Abstract
Remarkable progress has been achieved in understanding the regulation of gene expression and protein translation, and how aberrancies in these template-driven processes contribute to disease pathogenesis. However, much of cellular physiology is controlled by non-DNA, nonprotein mediators, such as glycans. The focus of this Translational Review is to highlight the importance of a specific glycan polymer—the glycosaminoglycan heparan sulfate (HS)—on lung health and disease. We demonstrate how HS contributes to lung physiology and pathophysiology via its actions as both a structural constituent of the lung parenchyma as well as a regulator of cellular signaling. By highlighting current uncertainties in HS biology, we identify opportunities for future high-impact pulmonary and critical care translational investigations.
Keywords: heparan sulfate, glycosaminoglycan, glycocalyx, proteoglycan
Clinical Relevance
Heparan sulfate (HS) is a linear glycosaminoglycan that significantly impacts lung structure and cellular signaling. By reviewing HS and its impact on lung development, homeostasis, and injury, this Translational Review highlights critical knowledge gaps and opportunities for novel translational investigations relevant to diseases such as acute lung injury and pulmonary fibrosis.
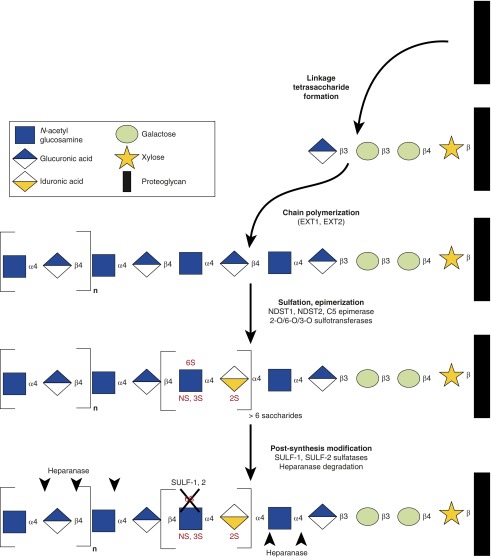
Heparan sulfate (HS) is a linear glycosaminoglycan composed of repeating disaccharide units of glucosamine and a hexuronic acid (glucuronic acid or its epimer, iduronic acid). HS synthesis occurs within the Golgi apparatus, where disaccharide polymerization extends from a protein backbone. Together, this HS–protein complex is known as an HS proteoglycan (HSPG). HS undergoes sequential steps of epimerization and/or sulfation, as governed by a system of epimerases and sulfotransferases targeted to specific sites on component HS disaccharides (Figure 1) (1). This complex process of synthesis and modification dictates the biologic functions of HS, which arise largely from the localized negative charge imparted by clusters of sulfation-enriched domains. These domains enable HS to not only bind positively charged residues of soluble ligands and cell surface receptors (2), but also to sequester water (3)—characteristics that allow HS to function both as a structural component of the lung parenchyma and as a regulator of signaling pathways.
Figure 1.
Heparan sulfate (HS) synthesis and modification. HS is synthesized within the Golgi apparatus via the actions of a complex biosynthetic machinery. After establishment of a linkage tetrasaccharide on a proteoglycan, heparan polymerization occurs via the sequential addition of uronic acid–glucosamine disaccharides. These disaccharides are rapidly modified by epimerization (glucuronic acid to iduronic acid) and/or targeted sulfation. Outside the Golgi apparatus, HS may be rapidly modified via endogenous sulfatases (e.g., SULF-1 and SULF-2, which desulfate 6-O HS) and heparanase (which generally acts to release highly sulfated fragments, although the exact target of cleavage remains the focus of active study). The coordination of this multistep process remains uncertain. EXT, exostosin glycosyltransferase; NDST, N-deacetylase/N-sulfotransferase; n, number of disaccharide repeats; NS, N-sulfation; S, sulfation at 2, 3, or 6 positions; SULF, sulfatase.
HS in Lung Development
Transgenic animal studies have demonstrated the importance of HS to proper organ development (4). As summarized in Table 1, the most severe pulmonary-relevant phenotypes described (embryonic lethal) arise from null mutants of Glact1, Ext1, and Ext2, which encode enzymes that build the initial unmodified HSPG. In addition, genes involved in the further modification of HS affect lung development. Null allele mutants of Ndst1, which encodes an enzyme that sulfates glucosamine at the N position, suffer from neonatal respiratory distress due to lung hypoplasia (5, 6). Genetic manipulations that result in HS containing less iduronic acid (Glce/Hsepi) or 6-O sulfation (H6st1) also produce animals that lack proper alveolar development (7, 8).
Table 1.
