Abstract
Nogo-B and its receptor (NgBR) are involved in blood vessel growth in developing lungs, but their role in pulmonary artery smooth muscle cell (PASMC) growth is unknown. We hypothesized that NgBR regulates growth of PASMCs by modulating the function of endoplasmic reticulum (ER) and formation of reactive oxygen species (ROS). In utero constriction of the ductus arteriosus created pulmonary hypertension in fetal lambs (hypertensive fetal lamb [HTFL]). PASMCs isolated 8 days after surgery were assessed for the alteration of protein levels by immunoblots and ROS formation by dihydroethidium and Cell ROX deep red fluorescence. NgBR small interfering RNA and plasmid DNA were used to manipulate NgBR levels. Proliferation and wound healing were assessed by cell counts and scratch recovery assay, respectively. Acute ER stress was induced by tunicamycin. Differences of mitogen-activated protein kinase and Akt pathway activation in HTFL versus control PASMCs were evaluated. Results showed that HTFL PASMCs had decreased NgBR levels and increased proliferation, wound healing, ER stress, and ROS formation compared with controls. Knockdown of NgBR in control PASMCs generated a phenotype similar to HTFL, and overexpression in HTFL restored the defective phenotype to control. Decreased NgBR levels were associated with increased ROS formation in HTFL PASMCs. Subsequently, scavenging ROS decreased proliferation and wound healing. Mechanistically, ROS formation decreases NgBR expression, which induces ER stress. This leads to extracellular signal–regulated kinase pathway activation and PASMC phenotype alteration. Our data suggest that decreased NgBR expression in pulmonary hypertension of the newborn contributes to increased PASMC proliferation and oxidative stress, which lead to the pathogenesis of lung injury.
Keywords: persistent pulmonary hypertension of the newborn, reactive oxygen species, endoplasmic reticulum stress, extracellular signal–regulated kinase pathway, pulmonary artery smooth muscle cell
Clinical Relevance
The study revealed that Nogo-B receptor (NgBR) modulates endoplasmic reticulum (ER) stress in pulmonary artery smooth muscle cells (PASMCs). Prolonged stress by reactive oxygen species decreases NgBR level and aggravates ER stress. Decreased NgBR levels promote the proliferation and migration of PASMCs.
Reticulons are a group of proteins that reside predominantly in the endoplasmic reticulum (ER) to influence ER–Golgi trafficking, vesicle formation, membrane morphogenesis (1), and intracellular calcium homeostasis (2). These proteins are highly conserved through evolution. Nogo-B (NgB) belongs to the reticulon family, and its increased levels are believed to be an important contributing factor to the development of idiopathic pulmonary hypertension (3). NgB receptor (NgBR) mediates the effects of ligand, NgB, on proliferation and migration of both vascular endothelial and smooth muscle cells (4). NgBR is distributed on the ER to play a role in the intracellular cholesterol trafficking (5). The receptor also serves a vital role in regulating dolichol monophosphate biosynthesis, which is important in glycosylation reactions located in the ER (6). Both NgB and NgBR are expressed in developing lungs, and decreased NgBR level in endothelial cells contributes to the development of intrauterine pulmonary hypertension (IPH) (7).
Persistent pulmonary hypertension of the newborn (PPHN) is a severe lung disorder affecting 1.9 in 1,000 live births, with a mortality rate of 11% (8, 9). Decreased vessel density and increased muscle wall thickness of pulmonary arteries are the two major contributors to high pulmonary vascular resistance in idiopathic PPHN (10, 11). Impairment of alveolarization in developing lungs is another structural change seen in idiopathic pulmonary hypertension (11, 12). Fetal lambs with in utero constriction of the ductus arteriosus develop the pathophysiology of idiopathic PPHN (13, 14). We previously reported that NgBR levels are decreased in both pulmonary artery endothelial cells (PAECs) and whole lung tissue in this model (7). Using PAECs from fetal lambs, we also observed that NgBR modulates angiogenesis through endothelial nitric oxide synthase coupling and Akt (protein kinase B) signaling pathway to promote angiogenesis in vitro (7). We demonstrated that decreased NgBR levels contribute to increased reactive oxygen species (ROS) formation and impaired angiogenesis of PAECs in PPHN (7). Unlike PAECs, pulmonary artery smooth muscle cells (PASMCs) from fetal lambs with chronic IPH are known to have increased proliferation (15). Increased formation of ROS is mechanistically linked to PASMC proliferation and remodeling (16, 17). The change of NgBR expression in PASMCs and its effects on ROS formation in hypertensive fetal lamb (HTFL) PASMCs remain unexplored.
Increased ROS formation is characteristic of PPHN (14). Multiple mechanistic pathways can regulate ROS formation in PASMCs, including ER stress (18–20), nicotinamide adenine dinucleotide phosphate reduced oxidase (15), xanthine oxidase, mitochondrial oxidative phosphorylation, et cetera (21). ROS promote vascular smooth muscle cell growth and migration via phosphorylation of Akt (22) and extracellular signal–regulated kinase (ERK) (23–25). Oxidative stress to the ER can further increase ROS formation secondary to unfolded protein response (UPR) (3, 20). The UPR is an adaptive response to ER stress for maintaining homeostasis (26). Thus, in the presence of oxidative stress, the upstream ER chaperone protein—glucose regulated protein 78, also known as binding Ig protein (BiP)—is up-regulated (19, 27). Protein kinase RNA-like ER kinase (PERK) and activating transcription factor (ATF)-6 are additional ER-resident transmembrane proteins that act as sensors to ER stress (28). Because NgBR is also localized to the ER, NgBR may play a role in regulating UPR. Here, we demonstrate that: (1) decreased NgBR expression in PASMCs contributes to increased ROS formation and smooth muscle cell layer thickening in PPHN; (2) NgBR modulates PASMC ER response and affects ROS formation; and (3) NgBR regulates PASMC proliferation through mitogen-activated protein kinase (MAPK)/ERK pathway.
Materials and Methods
IPH was created by constricting the ductus arteriosus as we previously reported (13) and detailed in the online supplement. Normal fetal lamb twin from the same gestation was used as control for comparison.
