Abstract
The airway epithelium constitutes a protective barrier against inhaled insults, such as viruses, bacteria, and toxic fumes, including cigarette smoke (CS). Maintenance of bronchial epithelial integrity is central for airway health, and defective epithelial barrier function contributes to the pathogenesis of CS-mediated diseases, such as chronic obstructive pulmonary disease. Although CS has been shown to increase epithelial permeability, current understanding of the mechanisms involved in CS-induced epithelial barrier disruption remains incomplete. We have previously identified that the receptor tyrosine kinase human epidermal receptor (HER) 2 growth factor is activated by the ligand neuregulin-1 and increases epithelial permeability in models of inflammatory acute lung injury. We hypothesized that CS activates HER2 and that CS-mediated changes in barrier function would be HER2 dependent in airway epithelial cells. We determined that HER2 was activated in whole lung, as well as isolated epithelial cells, from smokers, and that acute CS exposure resulted in HER2 activation in cultured bronchial epithelial cells. Mechanistic studies determined that CS-mediated HER2 activation is independent of neuregulin-1 but required upstream activation of the epidermal growth factor receptor. HER2 was required for CS-induced epithelial permeability as knockdown of HER2 blocked increases in permeability after CS. CS caused an increase in IL-6 production by epithelial cells that was dependent on HER2-mediated extracellular signal-regulated kinases (Erk) activation. Finally, blockade of IL-6 attenuated CS-induced epithelial permeability. Our data indicate that CS activates pulmonary epithelial HER2 and that HER2 is a central mediator of CS-induced epithelial barrier dysfunction.
Keywords: cigarette smoke, human epidermal receptor 2, epidermal growth factor receptor, pulmonary epithelium, permeability
Clinical Relevance
We have identified that the pulmonary epithelial human epidermal receptor (HER) 2 receptor is activated by cigarette smoke (CS) and that HER2 is required for CS-induced epithelial permeability. Our findings suggest that HER2 might be a new target for smoking related lung diseases.
Chronic obstructive pulmonary disease (COPD) is a chronic, debilitating disease marked by derangements at the airway and alveolar level, resulting in progressive, largely irreversible, airflow obstruction. Exposure to direct or second-hand cigarette smoke (CS) is the most common and significant risk factor for COPD. However, despite rising public awareness of the concrete health risks of CS, trends in smoking have not dramatically changed over the past few years (1). This is reflected in epidemiologic trends for COPD; COPD affects up to 24 million people in the United States and over 200 million people worldwide (2). Currently the fourth leading cause of death worldwide, expectations are that mortality rates will increase over the next decade (2). Clearly, improved understanding of disease pathogenesis, with an eye toward how CS induces and propagates organ dysfunction CS, is needed.
As the first line of defense against inhaled insults to the lung, the pulmonary epithelium is a central component of the proinflammatory response to inhaled toxins in the lung, and epithelial injury can lead directly to organ dysfunction. Airways disease caused by CS is marked by a robust and persistent inflammatory response that results in structural alterations, including epithelial disruption, bronchial submucosal gland hypertrophy and hyperplasia, and airway fibrosis (3, 4). Increased airway fibrosis and wall thickness correlates with decreases in FEV1 and it is now understood that narrowed, scarred small airways are the principal site of airflow obstruction in COPD (4, 5). Although understanding of the mechanisms of airways disease in COPD is incomplete, data suggest that repeated CS-mediated epithelial injury, resulting in loss of epithelial integrity and decreased barrier function, contributes to this process (6). However, knowledge of the mechanisms of CS-mediated effects on epithelial barrier function is limited.
The tyrosine kinase receptor, human epidermal receptor (HER) 2, is expressed by pulmonary bronchial and alveolar epithelial cells and is involved in multiple physiologic processes, including cell proliferation and wound repair. The HER receptor family consists of four type-1, membrane-bound tyrosine kinase receptors: HER1 or epidermal growth factor receptor (EGFR), HER2, HER3, and HER4 (7). The receptors exist in a quiescent state as single monomers, but ligand binding induces homo/heterodimerization, activation by auto- and transphosphorylation, and initiation of intracellular signaling. In the adult lung, EGFR, HER2, and HER3 are known to participate in epithelial injury and repair (8, 9), whereas HER4 is critical to epithelial cell proliferation and function in lung development (10). HER2 has no known ligand, yet partners with other HER family members. For example, we have previously identified that HER2 dimerizes with HER3 after HER3 binding with the ligand neuregulin (NRG)-1, which leads to HER2-dependent increases in pulmonary epithelial permeability (11, 12), and the NRG-1–HER2/3 signaling cascade participates in the pathogenesis of acute lung injury and the acute respiratory distress syndrome (7, 8, 11). Although HER2 is known to participate in epithelial barrier dysfunction in air–liquid interface (ALI), it has been unstudied in models of CS exposure.
We hypothesized that CS would induce HER2 activation leading to HER2-dependent changes in epithelial permeability. We determined that HER2, as well as EGFR and HER3, were activated in whole lung, as well as isolated airway and alveolar epithelial cells, from smokers. Acute CS exposure resulted in HER2, HER3, and EGFR activation in cultured bronchial epithelial cells. Mechanistic studies revealed that CS-mediated HER2 activation was independent of NRG-1 and HER3, but required upstream activation of the EGFR. Knockdown of either EGFR or HER2 using specific shRNAs blocked increases in permeability after CS, indicating that CS-mediated barrier dysfunction is EGFR and HER2 dependent. Finally, HER2-mediated barrier dysfunction occurs through extracellular signal-regulated kinases (Erk)-dependent epithelial IL-6 release. Our data outline a previously undescribed signaling cascade triggered by CS, whereby EGFR activation leads to HER2 activation and subsequent HER2-dependent increases in epithelial IL-6 production, and that this EGFR–HER2–IL-6 pathway increases epithelial permeability.
Materials and Methods
Chemicals and Reagents
Antibodies targeting HER2 (C-18) and phosphorylated (p) HER2 (Tyr1248), HER3, and pHER3, Erk, and pErk, and were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies directed against pEGFR, and EGFR were from Cell Signaling Technology (Danvers, MA). β-Actin antibody was from Sigma Chemical Co. (St. Louis, MO). Lapatinib ditosylate (GW-572016) was obtained from Biovision Inc. (Milpitas, CA). Neutralizing IL-6 antibody was from R&D Systems (Minneapolis, MN), and the isotype, control antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Primary Human Lung and Isolation of Human ATII Cells
Primary human alveolar epithelial cells were harvested as previously described (13). We obtained deidentified human lungs not suitable for transplantation and donated for medical research from the National Disease Research Interchange (Philadelphia, PA) and the International Institute for the Advancement of Medicine (Edison, NJ). We selected donors with reasonable lung function with an arterial oxygen pressure:fraction of inspired oxygen ratio of greater than 225, a clinical history and X-ray that did not indicate infection, and limited time on a ventilator. We analyzed the age, sex, race, smoking history, medical information, and cause of death. Smokers were individuals who smoked 10–25 cigarettes per day, nonsmokers included those who had never smoked, whereas individuals with COPD were smokers. The Committee for the Protection of Human Subjects at National Jewish Health (Denver, CO) approved this research.
Bronchoalveolar Lavage NRG-1
Bronchoalveolar lavage (BAL) was taken from COPD and control subjects according to protocols approved by the Institutional Review Board at National Jewish Health, as previously described (14). NRG-1 was assessed by enzyme-linked immunosorbant assay (ELISA) as previously described (8).
Cell Culture and Treatments
NuLi-1 cells (ATCC, Manassas, VA), a nontransformed, human airway epithelial cell line, and normal human bronchial epithelial (NHBE) cells were obtained and maintained as previously described (11). In all experiments, cells were grown to greater than 75% confluence in normal media at ALI and cultured overnight in low-serum media before use.
