Abstract
Pathologic accumulation of fibroblasts in pulmonary fibrosis appears to depend on their invasion through basement membranes and extracellular matrices. Fibroblasts from the fibrotic lungs of patients with idiopathic pulmonary fibrosis (IPF) have been demonstrated to acquire a phenotype characterized by increased cell-autonomous invasion. Here, we investigated whether fibroblast invasion is further stimulated by soluble mediators induced by lung injury. We found that bronchoalveolar lavage fluids from bleomycin-challenged mice or patients with IPF contain mediators that dramatically increase the matrix invasion of primary lung fibroblasts. Further characterization of this non–cell-autonomous fibroblast invasion suggested that the mediators driving this process are produced locally after lung injury and are preferentially produced by fibrogenic (e.g., bleomycin-induced) rather than nonfibrogenic (e.g., LPS-induced) lung injury. Comparison of invasion and migration induced by a series of fibroblast-active mediators indicated that these two forms of fibroblast movement are directed by distinct sets of stimuli. Finally, knockdown of multiple different membrane receptors, including platelet-derived growth factor receptor-β, lysophosphatidic acid 1, epidermal growth factor receptor, and fibroblast growth factor receptor 2, mitigated the non–cell-autonomous fibroblast invasion induced by bronchoalveolar lavage from bleomycin-injured mice, suggesting that multiple different mediators drive fibroblast invasion in pulmonary fibrosis. The magnitude of this mediator-driven fibroblast invasion suggests that its inhibition could be a novel therapeutic strategy for pulmonary fibrosis. Further elaboration of the molecular mechanisms that drive non–cell-autonomous fibroblast invasion consequently may provide a rich set of novel drug targets for the treatment of IPF and other fibrotic lung diseases.
Keywords: idiopathic pulmonary fibrosis, pathogenesis, fibroblast, invasion, migration
Clinical Relevance
Accumulation of fibroblasts in pulmonary fibrosis appears to depend on their invasion through basement membranes and extracellular matrices. Here, we demonstrate that bronchoalveolar lavage fluids from bleomycin-challenged mice or patients with idiopathic pulmonary fibrosis contain mediators that dramatically increase the matrix invasion of primary lung fibroblasts. The magnitude of this mediator-driven fibroblast invasion suggests that its inhibition could be a novel therapeutic strategy for pulmonary fibrosis.
Idiopathic pulmonary fibrosis (IPF) is a disease of great unmet medical need. Aberrant or overexuberant wound healing responses to chronic lung injury are now thought to be responsible for the excessive fibroblast accumulation and extracellular matrix deposition that distort the lung’s architecture and compromise its function in IPF (1, 2). Better characterization of these abnormal responses to lung injury should provide a rich set of therapeutic targets for novel IPF therapies.
The abnormal accumulation of fibroblasts is a central hallmark of fibrotic tissues, including the lung in IPF. In addition to fibroblast proliferation, fibroblast accumulation in pulmonary fibrosis appears to fundamentally depend on: (1) the migration of these cells to sites of tissue injury; and (2) their ability to invade extracellular matrix. Although migration and invasion share some fundamental mechanisms (e.g., both are powered by polarized actomyosin-driven shape change of the cell body [3]), these two modes of cell movement are distinct processes. Cell invasion, but not migration, involves activation of cell surface proteases that can remodel extracellular matrix, generating gaps through which invading cells can move (4). Cell leading edge structures also differ in migration versus invasion. Lamellipodia protrude from the leading edge of migrating cells, whereas different protrusions, termed invadopodia or podosomes, lead invading cells (5). With the exception of leukocytes, cells normally migrate along extracellular matrix, but do not invade through interstitial tissues (5).
Acquisition of the ability to invade through basement membranes and interstitial matrices by nonleukocytes has long been appreciated to be central to cancer biology, and fibroblast acquisition of this ability has recently gained attention in pulmonary fibrosis. This invasive capacity is the most important feature that distinguishes benign from malignant lesions, and the pathways leading to the acquisition of this capacity consequently have been recognized as an important source of novel targets for cancer therapies (6). An invasive phenotype has also recently been shown to distinguish lung fibroblasts isolated from patients with IPF and control subjects (7), and to distinguish lung fibroblasts isolated from bleomycin-injured mice and those from unchallenged mice (7, 8). The pathologic consequences of fibroblast invasiveness in fibrotic lung diseases were documented in a large study of lung biopsies and autopsy specimens from 373 patients with a wide variety of fibrotic lung disorders, including IPF (9). Fibroblasts and fibrotic tissue were present in the lumens of the alveoli, the alveolar ducts, and/or distal bronchioles in these diseases, suggesting that fibroblast invasion from the lung interstitium into the airspaces is a general feature of the development of pulmonary fibrosis. Therapies targeting the mediators and pathways of fibroblast invasion consequently could benefit patients with IPF and a broad array of other fibrotic lung diseases. Some of the molecules required for the invasive phenotype of fibrotic fibroblasts have already been identified, including hyaluronan synthase 2, CD44, and β-arrestin 1 and 2 (7, 8). Targeting these molecules has been shown to limit fibroblast invasion, but not migration, and to protect mice from bleomycin-induced pulmonary fibrosis (7, 8), providing important proof-of-concept evidence that inhibiting fibroblast invasion can be an effective therapeutic strategy for lung fibrosis.
Studies to date of the invasive phenotype of fibrotic lung fibroblasts from patients with IPF and bleomycin-challenged mice have demonstrated the increased cell-autonomous invasion of these cells (i.e., their increased ability to invade through extracellular matrix in the absence of added stimuli) (7, 10). Here, we investigated whether fibrogenic lung injury also induces increased non–cell-autonomous fibroblast invasion (i.e., fibroblast invasion driven by extrinsic mediators induced by lung injury). Bronchoalveolar lavage (BAL) from patients with fibrotic lung diseases, such as IPF and scleroderma-associated interstitial lung disease, has long been known to contain soluble mediators of fibroblast migration (11). We have previously demonstrated that the fibroblast chemoattractant activity of IPF BAL is largely attributable to lysophosphatidic acid (LPA) that is induced by lung injury (12). Here, we present evidence that soluble mediators, which potently direct fibroblast invasion, are also induced by fibrogenic lung injury, and are present in the airspaces of, and BAL recovered from, both bleomycin-injured mice and patients with IPF. This non–cell-autonomous invasion induced by these extrinsic BAL mediators was substantially greater than the cell-autonomous invasion of fibrotic lung fibroblasts in both humans and mice. Additional studies in mice indicated that BAL mediators of fibroblast invasion were induced to a substantially greater extent by fibrogenic bleomycin injury than by nonfibrogenic LPS injury, and that mediators driving fibroblast invasion can differ from those driving fibroblast migration. Taken together, we believe that these data suggest that targeting the non–cell-autonomous fibroblast invasion induced by lung injury may represent an effective new therapeutic strategy for IPF in particular, and for fibrotic lung diseases in general.
Materials and Methods
Bleomycin and LPS Mouse Models
Fibrosis and acute lung injury were induced by a single intratracheal injection of bleomycin (Fresenius Kabi, Lake Zurich, IL) or 4 mg/kg LPS (Escherichia coli 026:B6; Sigma, St. Louis, MO), respectively, to anesthetized 6- to 8-week-old male C57Bl/6 mice (NCI-Frederick, Frederick, MD), as previously described (12–16). All animal protocols were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care (Massachusetts General Hospital, Boston, MA), and all mice were maintained in a specific pathogen-free environment certified by the American Association for Accreditation of Laboratory Animal Care.