Histology for Lung and Pulmonary Artery
Preparation of histology slides is detailed in the online supplement. Images were obtained under a fluorescence microscope (Olympus IX51, Center Valley, PA) with the appropriate filter. Radial alveolar count, mean linear intercept, and pulmonary artery smooth cell layer thickness ratio were measured on hematoxylin and eosin staining of tissue sections.
NgBR Small Interfering RNA, Plasmid with NgBR cDNA, and Primers for PCR
Small interfering RNA (no. J-062667-09; Qiagen, Valencia, CA) matched to sheep NgBR mRNA (nucleotides 399∼417). Nonsilencing interfering RNA was used for control of knockdown (KD) experiments (see Figure E1 in the online supplement). Human NgBR cDNA was cloned into pIRES–neo vector (Clontech) for NgBR overexpression experiments. NgBR was overexpressed or knocked down in PASMCs as previously reported (7) and detailed in the online supplement. Primers for real-time RT-PCR were designed according to sheep NgBR messenger RNA sequence (XM_004011168.1) with the product length of 203 nucleotides.
Western Blotting
Proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with specific antibodies overnight. The signal from horseradish peroxidase–conjugated secondary antibody (1:10,000) was detected with enhanced chemiluminescence solution and recorded on Xposure films (ThermoFisher, Grand Island, NY). Integrated optical density was measured by ImageJ (National Institutes of Health, Bethesda, MD), and β-actin was used as a house keeping control.
Cell Proliferation
Cell proliferation was measured by counting cells at various time intervals. Treatment groups of KD, overexpression, polyethylene glycol–Cu,Zn-superoxide dismutase (SOD) and/or catalase (CAT) treatment were compared with their respective controls. Cells were dislodged using TrypLE (ThermoFisher, Grand Island, NY), mixed with 1:1 vol ratio of Trypan blue (Bio-Rad, Hercules, CA), and counted twice using a TC10 Cell Counter (Bio-Rad). The average counts were used for analysis.
Monolayer Wound Healing Assay
Monolayer wound healing assay was used to determine the migration of PASMCs. Images of the narrowest distance between the front lines of recovery were captured by a coauthor blinded to the assay, and measurements were obtained using ImageJ.
Quantification of ROS Formation
Both dihydroethidium (DHE) and Cell ROX deep red fluorescence were used to evaluate ROS production. Fluorescence was photographed and quantified by personnel blinded to the treatments using MetaView software (Universal Imaging Corporation, West Chester, PA) and expressed as relative light units.
NgBR Expression and ROS Formation in Response to ER Stress
The expression of NgBR, BiP, and ROS formation were examined in ER stress. ER stress was induced by exposure of cells to tunicamycin (TM; 0.1 μg/ml) and the effect of NgBR level on ROS production was assessed. ROS formation was assessed with DHE fluorescence.
Statistical Analysis
Data are shown as mean (±SE). Student’s t test and Mann-Whitney U test were used for comparisons between two groups. ANOVA with Student-Newman-Keuls post hoc test was used for comparison of more than two groups. A P value of less than 0.05 was considered statistically significant.
Results
Impaired Alveolarization and Thickening of the PASMC Layer in HTFL
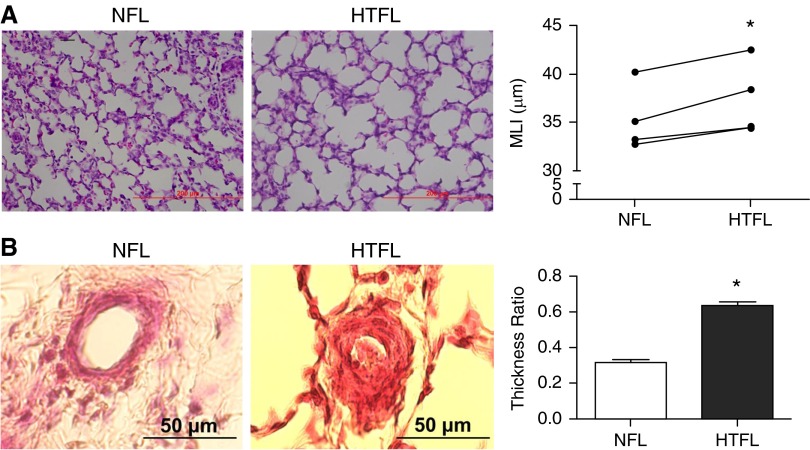
Mean linear intercept was significantly higher (Figure 1A), whereas, radial alveolar count was lower in HTFL lungs. The decreased alveolar count and malformation of alveolar structure that we observed in HTFL lungs were similar to those in a previous report from Grover and colleagues (12) in a similar animal model. We also observed increased thickness of smooth muscle cell layer in HTFL pulmonary arterial wall compared with normotensive fetal lung (NFL) twin controls (Figure 1B).
Figure 1.
Intrauterine pulmonary hypertension (hypertensive fetal lamb [HTFL]) impairs alveolarization and increases the smooth muscle cell layer thickness compared with normotensive co–twin fetal lung (NFL). (A) Mean linear intercept (MLI) in HTFL is higher than in NFL, indicating fewer and larger airspaces (individual data shown for n = 4). (B) The smooth muscle cell layer in HTFL pulmonary artery is thicker than in NFL (mean ± SEM; n = 3). *P < 0.05 compared with NFL. Scale bars: 200 μm in A and 50 μm in B.
