To the Editor:
The use of mass cytometry (inductively coupled plasma mass spectroscopy performed on rare metal-labeled single cells) has significantly expanded the ability to identify unique cell subsets in samples based on the multiplicity of surface markers that can be queried simultaneously (1, 2). Traditional flow cytometry on lung specimens, particularly alveolar macrophages, is significantly hindered by autofluorescence that limits the number of useful channels and dynamic range of cell protein evaluation. In addition, macrophage autofluorescence is enhanced by common environmental exposures, such as smoke, degrading the ability to compare samples from smoking and nonsmoking subjects. Mass cytometry overcomes these limitations by using rare metal isotopes covalently coupled to antibodies, rather than fluorophores. For this reason, mass cytometry has incredible potential for defining novel macrophage subsets in human samples. A wide range of rare metal tags and lack of interference from freezing or fixation means existing small-quantity samples banked from prior studies or clinical trials can produce multidimensional data for novel analysis of macrophage subsets.
Mass cytometry uses an atomic mass window from ∼100 to 200 Da, which includes lanthanides and other rare earth elements but few naturally occurring elements. Mass cytometry has been applied extensively to human mononuclear cells and, recently, to mouse myeloid lung cells (3), but less has been reported on human alveolar macrophages. In this letter, we report on the potential translational use of mass cytometry but advise caution regarding the use of this assay on samples from subjects with recent amiodarone exposure.
To define surface marker expression patterns of lung myeloid cells, we analyzed banked frozen human mononuclear cells, bronchoalveolar lavage (BAL) cells, and digested whole-lung specimens. Mononuclear cells and digested lungs were acquired as part of a National Institutes of Health–funded specialized center for clinical research grant in chronic obstructive pulmonary disease; BAL cells were collected as part of an ongoing project related to allograft rejection after lung transplantation. All samples were obtained from consented human subjects under human subject protocols approved by the Washington University Human Research Protection Office. All mononuclear cells and lung digests were frozen and stored for more than 2 years before use; BAL cell pellets were frozen for less than 3 months before use. Labeled cell preparations were evaluated using a CyTOF2 machine. All monoclonal antibodies were commercially metal conjugated (Fluidigm, San Francisco, CA).
Cytometry evaluation of lung digests demonstrated abundant myeloid cells with a broad dynamic range of cell surface protein expression. However, one specimen was interrupted as a result of significant contamination with environmental iodine. The iodine contamination was sufficiently robust, and there was concern for overheating the mass detector and excessive wear on the 127I detector. Multiple flushes were required to remove all traces of iodine from the cytometer.
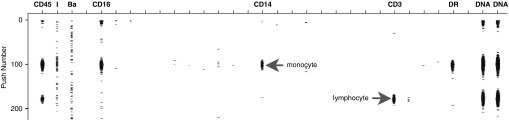
During mass cytometry, individual cells enter the detector as an ion cloud that is pushed through the spectrometer over a short period of time. Typically, cells are identified as a series of contiguous positive readings in a mass channel, (e.g., an 191Ir DNA intercalator). Events (cells) are separated from noise based on the time a channel demonstrates a continuous signal (Figure 1). In our sample, there was a continuous signal at 127 Da unrelated to DNA content, consistent with contamination by a stable isotope of iodine sufficient that no data could be collected. Our assumption was iodine was introduced in the lung collection procedure, possibly as a result of an iodinated cleaning solution.
Figure 1.
Normal mass cytometry result with mild contamination. Typical mass cytometry results demonstrate signal (dots) in one of 30+ activated detectors as the ion cloud of individual cells are pushed through the detector. On the y axis is the push number, and on the x axis is the individual mass channels. This sample is a human mononuclear preparation labeled with metal-conjugated antibodies for CD45, CD14, CD16, HLA-DR, and two DNA intercalators. A CD45+CD14+CD16+HLA-DR+ monocyte and CD45+CD3+ lymphocyte are pictured. The two detector lanes (I, Ba) next to the CD45 lane demonstrate mild environmental contamination, with 127I and 138Ba, respectively, resulting in continuous signal that is not cell associated.