Fibroblast Invasion and Migration Assays
To assess invasion, fibroblasts were labeled with 1,1′-didodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DilC12) (3) fluorescent dye (BD Biosciences, Franklin Lakes, NJ) by incubation for 1 hour prior, and then transferred to a 96-well BioCoat Tumor Invasion System (Corning, Corning, NY; Figure 1A), in which transwell inserts are coated with BD Matrigel. Invasion through Matrigel is used to model invasion through an extracellular matrix similar in composition to basement membranes. A caveat to using these invasion systems to model fibroblast invasion in IPF is that, in the disease setting, lung injury may also create gaps in basement membranes (17), which may allow fibroblasts to enter alveolar spaces by migration rather than invasion. To assess migration, fibroblasts were transferred to a 96-well Corning FlouroBlock Insert System (Corning), in which there is no barrier to migration through insert pores. The transwells in these migration systems were precoated with fibronectin at 10 μg/ml (Sigma) to facilitate cell attachment. In both assays, fibroblasts were added at a concentration of 50,000 cells per well in serum-free Dulbecco’s modified Eagle's medium. Chemoattractants in Dulbecco’s modified Eagle's medium, including platelet-derived growth factor-AA (PDGF-AA), PDGF-AB, PDGF-BB, epidermal growth factor (EGF), fibroblast growth factor 1 (FGF-1) and -2 (all from PeproTech, Rocky Hill, NJ), LPA (Avanti Polar Lipids, Alabaster, AL), and thrombin, keratinocyte growth factor (KGF), and transforming growth factor (TGF)-β1 (all from R&D Systems, Minneapolis, MN) were placed in the wells of both the invasion and migration assays. After 24 hours, fibroblasts were quantified with a Fluoroskan Ascent FL (Thermo, Waltham, MA) set to read from plate bottoms.
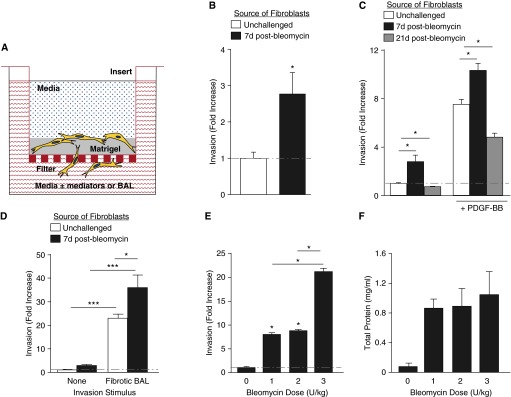
Figure 1.
Fibrogenic lung injury increases lung fibroblast invasion. (A) Schematic drawing of the filter-based assay used to model fibroblast invasion through basement membrane. We assessed primary lung fibroblast invasion through Matrigel covering polyethylene filters with 8-μm pores, in response to media with or without defined mediators or bronchoalveolar lavage (BAL). (B) Lung fibroblasts isolated from mice at Day 7 after bleomycin challenge demonstrated increased cell-autonomous invasion compared with lung fibroblasts from unchallenged mice. Data are presented as mean (±SEM) fold increase in number of invading cells, normalized to invasion of lung fibroblasts from unchallenged mice (n = 5 experiments; *P < 0.05). (C) Lung fibroblasts isolated from mice at Day 21 after bleomycin challenge demonstrated decreased cell-autonomous and non–cell-autonomous invasion compared with lung fibroblasts from unchallenged mice. Non–cell-autonomous invasion was induced by platelet-derived growth factor-BB (PDGF-BB) (10−9 M). Data are presented as mean (±SEM) fold increase in invasion, normalized to lung fibroblasts from unchallenged mice (n = 3 mice per group; *P < 0.05). (D) BAL recovered from mice at Day 7 after 1.2 U/kg bleomycin challenge induced non–cell-autonomous invasion of lung fibroblasts isolated from either unchallenged or bleomycin-challenged mice, which greatly exceeded the cell-autonomous invasion of these cells. Data are presented as mean (±SEM) fold increase in invasion, normalized to the invasion of lung fibroblasts from unchallenged mice in the absence of an exogenous stimulus (n = 5 experiments; *P < 0.05, ***P < 0.001). (E) BAL collected from mice at Day 7 after challenge with 3 U/kg bleomycin induced greater non–cell-autonomous invasion of unchallenged mouse lung fibroblasts than did BAL collected from mice at Day 7 after challenge with 1 or 2 U/kg bleomycin. Data are presented as mean (±SEM) fold increase in invasion, normalized to the cell-autonomous invasion of lung fibroblasts from unchallenged mice (n = 5 mice per group as the source of lung fibroblasts, and n = 5 mice per group as the source of BAL; *P < 0.05). (F) Higher-dose bleomycin did not increase vascular leak at Day 7 after challenge, as assessed by BAL total protein concentration (n = 5 mice per group as the source of BAL).
Human Lung Fibroblasts
Fibroblasts isolated from the lungs of patients diagnosed with IPF or normal subjects were kindly provided by Dr. Carol Feghali-Bostwick (Division of Rheumatology and Immunology, Medical University of South Carolina, Charleston, SC), and used at passages 3–5. These fibroblasts were cultured from the explanted lungs of patients with IPF who underwent lung transplantation at the University of Pittsburgh Medical Center (Pittsburgh, PA), under a protocol approved by the University of Pittsburgh Institutional Review Board, or from the normal lungs of potential organ donors considered, but rejected, for lung transplantation.
Human BAL
Supernatants of BAL samples recovered from patients with IPF and control subjects were kindly provided by Dr. Moisés Selman (Instituto Nacional de Enfermedades Respiratorias, Mexico City, Mexico) and Dr. Annie Pardo (Universidad Nacional Autonoma de Mexico, Mexico City, Mexico). These samples were recovered after instillation of sterile 0.9% saline by flexible fiberoptic bronchoscopy, performed at the Instituto Nacional de Enfermedades Respiratorias (Mexico City, Mexico), as previously described (18). These studies were approved by the Instituto Nacional de Enfermedades Respiratorias Ethics Committee, and informed consent was obtained from all participants.
Statistical Analysis
Differences between groups were analyzed with two-tailed Student’s t tests, using Microsoft Excel software (Microsoft, Redmond, WA). Additional descriptions of materials and methods are contained in the online supplement.
Results
Lung Fibroblasts Demonstrate Increased Invasion Early, but Not Late, after Fibrogenic Lung Injury
To quantify fibroblast invasion, we measured the emission of fluorescently labeled cells that had invaded through a Matrigel invasion chamber (Figure 1A), which correlated closely with the number of invaded cells when manually counted (r2 = 0.996; P < 0.001, data not shown). In the absence of exogenously added “chemoinvasants” (i.e., soluble mediators that stimulate cell invasion), there was a 2.7-fold increase in cell-autonomous invasion by fibroblasts isolated from mice 7 days after bleomycin challenge (with 1.2 U/kg, our standard dose to produce pulmonary fibrosis) compared with fibroblasts isolated from unchallenged mice (Figure 1B). These results demonstrate that fibroblasts from bleomycin-injured lungs acquire an invasive phenotype early after fibrogenic injury, before the development of measurable pulmonary fibrosis in this model (19).
In contrast to Day 7, pulmonary fibrosis is well established at Day 21 after bleomycin challenge, at which time measurable increases in lung collagen are present (19). Interestingly, at this later time point, fibroblasts from bleomycin-injured lungs no longer demonstrated an invasive phenotype. In the absence of exogenous chemoinvasants, cell-autonomous invasion of fibroblasts isolated from mice 21 days after 1.2 U/kg bleomycin challenge was actually decreased to 70% of the invasion observed with fibroblasts isolated from unchallenged mice (Figure 1C).
