Abstract
Lung epithelial cells play critical roles in initiating and modulating immune responses during pulmonary infection or injury. To better understand the spectrum of immune response–related proteins present in lung epithelial cells, we developed an improved method of isolating highly pure primary murine alveolar type (AT) II cells and murine tracheal epithelial cells (mTECs) using negative selection for a variety of lineage markers and positive selection for epithelial cell adhesion molecule (EpCAM), a pan-epithelial cell marker. This method yielded 2–3 × 106 ATII cells/mouse lung and 1–2 × 104 mTECs/trachea that were highly pure (>98%) and viable (>98%). Using these preparations, we found that both ATII cells and mTECs expressed the Lyn tyrosine kinase, which is best studied as an inhibitory kinase in hematopoietic cells. However, we found little or no expression of Syk in either ATII cells or mTECs, which is in contrast to earlier published reports. Both cell types expressed C-type lectin receptors, anaphylatoxin receptors, and various Toll-like receptors (TLRs). In addition, stimulation of ATII cells with TLR ligands led to secretion of various cytokines and chemokines. Interestingly, lyn−/− ATII cells were hyperresponsive to TLR3 stimulation, suggesting that, as in hematopoietic cells, Lyn might be playing an inhibitory role in ATII cells. In conclusion, the improved isolation method reported here, along with expression profiles of various immune defense proteins, will help refocus investigations of immune-related signaling events in pulmonary epithelium.
Keywords: lung epithelial cells, Syk, Lyn, tyrosine kinases, Toll-like receptors
Clinical Relevance
Lung epithelium is the first barrier to defense against pathogens and allergens. This report defines the repertoire of immune regulatory molecules present in alveolar epithelial type II cells and murine tracheal epithelial cells, isolated by an improved cell-sorting method.
The large internal surface of the lungs is constantly exposed to a wide variety of micro-organisms, some of which are potentially pathogenic. Although an immune response in the lungs is required to keep such pathogen colonization in check, the levels of inflammation must be tightly regulated to prevent host tissue damage and consequent reduction in efficient respiration.
The epithelial cells lining the inner tracheal and lung surfaces are among the first cells to encounter incoming pathogens; hence, they play important roles in initiating and regulating the ensuing immune response in the lung (1). The trachea and conducting airways are lined by club cells (previously known as “Clara” cells) and ciliated cells, which are armed with several defense mechanisms (2, 3). Although club cells secrete the major components of the airway mucus layer, which capture many of the micro-organisms coming in, the ciliated cells keep the mucus moving upward and outward in what is often referred to as the large airway mucociliary “escalator” (4, 5). Club cells also produce and secrete several broad acting antimicrobial proteins and peptides into the mucus layer, which provide a first line of host defense (6).
The alveoli are lined by alveolar type (AT) I and ATII cells, which perform distinct functions (7, 8). ATI cells are highly flattened to allow gas exchange across their cell bodies into the blood vessels. They are also thought to regulate alveolar fluid levels. However, few immune functions have been ascribed to ATI cells. ATII cells are more numerous than ATI cells, and are known for their roles in surfactant production and secretion, for serving as distal progenitors for ATI cell development, and in immune responses within the alveoli. However, much remains unknown about the immune functions of ATII cells and the pathways involved therein.
Given our laboratory’s long-standing interest in studying immune signaling pathways in hematopoietic cells, we were intrigued by reports of the expression of spleen tyrosine kinase (Syk) in both human and murine lung epithelial cells (9–11). We attempted to confirm the presence of Syk in mouse lung epithelial cells using highly purified cell preparations, rather than whole lung tissue, which is a highly vascular organ containing hematopoietic cells expressing high levels of Syk that could ultimately yield false-positive results. To obtain highly pure lung epithelial cell preparations, we developed a cell-sorting strategy that is an improvement over existing methods that yielded preparations contaminated with mesenchymal cells, endothelial cells, and erythroid cells among others (12–14). Using our improved cell-sorting method, gene expression profiling for Syk and related immune defense molecules could be reliably performed. In contrast to earlier reports (9–11), we found no expression of Syk protein in ATII cells, and only very faint mRNA signal in both ATII cells and murine tracheal epithelial cells (mTECs). In addition, we found expression of some members of the other families of related immune defense proteins, such as Lyn kinase, which opens up exciting avenues for further exploring the function of those proteins in lung epithelial cells both in vitro and in vivo. We also found that specific ligand-mediated stimulation of the Toll-like receptors (TLRs) found to be expressed in ATII cells led to a cytokine and chemokine response. Finally, Lyn was shown to mediate inhibition of chemokine production downstream of TLR3 stimulation, providing clues into regulatory networks involved in pulmonary immune defense mechanisms.
Materials and Methods
Mice, Reagents, and Antibodies
Wild-type (WT) C57BL/6 mice (Charles River Laboratories, Hollister, CA), lyn−/− mice (15) aged 8–10 weeks, and syk−/− bone marrow chimeric mice (16, 17) aged 16–18 weeks were maintained in a specific pathogen-free facility at the University of California, San Francisco. For reagents and antibodies used to isolate lung epithelial cells, please refer to the online supplement.
Isolation of Highly Pure Murine ATII Cells
Fluorescence-activated cell sorting (FACS) was used to obtain highly pure ATII cells. Lineage (Lin) markers CD45, CD16/32, CD31, Ter119, and integrin β4 were used to deplete hematopoietic cells, endothelial cells, erythroid cells, distal lung progenitor cells, and club cells (18), respectively. Epithelial cell adhesion molecule (EpCAM) was used to positively select for ATII cells. Full details are provided in the online supplement.