Lung Developmental Defects in Heparan Sulfate Biosynthesis Genetic Mutants
| Gene | Role in HS Biosynthesis | Effect on Lung Development (Reference) |
|---|---|---|
| Glact1 | Forms HS/CS/DS–protein tetrasaccharide link | Null allele: embryonic lethal (56) |
| Ext1/Ext2 | Elongates HS chain | Null allele: embryonic lethal; aberrant endoderm development (57, 58) |
| Ndst1 | Deacetylates and sulfates glucosamine residues at the N position | Null allele: perinatal lethal; pulmonary hypoplasia and neonatal respiratory distress (5, 6) |
| H6st1 | Sulfates glucosamine residues at the 6 position | Null allele: embryonic/perinatal lethal; enlarged alveoli (7) |
| Glce (Hsepi) | Epimerizes glucuronic acid to iduronic acid | Targeted disruption: perinatal lethal; neonatal respiratory distress and thickened poorly inflated alveoli (8) |
Definition of abbreviations: CS, chondroitin sulfate; DS, dermatan sulfate; HS, heparan sulfate.
The importance of HS biosynthesis to lung development likely reflects the necessity of specific HS sulfation patterns (such as N sulfation) to enable interaction of HS with soluble ligands and their cognate cell surface receptors. Through these sulfation-dependent interactions, HS regulates growth factor and morphogen signaling that contributes to airspace and vascular lung development. Nearly complete desulfation of lung HS prevents fibroblast growth factor (FGF) 10 from binding to distal epithelial cells, inhibiting airway branching (9). More specifically, N-sulfated HS is required to bind bone morphogenic protein 2 and 4, regulating lung epithelial proliferation and alveolar epithelial type (AT) II to ATI cell differentiation (10). HS also contributes to lung vascular development by interacting with two murine isoforms of vascular endothelial growth factor regulating blood–air barrier formation and proper vessel density (11). These interactions described are only a few of the many HS-dependent signaling pathways important for lung development.
HS in Lung Physiology
HS as a Structural Molecule
Sulfated glycosaminoglycans (such as HS) readily sequester water, forming large, gel-like structures with measurable rigidity (12). In addition, sulfated regions of HS bind both cell matrix proteins and cell adhesion proteins, allowing HS to contribute to structures within the pulmonary vasculature, interstitium, and alveolar epithelium (13) (Figure 2). Interestingly, there is significant heterogeneity of HS sulfation across different compartments within the lung, potentially reflecting context-specific contributions of this glycosaminoglycan to pulmonary function (14).
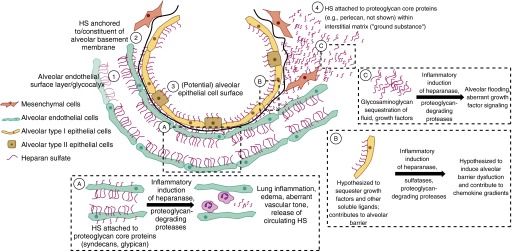
Figure 2.
HS in the alveolus. HS is abundant within the alveolus, contributing to: (1) the pulmonary microvascular glycocalyx/endothelial surface layer; (2) the alveolar basement membrane; (3) the alveolar epithelial surface layer; and (4) the lung interstitial extracellular matrix. Although the size of the pulmonary endothelial surface layer has been measured in vivo, its size relative to other HS-containing structures in the lungs is unknown. Indeed, even the existence of an alveolar epithelial surface layer is uncertain. During inflammation, HS degradation and/or modification at various alveolar sites contributes to lung injury (A–C).
HS in the pulmonary endothelial glycocalyx/endothelial surface layer
The endothelial glycocalyx is an intraluminal matrix enriched in glycoproteins and proteoglycans. In vivo, glycocalyx glycosaminoglycans (including HS, chondroitin sulfate, and hyaluronic acid) interact with plasma proteins and avidly sequester water, forming an endothelial surface layer (ESL) of substantial thickness. This gel-like, fully hydrated ESL contributes to the endothelial barrier to fluid and protein, transduces vascular shear stress into endothelial nitric oxide (NO) synthesis, and regulates the availability of cell membrane adhesion molecules to circulating leukocytes (15). Accordingly, enzymatic HS degradation causes collapse of pulmonary ESL thickness (16), leading to lung edema (17, 18), aberrant pressure-induced endothelial NO synthesis (17, 18), and lung inflammation (16).