Similar to the differences in cell-autonomous invasion, we found that non–cell-autonomous invasion of lung fibroblasts was increased early, but decreased late after fibrogenic injury. Compared with their cell-autonomous invasion, addition of the exogenous chemoinvasant, PDGF-BB, to the invasion chamber wells below lung fibroblasts from unchallenged mice increased their invasion 7.5-fold. In contrast, PDGF-BB increased the invasion of lung fibroblasts isolated 7 days after bleomycin challenge 10.3-fold over the cell-autonomous invasion of lung fibroblasts from unchallenged mice, whereas it increased the invasion of lung fibroblasts isolated 21 days after bleomycin challenge only 4.8-fold (Figure 1C). These data indicate that fibroblasts isolated early after fibrogenic lung injury are characterized by increased cell-autonomous and non–cell-autonomous invasion, whereas fibroblasts isolated after fibrosis has been established demonstrate decreased cell-autonomous and non–cell-autonomous invasion.
Potent Soluble Mediators of Non–Cell-Autonomous Fibroblast Invasion Are Induced by Lung Injury
Given the ability of PDGF-BB to further stimulate invasion by fibroblasts that already demonstrated increased cell-autonomous invasion after fibrogenic injury, we investigated whether mediators with similar activity (i.e., chemoinvasants) are induced in the fibrosing lung. We found that BAL isolated from mice 7 days after bleomycin injury potently induced fibroblast invasion, and that this non–cell-autonomous invasion greatly exceeded the cell-autonomous invasion induced by the same lung injury. Using lung fibroblasts from unchallenged mice, BAL isolated from mice 7 days after 1.2 U/kg bleomycin increased the invasion of these cells by 23-fold over the extent of their cell-autonomous invasion. Using lung fibroblasts isolated from mice 7 days after bleomycin challenge, BAL isolated from mice 7 days after 1.2 U/kg bleomycin increased the invasion of these cells by 36-fold over the extent of their cell-autonomous invasion (Figure 1D).
To determine if the induction of soluble mediators of fibroblast invasion exhibited a dose–response relationship to fibrogenic lung injury, we investigated the non–cell-autonomous fibroblast invasion induced by BAL from mice challenged with different doses of bleomycin. We collected BAL from unchallenged mice, and from mice 7 days after intratracheal injection of 1, 2, and 3 U/kg bleomycin. Compared with the BAL from unchallenged mice, there was significantly greater fibroblast invasion induced by BAL from mice challenged with 1 or 2 U/kg bleomycin, but the extent of invasion was similar between these two challenge doses. There was a dramatically greater increase in fibroblast invasion induced by BAL from mice challenged with 3 U/kg bleomycin, however, which induced a 21-fold increase in fibroblast invasion compared with BAL from unchallenged mice versus 7.9- and 8.7-fold increases induced by BAL from mice challenged with 1 or 2 U/kg bleomycin, respectively (Figure 1E). Of note, there were no significant differences in BAL total protein levels between mice challenged with the different bleomycin doses (Figure 1F), suggesting that increased local production of chemoinvasants, rather than increased leak of circulating chemoinvasants produced outside the lung, was responsible for the increased fibroblast invasion induced by BAL from mice challenged with the highest dose. Taken together, these data indicate that potent mediators of non–cell-autonomous fibroblast invasion are induced early in the lung after fibrogenic injury.
TGF-β1 Pretreatment Decreases Lung Fibroblast Cell-Autonomous and Non–Cell-Autonomous Migration and Invasion
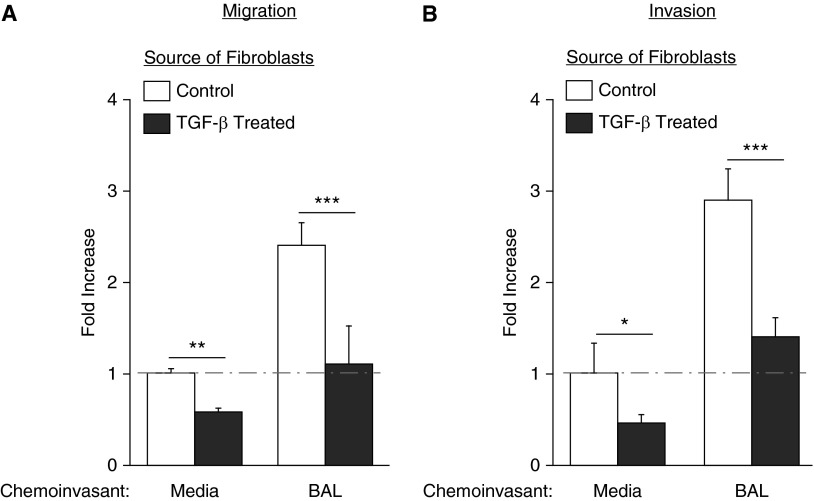
To further investigate the decrease in cell-autonomous invasion of lung fibroblasts isolated 21 days versus 7 days after bleomycin injury, we investigated the effects of TGF-β1 pretreatment on lung fibroblast cell–autonomous and non–cell-autonomous migration and invasion. TGF-β1 is the archetypal mediator of fibroblast differentiation to myofibroblasts, which have a contractile and matrix-synthetic effector phenotype, and accumulate as lung fibrosis progresses in both patients with IPF and mouse lung fibrosis models (20). We found that fibroblasts treated with TGF-β1 for 48 hours demonstrated decreased cell-autonomous and non–cell-autonomous migration (Figure 2A), and decreased cell-autonomous and non–cell-autonomous invasion (Figure 2B), compared with unstimulated fibroblasts. We confirmed that TGF-β1 exposure increased collagen I production, as assessed by mRNA expression (see Figure E1A in the online supplement), and myofibroblast differentiation, as determined by α-smooth muscle actin staining (Figure E1B). Taken together, these data suggest that myofibroblasts, which are increasingly prevalent at later time points after bleomycin challenge, are less invasive, as well as less migratory, than the less-activated fibroblasts that predominate at earlier time points after injury.
Figure 2.
Transforming growth factor (TGF)-β1 stimulation decreases migration and invasion of lung fibroblasts. (A) Primary lung fibroblasts pretreated with TGF-β1 for 48 hours had decreased migration (A) and invasion (B), both in the absence and presence of BAL from injured mice. Data are presented as mean (±SEM) fold increase in migration or invasion, normalized to the control fibroblast to media condition (n = 3 mice as source of lung fibroblasts and BAL; *P < 0.05, **P < 0.01, ***P < 0.001).
Fibrogenic Bleomycin Injury Induces Greater Fibroblast Invasion than Nonfibrogenic LPS Injury
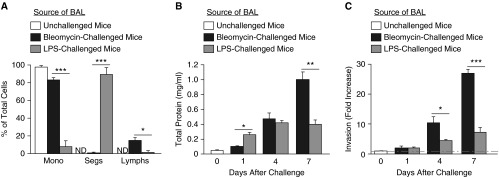
To investigate whether induction of fibroblast invasion is specific to fibrogenic lung injury, we compared invasion induced by BAL from mice after bleomycin versus LPS challenge. We collected BAL from mice 1, 4, and 7 days after intratracheal challenge with either 1.2 U/kg bleomycin or 4 mg/kg LPS. We first compared BAL leukocyte differential counts 1 day after these different challenges, and BAL total protein levels at 1, 4, and 7 days. At 1 day after LPS challenge, the great majority of BAL leukocytes were neutrophils, in contrast to BAL from the bleomycin-challenged mice, which contained mostly macrophages and some lymphocytes (Figure 3A). The kinetics of vascular leak also differed in the two models. The amount of BAL total protein in the LPS model increased at Day 1, and increased further reaching a plateau at Day 4. Total protein in BAL after bleomycin was lower than BAL after LPS BAL at Day 1, similar at Day 4, and greater than BAL after LPS at Day 7 (Figure 3B). The ability of BAL after bleomycin to induce non–cell-autonomous fibroblast invasion increased over time as well. BAL collected at Day 1 after either bleomycin or LPS challenge failed to induce much fibroblast invasion, but BAL after bleomycin, collected at Days 4 and 7, induced significantly greater fibroblast invasion than BAL after LPS collected at these time points (Figure 3C). These data indicate that mediators of non–cell-autonomous fibroblast invasion are induced in the lungs to a greater extent by fibrogenic rather than nonfibrogenic injury.
