (32) (Acns, Slc7a5, Slc7a11, Slc6a9, and Wars) were also increased in cGrp78f/f mice (Table E2). In microarray analysis (Table E2), Grp78 expression appears increased in cGrp78f/f lungs, presumably due to the fact that the Illumina probe used was located upstream of the portion of the gene deleted by Cre (exon 5–7). Although the remaining truncated Grp78 mRNA is up-regulated, probably due to compensatory mechanisms induced by ER stress as result of Grp78 KO, it is not translated into a detectable protein (11). All microarray data used in this study are publically available in Gene Expression Omnibus record GSE70208.
TUDCA Inhibits AEC Apoptosis in cGrp78f/f Lungs in Ex Vivo Culture
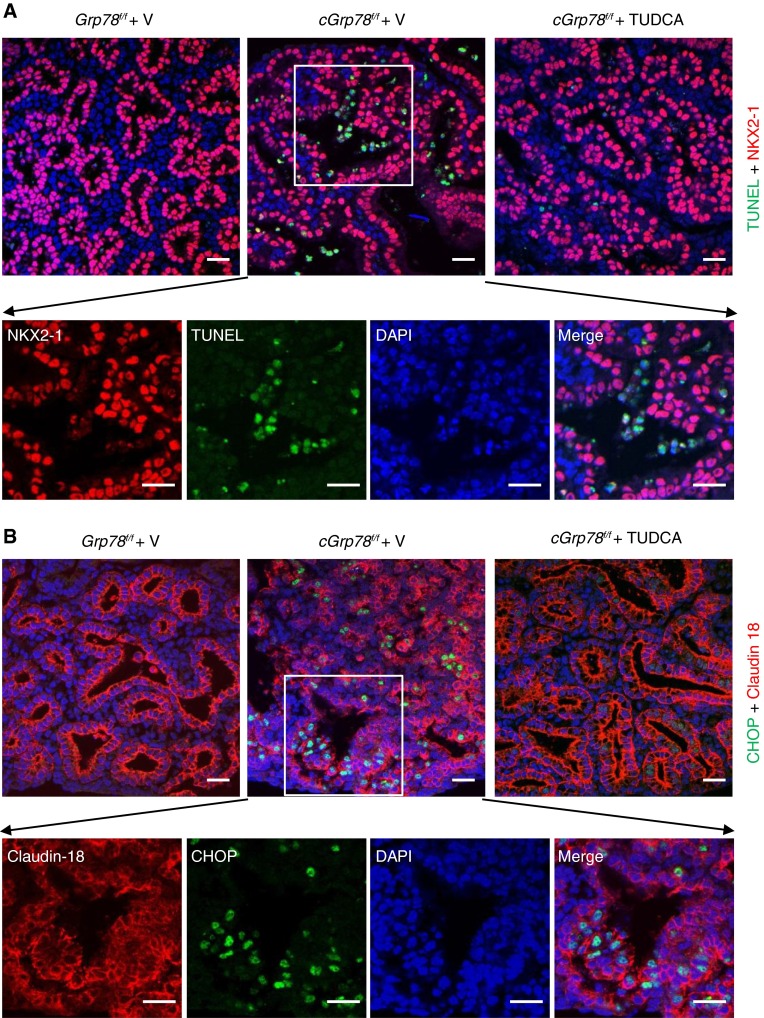
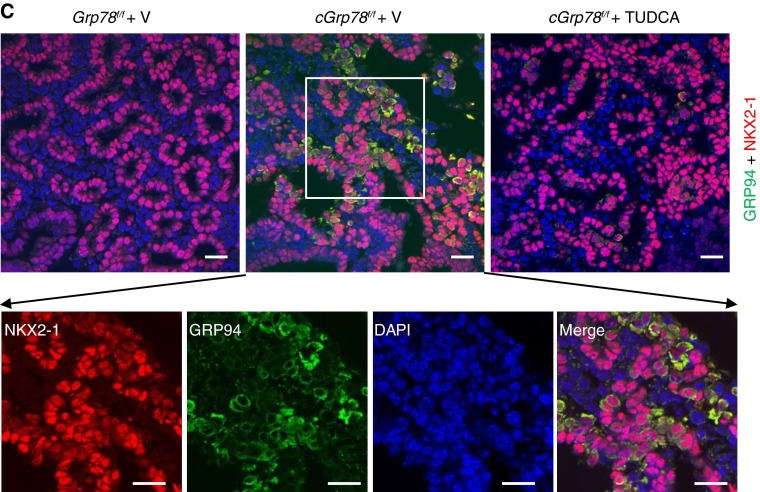
To examine whether inhibition of ER stress blocks AEC apoptosis, we cultured E12 lung explants (33) for 6 days in the presence or absence of the chemical chaperone and ER stress inhibitor, TUDCA (34, 35). Lungs of cGrp78f/f mice were normal at E12 and largely appeared to grow similarly to control lungs during 6 days in culture (Figure E11). However, TUNEL assay and immunostaining showed increased apoptosis and expression of GRP94 and CHOP in cGrp78f/f lungs cultured without TUDCA, whereas TUDCA treatment decreased TUNEL staining and expression of GRP94 and CHOP in cGrp78f/f epithelial cells (Figures 7A–7C). These data indicate that ER stress/UPR signaling mediates AEC apoptosis in cGrp78f/f mice.