NgBR Expression Is Decreased in HTFL Pulmonary Arteries and PASMCs
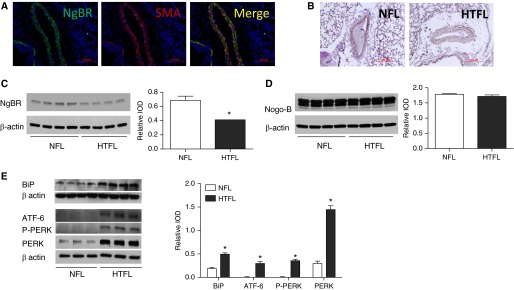
Immunofluorescence staining results showed that NgBR protein was colocalized with smooth muscle cell α-actin in lung blood vessels, indicating the presence of NgBR in PASMCs (Figure 2A). Immunohistochemistry staining further showed decreased intensity of NgBR in the smooth muscle cell layer of HTFL pulmonary arteries as compared with NFL (Figure 2B). We noted a similar change in PASMCs similar to our previous observation that NgBR protein level was decreased in both whole lung tissue and PAECs (7). The level of NgBR in HTFL PASMCs was approximately 60% of NFL PASMCs (Figure 2C). However, there was no difference in the levels of ligand, NgB, between NFL PASMCs and HTFL PASMCs (Figure 2D). BiP is a chaperone protein that binds ER membrane–associated proteins—PERK, inositol-requiring enzyme 1 (IRE1), and ATF6 (27). As shown in Figure 2E, all ER stress markers were elevated in HTFL PASMCs. These data suggest that ER stress is increased in HTFL PASMCs.
Figure 2.
The localization of Nogo-B (NgB) receptor (NgBR) in pulmonary arteries. (A) NgBR localizes to the smooth muscle cell (PASMC) layer of pulmonary arteries in lung section, as indicated by colocalization of NgBR (green) with smooth muscle cell α-actin (red) in pulmonary arteries. (B) NgBR staining intensity is decreased in HTFL pulmonary arteries compared with NFL pulmonary arteries. (C) NgBR protein levels are decreased in HTFL PASMCs compared with NFL twin (n = 4). (D) No difference is observed in the protein levels of ligand NgB between NFL and HTFL PASMCs (n = 4). (E) Markers for endoplasmic reticulum (ER) stress (binding Ig protein [BiP] n = 4; activating transcription factor [ATF]-6, P-protein kinase RNA-like ER kinase [PERK], n = 3) are all increased in HTFL PASMCs. IOD, integrated optic density; SMA, smooth muscle actin. *P < 0.05 compared with NFL. Data are shown as mean (±SE). Scale bars for A: 100 μm and scale bars for B: 200 μm.
Cell Proliferation, Wound Healing, and ROS Formation Are Increased in HTFL PASMCs
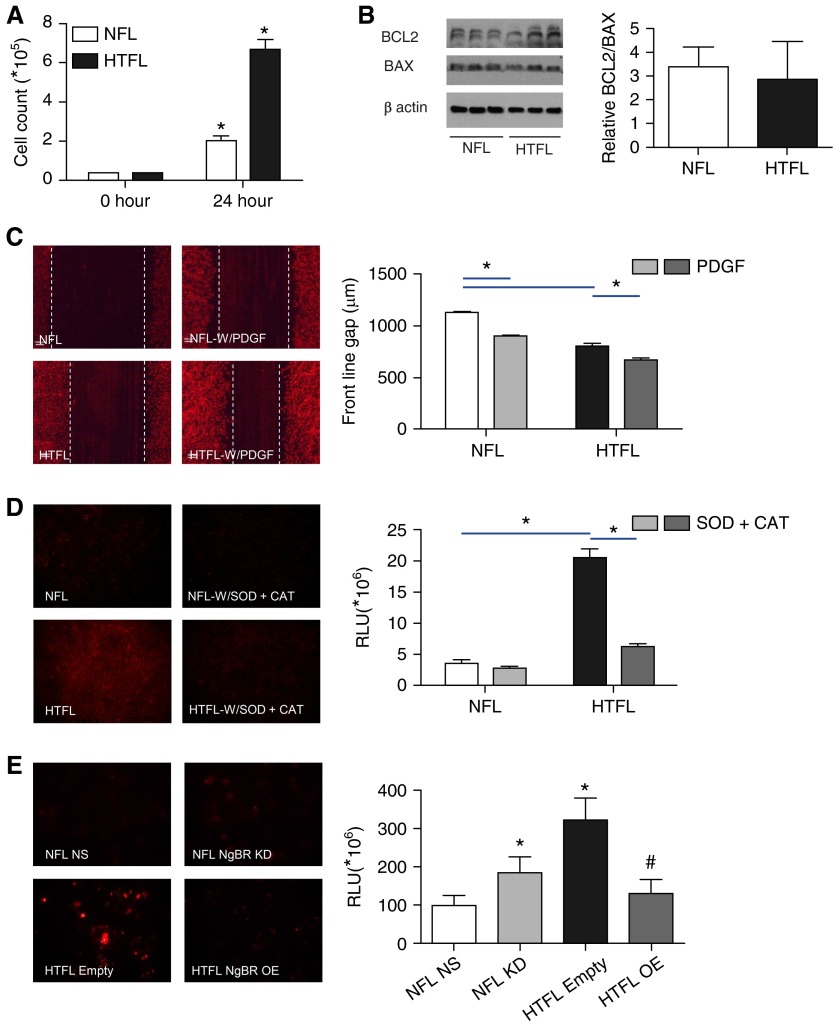
As shown in Figures 3A and 3C, PASMC proliferation and wound healing were significantly increased in HTFL PASMCs compared with NFL PASMCs. Cell counts for HTFL showed a large increase after 24 hours compared with NFL cells. B cell lymphoma protein (BCL) 2 is a marker for antiapoptosis, and BCL2-associated X protein is a proapoptosis marker (29). The ratio of BCL2 to BCL2-associated X protein, an index of apoptosis signaling, did not show a significant difference between NFL and HTFL (Figure 3B). These data suggest that the increased smooth muscle layer thickness in HTFL pulmonary arteries may be due to increased PASMC proliferation and migration, and not due to decreased apoptosis.
Figure 3.