Figure 3.
Comparison of fibroblast invasion induced by profibrotic bleomycin injury with proinflammatory LPS injury. (A) BAL recovered from mice at Day 1 after 4 mg/kg LPS challenge had greater percentages of neutrophils (Segs) than BAL recovered from mice at Day 1 after 1.2 U/kg bleomycin challenge, whereas BAL from bleomycin-challenged mice had greater percentages of macrophages (Mono) and lymphocytes (Lymphs). Data are presented as mean (±SEM) percentage (n = 3 mice per group; *P < 0.05, ***P < 0.001). (B) Determination of total protein concentrations in BAL from unchallenged mice and from mice at Days 1, 4, and 7 after 1.2 U/kg bleomycin or 4 mg/kg LPS challenge, indicated protein concentration was greater in LPS-challenged mice than in bleomycin-challenged mice at Day 1, similar between these groups at Day 4, and greater in bleomycin-challenged mice than in LPS-challenged mice at Day 7. Data are presented as mean (±SEM) total protein concentration (n = 3 mice per group; *P < 0.05, **P < 0.01). (C) BAL from mice at Days 4 and 7 after 1.2 U/kg bleomycin challenge induced greater non–cell-autonomous invasion of lung fibroblasts isolated from unchallenged mice than did BAL from mice at these days after 4 mg/kg LPS challenge. Data are presented as mean (±SEM) fold increase in invasion, normalized to the cell-autonomous invasion of lung fibroblasts from unchallenged mice (n = 3 mice per group as the source of lung fibroblasts, and n = 3 mice per group as the source of BAL; *P < 0.05, ***P < 0.001). ND, none detected.
Fibroblast-Active Mediators Have Different Effects on Chemotaxis and Invasion
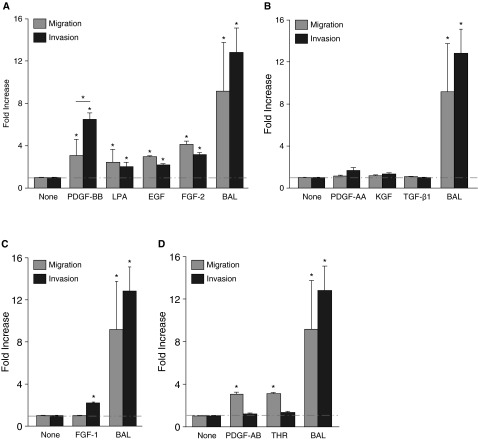
To investigate which mediators might contribute to the fibroblast invasion induced by BAL from bleomycin-challenged mice, we compared the migration versus invasion induced by multiple cytokines and other mediators known to be active on fibroblasts. Some mediators induced both activities (PDGF-BB, LPA, EGF, and FGF-2; Figure 4A), whrereas others induced neither (PDGF-AA, KGF, and TGF-β1; Figure 4B). Interestingly, we found that some mediators had discordant abilities to mediate these activities, consistent with these two modes of cell movement having some shared, but other distinct, mechanisms. FGF-1 specifically induced fibroblast invasion, but not migration (Figure 4C), whereas PDGF-AB and thrombin specifically induced fibroblast migration, but not invasion (Figure 4D). Of note, BAL from bleomycin-challenged mice was a more potent inducer of non–cell-autonomous invasion than any individual mediator tested (Figures 4A–4D).
Figure 4.
Different effects of fibroblast-active mediators on migration versus invasion. Assessment of the migration and invasion of lung fibroblasts from unchallenged mice induced by multiple fibroblast-active mediators indicated that: (A) PDGF-BB, lysophosphatidic acid (LPA), epidermal growth factor (EGF), and fibroblast growth factor 2 (FGF-2) induced both migration and invasion; (B) PDGF-AA, keratinocyte growth factor (KGF), and TGF-β1 induced neither migration nor invasion; (C) FGF-1 induced invasion, but not migration; and (D) PDGF-AB and thrombin induced migration but not invasion. BAL from mice at Day 7 after 1.2 U/kg bleomycin challenge induced greater invasion and migration than any individual mediator tested (A–D). The mediator concentrations used were 10−9 M PDGF-BB, 10−7 M LPA, 100 ng/ml EGF, 15 ng/ml FGF-2, 50 ng/ml PDGF-AA, 100 ng/ml KGF, 5 ng/ml TGF-β1, 20 ng/ml FGF-1, 10 ng/ml PDGF-AB, 10 ng/ml and thrombin, in serum-free Dulbecco’s modified Eagle's medium media, as determined by pilot dose–response studies (data not shown). Each of the panels shares the same BAL condition. Data are presented as mean (±SEM) fold increase in invasion, normalized to the cell-autonomous invasion of lung fibroblasts from unchallenged mice (n = 3 mice as the source of lung fibroblasts, and n = 3 as the source of BAL; *P < 0.05). THR, thrombin.
Molecular Mediators Contributing to Fibroblast Invasion Induced by BAL after Bleomycin Injury
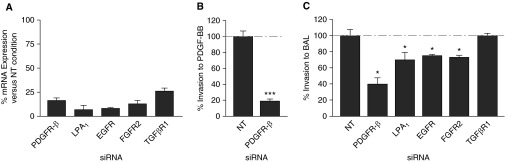
To investigate the mechanisms of non–cell-autonomous fibroblast invasion induced by BAL from bleomycin-challenged mice, we developed the ability to knockdown gene expression in fibroblasts by small interfering RNA (siRNA) transfection performed directly in the invasion plates, and to then assess invasion of the transfected fibroblasts. We first confirmed the ability of the siRNAs to effectively knockdown mRNA expression of the gene of interest (Figure 5A). We then tested the effectiveness of this approach by assessing the PDGF-BB–induced invasion of fibroblasts transfected with siRNA targeting platelet-derived growth factor receptor-β (PDGFRβ) or with nontargeting (siRNA). We found that siRNA targeting PDGFRβ, which decreased PDGFRβ mRNA by over 80%, decreased PDGF-BB–induced fibroblast to a very similar extent (i.e., by >80%; Figure 5B). We then found that transfection of fibroblasts with siRNAs targeting PDGFRβ, LPA1, epidermal growth factor receptor (EGFR), and fibroblast growth factor receptor 2 (FGFR2) significantly decreased their invasion to BAL collected at Day 7 from 1.2 U/kg bleomycin-challenged mice, compared with NT siRNA-transfected fibroblasts (Figure 5C). These results suggest that PDGF-BB, LPA, EGF, and FGF-1 and/or -2 may contribute to non–cell-autonomous fibroblast invasion after fibrogenic lung injury, consistent with our findings that each of these mediators can induce fibroblast invasion in vitro. In contrast, transfection of fibroblasts with siRNA targeting TGF-βR1 had no effect on their BAL-induced invasion, consistent with our finding that TGF-β1 did not induce non–cell-autonomous fibroblast invasion in vitro.
Figure 5.
