Abstract
Airway remodeling is strongly correlated with the progression of chronic obstructive pulmonary disease (COPD). In this study, our goal was to characterize progressive structural changes in site-specific airways, along with the temporal and spatial expression of transforming growth factor (TGF)-β in the lungs of male spontaneously hypertensive rats exposed to tobacco smoke (TS). Our studies demonstrated that TS-induced changes of the airways is dependent on airway generation and exposure duration for proximal, midlevel, and distal airways. Stratified squamous epithelial cell metaplasia was evident in the most proximal airways after 4 and 12 weeks but with minimal levels of TGF-β–positive epithelial cells after only 4 weeks of exposure. In contrast, epithelial cells in midlevel and distal airways were strongly TGF-β positive at both 4 and 12 weeks of TS exposure. Airway smooth muscle volume increased significantly at 4 and 12 weeks in midlevel airways. Immunohistochemistry of TGF-β was also found to be significantly increased at 4 and 12 weeks in lymphoid tissues and alveolar macrophages. ELISA of whole-lung homogenate demonstrated that TGF-β2 was increased after 4 and 12 weeks of TS exposure, whereas TGF-β1 was decreased at 12 weeks of TS exposure. Airway levels of messenger RNA for TGF-β2, as well as platelet-derived growth factor-A, granulocyte-macrophage colony–stimulating factor, and vascular endothelial growth factor-α, growth factors regulated by TGF-β, were significantly decreased in animals after 12 weeks of TS exposure. Our data indicate that TS increases TGF-β in epithelial and inflammatory cells in connection with airway remodeling, although the specific role of each TGF-β isoform remains to be defined in TS-induced airway injury and disease.
Keywords: chronic obstructive pulmonary disease, transforming growth factor-β, tobacco smoke, airway epithelium, spontaneously hypertensive rats
Clinical Relevance
The temporal and spatial expression of transforming growth factor-β during airway remodeling in a rodent model of chronic obstructive pulmonary disease (COPD) with progressive exposure to tobacco smoke (TS) can provide unique insights to better understanding COPD in humans. The study of the genesis and progression of TS-induced airway injury through the examination of site-specific airway epithelial, smooth muscle, and extracellular matrx (collagen) changes can provide new insights into the complex interactions between epithelial/mesenchymal target cells for potential therapeutic intervention in humans suffering from COPD.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the United States, with the majority of COPD cases attributed to smoking (1). In fact, tobacco smoke (TS) is the most common risk factor for COPD. The disease is characterized by progressive and poorly reversible airflow limitation, due to the loss of alveolar septal tissues and remodeling of the airways in connection with airway inflammation, epithelial cell metaplasia, mucus hypersecretion, and airway wall thickening. Many of the molecular mechanisms involved in the genesis and progression of COPD-like characteristics are not yet understood, but may hold promise for therapeutic intervention.
Researchers over the years have gained considerable knowledge about how the parenchyma is injured by TS via protease–antiprotease imbalance and oxidative stress (2). Clinical studies have shown strong correlations between the progression of COPD and airway remodeling. The progression of COPD, as defined by the Global Initiative for Chronic Obstructive Lung Disease, is strongly associated with increased wall thickening and inflammatory mucous exudates in the lumen of the small airways, suggesting that small airways are major sites for airflow limitation in COPD (3, 4). However, the genesis of these changes still remains poorly understood, and is thus the focus of this study.
In response to TS exposure, epithelial cells of the airways and alveoli, as well as inflammatory cells recruited to the lungs, release a plethora of cytokines that contribute to airway epithelial damage and airspace enlargement. Among these cytokines, transforming growth factor (TGF)-β may play a critical role in mediating TS-induced lung injury. This multifunctional cytokine has the remarkable ability to mediate numerous cellular behaviors, including cell proliferation, differentiation, immune modulation, carcinogenesis, and extracellular matrix production (5, 6). There are three known mammalian TGF-β isoforms: TGF-β1, TGF-β2, and TGF-β3. Each isoform shares 60–80% sequence homology (7). Among them, TGF-β1 is the most highly expressed TGF-β isoform in the human lung, which regulates various aspects of respiratory physiology and pathology (8). TGF-β1 knockout mice die in utero due to extensive inflammation in multiple organs (9). In studies of pulmonary fibrosis, TGF-β1 is a well known profibrotic factor that regulates the expression of extracellular matrix proteins, such as procollagen I and III, fibronectin, versican, and tenascin. An abnormal level of TGF-β1 in the lungs is associated with pulmonary fibrosis and is characterized by an excessive amount of extracellular matrix proteins in the lungs.
Compared with TGF-β1, the roles of TGF-β2 and TGF-β3 are less-well understood. TGF-β2 knockout mice die shortly after birth, due to respiratory failure, and exhibit collapsed distal airways in the presence of dilated proximal conducting airways (10). TGF-β2, but not TGF-β1, is implicated in bronchial epithelial mucin expression in asthma (11). TGF-β2 is also up-regulated in eosinophils found in subjects with severe asthma and is associated with increased profibrotic responses (12). TGF-β3 plays an essential role in lung development, as noted in TGF-β3 knockout mice, which die shortly after birth, due to insufficient pulmonary growth, including alveolar hypoplasia and the lack of alveolar septal formation (13).
Variation in the TGF-β1–encoding gene has been suggested to be one of the genetic determinants of COPD (14). Previous reports have also shown that TGF-β1 expression is increased in airway epithelium, airway smooth muscle, and macrophages in lungs of patients with COPD (15–17). However, very little is known regarding the temporal or spatial expression of TGF-β1 during the genesis of COPD. Moreover, TGF-β type II receptor expression is decreased in the bronchial glands of smokers with COPD (18). Some studies have reported an up-regulation of TGF-β1 expression in the lungs of TS-exposed animals. Previously, Bracke and colleagues (19) reported increased levels of TGF-β1 protein in the lung lavage of C57BL/6 mice exposed to TS for 1 month. Moreover, in vitro exposure of rat tracheal explants to TS caused an increase in mRNA levels of TGF-β1 (20). TGF-βs could be critical in the development of COPD, due to its ability to regulate both immune cells and airway structural cells in the lungs. However, to date, there are no studies that have specifically investigated the temporal and spatial distribution of TGF-βs in the lungs with progressive exposure to TS in either animals or humans during the genesis of COPD.
Exposure of spontaneously hypertensive (SH) rats to TS has been shown to produce many of the classic pathophysiological features found in patients with COPD, including chronic lung inflammation with significant increases in neutrophil number, pulmonary cytokines, squamous and mucous metaplasia of the airways, alveolar airspace enlargement, and weight loss (21–23). These past studies show SH rats to be an ideal model for COPD research. Therefore, we exposed SH rats to progressive TS and examined the lungs at acute (3 d), subchronic (4 wk), and chronic (12 wk) time points as a means to study the distribution of TGF-βs, which have been implicated to be important in the pathogenesis of COPD, as well as other selected growth factors that may be regulated by TGF-βs. Therapeutic interventions designed to target TGF-βs have been considered as a means to reduce airway remodeling caused by inflammation and fibrosis. Minagawa and colleagues (24) reported that inhibition of TGF-β activation in transgenic mice, using an antibody to human αvβ8, blocks fibroinflammatory responses induced by TS. Therefore, we hypothesize that TS increases TGF-βs and associated growth factors in epithelial and inflammatory cells in highly site-specific regions of the lungs, as noted in the lungs of patients with COPD.