Isolation of Highly Pure mTECs by FACS Sorting
mTECs were isolated similar to ATII cells. Please refer to the online supplement for full details.
Surface and Intracellular Staining for Flow Cytometric Analysis
Methods for staining for cell surface proteins (Lin markers, EpCAM, major histocompatibility antigen [MHC] II, or isotype controls), intracellular proteins (pro-surfactant protein [SP]-C, club cell secretory protein [CCSP], pancytokeratin, cytokeratin-8, or rabbit IgG), or determining intracellular alkaline phosphatase enzymatic activity are described in detail in the online supplement.
Immunofluorescent Staining and Microscopy
Methods used for immunofluorescent staining and microscopic analysis of cells cytospun onto slides for pro–SP-C, CCSP, pancytokeratin, cytokeratin-8, MHCII, E-cadherin, Syk, and Lyn are described in detail in the online supplement.
RNA Isolation and RT-PCR
RNA isolation methods and RT-PCR detection of various immune-related proteins in sorted ATII cells and mTECs are described in detail in the online supplement. Primer pairs used for RT-PCR analysis are listed in Table 1.
Table 1.
RT-PCR Primer Pairs
| Gene | Forward Primer | Reverse Primer | Product (bp) | References |
|---|---|---|---|---|
| Src | 5′ GACTCCATCCAGGCTG 3′ | 3′ TTGCACACCAGGTTCTC 5′ | 250 | 47 |
| Yes | 5′ TGGCATGGCGTATATTGAAA 3′ | 3′ CAGGATCCTTCTTCCAGCAA 5′ | 400 | N/A* |
| Fyn | 5′ ATGGGCTGTGTGCAATGTAAGG 3′ | 5′ TTCCGTCCGTGCTTCATAGT 3′ | 294 | N/A* |
| Lck | 5′ GCACCAGAAGCCATTAACTATG 3′ | 5′ GGCTGTGTGAAGAAGTCATCCAGAAC 3′ | 279 | 49 |
| Hck | 5′ TAGCCCGCAAGTCTTCGTCG 3′ | 5′ CGGTGAATAGCCTCATAGTCGTA 3′ | 339 | 50 |
| Lyn | 5′ ATGCATCAGTCCCAAACCTC 3′ | 5′ GACCAGGACGTTAGCAGCTC 3′ | 451 | N/A* |
| Blk | 5′ AACCCTGAGGTCATCCGTAGC 3′ | 5′ CACCATTCTTCCCTGATTCTGC 3′ | 317 | 50 |
| Fgr | 5′ TAAGATCCGAAAGCTGGACACG 3′ | 5′ CGACACCACCGCATACAGC 3′ | 385 | 50 |
| Zap-70 | 5′ GCACATATGCACTGTCCCTGGTCTA 3′ | 5′ GGGTCGCTGTAGGGACTCTCGTACA 3′ | 350 | 51 |
| Syk | 5′ GTGGCTGTGAAAATCCTGAAG 3′ | 5′ GAAATCGCTGATCTTGGCATA 3′ | 345 | N/A* |
| C3aR | 5′ TAACCAGATGAGCACCACCA 3′ | 5′ TGTGAATGTTGTGTGCATGG 3′ | 280 | 52 |
| C5aR | 5′ GAAGCGGCAACCTGGGGATGT 3′ | 5′ CGTCTGGCTCGAAGGCTGTCAC 3′ | 150 | 53 |
| Dectin-1 | 5′ ATCAGCATTCTTCCCCAACTCG 3′ | 5′ CAGTTCCTTCTCACAGATACTGTATGA 3′ | 280 | 54 |
| Dectin-2 | 5′ GGGGGCTCATCTGGTGGTG 3′ | 5′ ATGCTCCCTGGCTTGCTCTTC 3′ | 631 | 55 |
| TLR1 | 5′ ATCGGTTTGGAACTGTCTAA 3′ | 5′ GAAATGGGCTAACTTGGGACG 3′ | 412 | 56 |
| TLR2 | 5′ GAGCGAGCTGGGTAAAGTAGAAA 3′ | 5′ AGCCGAGGCAAGAACAAAGA 3′ | 528 | 56 |
| TLR3 | 5′ TCTCTGGGCTGAAGTGGACAA 3′ | 5′ AGCAAGGGAGAATGAGCAAGTGAC 3′ | 427 | 56 |
| TLR4 | 5′ CAGTGGGTCAAGGAACAGAAGC 3′ | 5′ GACAATGAAGATGATGCCAGAGC 3′ | 540 | 56 |
| TLR5 | 5′ CGGCCTCTGTTGGGATGTT 3′ | 5′ GACCGCATGGCTTCCTCTTC 3′ | 438 | 56 |
| TLR6 | 5′ GAAGCATGACCCCGTTCTCTAAT 3′ | 5′ AGGTTGCCAAATTCCTTACACAC 3′ | 367 | 56 |
| TLR7 | 5′ GGGGTCCAAAGCCAATGTGT 3′ | 5′ CGAGGGCAATTTCCACTTAGG 3′ | 471 | 56 |
| TLR8 | 5′ AATGGCATTTACACCCTCACAGA 3′ | 5′ AGCCAGCTTCGAAAATCACTTATG 3′ | 581 | 56 |
| TLR9 | 5′ ACAGTATCGTCTCTGTGGTC 3′ | 5′ CAGAGATGGTGCAGTATAGG 3′ | 348 | 56 |
| Collagen 1 | 5′ TTCTCCTGGCAAAGACGGACTCAA 3′ | 5′ AGGAAGCTGAAGTCATAACCGCCA 3′ | 350 | 57 |
| Vimentin | 5′ CAGCAGTATGAAAGCGTGG 3′ | 5′ GGAAGAAAAGGTTGGCAGAG 3′ | 441 | 58 |
Definition of abbreviation: TLR, Toll-like receptor.