CS Exposure
Cells were exposed to smoke as previously described (15). Briefly, one research-grade cigarette (2R4F; University of Kentucky, Lexington, KY) was combusted using a Master Flex L/S Economy Drive mini pump (Fisher Scientific, Pittsburgh, PA), which drew the smoke into a 1,000-ml flask and then into a smoking chamber, where bronchial epithelial cells were exposed to smoke or air (control) for 10 minutes. Previous experiments have determined that this culture system adequately mimics the human airway after exposure to CS (15).
In Vivo Smoke Exposure Model
Mice (C57Bl6/J) were exposed 6 h/d, 5 d/wk (Monday–Friday) using TE-10z smoking machines (Teague Enterprises, Davis, CA). Smoke was composed of 11% mainstream smoke and 89% side-stream smoke, and delivered at a concentration of 100 mg/m3 total particulate matter that approximates the exposure of primary smokers. Control mice were placed in the same room, but not in the smoking chamber.
Western Blotting
Briefly, 10 μg of total protein was loaded on 4–12% NuPage Bis-Tris gradient gels (Invitrogen Inc., Grand Island, NY). Resolved protein was transferred on to nitrocellulose membranes and then blocked and probed in 1% milk in TBS-T. After washing three times with TBS-T, images were captured using a FuorChem Q photography system from Protein Simple Inc. (Santa Clara, CA). Densitometry was performed and change in phosphorylated levels compared with total levels (phosphoreceptor/total receptor) expressed graphically. Actin served as a loading control.
Generation of HER2, HER3, EGFR, and NRG-1 Knockdown NuLi-1 Cell Lines
NuLi-1 cells were transduced with either nontargeting (NT) shRNA or specific shRNA targeting HER2, HER3, EGFR, or NRG-1 in a lentiviral vector obtained from Functional Genomics Core, University of Colorado (Boulder, CO). Cells were pretreated with 1 μg/ml of polybrene for 30 minutes. Viral particle suspension was also treated with 1 μg/ml of polybrene for 15 minutes and then added to cells. Infected cells were selected with 1 μg/ml of puromycin for 72 hours. Knockdown of the proteins was confirmed by Western blotting lysates of puromycin-resistant cells. Cells were subjected to puromycin selection regularly, once every 3 weeks.
Electrical Conductance Measurement
Electrical resistance was determined using an ohmmeter (EVOM; World Precision Instruments, Sarasota, FL) as previously described (11). After cells were exposed to smoke, transwells were transferred to new 12-well plates with fresh media.
Statistical Analysis
Values are expressed as means (±SEM). Groups with more than two groups were compared using an ANOVA followed by a Bonferroni posttest. Comparison of two groups was performed using a Student’s t test. P less than 0.05 was considered significant.
Results
Pulmonary Epithelial HER2, HER3, and EGFR Are Activated in Smokers
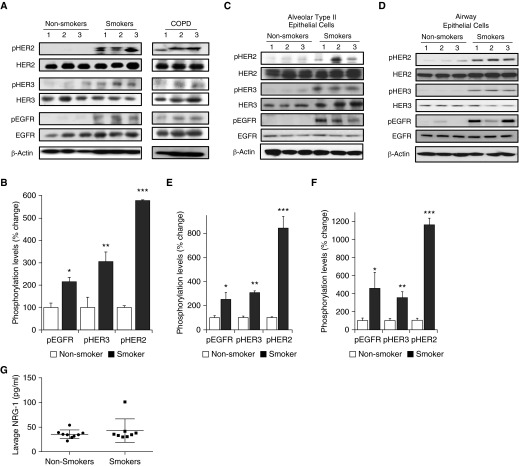
Initial studies sought to determine if HER2 was activated in lungs from smokers when compared with nonsmokers. Phosphorylation of HER2 in protein isolates from total lung was assessed by Western blot (Figures 1A and 1B). We identified that, compared with nonsmokers, lungs from smokers had a roughly fivefold increase in HER2 phosphorylation (P < 0.005) as well as a two- and threefold increase in phosphorylation of EGFR and HER3, respectively (P < 0.02 and P < 0.003). HER2, HER3, and EGFR were also activated in lungs from patients with COPD (though this was not directly compared with smokers without COPD or nonsmokers).
Figure 1.
Pulmonary epithelial human epidermal receptor (HER) 2, HER3 and epidermal growth factor receptor (EGFR) are activated in smokers. (A) Western blot of HER2, HER3, and EGFR phosphorylation from lungs of nonsmokers, smokers, and individuals with chronic obstructive pulmonary disease (COPD). (B) Densitometry of phosphorylation of EGFR (pEGFR), HER3, and HER2 from lungs of nonsmokers compared with smokers (*P < 0.02, **P < 0.003, ***P < 0. 0005; n = 3). (C and D) Western blot and densitometry of EGFR, HER3, and HER2 in alveolar type II epithelial cells isolated from nonsmokers and smokers (*P < 0.01 control pEGFR versus smoker pEGFR, **P < 0.01 control phosphorylated HER [pHER] 3 versus smoker pHER3, ***P < 0.005 control pHER2 versus smoker pHER2; n = 3). (E and F) Western blot and densitometry of EGFR, HER3, and HER2 in airway epithelial cells isolated from nonsmokers and smokers (*P < 0.02 control pEGFR versus smoker pEGFR, **P < 0.01 control pHER3 versus smoker pHER3, ***P < 0.005 control pHER2 versus smoker pHER2); n = 3 for all experiments. (G) Bronchoalveolar lavage of neuregulin (NRG)-1 from smokers (n = 8) versus nonsmokers (n = 9). Values are expressed as means (±SEM).
As the epithelium is the initial site of contact for CS with the lung, we next sought to determine if HER2 was activated in pulmonary epithelial cells from smokers. Alveolar type II (ATII) and bronchial epithelial cells were harvested from lung of smokers and nonsmokers as previously described (13, 16), and HER2 activation was measured by Western blot (Figures 1C and 1D). Similar to what was observed in whole lung, there was significantly increased activation of HER2, HER3, and EGFR in both ATII and airway epithelial cells from smokers when compared with nonsmokers. In ATII cells, HER2, HER3, and EGFR phosphorylation were increased approximately eightfold (P < 0.001), threefold (P < 0.01), and threefold (P < 0.05) (Figure 1E). In airway epithelial cells, HER2, HER3, and EGFR phosphorylation were increased approximately 11-fold (P < 0.001), threefold (P < 0.001), and fourfold (P < 0.01) (Figure 1F).
Given that NRG-1 is elevated in BAL from patients with acute lung injury, we next assessed if there were differences in lavage NRG-1 in smokers versus nonsmokers. In contrast to acute lung injury, there was no difference in NRG-1 levels in BAL from smokers and nonsmokers (Figure 1G).