Effects of fibroblast receptor knockdown on fibroblast non–cell-autonomous invasion. (A) Quantitative PCR demonstrating the mRNA expression of the relevant genes after small interfering RNA (siRNA)-mediated receptor knockdown. (B) Transfection with siRNA targeting platelet-derived growth factor receptor-β (PDGFRβ) reduced the invasion of lung fibroblasts from unchallenged mice that was induced by PDGF-BB. (C) Transfection with siRNAs targeting PDGFRβ, LPA1, epidermal growth factor receptor (EGFR), and fibroblast growth factor receptor 2 (FGFR2), but not transforming growth factor-β receptor 1 (TGF-βR1), reduced the invasion of lung fibroblasts from unchallenged mice that was induced by BAL recovered from mice at D7 after challenge with 1.2 U/kg bleomycin. Data are presented as percentage of the invasion of fibroblasts transfected with nontargeting (NT) siRNA that was induced by the same BAL samples acting on fibroblasts transfected with targeting siRNAs (n = 3 mice as the source of lung fibroblasts, and n = 3 as the source of BAL; *P < 0.05, ***P < 0.001).
To further investigate the potential contributions of PDGF-BB, EGF, and FGF-2 to non–cell-autonomous fibroblast migration induced by bleomycin injury, we compared the levels of these three mediators in BAL collected from unchallenged mice and mice challenged with bleomycin or LPS. We found increased levels of each of these mediators in BAL from injured versus uninjured mice, but no differences in the extents to which they were induced in the bleomycin and LPS-injury models (Figure E2). These data are consistent with PDGF-BB, EGF, and FGF-2 being partially responsible for non–cell-autonomous fibroblast invasion induced by lung injury, but not with their being responsible for the differential extent to which this form of fibroblast invasion is induced by fibrogenic (i.e., bleomycin versus nonfibrogenic [i.e., LPS, lung injury]).
Cell-Autonomous and Non–Cell-Autonomous Invasion of IPF Lung Fibroblasts
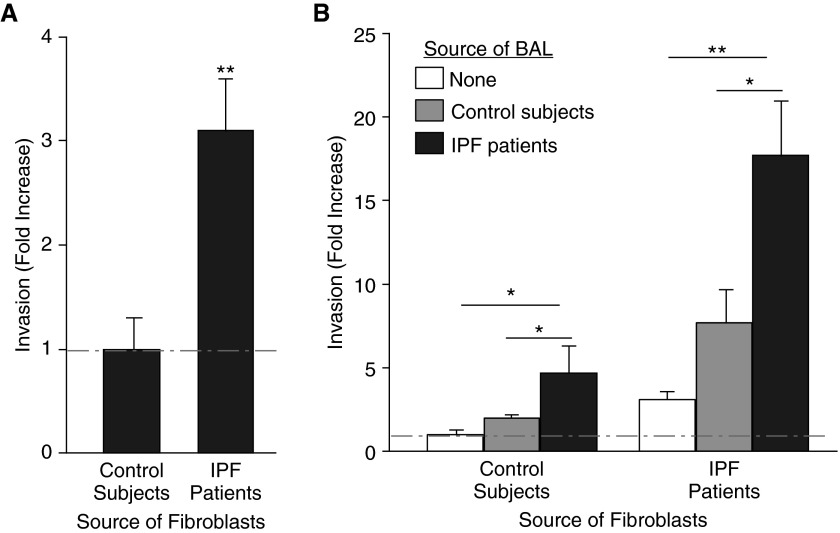
To determine whether our findings in the bleomycin mouse model were relevant to human disease, we investigated the cell-autonomous and non–cell-autonomous invasion of IPF lung fibroblasts. Consistent with our findings in the bleomycin model, and with those of other investigators (7), we found that fibroblasts isolated from lung tissues of patients with IPF demonstrated greater cell-autonomous invasion than fibroblasts isolated from control lung tissues (a 3.1-fold increase; Figure 6A). Also consistent with our findings in the mouse model, we found that BAL from patients with IPF potently induced fibroblast invasion, and that this non–cell-autonomous invasion greatly exceeded the cell-autonomous invasion of IPF lung fibroblasts (Figure 6B). BAL from patients with IPF increased the invasion of IPF lung fibroblasts 17.7-fold, and increased the invasion of control lung fibroblasts 4.7-fold. BAL from control subjects also induced invasion of both IPF and control lung fibroblasts, albeit to a significantly lower extent than IPF BAL (∼twofold greater than the cell-autonomous invasion of these cells; Figure 6B). Taken together, these data indicate that potent soluble mediators of fibroblast invasion, or fibroblast chemoinvasants, are also induced in the human lung during the development of IPF.
Figure 6.
BAL from patients with idiopathic pulmonary fibrosis (IPF) induces non–cell-autonomous invasion of lung fibroblasts from patients with IPF. (A) Lung fibroblasts isolated from patients with IPF demonstrated increased cell-autonomous invasion compared with lung fibroblasts from control subjects. (B) BAL recovered from patients with IPF induced non–cell-autonomous invasion of lung fibroblasts isolated from either patients with IPF or control subjects, which greatly exceeded the cell-autonomous invasion of these cells. BAL from control subjects also induced invasion of both IPF and control lung fibroblasts but to a significantly lesser extent than IPF BAL. Data are presented as mean (±SEM) fold increase in invasion, normalized to the cell-autonomous invasion of lung fibroblasts from control subjects (n = 5 persons with IPF and n = 5 control subjects as the source of lung fibroblasts; n = 3 persons with IPF and n = 3 control subjects as the source of BAL; *P < 0.05, **P < 0.01).
Discussion
IPF is thought to be a disease of aberrant responses to persistent lung injury (1), in which the biological processes of normal wound healing are dysregulated and lead to fibrosis rather than the restoration of normal lung structure and function. Fibroblast migration to sites of tissue injury, invasion into the provisional wound matrix, differentiation into myofibroblasts, and wound matrix contraction are all components of normal wound healing (21). Many of these processes appear to be excessive in IPF, however, including fibroblast invasion. The acquisition by cells of the ability to invade across tissue boundaries has been extensively studied in cancer (22–25), in which cell invasiveness is a hallmark of the metastatic process. The increased ability of fibroblasts to invade through extracellular matrix in IPF has recently been recognized as well. Compared with normal lung fibroblasts, which are poorly invasive, lung fibroblasts from patients with IPF have previously been demonstrated to have a robust capacity to invade through extracellular matrix (7, 10, 26). Fibroblast invasiveness may contribute to other fibrotic conditions as well, such as remodeling of the airways in asthma and remodeling of the joints in rheumatoid arthritis. Airway fibroblasts from patients with asthma are more invasive than those from control subjects (27), and fibroblast-like synoviocytes from patients with rheumatoid arthritis are particularly able to invade and destroy joints (28–30).
Careful histological observations of the architecture of fibrosis also suggest an important contribution of fibroblast invasion to IPF pathogenesis. Although the synthetically active fibroblasts in IPF fibroblastic foci are typically covered by poorly adherent hyperplastic alveolar epithelium in IPF lungs, immunohistochemical and electron microscopy studies indicate that these foci are frequently located distal to the original alveolar basement membranes or their remnants (9, 31). The location of these foci in collapsed alveoli indicates that the fibroblasts comprising them had previously invaded through alveolar basement membranes into the provisional matrices that develop in alveoli after lung injury. IPF is also characterized by areas of basement membrane destruction (17), and loss of basement membrane integrity in these areas may allow fibroblasts to enter alveolar spaces by migrating through basement membrane gaps, in addition to invading through basement membranes. In β-arrestin-1– and 2–deficient mice, however, protection from bleomycin-induced pulmonary fibrosis was associated with loss of invasiveness of lung fibroblasts after injury, despite preservation of their migratory capacities, suggesting a specific requirement for fibroblast invasion in fibrogenesis (8).