Materials and Methods
See the online supplement for method details.
Animals
Male SH rats (12 wk old) were purchased from Charles River Laboratories (Portage, MI). Animals were handled according to the standards established by the U.S. Animal Welfare Acts. Procedures were approved by the University of California, Davis (Davis, CA), Animal Care and Use Committee.
Exposure
Rats were exposed to filtered air (FA) or TS (90 mg/m3 total suspended particulate) at 6 h/d, 3 d/wk, for 3 days, 4 weeks, or 12 weeks (n = 6/time point). Whole-body TS exposure occurred in a smoking system (25) that combusted 2R4F research cigarettes (Tobacco and Health Research Institute, University of Kentucky, Lexington, KY).
Tissue Preparation
After killing of the rats, the right lung was lavaged and frozen in liquid nitrogen for protein isolation and analysis. The left lung was fixed with 4% paraformaldehyde and cut into transverse serial slices to capture the different intrapulmonary airway generations, with proximal as the third to fourth generation, midlevel as eighth to tenth, and distal as the level of the terminal bronchiole, when using the trachea as the first airway generation for the tracheobronchial tree. Each slice was embedded in paraffin and cut into 5-μm sections.
Histopathology
Lung tissue sections containing the first, second, and third intrapulmonary airway generations were deparaffinized and stained to detect mucosubstances (Alcian blue [AB]/periodic acid–Schiff [PAS]), squamous metaplasia (hematoxylin and eosin), and airway smooth muscle fiber and connective tissue (Masson’s trichrome).
Immunohistochemistry for TGF-β
Immunohistochemistry was performed for TGF-β protein using a rabbit anti-rat polyclonal antibody that stains all three isoforms of TGF-β (catalog number ab66043, stock concentration 0.5 mg/ml; Abcam, San Francisco, CA).
Morphometric Assessment of Epithelium, Smooth Muscle, and Collagen Volume
To measure the volume of epithelium, smooth muscle, and collagen for the proximal and midlevel airways, four images for each airway level were captured and used to determine the volume per basal lamina surface for each of these tissue compartments using Imagine J software (National Institutes of Health, Bethesda, MD).
Quantification of TGF-β–Positive Cells
The area of all TGF-β–positive epithelial cells and the length of the corresponding basal lamina for each airway were measured using ImageJ software. TGF-β was measured in sections containing proximal midlevel and distal (terminal) airways. The expression of TGF-β in lymphoid aggregates was scored using a semiquantitative system that considers both frequency and staining intensity ranging from 0 (no staining) to 5 (intense, dark staining).
Determination of Whole-Lung TGF-β Protein by ELISA
ELISA was performed on right accessory lung lobes for TGF-β1, -β2, and -β3 to determine protein expression for the entire lung lobe.
Quantitative RT-PCR for the Airways after 12-Week TS Exposure
The airways from the right caudal lung lobe of male SH rats exposed to FA or TS for 12 weeks (n = 5) were isolated by microdissection to determine mRNA expression in the airways. These isolated airways were generations 4–10 relative to the trachea identified as generation 1. Total RNA was purified and quantitative RT-PCR was performed. Primer sequences for each gene are listed in Table 1. Results are presented as ratios of target mRNA normalized to β-actin mRNA.
Table 1.
Genes and Primers Selected for RT-PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | AGCCATGTACGTAGCCATCC | CTCTCAGCTGTGGTGGTGAA |
| TGF-β1 | CGAGGTGACCTGGGCACCATCCATGAC | CTGCTCCACCTTGGGCTTGCGACCCAC |
| TGF-β2 | CTCCACATATGCCAGTGGTG | CTAAAGCAATAGGCGGCATC |
| TGF-β3 | GCAACTTGGAGGAGAACTGC | GTCAGAGGCTCCAGGTCTTG |
| FGF 2 | GAACCGGTACCTGGCTATGA | CCGTTTTGGATCCGAGTTTA |
| PDGF-A | ATGCCTTGGAGACAAACCTG | GTCAAGAAGTTGGCCGATGT |
| CTGF | TAGCAAGAGCTGGGTGTGTG | TTCACTTGCCACAAGCTGTC |
| Insulin-like GF 1 | GCTGAAGCCGTTCATTTAGC | GAGGAGGCCAAATTCAACAA |
| TGF-α | GCAAGTTCTGCCTGTTCCTC | GCACTGAACCAACCCACTTT |
| EGF | ACACCGAAGGTGGCTATGTC | TAGAGTCAGGGCAAGGCAGT |
| GM-CSF | TCCTAAATGACATGCGTGCT | GCCATTGAGTTTGGTGAGGT |
| VEGF-α | GCCCATGAAGTGGTGAAGTT | ACTCCAGGGCTTCATCATTG |
| Neurotrophin 3 | GATCCAGGCGGATATCTTGA | AGCGTCTCTGTTGCCGTAGT |
Definition of abbreviations: CTGF, connective tissue growth factor; EGF, epidermal growth factor; FGF, fibroblast growth factor; GF, growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; PDGF-A, platelet-derived growth factor α chain; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
Statistical Analysis
Data are expressed as mean (±SEM). Effects of TS and exposure time point were assessed by two-way ANOVA. Differences among experimental groups were examined by one-way ANOVA followed by Bonferroni’s post hoc test. A P value of less than 0.05 was considered significant.