HTFL PASMCs show increased cell proliferation, migration, and reactive oxygen species (ROS) formation. (A) Cell count is significantly higher in HTFL compared with NFL after 24 hours of growth in culture (n = 6). (B) Apoptosis index (B cell lymphoma [BCL] 2/BCL2-associated X protein [BAX]) does not show a significant difference between NFL and HTFL. (C) Cell migration into wound margins is greater in HTFL compared with NFL at basal level and in the presence of platelet-derived growth factor (PDGF)-BB (n = 10). (D) Dihydroethidium (DHE) fluorescence is increased in HTFL compared with NFL; polyethylene glycol–superoxide dismutase (SOD) plus catalase (CAT) decreases the fluorescence intensity, indicating that increased ROS accounts for increased DHE fluorescence in HTFL (n = 10). (E) HTFL PASMCs treated with empty plasmid (Empty) had a threefold increase in ROS formation compared with NFL treated with nonsilencing (NS) small interfering RNA (siRNA) (n = 10). ROS formation increased twofold when NgBR was knocked down in NFL (n = 10). HTFL NgBR overexpression (OE) decreased ROS formation by threefold (n = 10). *P < 0.05 between NFL and HTFL groups. *P < 0.05 when comparing NFL NS to NFL knockdown (KD) and to HTFL Empty. #P < 0.05 when comparing HTFL Empty to HTFL OE. RLU, relative light units (mean integrated fluorescence). Data are shown as mean (±SE).
Given increased ER stress in HTFL, we further examined the ROS and PASMC function change. ROS formation was quantified by DHE (Figure 3D) and Cell ROX deep red fluorescence (Figure 3E). DHE fluorescence increased significantly in HTFL cells (Figure 3D). Cell ROX deep red staining of HTFL cells showed a threefold increase in fluorescence compared with NFL (Figure 3E).
NgBR Regulates Proliferation, Wound Healing, and ROS Formation in PASMCs
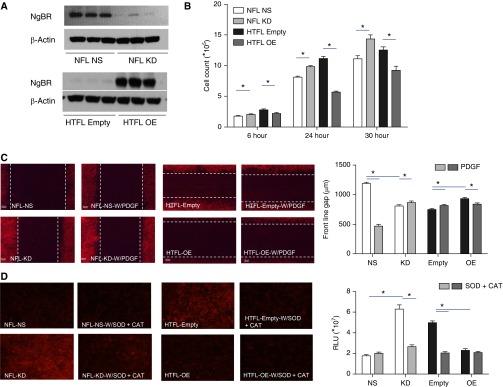
We knocked down NgBR in NFL PASMCs to determine whether a decrease in NgBR in HTFL cells contributes to increased ROS formation, as well as PASMC proliferation and migration. We verified the efficiency of NgBR KD by western blot (Figure 4A) and real-time RT-PCR (Figure E1). NgBR KD did not change the levels of ligand, NgB. NgBR KD in NFL PASMCs induced phenotypic changes that are similar to PPHN PASMCs. Cell counts increased after NgBR KD in NFL PASMCs, with the greatest difference observed at 30 hours (Figure 4B). Wound healing (Figure 4C), and ROS formation quantified by DHE (Figure 4D) and Cell ROX fluorescence (Figure 3E), were also increased with NgBR KD in NFL PASMCs. Cell ROX staining showed a twofold increase of fluorescence signal in KD compared with control PASMCs.
Figure 4.
Decreased expression of NgBR results in increased proliferation, migration, and ROS formation of PASMCs. (A) NgBR KD decreases its protein level in NFL, and OE increases NgBR protein in HTFL PASMCs (n = 3). (B) Cell counts were higher after NgBR KD in NFL PASMCs compared with NS siRNA–treated cells at all time points. NgBR OE decreases cell counts at all time points in HTFL PASMCs (n = 8). (C) NgBR OE in HTFL PASMCs leads to decreased migration when compared with controls, both at basal levels and in response to stimulation with PDGF. NgBR KD in NFL PASMCs leads to increased basal and PDGF-stimulated migration when compared with control (n = 10). (D) NgBR KD in NFL PASMCs increases DHE fluorescence intensity, whereas NgBR OE in HTFL PASMCs decreases DHE fluorescence intensity (n = 10). *P < 0.05 between two groups. Data are shown as mean (±SE).
As a rescue experiment, NgBR overexpression in HTFL PASMCs restored these defective phenotypes, similar to NFL PASMCs (Figure 4A). Cell counts of HTFL PASMCs overexpressing NgBR decreased compared with transfection with empty plasmid (Figure 4B). HTFL PASMCs overexpressing NgBR decreased wound healing (Figure 4C), as well as ROS formation quantified by DHE (Figure 4D) and Cell ROX fluorescence (Figure 3E), when compared with HTFL cells with empty vector plasmid DNA. Cell ROX staining decreased threefold in the NgBR overexpression group compared with the empty vector group.
ROS Alters NgBR Expression, Cell Proliferation, and Wound Healing
To further understand the mechanism of NgBR down-regulation in HTFL, hydrogen peroxide and N-acetylcysteine were added to NFL and HTFL PASMCs, respectively, for 72 hours. The treatment of hydrogen peroxide decreased NgBR expression in NFL PASMCs, whereas N-acetylcysteine increased NgBR expression in HTFL PASMCs (Figure E2). This suggests the down-regulation of NgBR expression in HTFL PASMCs by ROS.