Listed are the sequences of the forward and reverse primers used for all the RT-PCRs performed in the study. Also given are the sizes of the amplified PCR product and the references for the sequences.
Self-designed primer sequence. For Fyn, reverse primer was self-designed.
Western Blot
Methods used to analyze Lyn and Syk protein expression by immunoblotting are described in detail in the online supplement.
ATII Cell Culture and Stimulation by TLR Ligands
Purified ATII cells were cultured on Matrigel and 1.5% collagen I (BD Biosciences, San Jose, CA) matrix mix in small airway growth medium (Lonza, Walkersville, MD) with or without TLR ligands. Supernatant was collected after 24 hours and analyzed for cytokines and chemokines by multiplex Luminex bead assays (Bio-Rad, Hercules, CA). Please see the online supplement for details.
Statistical Analysis
The statistical analysis is detailed in the online supplement.
Results
Flow-Based Cell Sorting Strategy to Isolate Highly Pure ATII Cells and mTECs
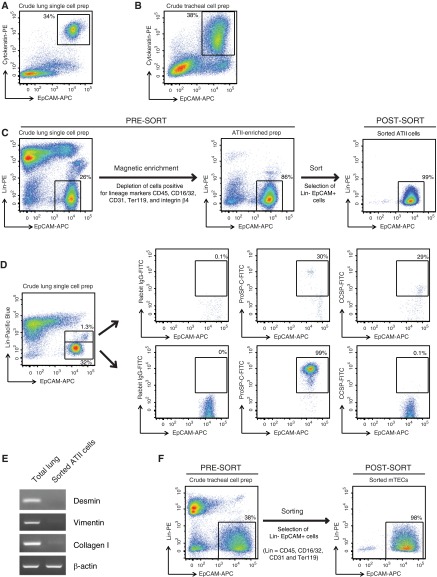
To reliably analyze expression of Syk and other related immune defense proteins in mouse primary lung epithelial cells, an improved FACS strategy was designed to isolate highly pure ATII cells and mTECs. To be able to sort live cells, the lung epithelial cells were marked by surface staining for EpCAM, a known pan-epithelial marker (19). In dispase-digested, crude lung cell preparations (Figure 1A), and also in dispase-digested, crude tracheal cell preparations (Figure 1B), prepared as described in Materials and Methods, we confirmed that EpCAM indeed marked all the epithelial cells, instead of a subpopulation, by costaining the cells intracellularly for cytokeratin, another known pan-epithelial cell marker, and finding that all cytokeratin-positive cells were also EpCAM positive.
Figure 1.
Cell-sorting strategy for isolating highly pure mouse alveolar type (AT) II cells and murine tracheal epithelial cells (mTECs). Representative flow cytometric dot plots of (A) a crude single-cell preparation of mouse lung cells or (B) tracheal cell preparations, stained on the surface for epithelial cell adhesion molecule (EpCAM)-allophycocyanin (APC) and intracellularly for cytokeratin-phycoerythrin (PE). (C) ATII cell–sorting strategy. Shown are representative dot plots obtained from flow cytometric analysis of lung cells collected at various stages of ATII cell isolation (pre- and post-magnetic enrichment, and post-sort) and stained for lineage (Lin) markers (CD45, CD16/32, CD31, Ter119, and integrin β4) versus EpCAM. Events displayed on the plots were 4′,6-diamidino-2-phenylindole–negative single cells. (D) Exclusion of club cell contamination during fluorescence-activated cell sorting for ATII cells. Representative flow cytometric dot plots of a crude single-cell preparation of lung cells stained for lineage markers (CD45, CD16/32, CD31, Ter119, and integrin β4) in the pacific blue channel and EpCAM-APC. The cells were also stained intracellularly for either rabbit isotype IgG, pro-surfactant protein (SP)-C or club cell secretory protein (CCSP), in the fluorescein isothiocyanate (FITC) channel and the distribution of this staining is shown in both the lower Lin− EpCAM+ gate, which was used to sort ATII cells, and the upper Linlow EpCAM+ gate. (E) RT-PCR analysis for the indicated genes was conducted on the highly pure, flow-sorted ATII cells, as described in Materials and Methods. Data shown are representative of three independent experiments. (F) Shown are flow cytometric dot plots from a representative cell sort performed for the isolation of highly pure mTECs.
Before ATII cell–sorting, the majority of the non-epithelial cells in dispase-digested, crude lung, single-cell preparations were magnetically depleted by staining for Lin markers CD45, CD16/32, CD31, Ter119, and integrin β4 to exclude hematopoietic cells, endothelial cells, erythroid cells, distal progenitor cells, and club cells, respectively (Figure 1C). Magnetic depletion of Lin+ cells lead to an enrichment of Lin− EpCAM+ cells from 25–30% to 80–90%. The enriched population was then subjected to flow-based cell sorting to produce a cellular isolate that was greater than 98% Lin− EpCAM+ (Figure 1C).