The contribution of HS to endothelial barrier function has been attributed to its physical presence as a charged meshwork overlying endothelial cells (15). In contrast, the mechanism by which an intact ESL regulates NO synthesis is less certain, with investigators speculating the importance of interactions between HSPGs (glypican) and endothelial NO synthase within endothelial caveoli (19). Furthermore, the impact of HS on leukocyte adhesion is complex. Loss of ESL thickness exposes endothelial surface adhesion molecules, facilitating neutrophil adhesion within alveolar capillaries (16). In contrast, endothelial surface HS can serve as an L-selectin ligand and regulator for chemotactic agent availability (20); as such, aberrance of pulmonary ESL HS structure or sulfation might be expected to decrease L-selectin–mediated neutrophil–endothelial interaction. It remains unclear how these seemingly disparate roles of ESL HS on leukocyte diapedesis are reconciled in vivo.
Alveolar basement membrane
The alveolar basement membrane is a structure enriched in HSPGs (including collagen XVIII, agrin, and perlecan) that can directly connect the alveolar endothelium and epithelium. Basement membrane HSPGs function not only to form a charged molecular “sieve” that contributes to the alveolocapillary barrier, but are also essential to the HS-dependent anchoring of endothelial and epithelial cells to extracellular matrix (ECM) proteins (3, 21, 22). These interactions, however, are complex. Perlecan HS, for example, demonstrates both adhesive and antiadhesive functions, and is necessary for mechanical stretch–induced alveolar epithelial signaling, indicating that HS facilitates matrix–cellular coupling (23, 24).
Pulmonary interstitium
Although a shared basement membrane can directly connect alveolar epithelium with capillary endothelium, these cellular compartments may additionally be separated by an interstitium enriched in nonfibrillary proteins, including chondroitin sulfate proteoglycans and, to a lesser extent, HSPGs (3, 25). These sulfated proteoglycans contribute to the “viscoelasticity” of the lung parenchyma (26), allowing for alveolar stability at low lung volumes (27). HSPGs also safeguard against lung edema formation by maintaining a noncompliant lung interstitium, which responds to fluid accumulation with a rapid increase in interstitial hydrostatic pressure that opposes ongoing vascular leak (3). Accordingly, experimental proteoglycan degradation triggers a transition from interstitial edema to alveolar flooding (28).
Alveolar epithelial surface layer
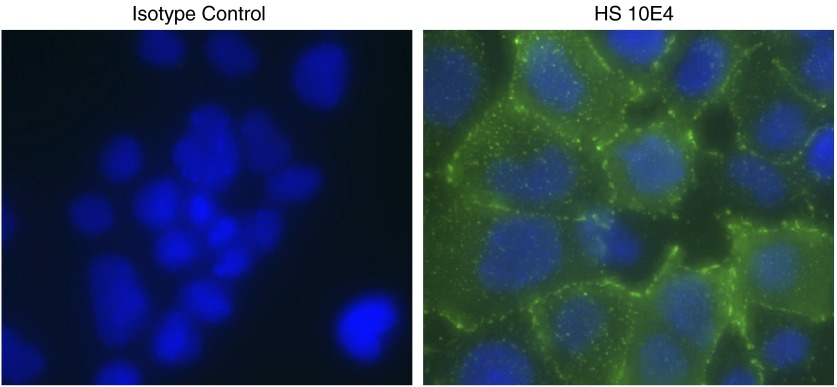
Although the importance of HS to the pulmonary ESL is well described, the structural significance (and very existence) of alveolar epithelial surface HS is uncertain. HS can be identified on the surface of mouse alveolar type II–like epithelial 12 cells (Figure 3) as well as in human ATII-like A549 cells (29). Although the liberation of HSPGs (including syndecan-1) into the alveolar space during lung injury implies the presence of HS lining the alveolar lumen, these findings are only suggestive, and the original source of the shed HSPGs in vivo has yet to be determined (30).
Figure 3.
Mouse alveolar type II–like epithelial 12 (MLE-12) cells express cell surface HS. Before fixation, live MLE-12 cells were treated with the HS 10E4 antibody at 4°C, which recognizes N-sulfated glucosamine residues of HS. This approach limits staining to the cell surface. An isotype antibody serves as a control. Z series images were taken, and a maximum intensity projection image was created. Green, HS 10E4; blue, 4',6-diamidino-2-phenylindole (DAPI).
HS in Cellular Signaling
Sulfation of HS is essential for its influence on cellular signaling, as the imparted negative charge allows HS to bind and regulate other bioactive ligands (Table 2). Some of the best-described interactions of HS are those with FGF ligands and FGF receptors. HSPGs on the cell surface may bind and oligomerize FGF ligands and provide a cis-scaffold for FGF ligand–receptor binding (31). Within the ECM, HS binds and stores FGF ligands, which can be released upon ECM degradation during injury (4). In a similar manner as with FGF signaling, HS binds and regulates many other soluble ligands that play critical roles in physiologic cellular signaling in the pulmonary vasculature and epithelium.