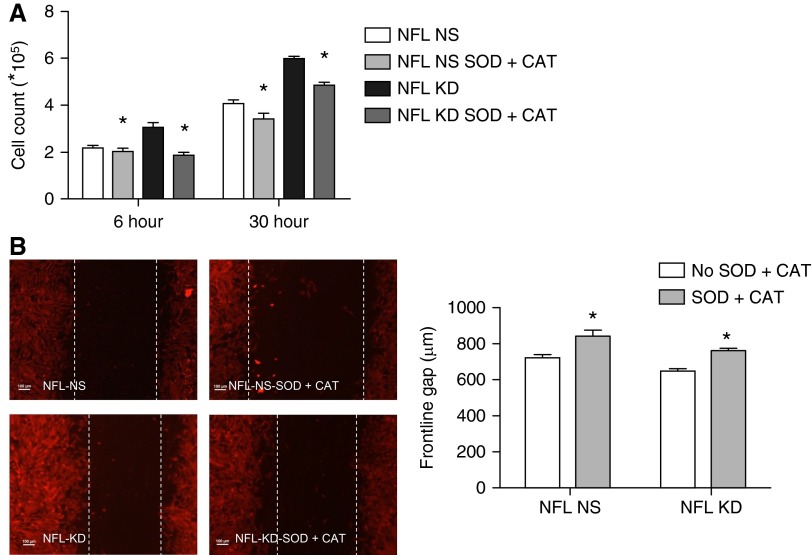
CAT, polyethylene glycol–SOD, or a combination of SOD and CAT (SOD + CAT) was used to scavenge ROS to determine its role in PASMC growth. SOD treatment alone did not affect cell counts (Figure E3); however, SOD + CAT diminished PASMC proliferation. Similar to previous studies (30), this finding supports the role of ROS formation in cell proliferation and wound healing. To determine the role of ROS in the stimulation of proliferation and wound healing by NgBR KD, both control and NgBR KD NFL PASMCs were treated with SOD + CAT. SOD + CAT treatment decreased the cell proliferation (Figure 5A) and wound healing (Figure 5B) of both control and NgBR KD NFL PASMCs. This suggests that stimulatory effects of NgBR deficiency on PASMCs are dependent on ROS formation.
Figure 5.
The addition of SOD + CAT to PASMCs decreases proliferation and migration. (A) The addition of SOD + CAT decreases cell counts in both control and NgBR KD NFL PASMCs at all time points (n = 6). (B) Wound healing is also decreased in both groups treated with SOD + CAT (n = 10), indicated by an increase in frontline gap distance. *P < 0.05 compared between nontreated and SOD + CAT–treated groups. Data are shown as mean (±SE).
NgBR Knockdown in PASMCs Increases the Phosphorylation of MAPK
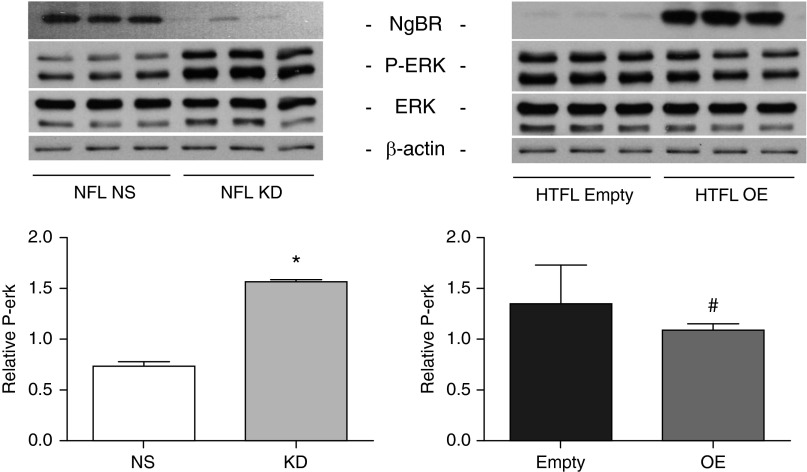
MAPKs (ERK and p38) and Akt regulate vascular smooth muscle cell growth (24, 25, 31). We examined whether they are also involved in the NgBR-regulated PASMC function. The regulatory role of NgBR was studied by examining phosphorylation of ERK, p38, and Akt. Phosphorylated (phospho-) ERK level was increased by twofold in PASMCs after NgBR KD (Figure 6). Overexpression of NgBR in HTFL PASMCs decreased the phospho-ERK level by approximately 20% (Figure 6). There was no difference in p38 and phospho-p38 levels with NgBR KD or overexpression (data not shown). Phospho-Akt and Akt levels also did not show a significant change with manipulation of NgBR in PASMCs. These data suggest that ERK phosphorylation is involved in NgBR signaling in PASMCs.
Figure 6.
Phosphorylated extracellular signal–regulated kinase (P-ERK) level increases in NgBR KD NFL and decreases in HTFL with NgBR OE (n = 3). *P < 0.05 compared with NFL with nonsilencing siRNA. #P < 0.05 compared with HTFL transfected with empty vector plasmid DNA. Data are shown as mean (±SE).
ER Stress Increases NgBR Expression and ROS Formation
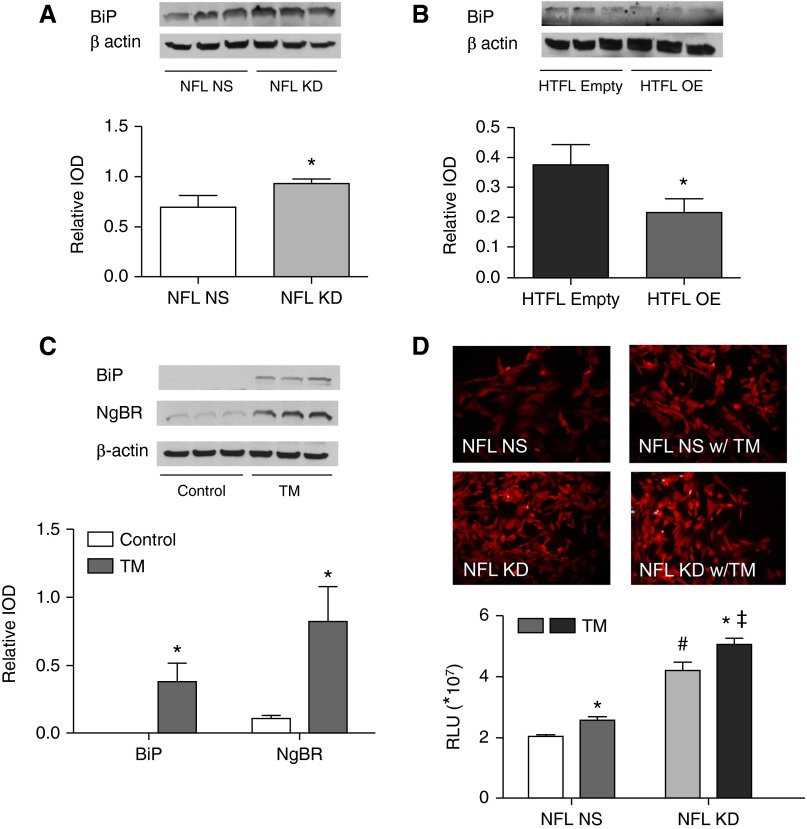
There were significant increases of BiP and PERK in NFL PASMCs when NgBR was knocked down (Figure 7A, Figure E4A). Both ER stress markers were decreased when NgBR was overexpressed in HTFL PASMCs (Figure 7B, Figure E4B). These findings indicate that loss of NgBR increases the ER stress response.