To validate that this sorting strategy resulted in minimal contamination of sorted ATII cell preparations by club cells, an abundant lung epithelial cell type present in mouse lungs, we performed intracellular staining for pro–SP-C, a specific ATII marker, and CCSP, which is specific for club cells. The Lin− EpCAM+ cells present in the sort gate stained for pro–SP-C (99%; Figure 1D, lower panels) and lacked CCSP expression. By contrast, the Linlow EpCAM+ cells contained a fraction of CCSP-expressing cells (29%; Figure 1D, top panels). The other cells in the Linlow EpCAM+ gate might include integrin β4–positive distal lung progenitor cells (18). Thus, by carefully gating out Linlow EpCAM+ cells, club cell contamination was avoided in the cell preparations.
The purity of the ATII epithelial cell preparation was also confirmed by RT-PCR analysis for a number of genes commonly expressed in mesenchymal cells. The purified ATII cells lacked expression of desmin, vimentin, and collagen type I (Figure 1E). Using these procedures, we routinely obtained 2–3 × 106 ATII cells/mouse lung. Cell viability was routinely 80–90% in crude lung preparations and greater than 98% after cell sorting.
A similar strategy was used to sort highly pure populations of mTECs (Figure 1F). However, because the total crude tracheal cell yield was small, a magnetic enrichment step was not performed before sorting. Dispase-digested crude tracheal cell preparations consisted of approximately 30–45% Lin− EpCAM+ mTECs. After sorting, the purity of the mTEC preparation was greater than 98%. The yield of purified mTECs was far less, averaging 1–2 × 104 cells/mouse trachea. Cell viability was routinely 75–80% in crude tracheal preparations and greater than 98% after cell sorting.
Purity and Further Characterization of Sorted ATII Cells
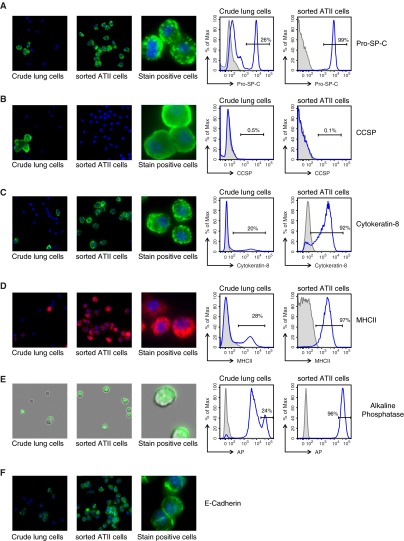
To assess purity, sorted Lin− EpCAM+ ATII cell preparations were examined for the expression of a number of proteins known to be present in ATII cells. Immunofluorescent staining of cytospun cells in addition to flow cytometric analysis allowed assessment of the cellular distribution of the markers and accurate quantitation. The purified ATII cells were routinely greater than 98% positive for pro–SP-C, which was found in intracellular vesicle-like structures, presumably lamellar bodies (Figure 2A). A small number of club cells, marked by positive staining for CCSP, were observed in multicell clumps in crude lung preparations (Figure 2B), but not in sorted ATII cell preparations. About 92% of sorted cells were also positive for cytokeratin-8 (Figure 2C), a marker commonly used to identify ATII cells. The immunostaining was distributed in foci under the plasma membrane, with finer filaments extending in the cytoplasm as reported previously (20). Interestingly, remarkably higher levels of cytokeratin-8 expression were observed in Linlow EpCAM+ cells and also in mTECs when compared with ATII cells (Figure E1A). Approximately 97% of sorted ATII cells were also positive for surface MHCII expression, as seen by surface staining and flow cytometric analysis. Immunofluorescent staining of cytospun ATII cells revealed that the majority of MHCII staining was localized in intracellular vesicles (Figure 2D), as previously described (21). Interestingly, the surface expression level of MHCII on ATII cells was slightly lower than that on Lin+ hematopoietic cells present in crude lung preparations (Figure E1B). Alkaline phosphatase, detected by its enzymatic activity on a chromogenic substrate, has also been used to stain for ATII cells (22). A highly sensitive fluorescent substrate-based flow cytometric analysis revealed that crude lung cell preparations contained a small fraction of cells that had no alkaline phosphatase activity, a large fraction that had intermediate activity, and approximately 26% cells that displayed high activity for alkaline phosphatase (Figure 2E). After cell sorting, approximately 96% of the cells displayed high alkaline phosphatase activity, as expected. The purified ATII cells also positively stained for E-cadherin, another well described epithelial cell protein found in tight junctions. As expected, the staining was found primarily along the plasma membrane (Figure 2F). These studies helped confirm the purity of the ATII cell isolations.
Figure 2.
Characterization of sorted Lin− EpCAM+ ATII cells. Shown are representative images from immunofluorescence microscopy of lung cells collected before magnetic enrichment and after sorting, cytospun, and stained for (A) Pro–SP-C, (B) CCSP, (C) cytokeratin-8, (D) major histocompatibility antigen (MHC) II, (E) alkaline phosphatase (AP), and (F) epithelial cadherin (E-cadherin). For quantitative analysis of stain-positive cells, representative histograms from flow cytometric analysis of lung cells collected pre-magnetic enrichment and post-sort are shown to the right. Percentages shown are representative of three to five independent experiments. Isotype controls are plotted in shaded gray; specific immunostains are shown in blue. In the case of AP staining, the shaded gray plot indicates unstained cells.