Results
Histopathological Evaluation of TS-Associated Remodeling in Pulmonary Airways
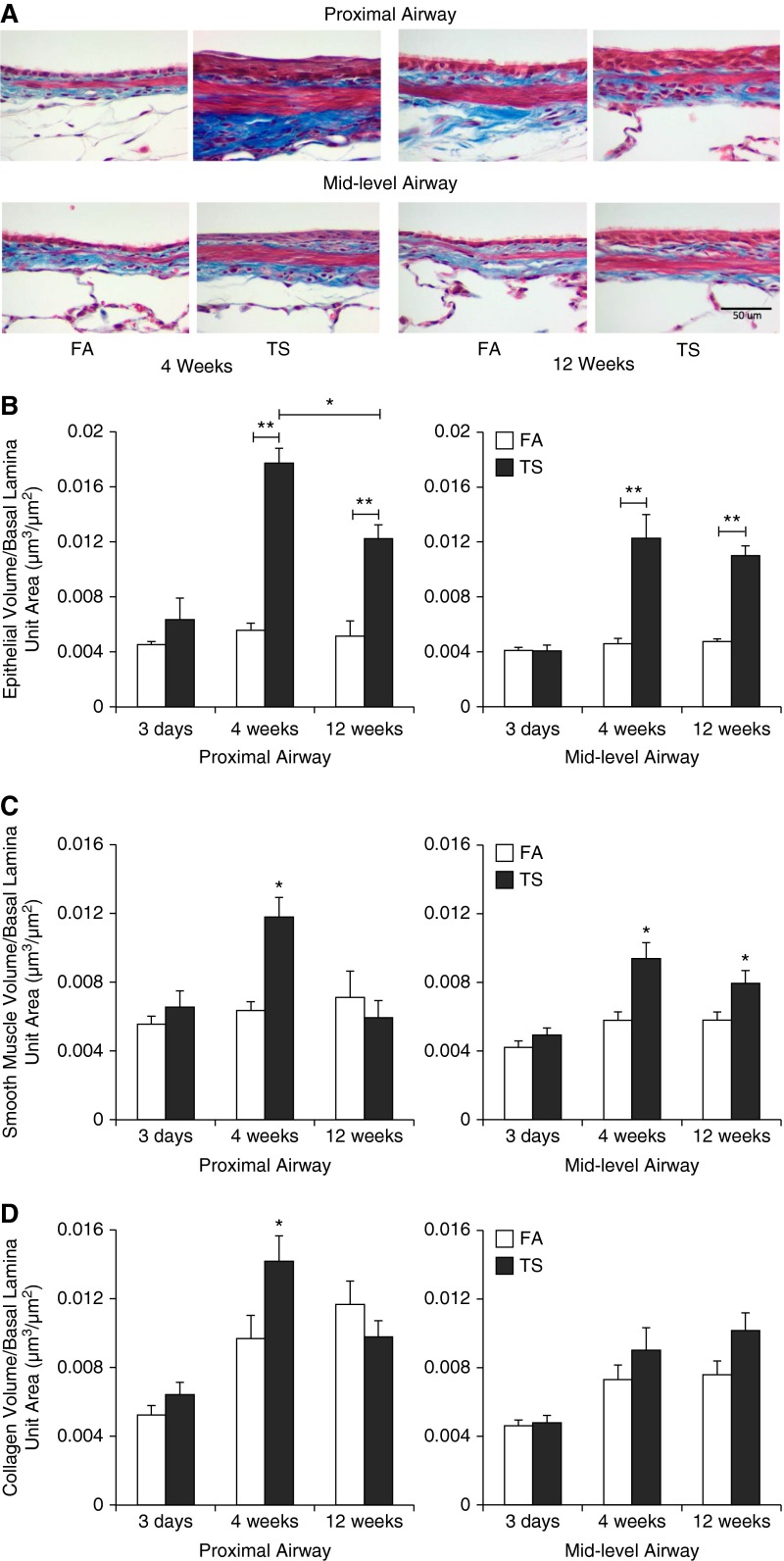
Although not shown, 3 days of smoke exposure induced epithelial cell injury in the proximal airways, with loss of ciliated epithelial cells and sloughing of airway epithelium. After 4 and 12 weeks of TS exposure, large areas of stratified squamous epithelial metaplasia along the proximal airways were noted. The midlevel airways of 4- and 12-week TS–exposed rats exhibited hypertrophic epithelial cells (Figure 1A). The significance of this hypertrophic state in the epithelium in response to smoke exposure was not known. The distal airways continued to demonstrate simple cuboidal epithelia, regardless of TS exposure. FA-exposed animals did not show squamation in the airway epithelium at any time point (Figure 1A).
Figure 1.
Histopathological evaluation of tobacco smoke (TS)–associated injuries in pulmonary airways. (A) Representative lung sections depicting changes in volume of airway epithelium, smooth muscle, and collagen; quantitative analysis of (B) epithelium volume, (C) airway smooth muscle volume, and (D) collagen volume at 3 days, 4 weeks, and 12 weeks of TS exposure in proximal and midlevel airways. Data are expressed as mean (±SEM) for six animals per group. Scale bar: 50 μm. *P < 0.05, **P < 0.001, ANOVA with Bonferroni’s post hoc test. FA, filtered air.
Quantitative measurement of epithelial volume shows that there was a significant increase in response to TS exposure compared with FA group at 4 and 12 weeks in both proximal and midlevel airways (Figure 1B). The volume of smooth muscle was significantly increased in the proximal airways after 4 weeks, but not 12 weeks. In contrast, the midlevel airways showed a significant increase in smooth muscle volume at both 4 and 12 weeks (Figure 1C). The volume of collagen showed a significant increase only at 4 weeks in the proximal airway. A trend for increased collagen volume was noted at 4 and 12 weeks for the midlevel airway, but not to a statistically significant level (Figure 1D). There was no significant change in epithelial, smooth muscle, or collagen volume in response to smoke exposure at 3 days in proximal and midlevel airways.
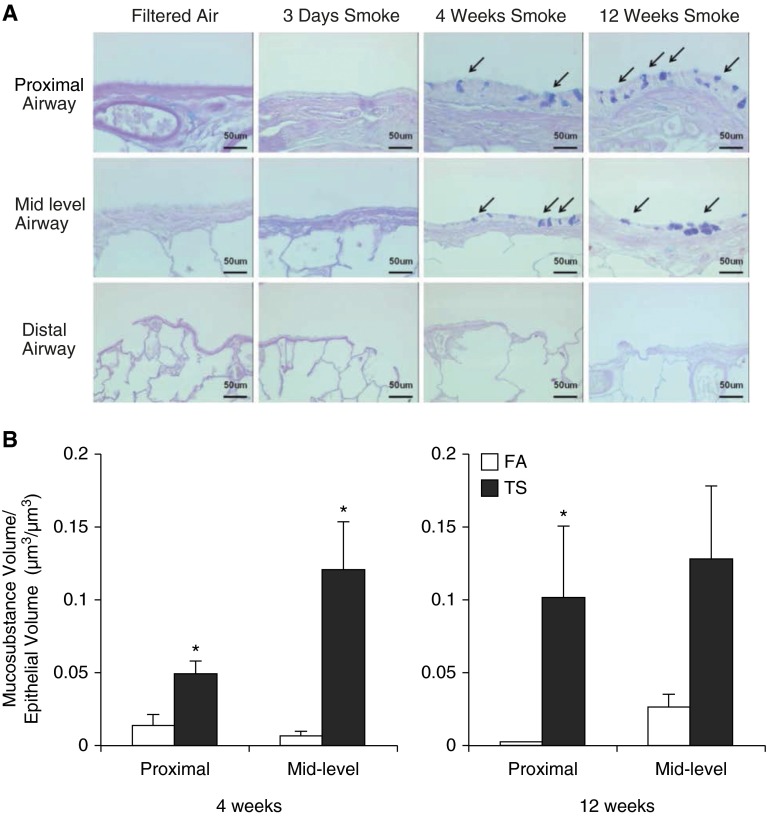
Intraepithelial mucosubstances were unaffected after 3 days of TS exposure. In contrast, after 4 and 12 weeks of TS exposure, SH rats demonstrated a significant increase in AB/PAS–stained epithelial cells in both proximal and midlevel airways compared with corresponding FA controls (Figures 2A and 2B). Staining for AB/PAS was not found in distal airways of rats exposed to TS at any time point (Figure 2A).