-
•
Pulmonary vasculature: basement membrane HSPGs serve as important regulators of angiogenesis and smooth muscle cell activation. Intact perlecan accelerates FGF2-induced angiogenesis, presumably via HS-mediated FGF2–FGF receptor 1 signaling (32). Accordingly, perlecan knockout mice exhibit aberrant vascular development (21). Interestingly, peptide fragments derived from basement membrane HSPGs, such as endorepellin (the C-terminus fragment of perlecan) and endotstatin (the C-terminus fragment of collagen XVIII) are strongly antiangiogenic, potentially due to their ability to bind cell surface HSPGs and limit endothelial interactions with proangiogenic ligands (33, 34).
-
•
Pulmonary epithelium: although it is unclear if alveolar epithelial cell surface HS contributes to an epithelial surface layer, epithelial HSPGs do influence alveolar intercellular signaling and epithelial cell phenotype. When added to ATII cells in combination with FGF1, heparin, a heavily sulfated form of HS, enhances the RNA expression of the ATII cell marker, surfactant protein B, and the ATI cell marker, aquaporin-5 (35). This effect is partially dependent on the degree of heparin sulfation (35). Furthermore, HS in both the ECM and on the epithelial cell surface affects epithelial cell viability and growth in a sulfation-dependent manner, consistent with influence on growth factor signaling (36).
Table 2.
Signaling Proteins Regulated by Heparan Sulfate that Influence Lung Development, Homeostasis, and Injury
| Binding Partner | Impact of Heparan Sulfate (References) |
|---|---|
| Growth factors | |
| FGF | Facilitates ligand–receptor binding, augmenting GF signaling (31, 59) |
| FGF10 influences airway branching during lung development (9) | |
| FGF2 induces angiogenesis (32) | |
| FGF1 and -2 enhances ATII cell proliferation (60) | |
| BMP2/4 | May inhibit signaling by preventing binding to the receptor, but also facilitates signaling by enhancing stability of ligand (10) |
| In the lung HS has been shown to inhibit BMP signaling during development and facilitate alveolar dilation (10) | |
| VEGF | Co-receptor for VEGFs/VEGFRs (61) |
| Contributes to vascular development and blood–air barrier formation (11) | |
| Cytokines/chemokines | |
| IL-6/IL-8 | HS binds to IL-8 in lung tissue (62) |
| Soluble heparin attenuates the secretion of IL-8 from endothelial cells when treated with LPS; however, cell-surface HS released by heparanase induces expression of IL-8 (40, 44) | |
| CXCL10 | Syndecan-4 binds CXCL10 and reduces fibroblast migration and fibrosis in animals treated with bleomycin (54) |
| Nuclear proteins | |
| Histones | Endothelial glycocalyx binds circulating histones and soluble heparin, competitively inhibits binding of histones to endothelial cells, and improves survival during sepsis (41, 42) |
| HMGB1 | Soluble heparin inhibits the binding of HMGB1 to macrophages and reduces mortality in a sepsis model (43) |
Definition of abbreviations: AT, alveolar epithelial type; BMP, bone morphogenic protein; CXCL10, C-X-C motif chemokine 10; FGF, fibroblast growth factor; GF, growth factor; HMGB1, high-mobility group box 1; HS, heparan sulfate; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
HS in Lung Injury
As with lung development and physiological homeostasis, HS can impart a multifactoral influence over pathogenic processes during both acute and chronic lung injury.
Acute Lung Injury
Known clinically as the acute respiratory distress syndrome (ARDS), acute lung injury is a heterogeneous entity representing the end result of any number of direct (e.g., pneumonia) or indirect (e.g., nonpulmonary sepsis) pulmonary insults. Interestingly, different ARDS-triggering events and pathophysiologic pathways may demonstrate unique patterns of HS dysfunction.
-
•
Indirect lung injury: systemic insults (such as nonpulmonary sepsis) can induce diffuse, pulmonary endothelial–centered lung injury. Accordingly, sepsis-induced lung injury is characterized by pulmonary ESL degradation, potentially mediated by induction of endothelial heparanase, an HS-specific endoglucuronidase (16). Other potential ESL “sheddases” include lysosomal constituents and matrix metalloproteinases (37–39). HS degradation rapidly reduces ESL size and rigidity, allowing neutrophils to access their cognate endothelial adhesion molecules, extravasate, and cause inflammatory lung injury (16). This injury may be additionally influenced by HS fragments released during ESL degradation. Soluble heparin has been shown to be antiinflammatory by inhibiting LPS-induced NF-κB signaling and IL-8 secretion from pulmonary endothelium, and by binding and sequestering circulating damage-associated molecular patterns, such as extracellular nuclear proteins, high-mobility group box 1 protein, and histones (40–43). Paradoxically, HS fragments released by enzymatic heparanase degradation may themselves propagate injury as a damage-associated molecular pattern (44).