Transient CS Activates HER2, HER3, and EGFR in Pulmonary Epithelial Cells
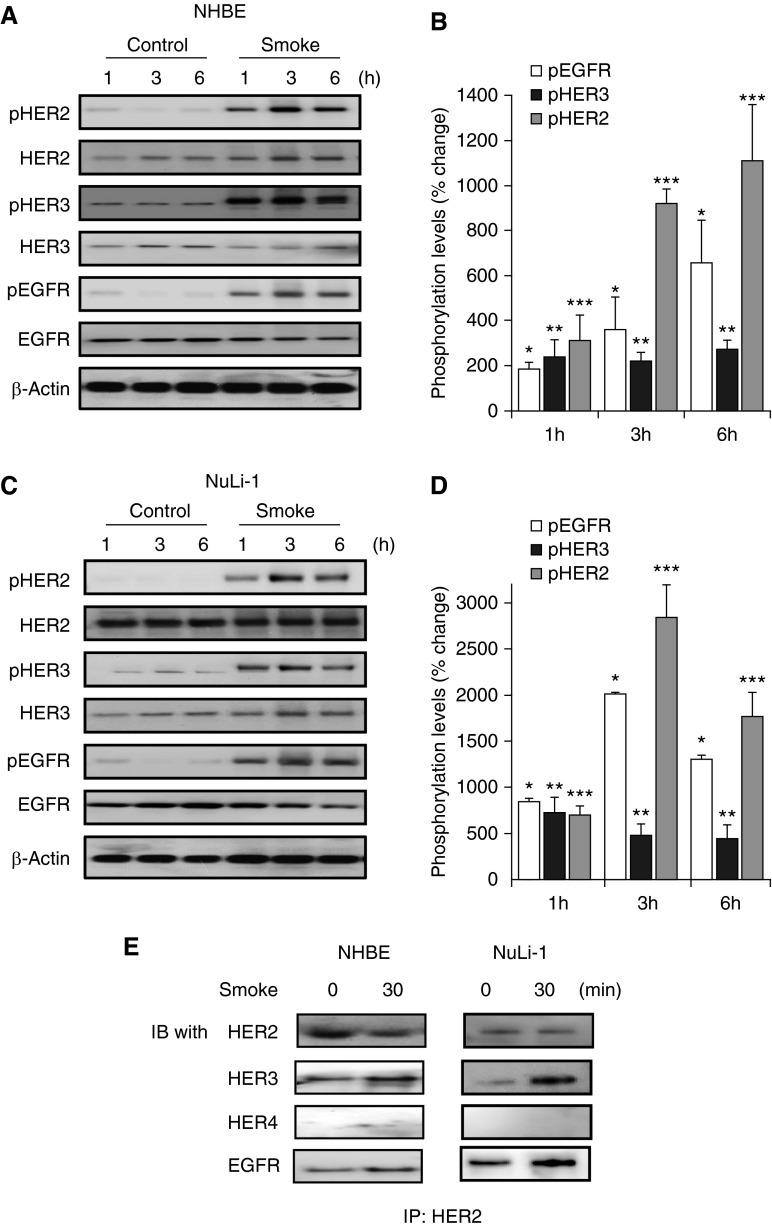
Generally, HER receptor activation occurs rapidly after ligand binding (11, 12). Given this, we next sought to determine if transient, acute CS induced activation of HER2 in cultured pulmonary epithelial cells. NHBE and NuLi-1 cells, a nontransformed, immortalized bronchial epithelial cell line, were cultured at ALI and exposed to CS for 10 minutes, after which media was replaced. Lysates were then collected at 1, 3, and 6 hours after CS exposure and HER2, HER3, and EGFR activation was measured. Although there were some differences in kinetics, at each time point, transient CS induced significant increases in phosphorylation of all three HER receptors (Figures 2A and 2B). In NHBE cells, CS increased HER2 phosphorylation 3-fold, 9-fold and 11-fold at 1, 3, and 6 hours. EGFR was increased twofold at 1 hour, fourfold at 3 hours, and roughly sixfold at 6 hours, and HER3 activation was increased twofold at 1 hour and threefold at both 3 and 6 hours, compared with cells exposed to ambient air. This effect was also observed in NuLi-1 cells (Figures 2C and 2D) with an increase in pHER2 of 7-, 28-, and 17-fold at 1, 3, and 6 hours compared with air-exposed cells. HER3 was increased 7-, 5-, and 4-fold at 1, 3, and 6 hours, whereas EGFR was increased 8-, 20-, and 13-fold at 1, 3, and 6 hours. HER receptors exist as monomers in a quiescent state, but activation includes formation of homo- and heterodimers. The receptor activation patterns observed after CS exposure suggest a physical association between HER2 with both HER3 and EGFR. To assess this, NHBE and NuLi-1 cells were exposed to CS and HER2 was immunoprecipitated, followed by Western blotting for EGFR, HER3, HER4, and HER2 (Figure 2E). HER2 was associated with HER3 and EGFR, but there was no evidence of an association with HER4. We (unpublished gene expression and RNA sequencing data of primary adult airway and alveolar epithelial cells) and others (17) have determined that all HER receptors, with the exception of HER4, are expressed in adult epithelial cells. In contrast, HER4 is expressed and known to play a critical role in epithelial cells of the developing lung (10).
Figure 2.
Cigarette smoke (CS) induces HER2, HER3, and EGFR activation in cultured pulmonary epithelial cells. (A and B) Western blot and densitometry of HER2, HER3, and EGFR phosphorylation in normal human bronchial epithelial cells (NHBE) cells grown at air–liquid interface (ALI) exposed to CS for 10 minutes and then placed in normal media. Lysates were collected after 1, 3, and 6 hours (*P < 0.05 pEGFR air versus CS 1 h, **P < 0.05 pHER3 air versus CS 1 h, ***P < 0.001 pHER2 air versus CS 1 h, *P < 0.01 pEGFR air versus CS 3 h, **P < 0.05 pHER3 air versus CS 3 h, ***P < 0.001 pHER2 air versus CS 3 h, *P < 0.001 pEGFR air versus CS 6 h, **P < 0.01 pHER3 air versus CS 6 h, ***P < 0.001 pHER2 air versus CS 6 h); n = 6. (C and D) Western blot and densitometry of HER2, HER3, and EGFR phosphorylation of CS-exposed NuLi-1 (*P < 0.05 pEGFR control versus 1 h, **P < 0.05 pHER3 control versus CS 1 h, ***P < 0.001 pHER2 control versus 1 h, *P < 0.01 pEGFR air versus smoke 3 h, **P < 0.05 pHER3 air versus smoke 3 h, ***P < 0.001 pHER2 air versus smoke 3 h, *P < 0.001 pEGFR air versus smoke 6 h, **P < 0.01 pHER3 air versus smoke 6 h, ***P < 0.001 pHER2 air versus smoke 6 h); n = 6. (E) Immunoprecipitation (IP) of HER2 in NuLi-1 and NHBE cells exposed to CS for 30 minutes, followed by immunoblotting (IB) for EGFR, HER3, HER4, and total HER2 (n = 3). Values are expressed as means (±SEM).
HER2 Activation by CS Occurs in Mice Exposed to CS
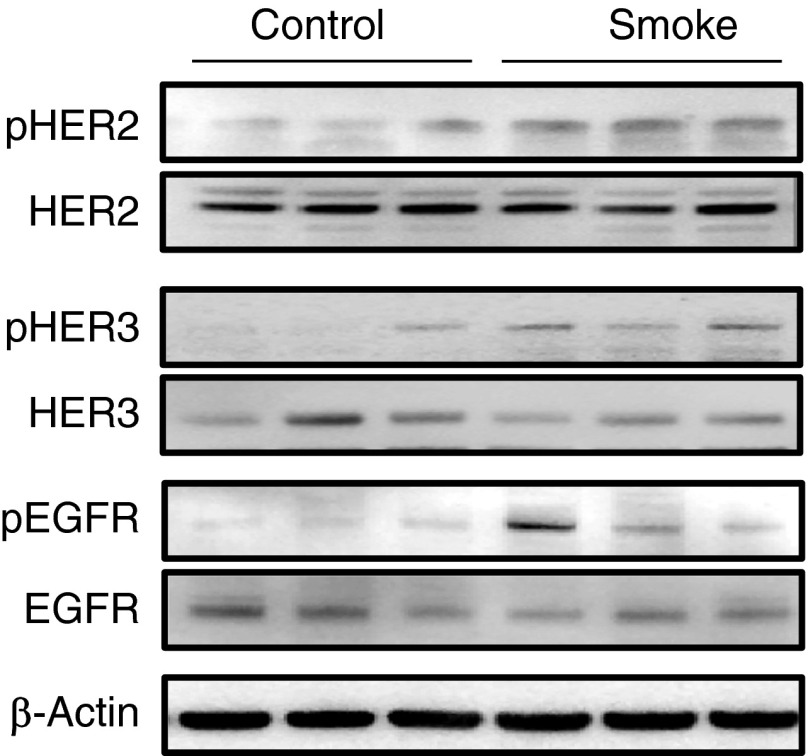
To determine if CS phosphorylated HER2 in vivo, C57Bl6/J mice were exposed to CS or air for 2 weeks, as this duration has been shown to induce an inflammatory response in the lung (18). Measurement of phosphorylated receptor from lungs of these mice demonstrated significantly increased HER2, HER3, and EGFR phosphorylation after CS exposure (Figure 3).