Figure 2.
Mucosubstance in the airway epithelium. (A) Representative lung sections depicting changes in mucosubstance in the epithelial cells of proximal and midlevel airways. Distal airway epithelium does not stain positive for Alcian blue (AB)/periodic acid–Schiff (PAS). Arrows indicate epithelium stained positive for mucosubstance using AB/PAS. (B) Volume fraction analyses for mucosubstance with AB/PAS. Data are expressed as mean (±SEM). Scale bars: 50 μm. *P < 0.05, ANOVA with Bonferroni’s post hoc test.
TGF-β Expression in the Airway Epithelium
TGF-β staining for all isoforms (TGF-β1, -β2, and -β3) was used to determine the temporal and spatial distribution of TGF-β in airway epithelium. After 3 days of TS exposure, TGF-β protein was not significantly up-regulated at any airway level. By 4 weeks, and again at 12 weeks, of TS exposure, TGF-β staining was increased in airway epithelium (e.g., ciliated cells, Clara cells, and basal cells) and found in all airway levels, including proximal, midlevel, and distal airways (Figure 3A). Quantitative measurement of TGF-β expression demonstrated that, at 4 and 12 weeks, epithelial cells in midlevel and distal airways had a significant elevation (Figure 3B). There was no change in TGF-β expression in the epithelial cells in the proximal airways at 4 weeks; however, proximal airways showed a significant elevation in TGF-β staining compared with the FA group at 12 weeks.
Figure 3.
Transforming growth factor (TGF)-β expression in the airway epithelium. (A) Representative lung sections depicting TGF-β expression in the epithelium of proximal, midlevel, and distal airways after progressive smoke exposure for 3 days, 4 weeks, and 12 weeks. (B) Quantitative measurement of TGF-β expression in proximal, midlevel, and distal airway epithelium from paraffin-embedded sections. Data are expressed as mean (±SEM) for six animals per group. Scale bar: 50 μm. *P < 0.001, ANOVA with Bonferroni’s post hoc test.
TGF-β Expression in Airway Lymphoid Aggregates
Clusters or aggregates of lymphoid cells in the airway wall of rats exposed to 4 or 12 weeks of smoke were present in the proximal and midlevel airways, and had significantly increased levels of TGF-βs compared with rats exposed to FA (Figure 4). TGF-β staining was found within the extracellular matrix of lymphoid aggregates, as well as inside the lymphocytes (Figure 4A). When scored for TGF-β–positive lymphoid aggregates, animals exposed to TS for 4 or 12 weeks had significantly higher scores than animals exposed to 3 days of TS (Figure 4B). The frequency of lymphoid aggregates was noted to increase at 4 weeks (FA:TS = 1:1.5) and to a statistically significant level by 12 weeks of TS exposure (FA:TS = 1:3.0, P < 0.05).
Figure 4.
TGF-β expression in the lymphoid aggregates. (A) Representative lung sections depicting TGF-β expression in the lymphoid aggregates after progressive smoke exposure for 3 days, 4 weeks, and 12 weeks. Arrows indicate areas of TGF-β–positive staining. After 4 and 12 weeks of TS exposure, lymphoid aggregates of spontaneously hypertensive (SH) rats demonstrated increased levels of TGF-β compared with lymphoid cells of rats exposed to FA. (B) Staining score for TGF-β expression in lymphoid aggregates from paraffin-embedded sections. Data are expressed as mean (±SEM) for four to six animals per group. Scale bars: 50 μm. *P < 0.05, **P < 0.0005, ***P < 0.001, ANOVA with Bonferroni’s post hoc test.
TGF-β Expression in the Alveolar Macrophages
Terminal bronchioles and adjacent alveolar structures in TS-exposed rats contained numerous clusters of activated macrophages in the airspaces that stained positively for TGF-β protein (Figure 5). Compared with 4 weeks of smoke exposure, rats exposed to TS for 12 weeks showed more intense staining for TGF-β protein in alveolar macrophages (Figure 5A). Similar to its temporal expression in the epithelium and lymphoid aggregates, TGF-β was not significantly increased after 3 days of smoke exposure. However, TGF-β expression was significantly increased in alveolar macrophages after both 4 and 12 weeks of TS exposure (Figure 5B). Animals exposed to TS for 4 and 12 weeks had a significantly higher frequency of TGF-β–positive macrophages compared with the FA control. In addition, with prolonged smoke exposure, the frequency of positive alveolar macrophages increased significantly (Figure 5B).
Figure 5.
TGF-β expression in the alveolar macrophages. (A) Representative lung sections depicting TGF-β expression in the alveolar macrophages after progressive smoke exposure for 3 days, 4 weeks, and 12 weeks. Arrows indicate TGF-β–negative macrophage. Arrowheads indicate TGF-β–positive macrophages. Terminal bronchiole and adjacent alveolar structures in rats exposed to smoke for 4 and 12 weeks contained numerous clusters of activated macrophages in the airspaces that stained strongly for TGF-β. Compared with rats exposed to TS, rats exposed to FA had fewer numbers of alveolar macrophages. (B) Measurement of frequency of TGF-β–positive alveolar macrophages from each total field in paraffin-embedded sections for the TS group at 4 weeks and 12 weeks. Data are expressed as mean (±SEM) for six animals per group. Scale bars: 50 μm. *P < 0.05, **P < 0.005, ANOVA with Bonferroni’s post hoc test.