Expression of Syk Family Tyrosine Kinases in Alveolar Epithelial and Tracheal Epithelial Cells
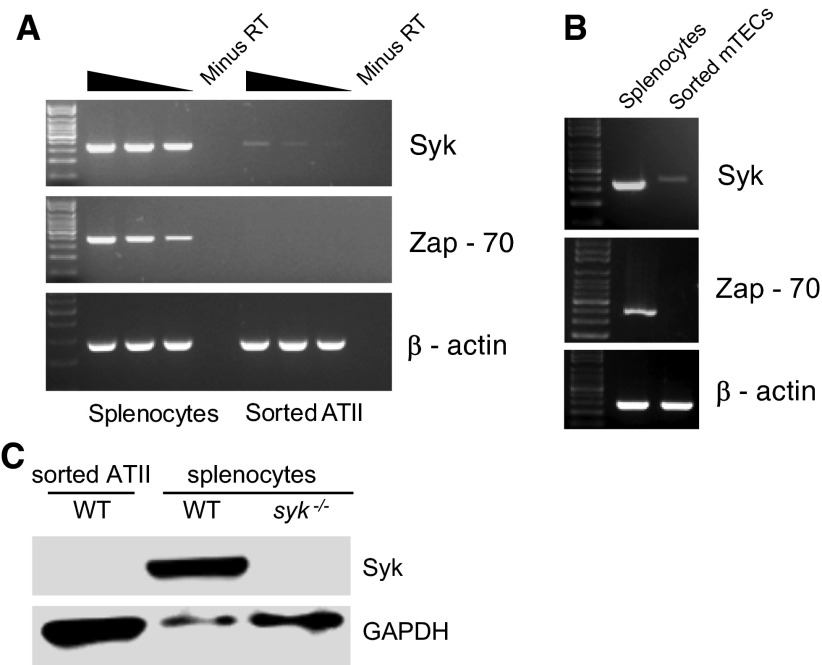
The Syk family of non-receptor protein tyrosine kinases is known to play significant roles in multiple immune signaling pathways in hematopoietic cells, and might be involved in immune responses mediated by lung epithelial cells (23). To analyze the expression of Syk and Zap-70, the two members of the Syk family tyrosine kinases, in ATII cells and mTECs, we performed RT-PCR using cDNA prepared from mRNA isolated from highly purified FACS sorted preparations of ATII cells and mTECs. For semiquantitative assessment of gene expression, ATII cDNA and positive control cDNA, synthesized from equal amounts of mRNA isolated from splenocytes, brain, or liver, were used either undiluted or at 1:3 and 1:9 dilutions for the various PCR reactions. Due to limited quantities of mTEC mRNA available, only a single concentration of cDNA was used for the PCR reactions. Positive control cDNA was used at the same concentration.
Of the two family members, very faint signal for Syk mRNA, but no expression of Zap-70 mRNA, was observed in both sorted ATII cells and mTECs (Figures 3A and 3B). Western blotting for Syk protein showed no expression in ATII cells (Figure 3C), even when ATII protein was loaded in excess compared with the splenocyte-positive control. Sorted and cytospun ATII cells also stained negative for Syk, whereas splenic B cells showed bright Syk staining (data not shown). Taken together, these data indicate that, in contrast to several previous reports (9–11), Syk family kinases are not expressed in either murine ATII cells or mTECs.
Figure 3.
Expression of Syk family kinases in sorted ATII cells and mTECs. RNA isolated from highly pure, flow-sorted ATII cells (A) and mTECs (B) was analyzed by RT-PCR, as described in Materials and Methods, to assess expression of Syk and Zap-70. RNA prepared from total splenocytes was used as a positive control. For analysis of ATII cells, undiluted, threefold, and ninefold dilutions of cDNA from ATII cells or positive control were used for each lane, as indicated by the gradient bar. For each PCR, a no–reverse transcriptase (minus RT) control was run alongside to demonstrate that the signal was not due to genomic DNA contamination. For analysis of mTECs, only a single cDNA concentration was tested, as RNA was limiting. As loading control, RT-PCR for β-actin was performed. Results shown are representative of three independent experiments. (C) Western blotting for Syk, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as loading control, was performed using sorted wild-type (WT) ATII cells, WT splenocytes, and syk−/− splenocytes harvested from spleens of syk−/− chimeric mice. WT ATII cell lysate was purposely overloaded to look for low-level Syk protein expression.