Figure 3.
HER2 activation by CS occurs in mice exposed to CS. C57Bl6/J mice were exposed to CS or air for two weeks (6 h/d 5 d/wk). Total and pHER2, HER3, and EGFR were measured in whole lung by Western blot (P < 0.01; n = 3).
HER2 Activation by CS Is Independent of NRG-1 and HER3
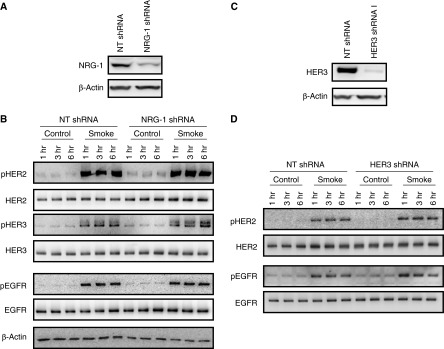
We have previously identified that the inflammatory cytokine, IL-1β, induces HER2 activation through a process dependent on the ligand NRG-1 binding HER3 and inducing HER2–HER3 heterodimerization and HER2 autophosphorylation (11), and that NRG-1 is elevated in bronchoalveolar lavage from patients with acute respiratory distress syndrome (8). Given this, we sought to determine if NRG-1 and HER3 were required for HER2 activation by CS. NuLi-1 cells were transduced with a specific shRNA for NRG-1, as well as an NT shRNA to achieve knockdown of at least 70% (Figure 4A). Knockdown cells were then grown to confluence at ALI and exposed to 10 minutes of CS, and receptor phosphorylation was assessed. Knockdown of NRG-1 with shRNA had no effect on the levels of total HER2, HER3 or EGFR (Figure 4B), and did not decrease activation of HER2, HER3 or EGFR after CS exposure (Figure 4B). In cells where HER3 was knocked down (Figure 4C), there was no effect on HER2 or EGFR levels or activation (Figure 4D). These findings demonstrate that CS-mediated activation of HER2 occurs independent of NRG-1 and HER3.
Figure 4.
HER2 activation by CS is independent of NRG-1 and HER3. (A) NRG-1 protein levels in NuLi-1 cells grown at ALI and transduced with an NRG-1 shRNA or nontargeting (NT) shRNA. (B) HER2, HER3, and EGFR phosphorylation in NuLi-1 cells transduced with NRG-1 or NT shRNA exposed to CS. (C) HER3 protein levels in NuLi-1 cells grown at ALI and transduced with an HER3 shRNA or NT shRNA. (D) HER2 and EGFR phosphorylation in NuLi-1 cells transfected with an HER3 or NT shRNA exposed to CS (n = 4). shRNA, small hairpin RNA.
HER2 and HER3 Activation by CS Are EGFR Dependent
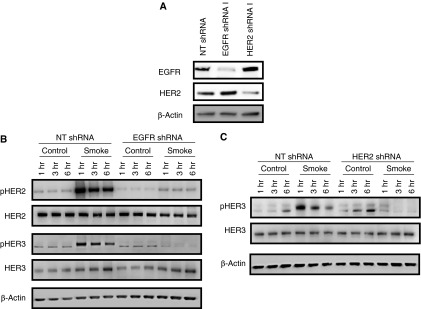
Given that HER2 and EGFR activation were independent of HER3 and NRG-1, we next sought to determine whether EGFR might participate in HER2 activation. NuLi-1 cells transduced with an EGFR-specific shRNA were grown at ALI, exposed to smoke for 10 minutes, and HER2 activation was measured by Western blot. As can be seen in Figure 5, EGFR knockdown resulted in loss of HER2 and HER3 activation after CS, indicating that EGFR is a required first step leading to HER2 and HER3 activation after CS. We observed HER2 and HER3 activation after CS that is not dependent on NRG-1 stimulation (Figures 5A and 5B), indicating that it is likely phosphorylated by a previously activated HER receptor, such as HER2 or EGFR. However, whether EGFR directly activates both HER2 and HER3 (in parallel), or activation of HER2 by EGFR is followed by HER2-dependent activation of HER3, is unknown. To address this, NuLi-1 cells transduced with an HER2 shRNA were exposed to CS, and HER3 phosphorylation was measured. In HER2 knockdown cells, HER3 activation was not observed, indicating that HER2 is a required partner and the likely kinase phosphorylating HER3 after CS exposure (Figure 5C).
Figure 5.
HER2 activation by CS in pulmonary epithelial cells is EGFR dependent. (A) HER2 and EGFR protein levels in NuLi-1 cells grown at ALI and transduced with HER2, EGFR, or NT shRNA. (B) HER2, HER3, and EGFR phosphorylation in NuLi-1 cells transduced with an NT or EGFR shRNA and exposed to CS. (C) HER3 activation in NuLi-1 cells transfected with an HER2 or NT shRNA.
CS-Mediated Epithelial Barrier Dysfunction Is HER2 Dependent
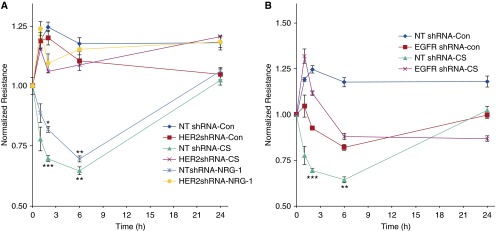
HER2 is known to modulate epithelial barrier function (11, 12), although whether it participates in epithelial barrier dysfunction after CS is unknown. To address this, NuLi-1 cells transfected with HER2 shRNA were grown to confluence (≥800 mΩ) at ALI, exposed to CS, and resistance was measured over 24 hours (Figure 6A). CS induced a significant, 45% decrease in resistance in cells that was rapid and reached a nadir at 6 hours. By 24 hours, resistance had returned to baseline. In comparison, in HER2 knockdown cells, there was no decrease in resistance after CS. Given that HER2 activation is EGFR dependent, we also exposed EGFR-deficient NuLi-1 cells to CS and measured resistance. Similar to HER2 knockdown cells, in EGFR knockdown cells there was no decrease in epithelial conductance (Figure 6B). These data indicate that CS-mediated changes in barrier function require both HER2 and EGFR.
Figure 6.
CS-mediated decreases in pulmonary epithelial resistance require HER2 and EGFR signaling. (A) NuLi-1 cells transduced with HER2 shRNA or NT shRNA were grown to confluence at ALI, and electrical resistance was measured after CS exposure. NRG-1 served as a positive control to induce HER2-dependent barrier dysfunction. NRG-1 decreased resistance 34% (*P < 0.05 NRG-1 versus control at 2 h) and 39% (**P < 0.001 NRG-1 versus control at 6 h). In NT cells, CS decreased resistance 44% (***P < 0.01 CS versus control at 2 h) and 45% (**P < 0.001 CS versus control at 6 h). There was no change in resistance in HER2 knockdown cells in CS-exposed versus air control cells (n = 3). (B) Resistance in NuLi-1 cells transduced with EGFR shRNA and exposed to CS. There was no change in resistance in EGFR knockdown cells in CS exposed versus air control cells (n = 3). Values are expressed as means (±SEM).