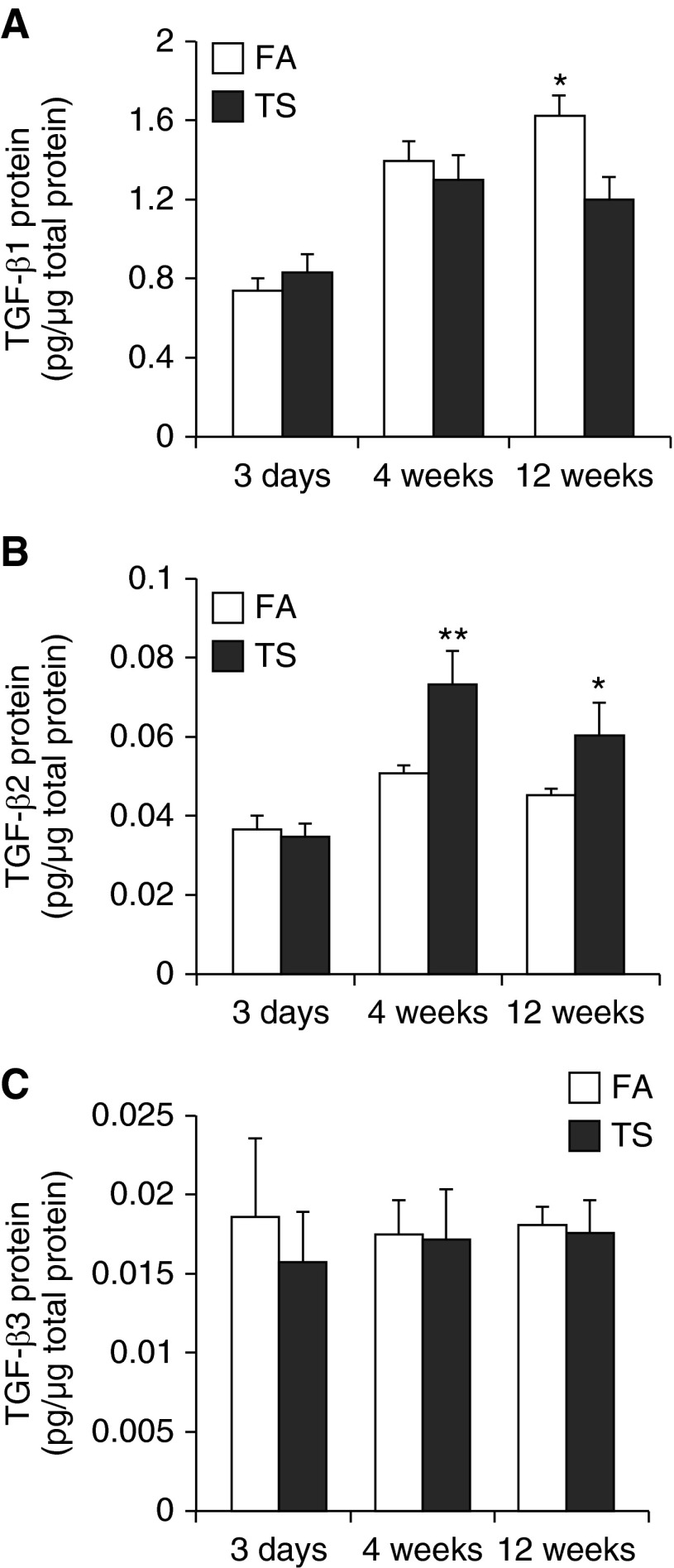
Lung Protein Levels Specific for TGF-β1, TGF-β2, and TGF-β3
To observe how the protein levels of TGF-β1, -β2, and -β3 change over time with progressive exposure to TS, we performed an ELISA to measure the protein levels in right accessory lobe homogenates of the same animals in which TGF-βs immunohistochemistry was performed on lung sections. TGF-β1 was not significantly changed after 3 days or 4 weeks of TS exposure (Figure 6A). In contrast, TGF-β1 was significantly reduced after 12 weeks of TS exposure. Interestingly, endogenous TGF-β1 appeared to increase with time in FA-treated animals (Figure 6A). Similar to TGF-β1, TGF-β2 was unchanged after 3-day acute smoke exposure (Figure 6B). However, TGF-β2 was significantly increased after 4 or 12 weeks of TS exposure. TGF-β3 was not changed after 3 days, 4 weeks, or 12 weeks of smoke exposure (Figure 6C).
Figure 6.
Whole accessory lobe–specific TGF-β1, -β2, and -β3 protein levels. (A) TGF-β1, (B) TGF-β2, and (C) TGF-β3 protein levels measured by ELISA in accessory lobe homogenates are reported. TGF-β1 protein was significantly reduced after 12 weeks of TS exposure. By contrast, TGF-β2 protein in lung homogenates was significantly elevated after 4 and 12 weeks of exposure to TS. TGF-β3 protein was not significantly changed in response to TS exposure at any time point. Data are expressed as mean (±SEM) for five to six animals per group.*P < 0.01, **P < 0.001, ANOVA with Bonferroni’s post hoc test.
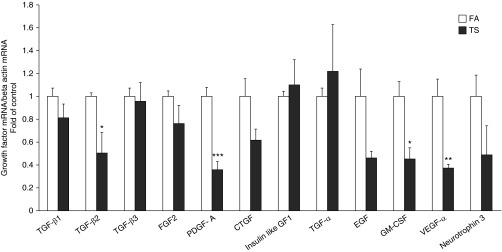
Airway-Specific Gene Expression of Growth Factors after 12 Weeks of Smoke Exposure in SH Rats
After 12 weeks of smoke exposure, exposed rats from this time point, along with matched FA controls, were used to analyze the gene expression of growth factors implicated in the process of airway remodeling in isolated, microdissected airways from the right caudal lobe (Figure 7). There was a significant trend for TS exposure to reduce mRNA levels for a number of growth factors, with significant decreases in TGF-β2, platelet-derived growth factor (PDGF)-A, granulocyte-macrophage colony–stimulating factor (GM-CSF), and vascular endothelial growth factor (VEGF)-α. Only insulin-like growth factor (IGF) 1 and TGF-α were up-regulated by smoke; however, the increase was not statistically significant.
Figure 7.
Airway-specific gene expression of growth factors in 12-week smoke-exposed SH rats. Effects of 12-week TS exposure on the gene expression of growth factors that may be implicated in the airway remodeling process. mRNA levels of growth factors measured by quantitative real-time RT-PCR in isolated airways of caudal lobes are reported. Data are expressed as mean (±SEM) for five animals per group. *P < 0.05, **P < 0.005, ***P < 0.0005, ANOVA with Bonferroni’s post hoc test. CTGF, connective tissue growth factor; EGF, epidermal growth factor; FGF, fibroblast growth factor; GF, growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; PDGF-A, platelet-derived growth factor α chain; VEGF, vascular endothelial growth factor.
Discussion
The airway remodeling process in the present study is consistent with that seen in human lung tissues as COPD severity increases. In the study by Hogg and colleagues (4), it was shown that, as COPD progresses, there was a significant increase in epithelial and smooth muscle volume per unit of surface area of basal lamina. In addition, the study by Hogg and colleagues showed that the progression of COPD was strongly correlated with an increase of macrophages and lymphoid aggregate frequency, consistent with what we observed in the present study. We also observed the thickening of the airways, as evidenced by a significant increase in epithelial and smooth muscle volume. The increase in collagen volume found in the proximal airways at 4 weeks also contributes to the airway thickening process.
Lung epithelial cells are the first cells to come into contact with toxic inhalants. Upon exposure to smoke, lung epithelial cells release inflammatory cytokines and chemokines to stimulate the recruitment of inflammatory leukocytes. These inflammatory mediators can potentially alter the interactions between the epithelium and mesenchymal tissues. For example, injury to the airway epithelium can influence the growth of fibroblasts and turnover of matrix proteins present in mesenchymal tissues. One study found that integrin αvβ8–mediated TGF-β activation in the epithelium caused IL-1β–dependent fibrosis in mesenchymal tissues, associated with small airway wall thickening in lung samples of patients with COPD (26). We found that TGF-β was present in the epithelium of midlevel and distal airways after 4 weeks of smoke exposure, and in proximal, midlevel, and distal airways after 12 weeks of smoke exposure.