Prior studies have highlighted fibroblast acquisition of increased cell-autonomous invasion in IPF and the bleomycin mouse model of pulmonary fibrosis (i.e., the increased ability of fibrotic lung fibroblasts to invade through extracellular matrix in the absence of exogenous mediators) (7, 8, 32). Consistent with these data, we found that fibroblasts isolated from the lungs of patients with IPF demonstrated increased cell-autonomous invasion compared with lung fibroblasts from control subjects, and lung fibroblasts isolated from mice 7 days after bleomycin injury demonstrated increased cell-autonomous invasion compared with lung fibroblasts from unchallenged mice. This challenge time point at 7 days after bleomycin occurs before the establishment of lung fibrosis in this model, as indicated by lung hydroxyproline levels. In contrast, mouse lung fibroblasts isolated 21 days after bleomycin challenge, at which time fibrosis in this model is well established (19), demonstrated decreased cell-autonomous invasion compared with lung fibroblasts from unchallenged mice.
Because we and others have demonstrated that soluble mediators of non–cell-autonomous lung fibroblast migration are induced in IPF and the bleomycin mouse model of fibrosis (11), we investigated whether soluble mediators of non–cell-autonomous lung fibroblast invasion are similarly induced. We found that such mediators are produced in the lung and present in BAL from both patients with IPF and bleomycin-injured mice, and that the magnitude of the non–cell-autonomous fibroblast invasion directed by these mediators is significantly greater than the increased cell-autonomous fibroblast invasion present in both human and mouse lung fibrosis. In experiments to investigate the nature of these soluble mediators of non–cell-autonomous fibroblast invasion, or chemoinvasants, we found evidence to suggest that these mediators: (1) are produced locally after lung injury rather than entering the lung from the circulation due to injury-increased pulmonary vascular permeability; (2) can be distinct from the mediators of fibroblast migration; and (3) are preferentially produced by fibrogenic rather than nonfibrogenic lung injury. Evidence that fibroblast chemoinvasants are produced locally in the lung after fibrogenic injury came from experiments escalating the challenge dose of bleomycin. Higher doses of bleomycin increased the invasion-inducing activity, but not the total protein concentration, of BAL after challenge. These data suggest that increased bleomycin doses resulted in greater chemoinvasant activity in BAL after challenge without further increasing lung vascular permeability, consistent with increased local production of the relevant chemoinvasants in the lung. We hypothesize that local production of these mediators is attributable to injured alveolar epithelial cells (AECs). Fibroblast activation and accumulation in IPF in general is now thought to be driven in a paracrine fashion by AEC-produced mediators (33). Areas of AEC apoptosis and foci of α-smooth muscle actin–positive myofibroblasts colocalize in the lungs of patients with IPF (34), consistent with the hypothesis that locally produced AEC mediators direct fibroblast behaviors as fibrosis develops. The ability of injured epithelial cells to affect fibroblast behaviors has also been directly demonstrated in cocultures of these two cell types in vitro (35). We consequently hypothesize that higher doses of bleomycin increased the invasion-inducing activity of BAL after challenge by increasing the numbers and/or extent of AECs injured, and consequently increasing the quantities of the fibroblast-active AEC-derived mediators produced.
Evidence that fibroblast invasion and migration can be directed by different mediators came from experiments assessing the invasion and migration induced by a series of fibroblast-active mediators. Although some mediators did have concordant effects on fibroblast invasion and migration (PDGF-BB, LPA, EGF, and FGF-2 induced both processes, whereas PDGF-AA, KGF, and TGF-β1 induced neither), other mediators had discordant abilities to mediate invasion and migration. FGF-1 induced fibroblast invasion, but not migration, whereas PDGF-AB and thrombin induced fibroblast migration, but not invasion. Of the mediators we found to induce invasion of primary mouse lung fibroblasts, PDGF-BB, EGF, and FGF-1 have been previously noted to induce invasion of fibroblasts isolated from other sources (32, 36–38), and LPA, FGF-1, and FGF-2 have been previously noted to induce cancer cell invasion (38–41). Consistent with our finding that these mediators are able to induce fibroblast invasion ex vivo, we found evidence to suggest that these mediators all contribute to the non–cell-autonomous fibroblast invasion induced in vivo in the bleomycin model: knockdown of lung fibroblast PDGFRβ, LPA1, EGFR, or FGFR2 expression each, by themselves, significantly decreased fibroblast invasion induced by BAL from bleomycin-challenged mice.
Evidence that fibroblast chemoinvasants are produced preferentially by fibrogenic, rather than nonfibrogenic, lung injury came from experiments comparing fibroblast invasion induced by a bleomycin challenge that produces fibrosis versus invasion induced by an LPS challenge that does not; despite comparable pulmonary vascular leak being produced by these two types of challenges, BAL from bleomycin-challenged mice induced significantly more fibroblast invasion than did BAL from LPS-challenged mice. We hypothesize that differing AEC responses to bleomycin versus LPS injury, in terms of the chemoinvasants that these cells produce, account for the differing extents to which these injuries induced fibroblast invasion. For the chemoinvasants that we were able to measure, PDGF-BB, EGF, and FGF-2, we saw no differences in their levels in BAL from bleomycin- versus LPS-challenged mice, suggesting that other chemoinvasants not measured, such as FGF-1 or LPA, are responsible for the differing extents of fibroblast invasion produced by these two different injuries.
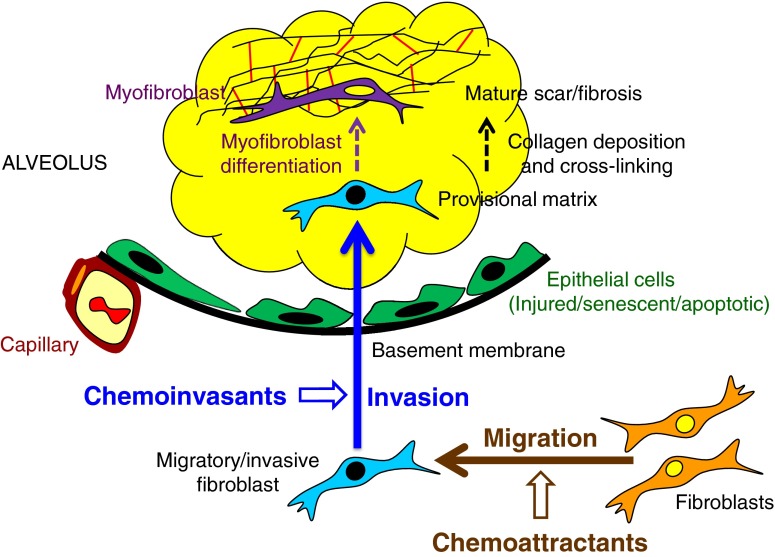
Taken together, the data presented in this study suggest a model of fibroblast/myofibroblast accumulation at sites of lung injury depicted in Figure 7. In this model, fibroblasts initially acquire a migratory/invasive phenotype characterized by cell-autonomous invasive capacity, and are guided by potentially differing sets of chemoattractant and chemoinvasant mediators to migrate to sites of injury, and then to invade into provisional matrices produced at those sites. After invasion into provisional matrices, fibroblasts lose this initial migratory/invasive phenotype and transition to the matrix-synthetic and contractile phenotype characteristic of myofibroblasts, directed by cytokines, such as TGF-β. After differentiation into myofibroblasts, these cells secrete increased collagen and other matrix proteins, and collagen cross-linking and wound contraction then convert provisional matrices into mature scar/fibrosis. Consistent with this model, we observed a reduction in fibroblast invasive capacity with myofibroblast differentiation induced by TGF-β1 pretreatment.
Figure 7.