Figure 7.
Tauroursodeoxycholic acid (TUDCA) inhibits AEC apoptosis in cGrp78f/f lung. (A) Staining for NKX2-1 (red) and TUNEL (green) shows increased TUNEL+ cells in cGrp78f/f (middle panel) compared with Grp78f/f lung (left panel). TUNEL+ cells in cGrp78f/f lung were decreased by TUDCA treatment (right panel). Scale bar: 20 μm. The four lower panels are individual and merged channels showing higher magnification of the rectangular area in the upper middle image. Scale bars: 20 μm. (B) Staining for tight junction protein claudin 18 (red) and CHOP (green) show increased CHOP+ cells in cGrp78f/f (middle panel) compared with Grp78f/f lung (left panel). CHOP+ cells in cGrp78f/f lung were decreased by TUDCA treatment (right panel). Scale bar: 20 μm. The four lower panels are individual and merged channels showing higher magnifications of the rectangular area in the upper middle image. Scale bar: 20 μm. (C) Staining for NKX2-1 (red) and GRP94+ (green) shows increased GRP94+ cells in cGrp78f/f lung (middle panel) compared with Grp78f/f lung (left panel). GRP94+ cells in cGrp78f/f lung were decreased by TUDCA treatment (right panel). Scale bars: 20 μm. The four lower panels are individual and merged channels showing higher magnifications of the rectangular area in the upper middle image. Scale bars: 20 μm.
Discussion
GRP78 modulates UPR activation and plays a crucial role in early embryogenesis. To examine the role of GRP78/ER stress in lung development and maturation/differentiation of distal lung epithelial cells, we generated cGrp78f/f mice with lung epithelial–specific Grp78 KO. cGrp78f/f mice died soon after birth from respiratory failure. Histological analysis revealed dilated airways in cGrp78f/f lungs at E14, whereas airspaces at E18 were enlarged and irregularly shaped, and contained considerable amounts of cell debris. Electron microscopy showed irregularly arranged and detaching AT2 cells with abnormal ER expansion, fewer lamellar bodies, and features of apoptosis. In addition, Grp78 KO resulted in reduced expression of AT2 and AT1 cell markers. Loss of Grp78 induced UPR activation at E14, which shifted to an apoptotic response at E18, as evidenced by greater induction of apoptotic ER stress marker, CHOP. Consistent with a role for GRP78 as a master regulator of ER stress/UPR signaling, these results suggest that GRP78 is required for maintenance of ER homeostasis and AEC survival during lung development. Together with activated oxidative stress signaling and TGF-β/Smad–mediated apoptotic signaling, these data suggest that interactions among UPR apoptotic response, oxidative stress, and TGF-β/Smad signaling pathways contribute to Grp78 KO–induced AEC apoptosis and resultant disruption of lung development in cGrp78f/f mice. AEC apoptosis, impaired AEC differentiation, and AT1 function, decreased surfactant protein expression, as well as oxidative stress, likely all contributed to postnatal respiratory failure.
The SFTPC-cre transgenic line used in this study has not been extensively characterized with regard to tissue specificity of Cre expression and activity. For this reason, we cannot exclude the possibility that deletion of Grp78 in other tissues might also contribute to perinatal lethality. Newborn KO pups featured clear signs of respiratory distress, including cyanosis, likely due to lung defects observed in these animals at E18. However, because our RT-PCR data showed Cre expression in brain and heart, defects in the brain and heart could also conceivably contribute to postnatal lethality in cGrp78f/f mice.