Expression of Src Family Tyrosine Kinases in Alveolar Epithelial and Tracheal Epithelial Cells
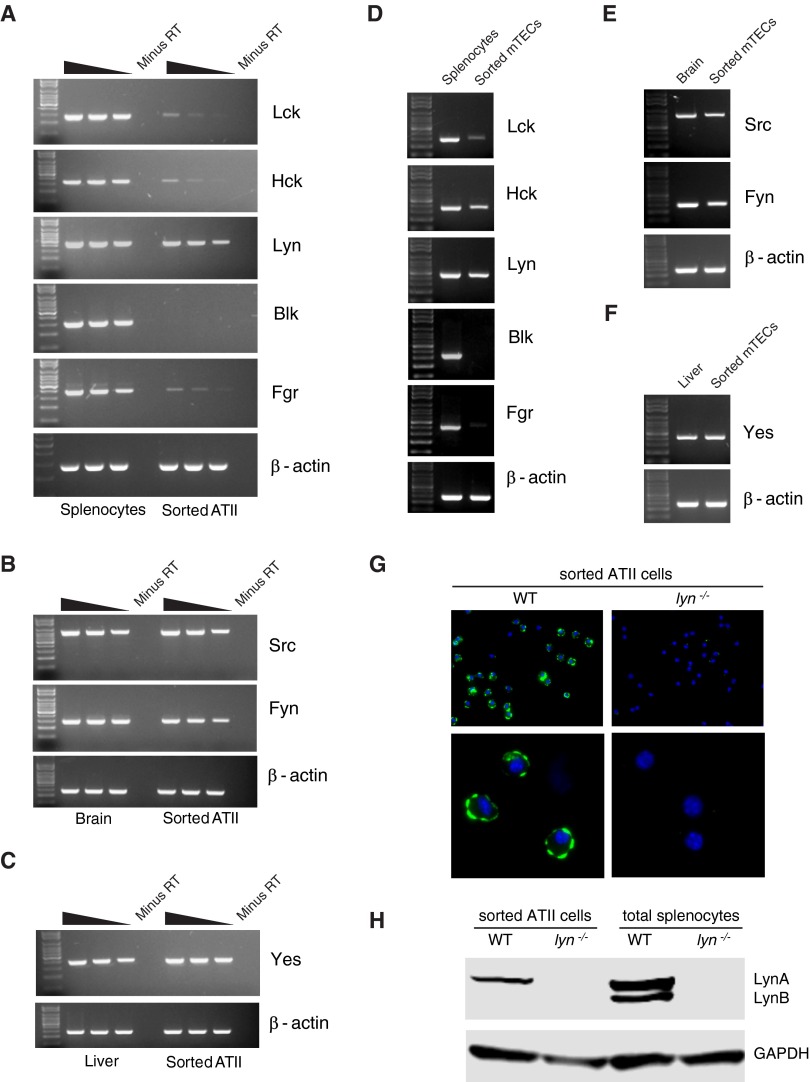
Next, we analyzed expression of the Src family of non-receptor tyrosine kinases that act upstream of the Syk family of tyrosine kinases and have multiple roles in physiological and pathological immune signaling (23, 24). Of the eight family members, Lck, Hck, Lyn, Blk, and Fgr are known to be predominantly expressed in hematopoietic cells. Of these, we observed surprisingly abundant expression of Lyn in both ATII cells and mTECs (Figures 4A and 4D). Low-level expression of Lck, Hck, and Fgr mRNA was also observed in both cell types (Figures 4A and 4D). No expression of Blk was observed in either cell type (Figures 4A and 4D). High-level expression of Src, Yes, and Fyn, which are known to be expressed more ubiquitously, was observed in both ATII cells and mTECs (Figures 4B, 4C, 4E, and 4F). In addition, we confirmed expression of Lyn in ATII cells at the protein level (Figures 4G and 4H). Cytospun preparations of highly pure, sorted ATII cells from WT mice, but not lyn−/− mice, stained for Lyn protein, which was found to be localized along the plasma membrane, likely within lipid rafts, as previously described in immune cells (25) (Figure 4G). Western blot analysis of total protein lysates prepared from sorted WT and lyn−/− ATII cells revealed expression of only the 56-kD LynA isoform in ATII cells, unlike splenocytes that expressed both the LynA (56 kD) and LynB (53 kD) isoforms. To our knowledge, Lyn protein has not been previously reported in murine ATII cells or mTECs.
Figure 4.
Expression of Src family kinases in sorted ATII cells and mTECs. Total RNA isolated from highly pure, flow-sorted ATII cells (A–C) or mTECs (D–F) was analyzed by RT-PCR for expression of all the members of the Src family kinases, as described in Materials and Methods. As positive controls, cDNA prepared from an equivalent amount of RNA from whole splenocytes, brain, or liver was used. Results shown are representative of three independent experiments. (G) Sorted ATII cells from WT and lyn−/− mice were cytospun and stained for Lyn protein, as described in Materials and Methods. (H) Western blotting for Lyn, with GADPH as loading control, was performed using WT and lyn−/−-sorted ATII cells and total splenocytes.
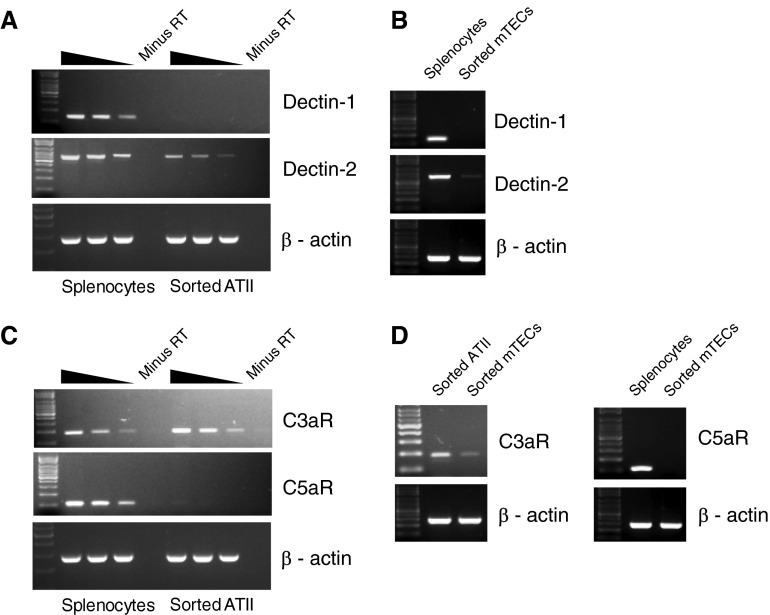
Expression of Dectin Receptors and Anaphylatoxin Receptors in Alveolar Epithelial and Tracheal Epithelial Cells
Sorted ATII cells and mTECs were analyzed for the expression of dectin-1 and dectin-2, which are C-type lectin receptors that bind fungal pattern recognition molecules and are involved in the first-line defense against fungal pathogens, such as Aspergillus fumigatus, a major causative agent of pulmonary invasive aspergillosis (26). We observed low-level expression of dectin-2 in both ATII cells and mTECs (Figures 5A and 5B). However, dectin-1 expression was not detectable for either of the cell types. We also analyzed the expression of the anaphylatoxin receptors, C3aR and C5aR, in ATII cells (Figure 5C) and mTECs (Figure 5D). Anaphylatoxin receptors are important mediators in the innate immune defense system, and are also known to modulate the phenotype of allergic and infectious lung diseases (27–30). Although C5aR expression has been reported in bronchial epithelial cells (28) by immunostaining, our RT-PCR analysis showed no expression of C5aR in either ATII cells or mTECs (Figures 5C and 5D). In contrast, high levels of expression of C3aR were observed in ATII cells (Figure 5C). C3aR was also expressed in mTECs (Figure 5D), albeit at lower levels compared with ATII cells. This is the first report of the expression of C3aR in murine ATII cells and mTECs, although C3aR expression in bronchial epithelial cells has been reported previously (28).