HER2 Activation by CS Results in Increased IL-6
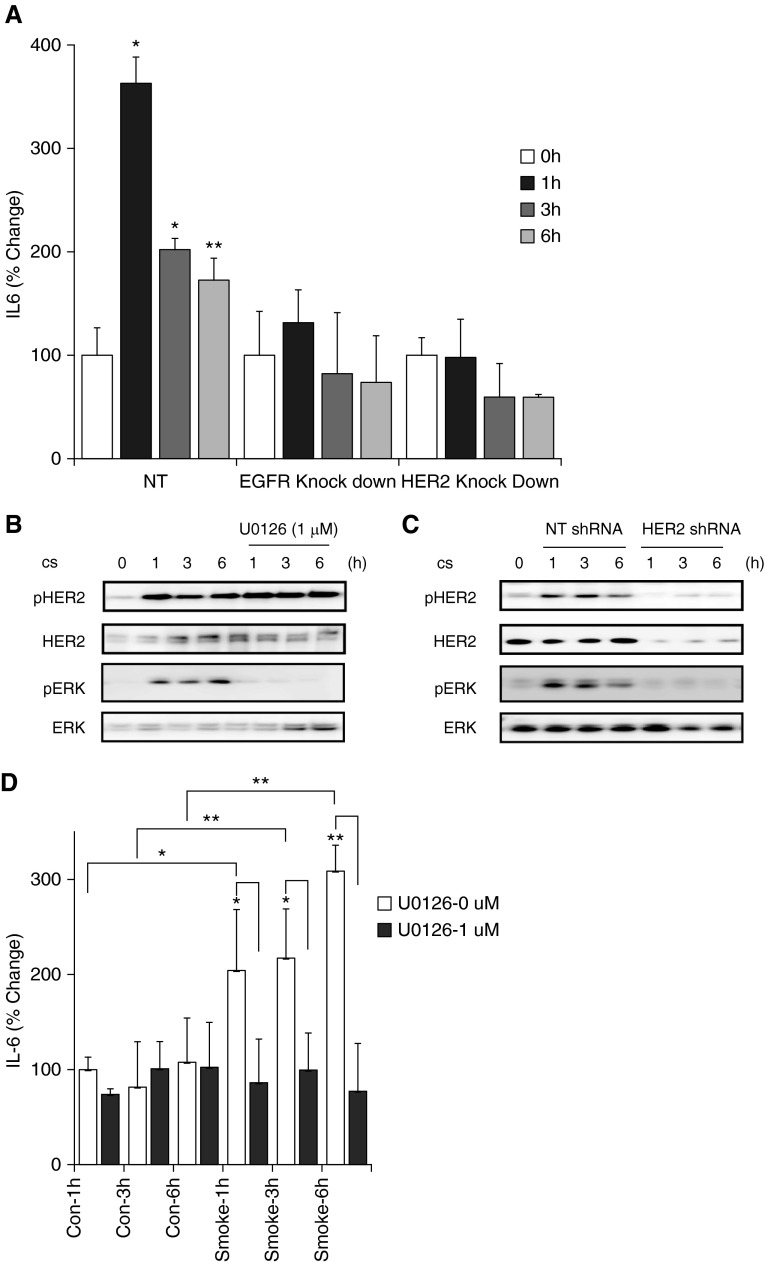
CS exposure results in a proinflammatory milieu in the airway, with an increase in IL-6 being a critical cytokine that increases after CS exposure. We sought to determine the role of HER2 in the increase in certain proinflammatory cytokines in pulmonary epithelial cells. IL-6 was measured in media from control, HER2 knockdown, and EGFR knockdown epithelial cells exposed to CS. Although CS induced a significant increase in both IL-6, knockdown of HER2 and EGFR completely abrogated the increase in these cytokines after CS (Figure 7A). As IL-6 production has been linked to Erk signaling in breast epithelial cells (19), we next sought to determine if Erk was required for HER2-dependent IL-6 production after CS. In our control pulmonary epithelial cells, Erk was activated in a time course similar to HER2. In HER2 knockdown cells, Erk activation was lost (Figure 7B). Use of the Erk inhibitor, U0126, blocked both Erk activation as well as IL-6 production after CS exposure (Figures 7C and 7D). Erk blockade had no effect on HER2 phosphorylation. Together, these data outline a signaling cascade, whereby CS leads to HER2 activation, HER2-dependent Erk activation, and subsequent IL-6 production.
Figure 7.
Epithelial IL-6 production after CS exposure is EGFR and HER2 dependent. (A) NuLi-1 cells transfected with HER2, EGFR, or NT shRNA were grown to confluence and exposed to CS, and in media IL-6 was measured by ELISA. In NT cells, CS increased IL-6 362% at 1 hour, 202% at 3 hours (*P < 0.001 versus control), and 172% at 6 hours (**P < 0.005 versus control). In EGFR knockdown cells, CS increased IL-6 131% at 1 hour, 82% at 3 hours, and 74% at 6 hours. In HER2 knockdown cells CS-induced increase in IL-6 was 97% at 1 hour, 59% at 3 hours, and 57% at 6 hours. (B) CS-induced HER2 phosphorylation at 1, 3, and 6 hours was not inhibited by ERK inhibitor U0126. (C) CS activates Erk in NuLi-1 cells but not in HER2 knockout cells. (D) NuLi-1 cells were exposed to CS in absence or presence of U0126, an ERK inhibitor, for 1, 3, and 6 hours. Level of IL-6 in media increased to 204%, 217% and 309% at 1, 3, and 6 hours, respectively, in absence of U0126 (*P > 0.05, **P < 0.01 versus control). In presence of U0126, the increase was limited to 86%, 97%, and 77% at 1, 3, and 6 hours, respectively, (P = NS versus control, *P > 0.05, **P < 0.01 versus corresponding time point). ERK, extracellular signal-regulated kinase; NS, not significant.
CS-Mediated Increases in Epithelial Permeability Are IL-6 Dependent
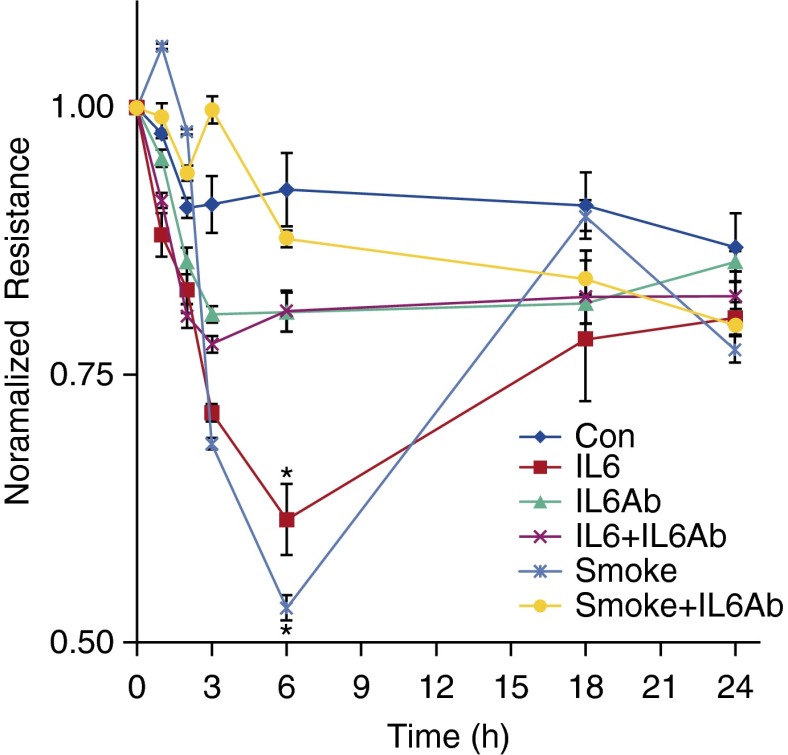
IL-6 has been demonstrated to participate in COPD pathogenesis and can modulate the epithelial barrier (20), but the effects of IL-6 in CS-mediated changes in epithelial barrier function has not been studied. To address if increases in epithelial permeability after CS exposure involved IL-6, we exposed NuLi-1 cells to IL-6 with and without an IL-6–blocking antibody. IL-6 induced a 61% decrease in epithelial resistance at 6 hours compared with untreated cells that was prevented with the blocking antibody (20% decrease), demonstrating that IL-6 can increase epithelial permeability. To specifically address the role of IL-6 in CS-mediated barrier modulation, resistance was measured in a monolayer of NuLi-1 cells exposed to CS in the presence of the IL-6–blocking antibody (Figure 8). Although CS decreased epithelial resistance 53%, pretreatment with the IL-6–blocking antibody significantly attenuated alterations in the epithelial barrier (20% change in resistance).