In this study, we found phenotypic effects of Grp78 gene deletion in the alveolar epithelium to be more severe compared with previously described GRP78 KDEL mutant mice (19). In this mutant, deletion of the KDEL domain makes the GRP78 protein unable to recycle back into the ER after performing protein chaperone and ER export functions. Despite decreased availability of functional GRP78 in the ER, GRP78 function is apparently sufficient to support normal development, and resultant ER stress is not severe enough to impair normal development in KDEL mutant embryos. However, postnatally, when production of surfactant protein increases, KDEL mutant GRP78 is unable to maintain ER stress homeostasis, leading to impaired SFTPC processing/production and resultant respiratory dysfunction. In the current study, chaperone function of GRP78 was completely abolished, inducing sustained ER stress in cGrp78f/f mice. This is likely the reason that we observed defects in cGrp78f/f mice already during embryonic development, whereas severe abnormalities did not appear until after birth in KDEL mutant mice.
Although the UPR is initially protective against ER stress, it promotes cell death when ER stress is severe and persistent. We observed apoptotic epithelial cells (Figures 5 and 6) in cGrp78f/f mice and strong expression of CHOP in E18, but not E14, lungs (Figure 4), despite UPR activation at E14. This suggests that a switch from an adaptive to an apoptotic UPR takes place in cGrp78f/f AT2 cells with sustained ER stress as lung development progresses. AT2 cells are specialized secretory cells for synthesis and secretion of surfactant proteins. During the saccular stage, production of surfactant proteins increases rapidly to prepare the lung for postnatal respiration. Thus, like other secretory cells (e.g., pancreatic β-cells and plasma cells [36, 37]), AT2 cells are highly dependent on ER homeostasis. Loss of GRP78 likely led to persistent ER stress (7) in AT2 cells of cGrp78f/f mice at E18, resulting in apoptosis and decreased surfactant protein production.
Grp78 loss has different effects in various tissues, including stem/progenitor cells. Tissue-specific Grp78 KO induced ER stress/apoptosis in Purkinje and liver cells (38, 39), but no abnormalities in prostate epithelial cells (40, 41). Grp78 KO induced apoptosis in inner cell mass and hematopoietic stem cells (11, 12), whereas, in intestine and esophagus, Grp78 KO forced stem/progenitor cell differentiation with no/low levels of apoptosis (13, 42). These data suggest that GRP78 regulates cell survival and function in a tissue-specific manner. In this study, unlike in stem/progenitor cells in intestine and esophagus, lung-specific Grp78 KO induced AEC apoptosis and prevented AEC differentiation, as evidenced by reduced expression of both AT2 and AT1 markers. AT2 cells are believed to be progenitors that can both self-renew and differentiate into AT1 cells. Thus, a decrease in both AT1 and AT2 cell marker expression suggests that loss of Grp78 induces apoptosis of AT2 cells, as well as arrested AT2 cell maturation and transdifferentiation into AT1 cells. Because both AT2 and AT1 cells have the Grp78 KO, AT1 cell function/survival might also be affected by ER stress caused by GRP78 deficiency, which could contribute to respiratory dysfunction after birth. Recent studies also reported the presence of bipotential progenitors at the tips of distal tubules that express both AT2 (SFTPC) and AT1 (T1α) cell markers, and have the capability to give rise to both AT1 and AT2 cell populations (43). Although not examined, we cannot exclude the possibility that bipotential epithelial progenitors also undergo ER stress–mediated apoptosis in cGrp78f/f mice. Interestingly, we did not observe UPR activation or apoptosis in bronchiolar epithelial cells, suggesting that loss of GRP78 in cGrp78f/f mice induced ER stress specifically in AECs. This difference is likely due to the fact that AT2 cells produce large amounts of surfactant protein, especially at the time of birth, making this cell type more sensitive to GRP78 deficiency from higher levels of ER stress.