Figure 7.
NgBR modulates ER stress in PASMCs. (A) BiP level increases in NFL PASMCs after NgBR KD (n = 3). (B) BiP level decreases in HTFL PASMCs after NgBR OE (n = 3). (C) Tunicamycin (TM)-induced ER stress leads to increased BiP and NgBR levels in NFL PASMCs compared with no treatment (n = 3). (D) Both TM treatment and NgBR KD increase ROS formation in PASMCs. Decreased NgBR levels further increase ROS formation in TM-treated NFL PASMCs (n = 24). *P < 0.05 compared between control and TM-treated groups; #P < 0.05 compared between NFL NS and NFL KD; ‡P < 0.05 compared between NFL KD with and without TM. Data are shown as mean (±SE).
As expected, the BiP levels (Figure 7C) and ROS formation increased when ER stress was induced with TM (Figure 7D). Interestingly, NgBR levels also increased after TM treatment (Figure 7C). NgBR KD resulted in an additional increase in ROS formation in NFL PASMCs treated with TM (Figure 7D). These observations suggest that NgBR is essential for preventing ER stress–induced ROS formation.
Discussion
Sustained increase in pulmonary vascular resistance (32) is a characteristic feature of PPHN (32, 33, 34). Thickening of the smooth muscle cell layer and decreased blood vessel density in lungs contribute to increased pulmonary vascular resistance and are mechanistically related to increased ROS formation (14, 15, 35). Intrauterine ductal constriction in fetal sheep is the most frequently used animal model to study idiopathic PPHN (13). We have gained important insights into the pathophysiology of PPHN from this animal model. Lung histology showed similarities to idiopathic pulmonary hypertension, demonstrated by impaired alveolarization and increased smooth muscle layer thickness in pulmonary arteries (12).
NgBR is widely distributed in the vascular system (4) and modulates blood vessel growth during early development (36). Using the fetal lamb model of PPHN, we investigated the roles of NgBR in PASMC function. We observed that NgBR is expressed in the smooth muscle layer of pulmonary arteries. Decreased NgBR staining of pulmonary arteries and decreased protein levels in HTFL PASMCs compared with NFL PASMCs is similar to what we found in our previous study investigating NgBR in PAECs (7). Sutendra and colleagues (3) showed PASMCs to have elevated levels of ligand, NgB, in pulmonary hypertension; however, in our model, we did not appreciate increased NgB expression. The discrepancy may be related to differences in our model of PPHN compared with idiopathic pulmonary hypertension in adults. In addition, NgBR KD in NFL PASMCs recapitulates the PPHN phenotype in HTFL PASMCs, and NgBR overexpression rescues the PPHN defect shown as increased ROS formation, cell growth, and migration. These findings support our hypothesis that NgBR alterations contribute to the phenotypic changes of HTFL PASMCs. Our studies strongly support the importance of NgBR in modulating ROS formation and the remodeling of pulmonary arteries in the developing lungs.
We found that NgBR expression is decreased in HTFL PAECs as well as PASMCs, and demonstrated the contribution of NgBR to the phenotype observed in PPHN. However, our data showed that NgBR has opposite cellular functions in PAECs and PASMCs. In our previous studies, loss of NgBR in PAECs led to decreased wound healing and proliferation of endothelial cells, which were associated with decreased nitric oxide levels and increased ROS formation (7). These changes led to impaired angiogenesis in endothelial cells (7). In our current study, we also observed that NgBR KD in PASMCs increased ROS formation. In contrast to the effects of NgBR deficiency on PAECs, NgBR KD in control PASMCs led to increased proliferation and wound healing without a change in apoptosis. Because ROS increased with NgBR KD in both cell types, our data suggest that ROS may contribute to the differential phenotype that we observed with decreased NgBR levels in PAECs and PASMCs. Previous reports from other laboratories have also shown that ROS impairs endothelial cell function and promotes the activation of vascular smooth muscle cells (15, 35). We conclude that the key differences in the effects of decreased NgBR levels on PAECs and PASMCs are ROS dependent, and NgBR has a reciprocal relationship with ROS formation.
We previously investigated the mechanism of increased ROS formation in PAECs from PPHN lambs and their role in decreased wound healing and proliferation (35). A variety of factors, such as endothelial nitric oxide synthase uncoupling (14), decreased GTP-cyclohydrolase-1 expression, tetrahydrobiopterin formation (37), decreased manganese SOD expression and activity (38), and increased nicotinamide adenine dinucleotide phosphate reduced oxidase activity (35, 39), are involved in increasing ROS formation in HTFL PAECs. Similarly, our current study with PASMCs showed that an increase in ROS formation in HTFL PASMCs is associated with increased proliferation, wound healing, and vessel thickening in vivo (32). The addition of ROS decreased NgBR expression, and scavenging of ROS increased cell proliferation and wound healing. This suggests that ROS may play a major role in PASMC proliferation and wound healing, as reported in other vascular smooth muscle cells (15, 21).
In addition to the role of ROS formation in PPHN, we investigated the signaling pathway involved in NgBR on PASMC phenotype. The three major pathways we investigated were MAPK, Akt/protein kinase B, and ER stress. Decreased NgBR levels and increased ROS formation in HTFL PASMCs were associated with increased phosphorylation of ERK (25), which is known to promote the growth of smooth muscle cells. Previous studies have shown cross-talk between MAPK and ROS formation (40). Runchel and colleagues (41) reported that ERK and p38 are activated by ROS. We found that phospho-ERK is increased in NgBR KD NFL PASMCs, whereas NgBR overexpression in HTFL PASMCs decreased phosphorylation of ERK. This suggests that NgBR decreases ROS formation and inhibits ROS-activated ERK, which then leads to decreased PASMC growth and migration. This pathway may be the mechanistic link for a reciprocal relationship between NgBR expression and ROS formation in regulating PASMC growth and migration.