-
•
Direct lung injury: in contrast to indirect/systemic lung injury, direct lung injury arises from insults that primarily target the lung epithelium. Accordingly, patients with pneumonia-induced respiratory failure have less circulating HS than patients with sepsis- or pancreatitis-induced respiratory failure (45). These differences may reflect insult-dependent roles of heparanase in lung injury: although heparanase knockout mice were protected from polymicrobial sepsis-induced lung injury (16), no such protection was enjoyed after intranasal LPS (46). In contrast, protease induction during direct lung injury onset sheds HSPGs into the alveolar space where they affect lung injury severity by releasing a sulfation-dependent chemokine gradient necessary for alveolar neutrophilic influx and by facilitating resolution of alveolar inflammation (30, 47, 48).
-
•
Ventilator-induced lung injury: although mechanical ventilation is necessary for the supportive care of patients with ARDS, improper use can induce injury across the alveolar endothelium, epithelium, and interstitium. High tidal volume ventilation induces matrix metalloproteinase activation, leading to proteoglycan cleavage and loss of whole-lung soluble and protein-bound glycosaminoglycan content (49). This fragmentation may be offset by induction of HSPG expression within the alveoli of high tidal volume–ventilated lungs (26). This degradation of interstitial proteoglycans removes a safeguard against alveolar flooding, as described previously here.
Chronic Lung Injury
Idiopathic pulmonary fibrosis (IPF) is thought to arise from chronic, unresolving lung injury. In comparison to the normal lung, HS is expressed in a different structural and spatial pattern during chronic injury, potentially regulating signaling pathways that contribute to the pathogenesis of fibrosis. During fibrosis, there is both increased amounts of lung HSPGs and alterations in HS sulfation (e.g., increased 6-O sulfation) (50, 51). This increased 6-O sulfation may contribute to IPF progression by enhancing pathogenic transforming growth factor-β signaling in both lung fibroblasts and ATII cells (29, 51). In addition, as observed during direct acute lung injury, HSPGs are shed into the alveolar space in animal models of pulmonary fibrosis and can be detected in the bronchoalveolar lavage fluid from patients with IPF (52); inhibition of this shedding with antioxidants attenuates fibrosis (53). Although HSPG shedding may enhance fibrosis, one HSPG, syndecan-4, appears to play an antifibrotic role, perhaps via sequestration of antifibrotic ligands, such as CXCL10 (54). Futhermore, heparin (in conjunction with FGF1) decreases collagen production and increases apoptosis and cell migration in IPF fibroblasts (55). These findings highlight the various and conflicting effects of HS on the pathogenesis of IPF, identifying the need for further investigation.
Conclusions
HS is critical to pulmonary homeostasis, maintaining parenchymal structure and facilitating cellular signaling necessary for lung development and function. The translational importance of HS is demonstrated by the critical contributions of changes in HS structure to pathologic events during acute and chronic lung injury. Accordingly, therapeutics targeted against HS degradation (16), sulfation (29), or modulation of HS–chemokine interactions (54) may have therapeutic value in ARDS or pulmonary fibrosis, warranting further translational study.
Footnotes
This work was supported by National Institutes of Health R01 HL125371 (E.P.S.).