Several studies have shown that PERK and IREs regulate the switch between adaptive and apoptotic UPRs. Activation of PERK upon ER stress regulates cell survival by attenuation of protein translation via Eukaryotic translation Initiation factor 2α (eIF-2α)-2α phosphorylation and promotes apoptosis via the PERK–eIF-2α–ATF4–CHOP pathway (5). CHOP up-regulates expression of genes encoding proapoptotic proteins (e.g., GADD34, death receptor 5 [DR5], ERO1β, and Tribbles 3 [TRB3]) and inhibits antiapoptotic proteins (e.g., B-cell lymphoma 2 [BCL2]) (4, 44). CHOP is also involved in regulation of oxidative stress. Up-regulation of GADD34, ERO1β, and PDI by CHOP promotes ROS generation, which, in turn, promotes apoptosis (6, 45). In addition, CHOP deletion in islet cells was associated with increased expression of genes encoding proteins with antioxidative functions (5). Recently, CHOP was found to interact with ATF4 to induce genes involved in protein synthesis, which resulted in increased ROS and cell death in stressed cells (32). In cGrp78f/f mice, CHOP was the most up-regulated gene, whereas both ATF4 and phospho (p)-eIF-2α were also up-regulated. Expression of genes involved in ER stress–mediated oxidative stress and cell death (e.g., Ero1β, Gadd34, Edem1, and Noxa1) was up-regulated, whereas expression of genes encoding antioxidant enzymes (e.g., Sod1, Sod2, Ucp2, Gpx1, Cat, and Nrf2) (Figures 4 and 6) was decreased at E18. These data suggest that ER stress–mediated AEC apoptosis in cGrp78f/f mice involves cross-talk between oxidative stress and the PERK–eIF-2α–ATF4–CHOP arm of UPR signaling, although other pathways, such as IRE1α-mediated activation of JNK signaling through apoptosis signal–regulating kinase 1/JNK may also be involved (4, 6). These data also suggest that activation of oxidative stress may increase sensitivity of cGrp78f/f mice to oxygen exposure at birth, contributing to early postnatal death. We did not find activation of autophagy in lungs of cGrp78f/f mice, as evidenced by unchanged number of autophagosomes by electron microscopy and absence of conversion of LC-3I to LC-3II. This is similar to observations in human embryonic kidney (HEK) 293 and HeLa cells after GRP78 knockdown, supporting the idea that GRP78 is required for stress-induced autophagy (9). Interestingly, in cGrp78f/f mice, Grp78 loss also increased p-Smad3, a key transducer of TGF-β signaling, and the downstream apoptosis regulators, p21 and GADD45A (46). TGF-β signaling is involved in the regulation of apoptosis in lung epithelial cells in addition to its role in branching and septation during lung development (47). Our results suggest that UPR and TGF-β/Smad signaling pathways may be tightly interconnected, contributing to AEC apoptosis, although the precise link between UPR and TGF-β/Smad signaling pathways requires further investigation.
Up-regulation of ER stress pathway components has been reported in neonatal human lungs with BPD (21). Although risk factors (e.g., hyperoxia, ventilator-induced lung injury, and antenatal infection) for BPD are well described, mechanisms underlying disrupted alveolar growth remain unclear. To date, no therapy has been identified that produces consistently efficient results (48). Our lung epithelial cell–specific cGrp78f/f mice feature arrest of alveolar development, suggesting that mice lacking Grp78 display a BPD phenocopy that is histologically consistent with what is observed in human neonates with BPD (49). Analysis of cGrp78f/f mice shows that perturbation of ER homeostasis induced AEC apoptosis via interaction of the UPR with oxidative stress and TGF-β/Smad pathways and impaired prenatal lung development/AEC differentiation, suggesting that imbalanced UPR signaling in the developing lung may contribute to BPD in neonates. Importantly, our data show that treatment with TUDCA alleviated ER stress/UPR activation, as evidenced by decreased expression of CHOP and GRP94 and reversal of ER stress–induced AEC apoptosis in cGrp78f/f lungs in ex vivo culture. TUDCA is a U.S. Food and Drug Administration–approved chemical chaperone, and has the ability to decrease ER stress and enhance the adaptive capacity of the ER by targeting PERK/CHOP and IRE/JNK pathways (35, 50). Recent evidence suggests that TUDCA protects the liver against injury and regeneration failure (34) and improves symptoms of obesity in a mouse model of severe obesity and type 2 diabetes associated with ER stress/UPR activation (35). Therefore, our data open a new perspective on the use of TUDCA as a potential therapeutic approach to modulation of ER stress/UPR signaling for treatment of BPD.