Figure 8.
CS-mediated increases in epithelial permeability are IL-6 dependent. NuLi-1 cells were grown to confluence at ALI, and resistance was measured after exposure to CS with and without pretreatment (30 min) with an IL-6–blocking antibody. Use of IL-6 served as a positive control and decreased electrical resistance 39% compared with a 19% drop in resistance at 6 hours in cells exposed to IL-6 and U0126. CS caused a 47% decrease in resistance to a 19% change in resistance at 6 hours in CS- and U0126-exposed cells (*P > 0.01 control [Con] versus smoke, smoke versus smoke plus IL-6–blocking antibody, control versus IL-6, IL-6 versus IL-6 plus IL-6–blocking antibody; n = 3 for each condition). Values are expressed as means (±SEM).
Discussion
In this study, we identified HER2 activation by CS and new mechanisms regulating epithelial barrier function after CS exposure. We determined that HER2 is phosphorylated in lungs, and specifically epithelial cells, from smokers and that CS rapidly activated HER2 in cultured pulmonary epithelial cells. This CS-mediated HER2 activation was independent of the ligand–receptor pair, NRG-1–HER3, but dependent on EGFR signaling. Interestingly, CS also activated HER3, which was NRG-1 independent, but dependent on both EGFR and HER2. Finally, CS decreased epithelial resistance through EGFR and HER2-dependent signaling. HER2 activation by CS has not been described previously, and these findings expand our understanding of inflammatory signaling in epithelial cells by CS and define a new signaling axis, CS–EGFR–HER2–IL6, which regulates epithelial barrier function in the context of CS.
The HER family is a complex system of ligands, receptors, and cytoplasmic adaptor proteins that modulate intracellular signaling. HER receptors exist in quiescent cells as single “closed” monomers, and ligand binding induces a change in confirmation to an “open” configuration. This allows homo or heterodimerization, leading to phosphorylation of intracellular tyrosine residues and activation of the kinase domain. The four HER receptors all share a similar structure, including an extracellular ligand-binding domain, a single transmembrane domain, and an cytoplasmic tyrosine kinase domain (7). However, certain structural distinctions influence how these monomers interact to form signaling-competent dimer pairs. Although HER2 has no ligand, it exists in a receptive “open” conformation, making it the preferred binding partner for other HER receptors. HER3 lacks an active kinase domain, but, when bound by its ligand, NRG-1, can readily dimerize with other HER receptors, be phosphorylated, and induce HER3 dependent signaling. We have previously identified that NRG-1 induces HER3-dependent activation of HER2 in airway and alveolar epithelial cells in the context of non–CS-mediated acute ling injury. Our current findings are notable in that HER3 was not required for HER2 activation by CS in pulmonary epithelial cells, and highlight the complexity of this signaling network. We have previously demonstrated that HER2 is activated in models of acute lung injury, a process that is felt to be IL-1β dependent. Similarly, in cultured epithelial cells, IL-1β–induced permeability is HER2 dependent. Interestingly, IL-1β requires NRG-1 and HER3 signaling upstream of HER2 activation, in contrast to the effects seen with CS, which are NRG-1 and HER3 independent. Notably, IL-1β plays a role in CS-mediated inflammation (21, 22), as well as emphysema and airways disease in mice (23); however, whether CS-mediated changes in epithelial permeability require IL-1β is unknown. CS does cause epithelial production of IL-1β; however, it is notable that, like IL-6, this requires Erk signaling (24). Given that the CS-mediated Erk–IL-6 production was HER2 dependent, it is possible that epithelial IL-1β release after CS exposure is HER2 dependent, although this was not specifically addressed in our experiments, nor was a specific requirement for IL-1β in CS-mediated HER2 activation.
We have previously identified that HER2 alters epithelial permeability via an interaction with the adherens protein, β-catenin (12). In our current study, we do not explore how HER2 interacts with adherens junction and tight junction proteins at points of cell–cell contact. Previous work has identified that CS alters the epithelial barrier through an interaction between P120-catenin and mucin-1 (MUC1) (25). Although EGFR has been linked to this effect, whether HER2 is a required partner remains unknown. CS also causes changes in tight junction proteins, including inducing a decrease in E-cadherin expression (6) and tyrosine phosphorylation of occludin, and decreased its association with zona occuldens-1 (ZO-1) (26), although the role of HER2 in these processes has not been studied.
This is the first description of an essential role for HER2 in CS-mediated effects on the epithelial barrier, and our findings identify new mechanisms, and possibly new therapeutic targets, regulating epithelial injury and permeability caused by CS. Epithelial injury and barrier disruption are significant consequences of CS exposure, which contribute to organ dysfunction and disease propagation. CS induces a rapid increase in pulmonary epithelial permeability in cultured epithelial cells, animal models, and humans (6, 27, 28). In addition, long-term smokers have a chronically permeable airway and alveolar epithelium (29). That HER2 is activated both rapidly after CS exposure and chronically in the epithelium of smokers supports its importance in CS-mediated barrier dysfunction.
Changes in epithelial cell–cell adhesion and permeability can have numerous consequences. Over time, alterations in epithelial barrier function stimulate a fibrotic response. Recurrent epithelial injury induces the recruitment and expansion of fibroblasts and myofibroblasts (30). The result is destruction of the original tissue architecture and deposition of excessive and disorganized extracellular matrix, including collagen and fibronectin. This dysregulated fibroblast proliferation and extracellular matrix production leads to the development of more diffuse pulmonary fibrosis with compromised lung function. It is apparent that deficient or delayed re-epithelialization of the basement membrane promotes fibrosis (31, 32), whereas rapid and complete re-epithelialization inhibits fibrosis (33). Increased airway fibrosis and wall thickness correlates with FEV1, and it is now understood that narrowed, scarred, small airways are the principal site of airflow obstruction in COPD. Although understanding of the mechanisms of airways disease in COPD is incomplete, data suggest that repeated CS-mediated epithelial injury resulting in loss of epithelial integrity and decreased barrier function contributes to this process (6). Beyond a possible effect on fibrogenesis, it is apparent that changes in epithelial barrier function caused by CS are pathologic in other pulmonary diseases, such as asthma (34, 35), allow for the passage of respiratory allergens (36), and are proinflammatory (30, 37). Increased permeability would result in transudation of plasma proteins into the alveolar space and small airways, causing inactivation of surfactant (38). In addition to maintaining alveolar surface tension, surfactant is antibacterial and antiviral, possibly further increasing the risk of infection or colonization in smokers. In addition, given that alterations in epithelial adhesion and polarity are hallmarks of epithelial malignancy (39), it is conceivable that changes in epithelial permeability influence future oncogenesis caused by CS. That HER2 is a critical mediator of barrier dysfunction after CS exposure has immediate translational potential, as HER2 inhibitors are currently available and in use in patients with breast cancer.
We have previously demonstrated that HER2 activation by NRG-1–HER3 can increase epithelial permeability (11), and EGFR has been linked to CS-mediated changes in epithelial resistance (40). However, that HER2 is a needed EGFR-binding partner mediating epithelial barrier disruption after CS likely has meaningful consequences. HER receptor signaling is influenced by numerous inputs, including ligand, dimerization partner, cell type, and existing intracellular signaling milieu. For example, depending on receptor expression patterns, NRG-1 can induce HER2/3 or EGFR/HER3 pairs with distinct intracellular signaling consequences (11, 41). HER2 enhances EGF-mediated activation of EGFR and prolongs EGFR signal transduction (42), and inhibits natural endocytosis of activated EGFR proteins (43, 44), thereby overcoming a regulator negative-feedback loop (45). In addition, although EGFR has been reported to directly activate HER3 (46), our finding that HER2 is required for EGFR-mediated activation of HER3 after CS exposure are consistent with previous reports outlining this kind of lateral transmission of signals among HER receptors (41). HER receptor activation is a dynamic process, and activated HER2 could dissociate from EGFR and subsequently bind and activate HER3, or could facilitate EGFR-mediated HER3 activation through formation of multiunit oligomers (47). The consequences of HER3 activation by CS in our system were not investigated, partially because HER3-deficient epithelial cells failed to achieve a competent monolayer (data not shown); however, HER3 activation has been implicated as an escape mechanism whereby cancer cells harboring an HER2 gene amplification become resistant to HER2 tyrosine kinase inhibitor (TKI) therapy (48).