Figure 5.
Expression of immune receptors in ATII cells and mTECs. RNA isolated from highly pure, flow-sorted ATII cells (A and C) and mTECs (B and D) was analyzed by RT-PCR to assess expression of dectin-1 and dectin-2 (A and B), members of the C-type lectin family of receptors, and also for the expression of complement anaphylatoxin receptors C3aR and C5aR (C and D), as described earlier in Figure 3. cDNA prepared from equivalent amounts of RNA from whole splenocytes was used as positive control. Note that, for the C3aR PCR for mTECs, cDNA from ATII cells was used as positive control. Results shown are representative of three independent experiments.
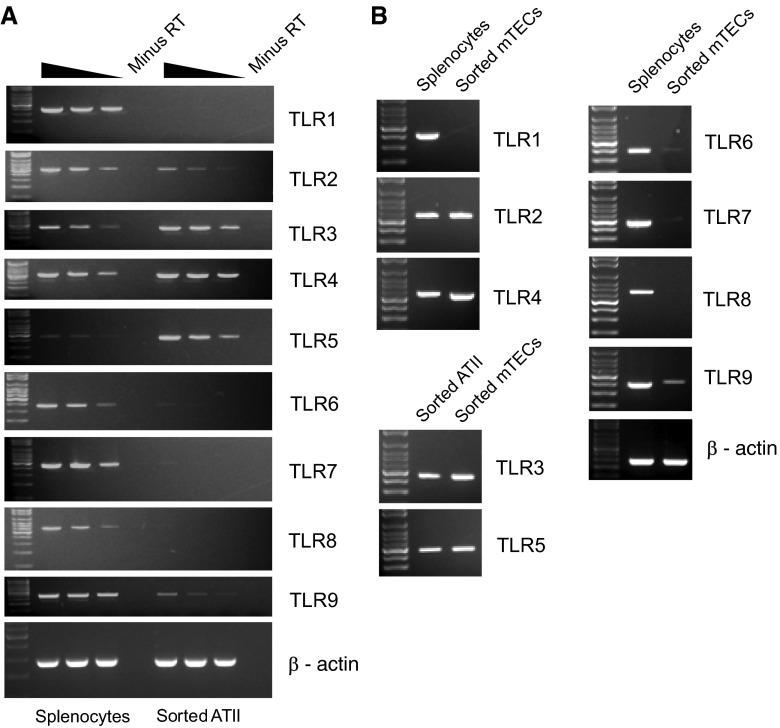
Expression of TLRs in Alveolar Epithelial and Tracheal Epithelial Cells
TLRs play critical roles in pathogen sensing and initiation of immune responses in hematopoietic cells (31). To determine if ATII cells and mTECs might also sense pathogens via TLRs, we examined the complete repertoire of TLR expression in these cell types by RT-PCR (Figure 6). Abundant expression of TLR3, -4, and -5 was observed in both ATII cells and mTECs. Low-level expression of TLR2 and TLR9 was observed in both ATIIs and mTECs.
Figure 6.
Expression of Toll-like receptors (TLRs) in sorted ATII cells and mTECs. RNA isolated from highly pure, flow-sorted ATII cells (A) and mTECs (B) was analyzed by RT-PCR to assess expression of TLRs 1–9, as described in Materials and Methods. RNA prepared from total splenocytes was used as a positive control, and RT-PCRs were conducted as described in Figure 3. Note that cDNA from ATII cells was used as positive control for TLR3 and TLR5 PCR for mTECs. Results shown are representative of three independent experiments.