Regulation of fibroblast/myofibroblast accumulation in pulmonary fibrosis. The data presented in this study support a model of fibroblast/myofibroblast accumulation at sites of lung injury depicted in this figure. In this figure, thick solid arrows represent cell movements, whereas thinner dashed arrows represent cell and matrix maturations. Early after lung injury, fibroblasts acquire a migratory/invasive phenotype characterized by the acquisition of cell-autonomous invasive capacity. Concurrent with the development of this intrinsic fibroblast phenotype, potent soluble mediators that markedly augment fibroblast migration (chemoattractants) and invasion (chemoinvasants) are induced in the lungs of patients with IPF and in the bleomycin mouse model of pulmonary fibrosis. After invasion into provisional matrices, fibroblasts lose their initial migratory/invasive phenotype and transition to the matrix-synthetic and contractile phenotype characteristic of myofibroblasts, directed by cytokines, such as TGF-β. Once they have differentiated into myofibroblasts, these cells remain in the provisional matrix, where they secrete increased amounts of collagen and other matrix proteins. Collagen cross-linking and wound contraction then convert the myofibroblast-rich provisional matrices into mature scar/fibrosis.
The magnitude of the mediator-driven, non–cell autonomous fibroblast invasion that is induced by BAL, both from bleomycin-challenged mice and patients with IPF, suggest that inhibition of this mechanism of fibroblast invasion could be a novel therapeutic strategy for fibrotic lung diseases. Prior research has demonstrated that targeting mediators of fibroblast migration can be an effective approach in limiting fibrosis (12, 42). Given emerging appreciation of the heterogeneity of IPF pathogenesis, our findings that multiple soluble mediators contribute to non–cell-autonomous fibroblast invasion raises the possibility that different individual chemoinvasants may be more or less important in different individual patients. Targeting molecular mechanisms common to fibroblast invasion driven by any of these mediators consequently may be a more broadly applicable therapeutic strategy than targeting the individual chemoinvasants themselves. Molecular mechanisms of non–cell-autonomous invasion may include activation of cell surface proteases (4), or formation of invadopodia or podosomes at the leading edge of invading cells (5). Prior studies have implicated two molecular mechanisms in cell-autonomous fibroblast invasion: signaling regulated by the β-arrestins (7, 8), and increased expression of hyaluronan synthase 2 (HAS2) (7, 8). In the second mechanism, the hyaluronan produced signals through fibroblast CD44 to induce increased expression of matrix metalloproteinases and decreased expression of tissue inhibitors of metalloproteinases, promoting invasion. The signal transduction pathways of PDGFRβ (43), LPA1 (44), and EGFR (45) have all previously been shown to involve the β-arrestins, suggesting that non–cell autonomous fibroblast invasion driven by these mediators will depend on β-arrestin1 and/or -2 as well. Similarly, PDGF-BB, FGF-2, and EGF have all been shown to induce HAS2 expression in human fibroblasts (46), as has LPA (47), suggesting that the HAS2–HA–CD44 pathway described for cell-autonomous invasion may also contribute to the non–cell-autonomous fibroblast invasion that we describe here. We believe that further elaboration of the common molecular mechanisms that drive non–cell-autonomous fibroblast invasion in pulmonary fibrosis has the potential to provide a rich set of drug targets for the treatment of IPF and other fibrotic lung diseases.
Acknowledgments
Acknowledgments
The authors gratefully acknowledge Jürgen Schymeinsky, Peter Seither, Paul Nicklin, Bärbel Lämmle, Stephanie Lefèvre, and Stefan-Lutz Wollin, all at Boehringer-Ingelheim, for very helpful discussions.
Footnotes
This work was supported by Boehringer-Ingelheim RNAi Screening Award (N.A. and A.M.T.), and by National Institutes of Health grants T32HL116275 (N.A.), P30AR061271 and K24AR060297 (C.F.-B.), and R01HL095732 and R01HL108975 (A.M.T.).
Author Contributions: Conception and design—N.A., D.L., and A.M.T.; data acquisition—N.A., P.E.G., B.M.M., and D.L.; analysis and interpretation—N.A., C.F.-B., A.P., M.S., D.L., and A.M.T.; drafting the manuscript—N.A. and A.M.T.; revising the manuscript for important intellectual content—N.A., P.E.G., B.M.M., C.F.-B., A.P., M.S., D.L., and A.M.T.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0040OC on November 24, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Selman M, King TE, Pardo A American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis: aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med. 2014;190:867–878. doi: 10.1164/rccm.201403-0509PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 4.Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20:931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis SF, Zahradka P. Vascular smooth muscle cell motility: from migration to invasion. Exp Clin Cardiol. 2010;15:e75–e85. [PMC free article] [PubMed] [Google Scholar]
- 6.Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51(18 suppl):5054s–5059s. [PubMed] [Google Scholar]
- 7.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovgren AK, Kovacs JJ, Xie T, Potts EN, Li Y, Foster WM, Liang J, Meltzer EB, Jiang D, Lefkowitz RJ, et al. β-arrestin deficiency protects against pulmonary fibrosis in mice and prevents fibroblast invasion of extracellular matrix. Sci Transl Med. 2011;3:74ra23. doi: 10.1126/scitranslmed.3001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basset F, Ferrans VJ, Soler P, Takemura T, Fukuda Y, Crystal RG. Intraluminal fibrosis in interstitial lung disorders. Am J Pathol. 1986;122:443–461. [PMC free article] [PubMed] [Google Scholar]
- 10.White ES, Thannickal VJ, Carskadon SL, Dickie EG, Livant DL, Markwart S, Toews GB, Arenberg DA. Integrin α4β1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med. 2003;168:436–442. doi: 10.1164/rccm.200301-041OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behr J, Adelmann-Grill BC, Krombach F, Beinert T, Schwaiblmair M, Fruhmann G. Fibroblast chemotactic response elicited by native bronchoalveolar lavage fluid from patients with fibrosing alveolitis. Thorax. 1993;48:736–742. doi: 10.1136/thx.48.7.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 13.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol. 2004;31:395–404. doi: 10.1165/rcmb.2004-0175OC. [DOI] [PubMed] [Google Scholar]