Author Contributions: Conception, analysis, and drafting of the manuscript were performed by S.M.H., Y.Y., and E.P.S.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0043TR on March 16, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kreuger J, Kjellén L. Heparan sulfate biosynthesis: regulation and variability. J Histochem Cytochem. 2012;60:898–907. doi: 10.1369/0022155412464972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu D, Esko JD. Demystifying heparan sulfate–protein interactions. Annu Rev Biochem. 2014;83:129–157. doi: 10.1146/annurev-biochem-060713-035314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negrini D, Passi A, Moriondo A. The role of proteoglycans in pulmonary edema development. Intensive Care Med. 2008;34:610–618. doi: 10.1007/s00134-007-0962-y. [DOI] [PubMed] [Google Scholar]
- 4.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3:pii: a004952. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ringvall M, Ledin J, Holmborn K, van Kuppevelt T, Ellin F, Eriksson I, Olofsson AM, Kjellen L, Forsberg E. Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J Biol Chem. 2000;275:25926–25930. doi: 10.1074/jbc.C000359200. [DOI] [PubMed] [Google Scholar]
- 6.Fan G, Xiao L, Cheng L, Wang X, Sun B, Hu G. Targeted disruption of NDST-1 gene leads to pulmonary hypoplasia and neonatal respiratory distress in mice. FEBS Lett. 2000;467:7–11. doi: 10.1016/s0014-5793(00)01111-x. [DOI] [PubMed] [Google Scholar]
- 7.Habuchi H, Nagai N, Sugaya N, Atsumi F, Stevens RL, Kimata K. Mice deficient in heparan sulfate 6-O-sulfotransferase-1 exhibit defective heparan sulfate biosynthesis, abnormal placentation, and late embryonic lethality. J Biol Chem. 2007;282:15578–15588. doi: 10.1074/jbc.M607434200. [DOI] [PubMed] [Google Scholar]
- 8.Li JP, Gong F, Hagner-McWhirter A, Forsberg E, Abrink M, Kisilevsky R, Zhang X, Lindahl U. Targeted disruption of a murine glucuronyl C5-Epimerase gene results in heparan sulfate lacking l-iduronic acid and in neonatal lethality. J Biol Chem. 2003;278:28363–28366. doi: 10.1074/jbc.C300219200. [DOI] [PubMed] [Google Scholar]
- 9.Izvolsky KI, Shoykhet D, Yang Y, Yu Q, Nugent MA, Cardoso WV. Heparan sulfate–FGF10 interactions during lung morphogenesis. Dev Biol. 2003;258:185–200. doi: 10.1016/s0012-1606(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 10.Hu Z, Wang C, Xiao Y, Sheng N, Chen Y, Xu Y, Zhang L, Mo W, Jing N, Hu G. NDST1-dependent heparan sulfate regulates BMP signaling and internalization in lung development. J Cell Sci. 2009;122:1145–1154. doi: 10.1242/jcs.034736. [DOI] [PubMed] [Google Scholar]
- 11.Galambos C, Ng YS, Ali A, Noguchi A, Lovejoy S, D’Amore PA, DeMello DE. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol. 2002;27:194–203. doi: 10.1165/ajrcmb.27.2.4703. [DOI] [PubMed] [Google Scholar]
- 12.Wiesinger A, Peters W, Chappell D, Kentrup D, Reuter S, Pavenstädt H, Oberleithner H, Kümpers P. Nanomechanics of the endothelial glycocalyx in experimental sepsis. PLoS One. 2013;8:e80905. doi: 10.1371/journal.pone.0080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Kuppevelt TH, Cremers FP, Domen JG, van Beuningen HM, van den Brule AJ, Kuyper CM. Ultrastructural localization and characterization of proteoglycans in human lung alveoli. Eur J Cell Biol. 1985;36:74–80. [PubMed] [Google Scholar]
- 14.Smits NC, Robbesom AA, Versteeg EM, van de Westerlo EM, Dekhuijzen PN, van Kuppevelt TH. Heterogeneity of heparan sulfates in human lung. Am J Respir Cell Mol Biol. 2004;30:166–173. doi: 10.1165/rcmb.2003-0198OC. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Schmidt EP. The endothelial glycocalyx: an important regulator of the pulmonary vascular barrier. Tissue Barriers. 2013;1:23494. doi: 10.4161/tisb.23494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dull RO, Mecham I, McJames S. Heparan sulfates mediate pressure-induced increase in lung endothelial hydraulic conductivity via nitric oxide/reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1452–L1458. doi: 10.1152/ajplung.00376.2006. [DOI] [PubMed] [Google Scholar]
- 18.Dull RO, Cluff M, Kingston J, Hill D, Chen H, Hoehne S, Malleske DT, Kaur R. Lung heparan sulfates modulate Kfc during increased vascular pressure: evidence for glycocalyx-mediated mechanotransduction. Am J Physiol Lung Cell Mol Physiol. 2012;302:L816–L828. doi: 10.1152/ajplung.00080.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007;355:228–233. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin– and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 21.Bix G, Iozzo RV. Novel interactions of perlecan: unraveling perlecan’s role in angiogenesis. Microsc Res Tech. 2008;71:339–348. doi: 10.1002/jemt.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong S, Cole GJ, Halfter W. Expression of collagen XVIII and localization of its glycosaminoglycan attachment sites. J Biol Chem. 2003;278:1700–1707. doi: 10.1074/jbc.M209276200. [DOI] [PubMed] [Google Scholar]