Although evident at multiple levels of the airways, the effect of TGF-β has been most recognized in medium-sized and small airways (16). Morphological lesions induced in the airway epithelium by TS included stratified squamous epithelial cell differentiation and mucus metaplasia. These changes in airway epithelium are also found in the lungs of patients with COPD, and can be correlated with increased severity of COPD (26). The work of Araya and colleagues (26) suggested that integrin αvβ8–mediated TGF-β activation enhances squamous differentiation. Moreover, TGF-β2 has been shown to induce mucin production in cultured epithelial cells obtained from subjects with asthma (11). In patients with COPD, TGF-β contributes to the production of the extracellular matrix, epithelial-to-mesenchymal transition, fibroblast differentiation, and smooth muscle dysfunction (14, 27). Future investigations are needed to further unravel the role of TGF-βs in epithelial/mesenchymal cell injury caused by exposure to TS.
Lymphoid aggregates, also known as bronchus-associated lymphoid tissues, are typically found at bifurcations of the bronchial airways. These regions of the airways are most susceptible to injury caused by toxic inhalants, because of their anatomical location as sites of highest particle deposition. An excess number of lymphocytes is one of the hallmarks of COPD (4). Animals exposed to TS have increased numbers of lymphoid aggregates compared with animals exposed to FA, as noted in this study and others (28). Lymphoid aggregates contain numerous lymphocytes, including B lymphocytes, natural killer cells, and T lymphocytes. The effects of TGF-β on lymphocytes in COPD pathogenesis are not yet understood. However, TGF-β1 has been shown to exert a variety of regulatory roles on T lymphocytes. For example, TGF-β1 stimulates the proliferation of T cells (29) and induces human T lymphocyte migration in vitro (30). Our study demonstrates the expression of TGF-β in lymphoid tissues containing an excess number of lymphocytes in TS-exposed animals, but we did not determine the lymphocyte phenotype present, suggesting that TGF-β may either play an important role in regulating the activities of lymphocytes or simply be a reflection of T lymphocyte differentiation during TS exposure. The mechanisms underlying this interaction between TGF-β and TS-induced lymphocyte activity is not yet known.
In addition to the epithelium and lymphoid aggregates, macrophages were also found to be a source of TGF-β production. Terminal bronchioles and adjacent alveolar structures in TS-exposed rats contained numerous clusters of activated macrophages in the airspaces that stained positive for TGF-β; very few macrophages were negative for TGF-β. Macrophages can account for up to 98% of inflammatory cells in the lungs of mice exposed to TS (31), and they produce high levels of matrix metalloproteinases (MMPs) 2, 9, and 12, which degrade the extracellular matrix and lead to emphysema (32). Mice lacking macrophage elastase (MMP-12) are protected against emphysema (33). It is unknown if TS-induced TGF-β1 modulates production of MMPs in alveolar macrophages. However, investigations using mice deficient in the β6 subunit of the epithelial integrin αvβ6–null mice and SMAD 3–null mice have shown interesting results. Morris and colleagues (34) showed that activation of TGF-β1 by epithelial-restricted integrin αvβ6 reduced MMP-12 expression. Bonniaud and colleagues (35) found that SMAD 3–null mice developed airspace enlargement along with up-regulated levels of MMP9 and MMP12. Animals were not exposed to smoke in these two studies. Feinberg and colleagues (36) showed that TGF-β1 inhibited MMP12 expression in alveolar macrophages in vitro. In addition to affecting MMPs, TGF-β1 was found to impair macrophage function by down-regulating its production of nitric oxide synthase and prostaglandin synthase (37, 38). It would be interesting to know if TGF-βs had the same effect on the macrophages of TS-exposed animals.
This study shows that TS had reduced TGF-β1 and increased TGF-β2 after 12 weeks of exposure. Up-regulation of TGF-β2 may be relevant to COPD pathogenesis, but its mechanism remains unknown. TGF-β1 and -β2 could play nonoverlapping roles in smoke-induced lung injury. Functions of TGF-β1 and -β2 may not be interchangeable; Yu and colleagues (39) reported that different TGF-βs may have distinctive activities depending on cell types and environment. There is no overlapping phenotype between TGF-β1 and -β2 knockout mice, indicating their mutually exclusive functions (10). Their functional difference suggests that a better understanding of TGF-β1 and -β2 activities in COPD is important.
Many cell types express receptors for TGF-β1 and -β2 (40). TGF-β activity is dependent on the relative expression of TGF-β receptors on the cells and the binding preference of the receptors that are on airway fibroblasts, smooth muscle, and epithelial cells. Previously, it was shown that TGF-β receptors on rat lung fibroblasts have higher affinity for TGF-β1 than TGF-β2 (40). The TGF-β receptors and TGF-β activity could play an important role in the airway remodeling process seen in the present study by mediating airway myofibroblasts to produce more collagen and causing smooth muscle cells to thicken, as well as causing airway epithelial cells to undergo mucous cell metaplasia.
A previous study by Gosselink and colleagues (41) found a pattern of expression of many tissue repair genes associated with emphysema. Furthermore, Campbell and colleagues (42) identified various changes in gene expression associated with emphysema to understand the processes underlying COPD. In our study, we looked at many growth factors that are associated with the growth of cellular components involved in the airway remodeling process. For example, the growth factors, PDGF-A and fibroblast growth factor 2, have been shown to influence the growth of fibroblasts and to regulate smooth muscle cell growth (43); GM-CSF induces the recruitment of neutrophils, monocytes, and lymphocytes (44), and VEGF-α controls angiogenesis and is a chemotactic factor for granulocytes (45). Connective tissue growth factor activates the synthesis of the extracellular matrix proteins (46). Neurotrophin 3 is involved in the regulation of neurogenesis (47). IGF1 is a stimulator of cell growth and proliferation, and a potent inhibitor of programmed cell death (48). Epidermal growth factor contributes to the proliferation of smooth muscle cell and mucus hypersecretion (49). TGF-α contributes to mucus hypersecretion (2). Because we saw that TS increased the expression of TGF-β in the airways, we asked whether TS also up-regulates other growth factors that may potentially lead to airway remodeling, as in extracellular matrix deposition, smooth muscle hyperplasia, and mucus hypersecretion. Therefore, mRNA levels were measured for PDGF-A, fibroblast growth factor 2, GM-CSF, VEGF-α, connective tissue growth factor, neurotrophin 3, IGF1, epidermal growth factor, and TGF-α in the airways of rats exposed to 12 weeks of smoke. In contrast to our hypothesis, and much to our surprise, TS reduced gene expression of most growth factors that may be implicated in the airway remodeling process. This disparity between TGF-β2 protein concentration and mRNA expression of TGF-β2 could be due to a difference in the half-life of cellular molecules at the time of measurement or missing the critical window of its expression. A further possibility might be a negative feedback control mechanism to reduce TGF-β1 protein, leading to decreased growth factor mRNA levels as an attempt to reduce further changes in cells and matrix, while resulting in a negative effect on epithelial repair.