Analysis of BAL cells also demonstrated a broad dynamic range of macrophage surface protein expression; however, three of the six BAL samples had significant iodine contamination. There was no evidence of iodine introduction via cleaning solutions in either the bronchoscopy laboratory or the research laboratory. Available clinical history revealed that one subject had intravenous iodinated contrast preprocedurally, but all contaminated subjects had prior amiodarone exposure for postoperative atrial arrhythmias, with amiodarone use in the last 3 months only present in BAL samples with iodine contamination. It is uncommon for individuals with severe lung disease to receive amiodarone, but postoperative atrial arrhythmias are not uncommon after transplantation, explaining this high rate in pulmonary patients. The kinetics of the amiodarone in the lung are well known, with accumulation in lipid membranes of alveolar cells and macrophages, resulting in a half-life of months. Subsequent samples were limited to individuals without a history of postoperative amiodarone. However, a subsequent assay that included two specimens from the same subject demonstrated iodine in the second BAL despite there being none in the first sample. On review, this subject developed an atrial arrhythmia between the first and second surveillance bronchoscopy and was placed on amiodarone 2 weeks before the second BAL. We believe this subject was sufficient to implicate amiodarone as the most likely source of iodine contamination that can prevent mass cytometry experiments. We do note, however, that there is no information on dietary iodine intake, thyroid supplement use, or any pretransplant history. On review of all subjects to date, samples at −155 and −345 days after amiodarone were adequate, but one at −140 days demonstrated interference.
Given the common use of amiodarone for atrial arrhythmias, it could affect translational research opportunities for mass cytometry in this cohort and donor organs. We cannot determine at what point there will be adequate decay of the cellular amiodarone to allow mass cytometry evaluation. Although we did not specifically evaluate, we predict amiodarone-derived iodine contamination could also affect liver macrophage or cardiac macrophage evaluation by mass cytometry. We do not know whether medicinal iodine contrast agents pose a similar problem. However, as gadolinium is in the evaluated mass window, recent contrast-enhanced magnetic resonance imaging may also exclude some subjects from this type of assay. In summary, we believe mass cytometry is a potentially powerful tool to better characterize macrophage subsets, particularly in smokers, but suggest excluding subjects with a history of amiodarone exposure to prevent medicinal iodine interference.
Footnotes
This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute grant U01HL121804 (J.J.A. and R.M.P.), and the Lottie Caroline Hardy Trust (J.J.A.).
Author Contributions: B.C.K. contributed to the acquisition of specimens, analysis and interpretation of the data, and revision of the draft and has approved this draft form of the letter; R.M.P. contributed to the design of the work and analysis of the data and has approved this draft form of the letter; D.E.B. contributed to the design of the work, acquisition of the specimens, interpretation of the data, and revision of the draft and has approved this draft form of the letter; J.J.A. conceived the study, contributed to the design of the work, authored the first draft, contributed to the analysis of the data, revised the draft, and has approved this draft form of the letter.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Bjornson ZB, Nolan GP, Fantl WJ. Single-cell mass cytometry for analysis of immune system functional states. Curr Opin Immunol. 2013;25:484–494. doi: 10.1016/j.coi.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spitzer MH, Gherardini PF, Fragiadakis GK, Bhattacharya N, Yuan RT, Hotson AN, Finck R, Carmi Y, Zunder ER, Fantl WJ, et al. IMMUNOLOGY. An interactive reference framework for modeling a dynamic immune system. Science. 2015;349:1259425. doi: 10.1126/science.1259425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becher B, Schlitzer A, Chen J, Mair F, Sumatoh HR, Teng KW, Low D, Ruedl C, Riccardi-Castagnoli P, Poidinger M, et al. High-dimensional analysis of the murine myeloid cell system. Nat Immunol. 2014;15:1181–1189. doi: 10.1038/ni.3006. [DOI] [PubMed] [Google Scholar]