- 23.Whitelock JM, Graham LD, Melrose J, Murdoch AD, Iozzo RV, Underwood PA. Human perlecan immunopurified from different endothelial cell sources has different adhesive properties for vascular cells. Matrix Biol. 1999;18:163–178. doi: 10.1016/s0945-053x(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 24.Jones JC, Lane K, Hopkinson SB, Lecuona E, Geiger RC, Dean DA, Correa-Meyer E, Gonzales M, Campbell K, Sznajder JI, et al. Laminin-6 assembles into multimolecular fibrillar complexes with perlecan and participates in mechanical-signal transduction via a dystroglycan-dependent, integrin-independent mechanism. J Cell Sci. 2005;118:2557–2566. doi: 10.1242/jcs.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludwig MS. Proteoglycans and pathophysiology. J Appl Physiol (1985) 2007;103:735–736. doi: 10.1152/japplphysiol.00751.2007. [DOI] [PubMed] [Google Scholar]
- 26.Al-Jamal R, Ludwig MS. Changes in proteoglycans and lung tissue mechanics during excessive mechanical ventilation in rats. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1078–L1087. doi: 10.1152/ajplung.2001.281.5.L1078. [DOI] [PubMed] [Google Scholar]
- 27.Cavalcante FS, Ito S, Brewer K, Sakai H, Alencar AM, Almeida MP, Andrade JS, Jr, Majumdar A, Ingenito EP, Suki B. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol (1985) 2005;98:672–679. doi: 10.1152/japplphysiol.00619.2004. [DOI] [PubMed] [Google Scholar]
- 28.Negrini D, Passi A, de Luca G, Miserocchi G. Pulmonary interstitial pressure and proteoglycans during development of pulmonary edema. Am J Physiol. 1996;270:H2000–H2007. doi: 10.1152/ajpheart.1996.270.6.H2000. [DOI] [PubMed] [Google Scholar]
- 29.Yue X, Lu J, Auduong L, Sides MD, Lasky JA. Overexpression of Sulf2 in idiopathic pulmonary fibrosis. Glycobiology. 2013;23:709–719. doi: 10.1093/glycob/cwt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 31.Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14:166–180. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79:1005–1013. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 33.Reis RC, Schuppan D, Barreto AC, Bauer M, Bork JP, Hassler G, Coelho-Sampaio T. Endostatin competes with bFGF for binding to heparin-like glycosaminoglycans. Biochem Biophys Res Commun. 2005;333:976–983. doi: 10.1016/j.bbrc.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Pozzi A, Zent R. Regulation of endothelial cell functions by basement membrane–and arachidonic acid–derived products. Wiley Interdiscip Rev Syst Biol Med. 2009;1:254–272. doi: 10.1002/wsbm.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leiner KA, Newman D, Li CM, Walsh E, Khosla J, Sannes PL. Heparin and fibroblast growth factors affect surfactant protein gene expression in type II cells. Am J Respir Cell Mol Biol. 2006;35:611–618. doi: 10.1165/rcmb.2006-0159OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Newman DR, Sannes PL. HSULF-1 inhibits ERK and AKT signaling and decreases cell viability in vitro in human lung epithelial cells. Respir Res. 2012;13:69. doi: 10.1186/1465-9921-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zullo JA, Fan J, Azar TT, Yen W, Zeng M, Chen J, Ratliff BB, Song J, Tarbell JM, Goligorsky MS, et al. Exocytosis of endothelial lysosome–related organelles hair-triggers a patchy loss of glycocalyx at the onset of sepsis. Am J Pathol. 2016;186:248–258. doi: 10.1016/j.ajpath.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipowsky HH. Protease activity and the role of the endothelial glycocalyx in inflammation. Drug Discov Today Dis Models. 2011;8:57–62. doi: 10.1016/j.ddmod.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol. 2015;80:389–402. doi: 10.1111/bcp.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Liu Y, Wang L, Li Z, Ma X. Unfractionated heparin attenuates LPS-induced IL-8 secretion via PI3K/Akt/NF-κB signaling pathway in human endothelial cells. Immunobiology. 2015;220:399–405. doi: 10.1016/j.imbio.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Freeman CG, Parish CR, Knox KJ, Blackmore JL, Lobov SA, King DW, Senden TJ, Stephens RW. The accumulation of circulating histones on heparan sulphate in the capillary glycocalyx of the lungs. Biomaterials. 2013;34:5670–5676. doi: 10.1016/j.biomaterials.2013.03.091. [DOI] [PubMed] [Google Scholar]