We have identified HER2 as a regulator of epithelial IL-6 production after CS exposure. Numerous reports have outlined IL-6 as a possible major contributor to COPD generation. IL-6 has been identified as a candidate gene for COPD, and certain polymorphisms are associated with development of emphysema in smokers (49). Blood and lavage IL-6 levels are increased in patients with COPD and decreases after inhaled steroid treatment (50), suggesting a possible causal relationship. Indeed, murine studies confirm that overexpression of IL-6 induces spontaneous air space enlargement, whereas knockout mice are protected from emphysema development after CS exposure in mice (51). IL-6 has been linked to barrier function in different organs, including the skin (52) and gut (53). In the lung, IL-6 regulates in vivo epithelial wound repair after naphthalene, and enhances wound repair in an in vitro scratch model via signal transducer and activator of transcription 3 (STAT3) signaling (54). In a competent monolayer, IL-6 increases permeability through changing expression of claudin-2 (55). Although the intracellular pathways governing IL-6 production are not fully elucidated, we identified that the mitogen-activated protein kinase Erk serves as an intermediate set linking HER2 to IL-6 production.
We have identified that CS leads to HER2 activation via an EGFR-dependent mechanism. HER2 in turn activates Erk, leading to epithelial IL-6 release, and this CS–EGFR–HER2–IL-6 pathway regulates the epithelial barrier. Epithelial injury, including squamous metaplasia, increased mucous production, and increased permeability, are hallmarks of COPD, and understanding the mechanisms of CS-mediated epithelial injury is critical to designing novel therapies. That HER2 is activated in smokers and patients with COPD suggests that targeting HER2 with currently available inhibitors might be beneficial in reducing epithelial injury and pulmonary dysfunction caused by CS.
Footnotes
This work was supported by National Institutes of Health (NIH) grant HL111674 (J.H.F.), Flight Attendants Medical Research Initiative (FAMRI) grant 113038 (J.H.F.), NIH grant P50 CA058187 (J.H.F.), NIH grant R01 HL118171 (B.K.), and by FAMRI (B.K.).
Author Contributions: R.M. and J.H.F. helped design, conduct, and analyze experiments and write the manuscript; D.F., V.T.V., and J.V.T. helped design, conduct, and analyze experiments; B.K. and H.W.C. helped design and analyze experiments and write the manuscript; R.P.B. helped design and analyze experiments; J.H.F. helped design, conduct, and analyze experiments and write the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2014-0437OC on November 24, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Centers for Disease Control and Prevention (CDC) Vital signs: current cigarette smoking among adults aged ≥ 18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1135–1140. [PubMed] [Google Scholar]
- 2.Soriano JB, Rodríguez-Roisin R. Chronic obstructive pulmonary disease overview: epidemiology, risk factors, and clinical presentation. Proc Am Thorac Soc. 2011;8:363–367. doi: 10.1513/pats.201102-017RM. [DOI] [PubMed] [Google Scholar]
- 3.Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974;291:755–758. doi: 10.1056/NEJM197410102911503. [DOI] [PubMed] [Google Scholar]
- 4.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salazar LM, Herrera AM. Fibrotic response of tissue remodeling in COPD. Lung. 2011;189:101–109. doi: 10.1007/s00408-011-9279-2. [DOI] [PubMed] [Google Scholar]
- 6.Forteza RM, Casalino-Matsuda SM, Falcon NS, Valencia Gattas M, Monzon ME. Hyaluronan and layilin mediate loss of airway epithelial barrier function induced by cigarette smoke by decreasing E-cadherin. J Biol Chem. 2012;287:42288–42298. doi: 10.1074/jbc.M112.387795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finigan JH, Downey GP, Kern JA.Human epidermal growth factor receptor signaling in acute lung injury Am J Respir Cell Mol Biol 201247395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finigan JH, Mishra R, Vasu VT, Silveira LJ, Nethery DE, Standiford TJ, Burnham EL, Moss M, Kern JA.Bronchoalveolar lavage neuregulin-1 is elevated in acute lung injury and correlates with inflammation Eur Respir J 201241396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kheradmand F, Folkesson HG, Shum L, Derynk R, Pytela R, Matthay MA. Transforming growth factor-α enhances alveolar epithelial cell repair in a new in vitro model. Am J Physiol. 1994;267:L728–L738. doi: 10.1152/ajplung.1994.267.6.L728. [DOI] [PubMed] [Google Scholar]
- 10.Zscheppang K, Dörk T, Schmiedl A, Jones FE, Dammann CE. Neuregulin receptor ErbB4 functions as a transcriptional cofactor for the expression of surfactant protein B in the fetal lung. Am J Respir Cell Mol Biol. 2011;45:761–767. doi: 10.1165/rcmb.2010-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finigan JH, Faress JA, Wilkinson E, Mishra RS, Nethery DE, Wyler D, Shatat M, Ware LB, Matthay MA, Mason R, et al. Neuregulin-1–human epidermal receptor-2 signaling is a central regulator of pulmonary epithelial permeability and acute lung injury. J Biol Chem. 2011;286:10660–10670. doi: 10.1074/jbc.M110.208041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finigan JH, Vasu VT, Thaikoottathil JV, Mishra R, Shatat MA, Mason RJ, Kern JA. HER2 activation results in β-catenin–dependent changes in pulmonary epithelial permeability. Am J Physiol Lung Cell Mol Physiol. 2015;308:L199–L207. doi: 10.1152/ajplung.00237.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol. 2007;36:661–668. doi: 10.1165/rcmb.2006-0410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moré JM, Voelker DR, Silveira LJ, Edwards MG, Chan ED, Bowler RP. Smoking reduces surfactant protein D and phospholipids in patients with and without chronic obstructive pulmonary disease. BMC Pulm Med. 2010;10:53. doi: 10.1186/1471-2466-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Case S, Bowler RP, Martin RJ, Jiang D, Chu HW. Cigarette smoke modulates PGE(2) and host defence against Moraxella catarrhalis infection in human airway epithelial cells. Respirology. 2011;16:508–516. doi: 10.1111/j.1440-1843.2010.01920.x. [DOI] [PubMed] [Google Scholar]
- 16.Messier EM, Bahmed K, Tuder RM, Chu HW, Bowler RP, Kosmider B. Trolox contributes to Nrf2-mediated protection of human and murine primary alveolar type II cells from injury by cigarette smoke. Cell Death Dis. 2013;4:e573. doi: 10.1038/cddis.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tollefson AK, Oberley-Deegan RE, Butterfield KT, Nicks ME, Weaver MR, Remigio LK, Decsesznak J, Chu HW, Bratton DL, Riches DW, et al. Endogenous enzymes (NOX and ECSOD) regulate smoke-induced oxidative stress. Free Radic Biol Med. 2010;49:1937–1946. doi: 10.1016/j.freeradbiomed.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol Cell. 2012;45:777–789. doi: 10.1016/j.molcel.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Sadi R, Ye D, Boivin M, Guo S, Hashimi M, Ereifej L, Ma TY. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS One. 2014;9:e85345. doi: 10.1371/journal.pone.0085345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauwels NS, Bracke KR, Dupont LL, Van Pottelberge GR, Provoost S, Vanden Berghe T, Vandenabeele P, Lambrecht BN, Joos GF, Brusselle GG. Role of IL-1α and the Nlrp3/caspase-1/IL-1β axis in cigarette smoke–induced pulmonary inflammation and COPD. Eur Respir J. 2011;38:1019–1028. doi: 10.1183/09031936.00158110. [DOI] [PubMed] [Google Scholar]