Conclusions
Exposure to TS in SH rats induced extensive damage to the pulmonary airways, as indicated by significant elevations in epithelial mucosubstances, squamous metaplasia, and smooth muscle; airway extracellular matrix also showed an increase, but did not achieve a level of statistical significance. Airway injury varied by exposure duration and airway level. The present study evaluated the cellular distribution and time-dependent expression of TGF-β in the lungs. Based on immunohistochemical findings, the expression of TGF-βs is up-regulated in the epithelium of the airways, bronchial-associated lymphoid aggregates, and alveolar macrophages of SH rats after 4 and 12 weeks of TS exposure. TGF-β was not changed after 3 days of TS exposure compared with control lung tissues.
The disparity in TGF-β isoform expression in lung homogenates after 4 and 12 weeks of TS exposure suggests that TGF-β1 and -β2 are likely to play nonoverlapping roles in TS-induced lung injury. Up-regulation of TGF-β2 may be relevant to COPD pathogenesis, but its mechanism remains unknown. Furthermore, TS reduced mRNA levels for a number of growth factors implicated in the airway remodeling process, possibly reflecting a negative-feedback control mechanism to minimize further change. However, the potential for changes in the expression of specific TGF-β isoforms to drive the progression of changes in the lungs after TS exposure merits further investigation. Such studies may provide a better understanding of the molecular mechanisms of COPD in the small airways of the lungs, along with identifying whether TGF-β could serve as a potential pharmaceutical target for the treatment of COPD.
Acknowledgments
Acknowledgments
Special thanks to Janice Peake, University of California, Davis, CA, for technical support with immunohistochemical methods. The authors appreciate the help of Dr. Benjamin Davis, University of California, Davis, CA, with exposure design and tissue collection.
Footnotes
This work was supported by National Institute of Environmental Health Sciences grants ES 011,634 and ES013932, California Tobacco-Related Disease Research Program grant 18XT-0154, and by AstraZeneca, and by National Institutes of Health training grant T32 HL007013 (Y.-h.S.).
Author Contributions: L.L.H., Y.-h.S., and K.E.P. designed and planned the research; L.L.H., Y.P.N., R.A., Y.-h.S., and K.E.P. conducted the study and statistical analysis and drafted the manuscript; L.L.H., Y.P.N., S.J.B., L.W., N.J.K., S.S.-J., and K.E.P. edited and critically reviewed the manuscript in preparation.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0119OC on December 4, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Balkissoon R, Lommatzsch S, Carolan B, Make B. Chronic obstructive pulmonary disease: a concise review. Med Clin North Am. 2011;95:1125–1141. doi: 10.1016/j.mcna.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 3.Baraldo S, Turato G, Saetta M. Pathophysiology of the small airways in chronic obstructive pulmonary disease. Respiration. 2012;84:89–97. doi: 10.1159/000341382. [DOI] [PubMed] [Google Scholar]
- 4.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 5.Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, Zheng W, Little PJ, Osman N. Transforming growth factor-β signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 2013;25:2017–2024. doi: 10.1016/j.cellsig.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Verrecchia F, Mauviel A. Transforming growth factor-β signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 7.Hinck AP. Structural studies of the TGF-βs and their receptors—insights into evolution of the TGF-β superfamily. FEBS Lett. 2012;586:1860–1870. doi: 10.1016/j.febslet.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Morty RE, Königshoff M, Eickelberg O. Transforming growth factor-β signaling across ages: from distorted lung development to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:607–613. doi: 10.1513/pats.200908-087RM. [DOI] [PubMed] [Google Scholar]
- 9.Letterio JJ, Böttinger EP. TGF-β knockout and dominant-negative receptor transgenic mice. Miner Electrolyte Metab. 1998;24:161–167. doi: 10.1159/000057365. [DOI] [PubMed] [Google Scholar]
- 10.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu HW, Balzar S, Seedorf GJ, Westcott JY, Trudeau JB, Silkoff P, Wenzel SE. Transforming growth factor-β2 induces bronchial epithelial mucin expression in asthma. Am J Pathol. 2004;165:1097–1106. doi: 10.1016/s0002-9440(10)63371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottoms SE, Howell JE, Reinhardt AK, Evans IC, McAnulty RJ. TGF-β isoform specific regulation of airway inflammation and remodelling in a murine model of asthma. PLoS One. 2010;5:e9674. doi: 10.1371/journal.pone.0009674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-β 3 indicates defects of epithelial–mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa E, Ruan J, Connett JE, Anthonisen NR, Paré PD, Sandford AJ. Transforming growth factor-β1 polymorphisms, airway responsiveness and lung function decline in smokers. Respir Med. 2007;101:938–943. doi: 10.1016/j.rmed.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 15.de Boer WI, van Schadewijk A, Sont JK, Sharma HS, Stolk J, Hiemstra PS, van Krieken JH. Transforming growth factor β1 and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:1951–1957. doi: 10.1164/ajrccm.158.6.9803053. [DOI] [PubMed] [Google Scholar]
- 16.Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, Okada Y, Yamasawa F, Nakahara K, Umeda A. Increased expression of transforming growth factor-β1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD) Am J Respir Crit Care Med. 2001;163:1476–1483. doi: 10.1164/ajrccm.163.6.9908135. [DOI] [PubMed] [Google Scholar]
- 17.Gohy ST, Detry BR, Lecocq M, Bouzin C, Weynand BA, Amatngalim GD, Sibille YM, Pilette C. Polymeric immunoglobulin receptor down-regulation in chronic obstructive pulmonary disease: persistence in the cultured epithelium and role of transforming growth factor-β. Am J Respir Crit Care Med. 2014;190:509–521. doi: 10.1164/rccm.201311-1971OC. [DOI] [PubMed] [Google Scholar]