- 42.Wildhagen KC, García de Frutos P, Reutelingsperger CP, Schrijver R, Aresté C, Ortega-Gómez A, Deckers NM, Hemker HC, Soehnlein O, Nicolaes GA. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood. 2014;123:1098–1101. doi: 10.1182/blood-2013-07-514984. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Ling Y, Huang M, Yin T, Gou SM, Zhan NY, Xiong JX, Wu HS, Yang ZY, Wang CY. Heparin inhibits the inflammatory response induced by LPS and HMGB1 by blocking the binding of HMGB1 to the surface of macrophages. Cytokine. 2015;72:36–42. doi: 10.1016/j.cyto.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Goodall KJ, Poon IK, Phipps S, Hulett MD. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PLoS One. 2014;9:e109596. doi: 10.1371/journal.pone.0109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt EP, Li G, Li L, Fu L, Yang Y, Overdier KH, Douglas IS, Linhardt RJ. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem. 2014;289:8194–8202. doi: 10.1074/jbc.M113.539452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris A, Wang B, Waern I, Venkatasamy R, Page C, Schmidt EP, Wernersson S, Li JP, Spina D. The role of heparanase in pulmonary cell recruitment in response to an allergic but not non-allergic stimulus. PLoS One. 2015;10:e0127032. doi: 10.1371/journal.pone.0127032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114:3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanino Y, Chang MY, Wang X, Gill SE, Skerrett S, McGuire JK, Sato S, Nikaido T, Kojima T, Munakata M, et al. Syndecan-4 regulates early neutrophil migration and pulmonary inflammation in response to lipopolysaccharide. Am J Respir Cell Mol Biol. 2012;47:196–202. doi: 10.1165/rcmb.2011-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moriondo A, Pelosi P, Passi A, Viola M, Marcozzi C, Severgnini P, Ottani V, Quaranta M, Negrini D. Proteoglycan fragmentation and respiratory mechanics in mechanically ventilated healthy rats. J Appl Physiol (1985) 2007;103:747–756. doi: 10.1152/japplphysiol.00056.2007. [DOI] [PubMed] [Google Scholar]
- 50.Venkatesan N, Ebihara T, Roughley PJ, Ludwig MS. Alterations in large and small proteoglycans in bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med. 2000;161:2066–2073. doi: 10.1164/ajrccm.161.6.9909098. [DOI] [PubMed] [Google Scholar]
- 51.Lu J, Auduong L, White ES, Yue X. Up-regulation of heparan sulfate 6-O-sulfation in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;50:106–114. doi: 10.1165/rcmb.2013-0204OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, Rosas I, Oury TD. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009;284:3537–3545. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kliment CR, Tobolewski JM, Manni ML, Tan RJ, Enghild J, Oury TD. Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung. Antioxid Redox Signal. 2008;10:261–268. doi: 10.1089/ars.2007.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, et al. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120:2049–2057. doi: 10.1172/JCI38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacKenzie B, Korfei M, Henneke I, Sibinska Z, Tian X, Hezel S, Dilai S, Wasnick R, Schneider B, Wilhelm J, et al. Increased FGF1-FGFRc expression in idiopathic pulmonary fibrosis. Respir Res. 2015;16:83. doi: 10.1186/s12931-015-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Izumikawa T, Kanagawa N, Watamoto Y, Okada M, Saeki M, Sakano M, Sugahara K, Sugihara K, Asano M, Kitagawa H. Impairment of embryonic cell division and glycosaminoglycan biosynthesis in glucuronyltransferase-I–deficient mice. J Biol Chem. 2010;285:12190–12196. doi: 10.1074/jbc.M110.100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin X, Wei G, Shi Z, Dryer L, Esko JD, Wells DE, Matzuk MM. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev Biol. 2000;224:299–311. doi: 10.1006/dbio.2000.9798. [DOI] [PubMed] [Google Scholar]
- 58.Stickens D, Zak BM, Rougier N, Esko JD, Werb Z. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development. 2005;132:5055–5068. doi: 10.1242/dev.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 60.Sannes PL, Khosla J, Cheng PW. Sulfation of extracellular matrices modifies responses of alveolar type II cells to fibroblast growth factors. Am J Physiol. 1996;271:L688–L697. doi: 10.1152/ajplung.1996.271.5.L688. [DOI] [PubMed] [Google Scholar]
- 61.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 62.Frevert CW, Kinsella MG, Vathanaprida C, Goodman RB, Baskin DG, Proudfoot A, Wells TN, Wight TN, Martin TR. Binding of interleukin-8 to heparan sulfate and chondroitin sulfate in lung tissue. Am J Respir Cell Mol Biol. 2003;28:464–472. doi: 10.1165/rcmb.2002-0084OC. [DOI] [PubMed] [Google Scholar]