- 22.Doz E, Noulin N, Boichot E, Guénon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180:1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 23.Churg A, Zhou S, Wang X, Wang R, Wright JL. The role of interleukin-1β in murine cigarette smoke–induced emphysema and small airway remodeling. Am J Respir Cell Mol Biol. 2009;40:482–490. doi: 10.1165/rcmb.2008-0038OC. [DOI] [PubMed] [Google Scholar]
- 24.Semlali A, Witoled C, Alanazi M, Rouabhia M. Whole cigarette smoke increased the expression of TLRs, HBDs, and proinflammory cytokines by human gingival epithelial cells through different signaling pathways. PLoS One. 2012;7:e52614. doi: 10.1371/journal.pone.0052614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Gallup M, Zlock L, Basbaum C, Finkbeiner WE, McNamara NA. Cigarette smoke disrupts the integrity of airway adherens junctions through the aberrant interaction of p120-catenin with the cytoplasmic tail of MUC1. J Pathol. 2013;229:74–86. doi: 10.1002/path.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivera D, Knall C, Boggs S, Seagrave J. Cytoskeletal modulation and tyrosine phosphorylation of tight junction proteins are associated with mainstream cigarette smoke–induced permeability of airway epithelium. Exp Toxicol Pathol. 2010;62:133–143. doi: 10.1016/j.etp.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Boucher RC, Johnson J, Inoue S, Hulbert W, Hogg JC. The effect of cigarette smoke on the permeability of guinea pig airways. Lab Invest. 1980;43:94–100. [PubMed] [Google Scholar]
- 28.Mason GR, Uszler JM, Effros RM, Reid E. Rapidly reversible alterations of pulmonary epithelial permeability induced by smoking. Chest. 1983;83:6–11. doi: 10.1378/chest.83.1.6. [DOI] [PubMed] [Google Scholar]
- 29.Jones JG, Minty BD, Lawler P, Hulands G, Crawley JC, Veall N. Increased alveolar epithelial permeability in cigarette smokers. Lancet. 1980;1:66–68. doi: 10.1016/s0140-6736(80)90493-6. [DOI] [PubMed] [Google Scholar]
- 30.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamson IY, Young L, Bowden DH. Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis. Am J Pathol. 1988;130:377–383. [PMC free article] [PubMed] [Google Scholar]
- 32.Bitterman PB. Pathogenesis of fibrosis in acute lung injury. Am J Med. 1992;92:39S–43S. doi: 10.1016/0002-9343(92)90606-c. [DOI] [PubMed] [Google Scholar]
- 33.Adamson IY, Hedgecock C, Bowden DH. Epithelial cell–fibroblast interactions in lung injury and repair. Am J Pathol. 1990;137:385–392. [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, Haitchi HM, Vernon-Wilson E, Sammut D, Bedke N, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–556. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 35.Rezaee F, Meednu N, Emo JA, Saatian B, Chapman TJ, Naydenov NG, De Benedetto A, Beck LA, Ivanov AI, Georas SN. Polyinosinic:polycytidylic acid induces protein kinase D–dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells. J Allergy Clin Immunol. 2011;128:1216–1224. doi: 10.1016/j.jaci.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gangl K, Reininger R, Bernhard D, Campana R, Pree I, Reisinger J, Kneidinger M, Kundi M, Dolznig H, Thurnher D, et al. Cigarette smoke facilitates allergen penetration across respiratory epithelium. Allergy. 2009;64:398–405. doi: 10.1111/j.1398-9995.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- 37.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 38.Zuo YY, Veldhuizen RA, Neumann AW, Petersen NO, Possmayer F. Current perspectives in pulmonary surfactant–inhibition, enhancement and evaluation. Biochim Biophys Acta. 2008;1778:1947–1977. doi: 10.1016/j.bbamem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 39.Gloushankova NA. Changes in regulation of cell–cell adhesion during tumor transformation. Biochemistry (Mosc) 2008;73:742–750. doi: 10.1134/s000629790807002x. [DOI] [PubMed] [Google Scholar]
- 40.Heijink IH, Brandenburg SM, Postma DS, van Oosterhout AJ. Cigarette smoke impairs airway epithelial barrier function and cell–cell contact recovery. Eur Respir J. 2012;39:419–428. doi: 10.1183/09031936.00193810. [DOI] [PubMed] [Google Scholar]
- 41.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartman Z, Zhao H, Agazie YM. HER2 stabilizes EGFR and itself by altering autophosphorylation patterns in a manner that overcomes regulatory mechanisms and promotes proliferative and transformation signaling. Oncogene. 2013;32:4169–4180. doi: 10.1038/onc.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haslekås C, Breen K, Pedersen KW, Johannessen LE, Stang E, Madshus IH. The inhibitory effect of ErbB2 on epidermal growth factor–induced formation of clathrin-coated pits correlates with retention of epidermal growth factor receptor–ErbB2 oligomeric complexes at the plasma membrane. Mol Biol Cell. 2005;16:5832–5842. doi: 10.1091/mbc.E05-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Offterdinger M, Bastiaens PI. Prolonged EGFR signaling by ERBB2-mediated sequestration at the plasma membrane. Traffic. 2008;9:147–155. doi: 10.1111/j.1600-0854.2007.00665.x. [DOI] [PubMed] [Google Scholar]
- 45.Stoscheck CM, Carpenter G. Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol. 1984;98:1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HH, Sierke SL, Koland JG. Epidermal growth factor–dependent association of phosphatidylinositol 3-kinase with the erbB3 gene product. J Biol Chem. 1994;269:24747–24755. [PubMed] [Google Scholar]
- 47.Lax I, Mitra AK, Ravera C, Hurwitz DR, Rubinstein M, Ullrich A, Stroud RM, Schlessinger J. Epidermal growth factor (EGF) induces oligomerization of soluble, extracellular, ligand-binding domain of EGF receptor: a low resolution projection structure of the ligand-binding domain. J Biol Chem. 1991;266:13828–13833. [PubMed] [Google Scholar]
- 48.Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sánchez V, Chakrabarty A, Dave B, Cook RS, Pao W, McKinely E, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci USA. 2011;108:5021–5026. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He JQ, Foreman MG, Shumansky K, Zhang X, Akhabir L, Sin DD, Man SF, DeMeo DL, Litonjua AA, Silverman EK, et al. Associations of IL6 polymorphisms with lung function decline and COPD. Thorax. 2009;64:698–704. doi: 10.1136/thx.2008.111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strandberg K, Palmberg L, Larsson K. Effect of budesonide and formoterol on IL-6 and IL-8 release from primary bronchial epithelial cells. J Asthma. 2008;45:201–203. doi: 10.1080/02770900801890372. [DOI] [PubMed] [Google Scholar]
- 51.Ruwanpura SM, McLeod L, Miller A, Jones J, Bozinovski S, Vlahos R, Ernst M, Armes J, Bardin PG, Anderson GP, et al. Interleukin-6 promotes pulmonary emphysema associated with apoptosis in mice. Am J Respir Cell Mol Biol. 2011;45:720–730. doi: 10.1165/rcmb.2010-0462OC. [DOI] [PubMed] [Google Scholar]
- 52.Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, Luster MI. Impaired cutaneous wound healing in interleukin-6–deficient and immunosuppressed mice. FASEB J. 2000;14:2525–2531. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- 53.Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 54.Kida H, Mucenski ML, Thitoff AR, Le Cras TD, Park KS, Ikegami M, Müller W, Whitsett JA. GP130-STAT3 regulates epithelial cell migration and is required for repair of the bronchiolar epithelium. Am J Pathol. 2008;172:1542–1554. doi: 10.2353/ajpath.2008.071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]