- 18.Baraldo S, Bazzan E, Turato G, Calabrese F, Beghé B, Papi A, Maestrelli P, Fabbri LM, Zuin R, Saetta M. Decreased expression of TGF-β type II receptor in bronchial glands of smokers with COPD. Thorax. 2005;60:998–1002. doi: 10.1136/thx.2005.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bracke KR, D'Hulst AI, Maes T, Demedts IK, Moerloose KB, Kuziel WA, Joos GF, Brusselle GG. Cigarette smoke–induced pulmonary inflammation, but not airway remodelling, is attenuated in chemokine receptor 5–deficient mice. Clin Exp Allergy. 2007;37:1467–1479. doi: 10.1111/j.1365-2222.2007.02808.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang RD, Wright JL, Churg A. Transforming growth factor-β1 drives airway remodeling in cigarette smoke–exposed tracheal explants. Am J Respir Cell Mol Biol. 2005;33:387–393. doi: 10.1165/rcmb.2005-0203OC. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Yang J, Guo L, Uyeminami D, Dong H, Hammock BD, Pinkerton KE. Use of a soluble epoxide hydrolase inhibitor in smoke-induced chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2012;46:614–622. doi: 10.1165/rcmb.2011-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolton SJ, Pinnion K, Oreffo V, Foster M, Pinkerton KE. Characterisation of the proximal airway squamous metaplasia induced by chronic tobacco smoke exposure in spontaneously hypertensive rats. Respir Res. 2009;10:118. doi: 10.1186/1465-9921-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong CY, Zhou YM, Pinkerton KE. NF-κB inhibition is involved in tobacco smoke–induced apoptosis in the lungs of rats. Toxicol Appl Pharmacol. 2008;230:150–158. doi: 10.1016/j.taap.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minagawa S, Lou J, Seed RI, Cormier A, Wu S, Cheng Y, Murray L, Tsui P, Connor J, Herbst R, et al. Selective targeting of TGF-β activation to treat fibroinflammatory airway disease. Sci Transl Med. 2014;6:241ra79. doi: 10.1126/scitranslmed.3008074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teague SV, Pinkerton KE, Goldsmith M, Gebremichael A, Chang S, Jenkins RA, Moneyhun JH. Sidestream cigarette smoke generation and exposure system for environmental tobacco smoke studies. Inhal Toxicol. 1994;6:79–93. [Google Scholar]
- 26.Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard D, et al. Squamous metaplasia amplifies pathologic epithelial–mesenchymal interactions in COPD patients. J Clin Invest. 2007;117:3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gohy ST, Hupin C, Fregimilicka C, Detry BR, Bouzin C, Gaide Chevronay H, Lecocq M, Weynand B, Ladjemi MZ, Pierreux CE, et al. Imprinting of the COPD airway epithelium for dedifferentiation and mesenchymal transition. Eur Respir J. 2015;45:1258–1272. doi: 10.1183/09031936.00135814. [DOI] [PubMed] [Google Scholar]
- 28.van der Strate BW, Postma DS, Brandsma CA, Melgert BN, Luinge MA, Geerlings M, Hylkema MN, van den Berg A, Timens W, Kerstjens HA. Cigarette smoke–induced emphysema: a role for the B cell? Am J Respir Crit Care Med. 2006;173:751–758. doi: 10.1164/rccm.200504-594OC. [DOI] [PubMed] [Google Scholar]
- 29.Kuwahara M, Yamashita M, Shinoda K, Tofukuji S, Onodera A, Shinnakasu R, Motohashi S, Hosokawa H, Tumes D, Iwamura C, et al. The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF-β and suppresses T(H)2 differentiation. Nat Immunol. 2012;13:778–786. doi: 10.1038/ni.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams DH, Hathaway M, Shaw J, Burnett D, Elias E, Strain AJ. Transforming growth factor-β induces human t lymphocyte migration in vitro. J Immunol. 1991;147:609–612. [PubMed] [Google Scholar]
- 31.Woodruff PG, Ellwanger A, Solon M, Cambier CJ, Pinkerton KE, Koth LL. Alveolar macrophage recruitment and activation by chronic second hand smoke exposure in mice. COPD. 2009;6:86–94. doi: 10.1080/15412550902751738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valença SS, da Hora K, Castro P, Moraes VG, Carvalho L, Porto LC. Emphysema and metalloelastase expression in mouse lung induced by cigarette smoke. Toxicol Pathol. 2004;32:351–356. doi: 10.1080/01926230490431466. [DOI] [PubMed] [Google Scholar]
- 33.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke–induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 34.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin α(v)β6–mediated TGF-β activation causes Mmp12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 35.Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-β–mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- 36.Feinberg MW, Jain MK, Werner F, Sibinga NE, Wiesel P, Wang H, Topper JN, Perrella MA, Lee ME. Transforming growth factor-β 1 inhibits cytokine-mediated induction of human metalloelastase in macrophages. J Biol Chem. 2000;275:25766–25773. doi: 10.1074/jbc.M002664200. [DOI] [PubMed] [Google Scholar]
- 37.Reddy ST, Gilbert RS, Xie W, Luner S, Herschman HR. TGF-β1 inhibits both endotoxin-induced prostaglandin synthesis and expression of the TIS10/prostaglandin synthase 2 gene in murine macrophages. J Leukoc Biol. 1994;55:192–200. doi: 10.1002/jlb.55.2.192. [DOI] [PubMed] [Google Scholar]
- 38.Ding A, Nathan CF, Graycar J, Derynck R, Stuehr DJ, Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990;145:940–944. [PubMed] [Google Scholar]
- 39.Yu L, Border WA, Huang Y, Noble NA. TGF-β isoforms in renal fibrogenesis. Kidney Int. 2003;64:844–856. doi: 10.1046/j.1523-1755.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- 40.Kalter VG, Brody AR. Receptors for transforming growth factor-β (TGF-β) on rat lung fibroblasts have higher affinity for TGF-β 1 than for TGF-β 2. Am J Respir Cell Mol Biol. 1991;4:397–407. doi: 10.1165/ajrcmb/4.5.397. [DOI] [PubMed] [Google Scholar]
- 41.Gosselink JV, Hayashi S, Elliott WM, Xing L, Chan B, Yang L, Wright C, Sin D, Paré PD, Pierce JA, et al. Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:1329–1335. doi: 10.1164/rccm.200812-1902OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell JD, McDonough JE, Zeskind JE, Hackett TL, Pechkovsky DV, Brandsma CA, Suzuki M, Gosselink JV, Liu G, Alekseyev YO, et al. A gene expression signature of emphysema-related lung destruction and its reversal by the tripeptide GHK. Genome Med. 2012;4:67. doi: 10.1186/gm367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonello MR, Khachigian LM. Fibroblast growth factor-2 represses platelet-derived growth factor receptor-α (PDGFR-α) transcription via ERK1/2-dependent Sp1 phosphorylation and an atypical cis-acting element in the proximal PDGFR-α promoter. J Biol Chem. 2004;279:2377–2382. doi: 10.1074/jbc.M308254200. [DOI] [PubMed] [Google Scholar]
- 44.Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, Zhang Y, Zhang L, Yuan ZR, Tan HS, et al. Granulocyte-macrophage colony–stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16:126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 45.Hattori Y, Yamamoto S, Matsuda N. Sympathetic control of VEGF angiogenic signaling: dual regulations by α2-adrenoceptor activation? Circ Res. 2007;101:642–644. doi: 10.1161/CIRCRESAHA.107.161855. [DOI] [PubMed] [Google Scholar]
- 46.Gao X, Li J, Huang H, Li X. Connective tissue growth factor stimulates renal cortical myofibroblast-like cell proliferation and matrix protein production. Wound Repair Regen. 2008;16:408–415. doi: 10.1111/j.1524-475X.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- 47.Gómez-Palacio-Schjetnan A, Escobar ML. Neurotrophins and synaptic plasticity. Curr Top Behav Neurosci. 2013;15:117–136. doi: 10.1007/7854_2012_231. [DOI] [PubMed] [Google Scholar]
- 48.Galvan V, Logvinova A, Sperandio S, Ichijo H, Bredesen DE. Type 1 insulin-like growth factor receptor (IGF-IR) signaling inhibits apoptosis signal-regulating kinase 1 (ASK1) J Biol Chem. 2003;278:13325–13332. doi: 10.1074/jbc.M211398200. [DOI] [PubMed] [Google Scholar]
- 49.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34(34) suppl:50s–59s. [PubMed] [Google Scholar]