We also investigated ER stress, because of the localization of NgBR to the ER membrane (5). Although we did not observe a change in the levels of NgBR ligand, NgB, in our model, previous studies have shown that increased NgB leads to ER stress and pulmonary hypertension (3). We speculate that changes in NgBR may precede the changes in NgB during development, contrasting PPHN to idiopathic pulmonary hypertension. Other than regulating intracellular cholesterol trafficking (5) and dolichol monophosphate biosynthesis (6), the role of NgBR on ER function is largely unexplored. We examined the ER chaperone protein, BiP, important in the UPR, and found it to be up-regulated in HTFL PASMCs. Other markers for ER stress, such as ATF-6, P-PERK, and PERK, also were increased in HTFL PASMCs. Furthermore, BiP and PERK were elevated in NgBR KD NFL PASMCs, suggesting that decreased NgBR expression increases ER stress. However, when we induced acute ER stress with TM, we observed elevated NgBR levels. One possible explanation is that NgBR functions as a protective mechanism in response to elevated ER stress to reduce ER stress–induced ROS formation. The decreased NgBR expression in HTFL PASMCs or NgBR KD PASMCs likely leads to elevated ER stress, which then loses the inhibitory effect on ROS formation, as evidenced by the increased ROS levels shown in our studies. The consequence of this altered regulation is PASMC growth and migration, as shown in previous studies of this PPHN model (16, 17, 35).
In conclusion, we have delineated the role and mechanistic pathway of NgBR in modulating the growth and migration of PASMCs in the context of our PPHN model. PPHN causes a phenotypical change in PASMCs with elevated levels of ROS formation, which decreases NgBR expression. Lower NgBR expression deprives the coping mechanism against stress to the ER, impairing the UPR that normally regulates ER homeostasis. Without this mechanism, there is further ROS formation, leading to PASMC hyperproliferation. This then activates the ERK pathway to increase PASMC proliferation and migration. By using our fetal lamb model, our study provides new insights into how NgBR regulates the response of PASMCs in persistent pulmonary hypertension by modulating ER stress and ROS formation. This novel observation may have implications for pulmonary artery remodeling in PPHN.
Footnotes
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) grant “Summer Research Program to Medical College of Wisconsin,” the Children’s Research Institute Start-Up Fund (Children’s Hospital of Wisconsin), NHLBI grant RO1 HL057268 and Muma Endowed Chair (G.G.K.), NHLBI grant RO1HL108938 (Q.R.M), 2013 Clinical and Translational Science Institute Pilot Award UL1TR000055 (“Advancing a Healthier Wisconsin”), and National Institute of Child Health and Human Development RO3HD073274 (R.-J.T.).
Author Contributions: Conception and design—Q.R.M. and R.-J.T.; analysis and interpretation—K.S.T., U.R., X.J., G.G.K., and R.-J.T.; drafting the manuscript for important intellectual content—K.S.T., G.G.K., Q.R.M., and R.-J.T.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0068OC on December 14, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 2.Jozsef L, Tashiro K, Kuo A, Park EJ, Skoura A, Albinsson S, Rivera-Molina F, Harrison KD, Iwakiri Y, Toomre D, et al. Reticulon 4 is necessary for endoplasmic reticulum tubulation, STIM1–Orai1 coupling, and store-operated calcium entry. J Biol Chem. 2014;289:9380–9395. doi: 10.1074/jbc.M114.548602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutendra G, Dromparis P, Wright P, Bonnet S, Haromy A, Hao Z, McMurtry MS, Michalak M, Vance JE, Sessa WC, et al. The role of Nogo and the mitochondria–endoplasmic reticulum unit in pulmonary hypertension. Sci Transl Med. 2011;3:88ra55. doi: 10.1126/scitranslmed.3002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao RQ, Gao Y, Harrison KD, Prendergast J, Acevedo LM, Yu J, Hu F, Strittmatter SM, Sessa WC. Identification of a receptor necessary for Nogo-B stimulated chemotaxis and morphogenesis of endothelial cells. Proc Natl Acad Sci USA. 2006;103:10997–11002. doi: 10.1073/pnas.0602427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison KD, Miao RQ, Fernandez-Hernándo C, Suárez Y, Dávalos A, Sessa WC. Nogo-B receptor stabilizes Niemann-Pick type C2 protein and regulates intracellular cholesterol trafficking. Cell Metab. 2009;10:208–218. doi: 10.1016/j.cmet.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison KD, Park EJ, Gao N, Kuo A, Rush JS, Waechter CJ, Lehrman MA, Sessa WC. Nogo-B receptor is necessary for cellular dolichol biosynthesis and protein N-glycosylation. EMBO J. 2011;30:2490–2500. doi: 10.1038/emboj.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng RJ, Rana U, Afolayan AJ, Zhao B, Miao QR, Konduri GG. Nogo-B receptor modulates angiogenesis response of pulmonary artery endothelial cells through eNOS coupling. Am J Respir Cell Mol Biol. 2014;51:169–177. doi: 10.1165/rcmb.2013-0298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, Verter J, Stoll BJ, Lemons JA, Papile LA, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105:14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 9.Clark RH, Huckaby JL, Kueser TJ, Walker MW, Southgate WM, Perez JA, Roy BJ, Keszler M Clinical Inhaled Nitric Oxide Research Group. Low-dose nitric oxide therapy for persistent pulmonary hypertension: 1-year follow-up. J Perinatol. 2003;23:300–303. doi: 10.1038/sj.jp.7210908. [DOI] [PubMed] [Google Scholar]
- 10.Haworth SG, Reid L. Persistent fetal circulation: newly recognized structural features. J Pediatr. 1976;88:614–620. doi: 10.1016/s0022-3476(76)80021-2. [DOI] [PubMed] [Google Scholar]
- 11.Ohara T, Ogata H, Tezuka F. Histological study of pulmonary vasculature in fatal cases of persistent pulmonary hypertension of the newborn. Tohoku J Exp Med. 1991;164:59–66. doi: 10.1620/tjem.164.59. [DOI] [PubMed] [Google Scholar]