In summary, GRP78 is required in lung epithelium for neonatal survival. Loss of GRP78 causes an ER stress response and dilation of the ER compartment, and alveolar simplification accompanied by AEC apoptosis and decreased expression of differentiation markers (including surfactant proteins) in distal lung epithelial cells. These observations highlight the general importance of a properly functioning UPR regulated by GRP78 in different tissues, including AEC. In addition, because AT2 cells function as progenitor cells for both AT2 and AT1 cells, decreased survival of AT2 cells will affect the entire alveolar epithelium in the developing lung, consistent with studies showing effects of GRP78 KO on stem cell survival/differentiation in other tissues (11–14).
Footnotes
This work was supported by National Institutes of Health (NIH) grant R01 HL114959 (B.Z.), American Heart Association grant 12BGIA12060329 (P.F.), Norris Comprehensive Cancer Center Core grant P30 CA014089, and the Hastings and Whittier Foundations. Histology and microscopy services were provided by the Cell and Tissue Imaging Core of the USC Research Center for Liver Diseases (NIH P30 DK048522). Electron microscopy service was provided by the University of Southern California/Norris Cell and Tissue Imaging Core (NIH P30 CA014089).
Author Contributions: P.F. generated transgenic mice, designed animal experiments, interpreted experimental data, and wrote the manuscript; C.L. interpreted experimental data and edited the manuscript; Y.L. performed Western blots, terminal deoxynucleotidyl transferase dUTP nick end labeling assays, and explant cultures; H.W. managed mouse colonies, and performed quantitative RT-PCR and antibody staining; C.N.M. performed microarray analysis and ingenuity pathway analysis; I.A.L.-O. interpreted microarray data and edited the manuscript; P.M. conceived experiments and edited the manuscript; A.S.L. provided Grp78f/f mice, conceived experiments, and critically reviewed the manuscript; B.Z. conceived and directed experiments, interpreted experimental data, and wrote the manuscript; all authors read and approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0327OC on January 27, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–818. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 5.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014;14:263–276. doi: 10.1038/nrc3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, Mao C, Ye R, Wang M, Pen L, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 10.Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, Talbot UM, Paton JC. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443:548–552. doi: 10.1038/nature05124. [DOI] [PubMed] [Google Scholar]
- 11.Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wey S, Luo B, Lee AS. Acute inducible ablation of GRP78 reveals its role in hematopoietic stem cell survival, lymphogenesis and regulation of stress signaling. PLoS One. 2012;7:e39047. doi: 10.1371/journal.pone.0039047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heijmans J, van Lidth de Jeude JF, Koo BK, Rosekrans SL, Wielenga MC, van de Wetering M, Ferrante M, Lee AS, Onderwater JJ, Paton JC, et al. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Reports. 2013;3:1128–1139. doi: 10.1016/j.celrep.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Rosekrans SL, Heijmans J, Büller NV, Westerlund J, Lee AS, Muncan V, van den Brink GR. ER stress induces epithelial differentiation in the mouse oesophagus. Gut. 2015;64:195–202. doi: 10.1136/gutjnl-2013-306347. [DOI] [PubMed] [Google Scholar]
- 15.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamson IY, Bowden DH. Derivation of type 1 epithelium from type 2 cells in the developing rat lung. Lab Invest. 1975;32:736–745. [PubMed] [Google Scholar]
- 17.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22:142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 18.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mimura N, Hamada H, Kashio M, Jin H, Toyama Y, Kimura K, Iida M, Goto S, Saisho H, Toshimori K, et al. Aberrant quality control in the endoplasmic reticulum impairs the biosynthesis of pulmonary surfactant in mice expressing mutant BiP. Cell Death Differ. 2007;14:1475–1485. doi: 10.1038/sj.cdd.4402151. [DOI] [PubMed] [Google Scholar]
- 20.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 21.Choo-Wing R, Syed MA, Harijith A, Bowen B, Pryhuber G, Janér C, Andersson S, Homer RJ, Bhandari V. Hyperoxia and interferon-γ–induced injury in developing lungs occur via cyclooxygenase-2 and the endoplasmic reticulum stress–dependent pathway. Am J Respir Cell Mol Biol. 2013;48:749–757. doi: 10.1165/rcmb.2012-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bridges JP, Wert SE, Nogee LM, Weaver TE. Expression of a human surfactant protein C mutation associated with interstitial lung disease disrupts lung development in transgenic mice. J Biol Chem. 2003;278:52739–52746. doi: 10.1074/jbc.M309599200. [DOI] [PubMed] [Google Scholar]