- 14.Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol. 2010;43:662–673. doi: 10.1165/rcmb.2009-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funke M, Zhao Z, Xu Y, Chun J, Tager AM. The lysophosphatidic acid receptor LPA1 promotes epithelial cell apoptosis after lung injury. Am J Respir Cell Mol Biol. 2012;46:355–364. doi: 10.1165/rcmb.2010-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagares D, Busnadiego O, García-Fernández RA, Kapoor M, Liu S, Carter DE, Abraham D, Shi-Wen X, Carreira P, Fontaine BA, et al. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum. 2012;64:1653–1664. doi: 10.1002/art.33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardo A, Selman M. Role of matrix metaloproteases in idiopathic pulmonary fibrosis. Fibrogenesis Tissue Repair. 2012;5(suppl 1):S9. doi: 10.1186/1755-1536-5-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selman M, Carrillo G, Estrada A, Mejia M, Becerril C, Cisneros J, Gaxiola M, Pérez-Padilla R, Navarro C, Richards T, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS One. 2007;2:e482. doi: 10.1371/journal.pone.0000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol. 2002;83:111–119. doi: 10.1046/j.1365-2613.2002.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 21.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 22.Grindel BJ, Martinez JR, Pennington CL, Muldoon M, Stave J, Chung LW, Farach-Carson MC. Matrilysin/matrix metalloproteinase-7 (MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. Matrix Biol. 2014;36:64–76. doi: 10.1016/j.matbio.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terai S, Fushida S, Tsukada T, Kinoshita J, Oyama K, Okamoto K, Makino I, Tajima H, Ninomiya I, Fujimura T, et al. Bone marrow derived “fibrocytes” contribute to tumor proliferation and fibrosis in gastric cancer. Gastric Cancer. 2015;18:306–313. doi: 10.1007/s10120-014-0380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Augoff K, Hryniewicz-Jankowska A, Tabola R, Czapla L, Szelachowski P, Wierzbicki J, Grabowski K, Sikorski AF. Upregulated expression and activation of membrane-associated proteases in esophageal squamous cell carcinoma. Oncol Rep. 2014;31:2820–2826. doi: 10.3892/or.2014.3162. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Qu C, Chen H, Xu L, Qi Q, Luo J, Wang K, Meng Z, Chen Z, Wang P, et al. Chinese herbal medicine suppresses invasion-promoting capacity of cancer-associated fibroblasts in pancreatic cancer. PLoS One. 2014;9:e96177. doi: 10.1371/journal.pone.0096177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karvonen HM, Lehtonen ST, Sormunen RT, Harju TH, Lappi-Blanco E, Bloigu RS, Kaarteenaho RL. Myofibroblasts in interstitial lung diseases show diverse electron microscopic and invasive features. Lab Invest. 2012;92:1270–1284. doi: 10.1038/labinvest.2012.95. [DOI] [PubMed] [Google Scholar]
- 27.Ingram JL, Huggins MJ, Church TD, Li Y, Francisco DC, Degan S, Firszt R, Beaver DM, Lugogo NL, Wang Y, et al. Airway fibroblasts in asthma manifest an invasive phenotype. Am J Respir Crit Care Med. 2011;183:1625–1632. doi: 10.1164/rccm.201009-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao W, Zhang C, Shi M, Zhang J, Li M, Xue X, Zhang Z, Shu Z, Zhu J, Mu N, et al. The DDR2-annexin A2-MMP13 loop promotes joint destruction in arthritis through promoting migration and invasion of fibroblast-like synoviocytes. Arthritis Rheumatol. 2015;290:30910–30923. doi: 10.1002/art.38696. [DOI] [PubMed] [Google Scholar]
- 29.Tong B, Wan B, Wei Z, Wang T, Zhao P, Dou Y, Lv Z, Xia Y, Dai Y. Role of cathepsin B in regulating migration and invasion of fibroblast-like synoviocytes into inflamed tissue from patients with rheumatoid arthritis. Clin Exp Immunol. 2014;177:586–597. doi: 10.1111/cei.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onuora S. Rheumatoid arthritis: SSAT1 inhibition slows synovial fibroblast invasion. Nat Rev Rheumatol. 2014;10:259. doi: 10.1038/nrrheum.2014.45. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn C, III, Boldt J, King TE, Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989;140:1693–1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- 32.Burgstaller G, Oehrle B, Koch I, Lindner M, Eickelberg O. Multiplex profiling of cellular invasion in 3D cell culture models. PLoS One. 2013;8:e63121. doi: 10.1371/journal.pone.0063121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakai N, Tager AM. Fibrosis of two: epithelial cell–fibroblast interactions in pulmonary fibrosis. Biochim Biophys Acta. 2013;1832:911–921. doi: 10.1016/j.bbadis.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, Selman M. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol. 1998;275:L1192–L1199. doi: 10.1152/ajplung.1998.275.6.L1192. [DOI] [PubMed] [Google Scholar]
- 35.Morishima Y, Nomura A, Uchida Y, Noguchi Y, Sakamoto T, Ishii Y, Goto Y, Masuyama K, Zhang MJ, Hirano K, et al. Triggering the induction of myofibroblast and fibrogenesis by airway epithelial shedding. Am J Respir Cell Mol Biol. 2001;24:1–11. doi: 10.1165/ajrcmb.24.1.4040. [DOI] [PubMed] [Google Scholar]
- 36.Banyard J, Anand-Apte B, Symons M, Zetter BR. Motility and invasion are differentially modulated by Rho family GTPases. Oncogene. 2000;19:580–591. doi: 10.1038/sj.onc.1203338. [DOI] [PubMed] [Google Scholar]
- 37.Scott LA, Vass JK, Parkinson EK, Gillespie DA, Winnie JN, Ozanne BW. Invasion of normal human fibroblasts induced by v-Fos is independent of proliferation, immortalization, and the tumor suppressors p16INK4a and p53. Mol Cell Biol. 2004;24:1540–1559. doi: 10.1128/MCB.24.4.1540-1559.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henriksson ML, Edin S, Dahlin AM, Oldenborg PA, Öberg Å, Van Guelpen B, Rutegård J, Stenling R, Palmqvist R. Colorectal cancer cells activate adjacent fibroblasts resulting in FGF1/FGFR3 signaling and increased invasion. Am J Pathol. 2011;178:1387–1394. doi: 10.1016/j.ajpath.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lungu G, Covaleda L, Mendes O, Martini-Stoica H, Stoica G. FGF-1–induced matrix metalloproteinase-9 expression in breast cancer cells is mediated by increased activities of NF-κB and activating protein-1. Mol Carcinog. 2008;47:424–435. doi: 10.1002/mc.20398. [DOI] [PubMed] [Google Scholar]
- 40.Sailer MH, Gerber A, Tostado C, Hutter G, Cordier D, Mariani L, Ritz MF. Non-invasive neural stem cells become invasive in vitro by combined FGF2 and BMP4 signaling. J Cell Sci. 2013;126:3533–3540. doi: 10.1242/jcs.125757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward JD, Ha JH, Jayaraman M, Dhanasekaran DN. LPA-mediated migration of ovarian cancer cells involves translocalization of Gαi2 to invadopodia and association with Src and β-pix. Cancer Lett. 2015;356:382–391. doi: 10.1016/j.canlet.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding Q, Cai GQ, Hu M, Yang Y, Zheng A, Tang Q, Gladson CL, Hayasaka H, Wu H, You Z, et al. FAK-related nonkinase is a multifunctional negative regulator of pulmonary fibrosis. Am J Pathol. 2013;182:1572–1584. doi: 10.1016/j.ajpath.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alderton F, Rakhit S, Kong KC, Palmer T, Sambi B, Pyne S, Pyne NJ. Tethering of the platelet-derived growth factor β receptor to G-protein-coupled receptors: a novel platform for integrative signaling by these receptor classes in mammalian cells. J Biol Chem. 2001;276:28578–28585. doi: 10.1074/jbc.M102771200. [DOI] [PubMed] [Google Scholar]
- 44.Li TT, Alemayehu M, Aziziyeh AI, Pape C, Pampillo M, Postovit LM, Mills GB, Babwah AV, Bhattacharya M. Beta-arrestin/Ral signaling regulates lysophosphatidic acid–mediated migration and invasion of human breast tumor cells. Mol Cancer Res. 2009;7:1064–1077. doi: 10.1158/1541-7786.MCR-08-0578. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Ahn S, Guo R, Daaka Y. Regulation of epidermal growth factor receptor internalization by G protein–coupled receptors. Biochemistry. 2003;42:2887–2894. doi: 10.1021/bi026942t. [DOI] [PubMed] [Google Scholar]
- 46.Nagaoka A, Yoshida H, Nakamura S, Morikawa T, Kawabata K, Kobayashi M, Sakai S, Takahashi Y, Okada Y, Inoue S. Regulation of hyaluronan (HA) metabolism mediated by HYBID (hyaluronan binding protein involved in HA depolymerization, KIAA1199) and HA synthases in growth factor-stimulated fibroblasts. J Biol Chem. 2015;290:30910–30923. doi: 10.1074/jbc.M115.673566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda-Sano K, Gotoh M, Morohoshi T, Someya T, Murofushi H, Murakami-Murofushi K. Cyclic phosphatidic acid and lysophosphatidic acid induce hyaluronic acid synthesis via CREB transcription factor regulation in human skin fibroblasts. Biochim Biophys Acta. 2014;1841:1256–1263. doi: 10.1016/j.bbalip.2014.05.004. [DOI] [PubMed] [Google Scholar]