- 12.Grover TR, Parker TA, Balasubramaniam V, Markham NE, Abman SH. Pulmonary hypertension impairs alveolarization and reduces lung growth in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2005;288:L648–L654. doi: 10.1152/ajplung.00288.2004. [DOI] [PubMed] [Google Scholar]
- 13.Morin FC., III Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res. 1989;25:245–250. doi: 10.1203/00006450-198903000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Konduri GG, Ou J, Shi Y, Pritchard KA., Jr Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2003;285:H204–H211. doi: 10.1152/ajpheart.00837.2002. [DOI] [PubMed] [Google Scholar]
- 15.Wedgwood S, Black SM. Role of reactive oxygen species in vascular remodeling associated with pulmonary hypertension. Antioxid Redox Signal. 2003;5:759–769. doi: 10.1089/152308603770380061. [DOI] [PubMed] [Google Scholar]
- 16.Rao GN, Berk BC. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992;70:593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- 17.Wedgwood S, Lakshminrusimha S, Czech L, Schumacker PT, Steinhorn RH. Increased p22phox/Nox4 expression is involved in remodeling through hydrogen peroxide signaling in experimental persistent pulmonary hypertension of the newborn. Antioxid Redox Signal. 2013;18:1765–1776. doi: 10.1089/ars.2012.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Pareja KA, Kaiser CA, Sevier CS. Redox signaling via the molecular chaperone BiP protects cells against endoplasmic reticulum–derived oxidative stress. eLife. 2014;3:e03496. doi: 10.7554/eLife.03496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhandary B, Marahatta A, Kim HR, Chae HJ. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci. 2012;14:434–456. doi: 10.3390/ijms14010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006;71:216–225. doi: 10.1016/j.cardiores.2006.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 23.Susa S, Wakabayashi I. Extracellular alkalosis activates ERK mitogen-activated protein kinase of vascular smooth muscle cells through NADPH-mediated formation of reactive oxygen species. FEBS Lett. 2003;554:399–402. doi: 10.1016/s0014-5793(03)01198-0. [DOI] [PubMed] [Google Scholar]
- 24.Hartney T, Birari R, Venkataraman S, Villegas L, Martinez M, Black SM, Stenmark KR, Nozik-Grayck E. Xanthine oxidase–derived ROS upregulate Egr-1 via ERK1/2 in PA smooth muscle cells; model to test impact of extracellular ROS in chronic hypoxia. PLoS One. 2011;6:e27531. doi: 10.1371/journal.pone.0027531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin C, Guo J, Qiu X, Ma K, Xiang M, Zhu X, Guo J. IGF-1 induces iNOS expression via the p38 MAPK signal pathway in the anti-apoptotic process in pulmonary artery smooth muscle cells during PAH. J Recept Signal Transduct Res. 2014;34:325–331. doi: 10.3109/10799893.2014.903417. [DOI] [PubMed] [Google Scholar]
- 26.Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71–92. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Wedgwood S, Black SM. Combined superoxide dismutase/catalase mimetics alter fetal pulmonary arterial smooth muscle cell growth. Antioxid Redox Signal. 2004;6:191–197. doi: 10.1089/152308604771978507. [DOI] [PubMed] [Google Scholar]
- 31.Stabile E, Zhou YF, Saji M, Castagna M, Shou M, Kinnaird TD, Baffour R, Ringel MD, Epstein SE, Fuchs S. Akt controls vascular smooth muscle cell proliferation in vitro and in vivo by delaying G1/S exit. Circ Res. 2003;93:1059–1065. doi: 10.1161/01.RES.0000105086.31909.1B. [DOI] [PubMed] [Google Scholar]
- 32.Teng RJ, Wu TJ. Persistent pulmonary hypertension of the newborn. J Formos Med Assoc. 2013;112:177–184. doi: 10.1016/j.jfma.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puthiyachirakkal M, Mhanna MJ. Pathophysiology, management, and outcome of persistent pulmonary hypertension of the newborn: a clinical review. Front Pediatr. 2013;1:23–30. doi: 10.3389/fped.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000;87:179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- 35.Teng RJ, Eis A, Bakhutashvili I, Arul N, Konduri GG. Increased superoxide production contributes to the impaired angiogenesis of fetal pulmonary arteries with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;297:L184–L195. doi: 10.1152/ajplung.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao B, Chun C, Liu Z, Horswill MA, Pramanik K, Wilkinson GA, Ramchandran R, Miao RQ. Nogo-B receptor is essential for angiogenesis in zebrafish via Akt pathway. Blood. 2010;116:5423–5433. doi: 10.1182/blood-2010-02-271577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teng RJ, Du J, Xu H, Bakhutashvili I, Eis A, Shi Y, Pritchard KA, Jr, Konduri GG. Sepiapterin improves angiogenesis of pulmonary artery endothelial cells with in utero pulmonary hypertension by recoupling endothelial nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol. 2011;301:L334–L345. doi: 10.1152/ajplung.00316.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afolayan AJ, Eis A, Teng RJ, Bakhutashvili I, Kaul S, Davis JM, Konduri GG. Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2012;303:L870–L879. doi: 10.1152/ajplung.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teng RJ, Du J, Welak S, Guan T, Eis A, Shi Y, Konduri GG. Cross talk between NADPH oxidase and autophagy in pulmonary artery endothelial cells with intrauterine persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302:L651–L663. doi: 10.1152/ajplung.00177.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct. 2011;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Runchel C, Matsuzawa A, Ichijo H. Mitogen-activated protein kinases in mammalian oxidative stress responses. Antioxid Redox Signal. 2011;15:205–218. doi: 10.1089/ars.2010.3733. [DOI] [PubMed] [Google Scholar]