- 23.Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- 24.Wert SE, Glasser SW, Korfhagen TR, Whitsett JA. Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev Biol. 1993;156:426–443. doi: 10.1006/dbio.1993.1090. [DOI] [PubMed] [Google Scholar]
- 25.Salminen A, Paananen R, Karjalainen MK, Tuohimaa A, Luukkonen A, Ojaniemi M, Jouppila P, Glasser S, Haataja R, Vuolteenaho R, et al. Genetic association of SP-C with duration of preterm premature rupture of fetal membranes and expression in gestational tissues. Ann Med. 2009;41:629–642. doi: 10.1080/07853890903186176. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schonthal AH. Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica (Cairo) 2012;2012:857516. doi: 10.6064/2012/857516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoo J, Ghiassi M, Jirmanova L, Balliet AG, Hoffman B, Fornace AJ, Jr, Liebermann DA, Bottinger EP, Roberts AB. Transforming growth factor-β–induced apoptosis is mediated by Smad-dependent expression of GADD45b through p38 activation. J Biol Chem. 2003;278:43001–43007. doi: 10.1074/jbc.M307869200. [DOI] [PubMed] [Google Scholar]
- 31.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, et al. ER-stress–induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Tuyl M, Groenman F, Wang J, Kuliszewski M, Liu J, Tibboel D, Post M. Angiogenic factors stimulate tubular branching morphogenesis of sonic hedgehog-deficient lungs. Dev Biol. 2007;303:514–526. doi: 10.1016/j.ydbio.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 34.Ben Mosbah I, Alfany-Fernández I, Martel C, Zaouali MA, Bintanel-Morcillo M, Rimola A, Rodés J, Brenner C, Roselló-Catafau J, Peralta C. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia–reperfusion. Cell Death Dis. 2010;1:e52. doi: 10.1038/cddis.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 37.Ye R, Mareninova OA, Barron E, Wang M, Hinton DR, Pandol SJ, Lee AS. Grp78 heterozygosity regulates chaperone balance in exocrine pancreas with differential response to cerulein-induced acute pancreatitis. Am J Pathol. 2010;177:2827–2836. doi: 10.2353/ajpath.2010.100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang M, Ye R, Barron E, Baumeister P, Mao C, Luo S, Fu Y, Luo B, Dubeau L, Hinton DR, et al. Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ. 2010;17:488–498. doi: 10.1038/cdd.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology. 2011;54:229–239. doi: 10.1002/hep.24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu G, Ye R, Jung DY, Barron E, Friedline RH, Benoit VM, Hinton DR, Kim JK, Lee AS. GRP78 plays an essential role in adipogenesis and postnatal growth in mice. FASEB J. 2013;27:955–964. doi: 10.1096/fj.12-213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P, Lee AS. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA. 2008;105:19444–19449. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heijmans J, Wielenga MC, Rosekrans SL, van Lidth de Jeude JF, Roelofs J, Groothuis P, Ederveen A, de Jonge-Muller ES, Biemond I, Hardwick JC, et al. Oestrogens promote tumorigenesis in a mouse model for colitis-associated cancer. Gut. 2014;63:310–316. doi: 10.1136/gutjnl-2012-304216. [DOI] [PubMed] [Google Scholar]
- 43.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 45.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamasaki M, Kang HR, Homer RJ, Chapoval SP, Cho SJ, Lee BJ, Elias JA, Lee CG. P21 regulates TGF-β1–induced pulmonary responses via a TNF-α–signaling pathway. Am J Respir Cell Mol Biol. 2008;38:346–353. doi: 10.1165/rcmb.2007-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sureshbabu A, Syed MA, Boddupalli CS, Dhodapkar MV, Homer RJ, Minoo P, Bhandari V. Conditional overexpression of TGFβ1 promotes pulmonary inflammation, apoptosis and mortality via TGFβR2 in the developing mouse lung. Respir Res. 2015;16:4. doi: 10.1186/s12931-014-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tropea K, Christou H. Current pharmacologic approaches for prevention and treatment of bronchopulmonary dysplasia. Int J Pediatr. 2012;2012:598606. doi: 10.1155/2012/598606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 50.Malo A, Krüger B, Seyhun E, Schäfer C, Hoffmann RT, Göke B, Kubisch CH. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol. 2010;299:G877–G886. doi: 10.1152/ajpgi.00423.2009. [DOI] [PubMed] [Google Scholar]