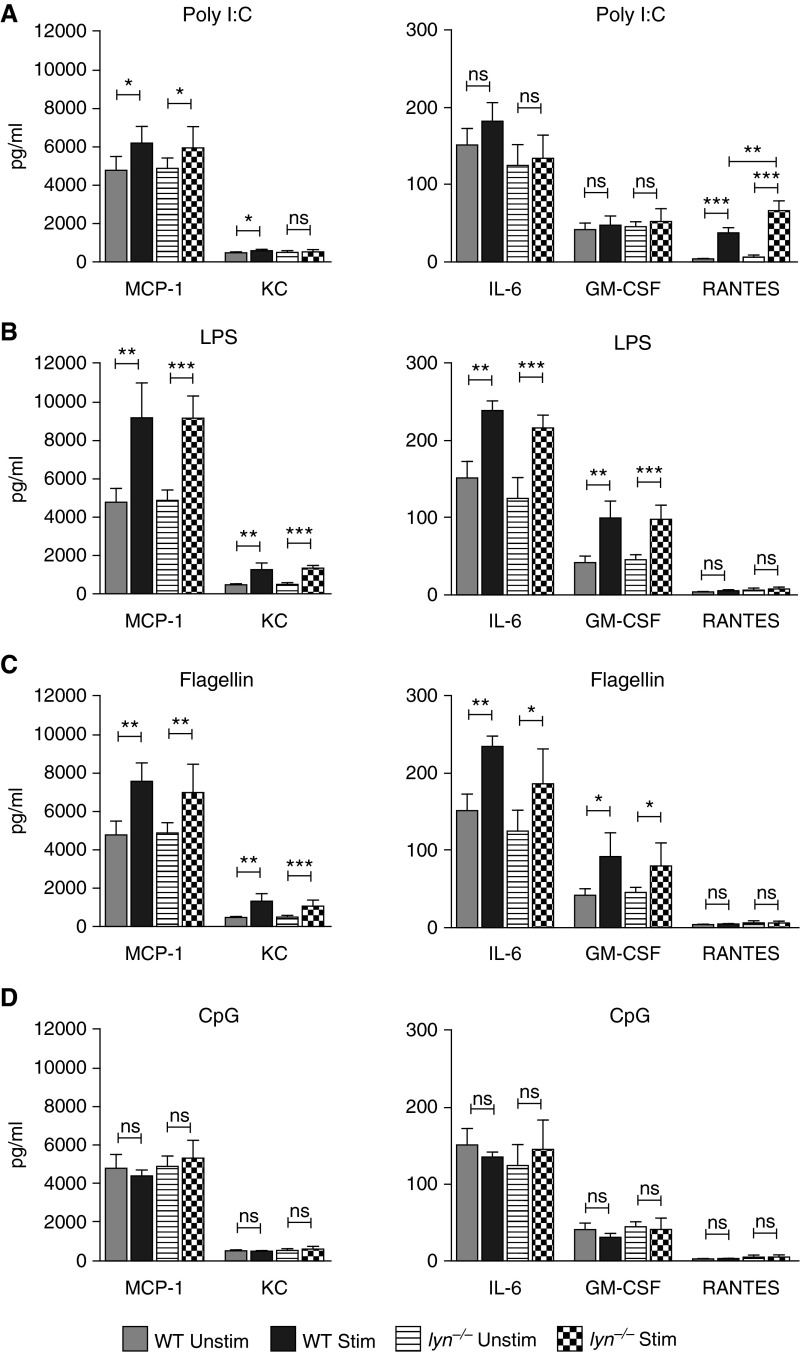
Cytokine and Chemokine Expression in Response to TLR Ligation
To validate that the TLR expression profile we observed for purified ATII cells correlated with functional responses, we stimulated purified ATII cells with TLR3 ligand polyinosinic:polycytidylic acid (poly I:C), TLR4 ligand LPS, TLR5 ligand flagellin, and TLR9 ligand CpG for 24 hours, then measured cytokine production in the cell culture supernatant using multiplexed Luminex cytokine and chemokine bead assays. Of the many cytokines and chemokines assayed (fibroblast growth factor [FGF]-basic, granulocyte/macrophage colony–stimulating factor [GM-CSF], IL-1β, IL-2, tumor necrosis factor [TNF]-α, IL-4, IL-6, macrophage inflammatory protein [MIP]-1α, IL-12 [p40/p70], IL-1α, interferon [IFN]-γ, IL-13, macrophage chemoattractant protein [MCP]-1, interferon γ-induced protein [IP]-10, monokine induced by interferon γ [MIG], keratinocyte-derived cytokine [KC], vascular endothelial growth factor [VEGF], regulated upon activation, normal T cell expressed and secreted [RANTES], IL-17, IL-10, IL-5, IL-25, and IL-33), most were undetectable both before and after stimulation. The TLR3 ligand poly I:C stimulated production of MCP-1 (CCL2) and KC (CXCL1) (Figure 7A). Interestingly, poly I:C was the only ligand that stimulated a significant amount of RANTES (CCL5) release. The TLR4 ligand, LPS, induced release of MCP-1, KC, IL-6, and GM-CSF in the cell culture supernatant (Figure 7B). Notably, compared with all the other stimulants used, LPS induced the highest levels of release of cytokines and chemokines in the cell supernatant. The TLR5 ligand, flagellin, stimulated production of MCP-1, KC, IL-6, and GM-CSF (Figure 7C). The TLR9 ligand, CpG, did not stimulate production of any of the cytokines or chemokines assayed (Figure 7D). Stimulation with the TLR2 ligand, Pam3CSK, also failed to stimulate production of cytokines/chemokines (data not shown). These data show that the TLRs found on highly purified primary ATII cells were active, although, in general, responses were much weaker than seen with macrophages or dendritic cells.
Figure 7.
TLR ligand stimulation–induced secretion of cytokines and chemokines by ATII cells purified from WT and lyn−/− mice. Equal numbers of purified WT or lyn−/− ATII cells were cultured on matrigel without or with TLR ligands, as described in Materials and Methods. Shown are the levels of macrophage chemoattractant protein (MCP)-1, keratinocyte-derived cytokine (KC), IL-6, granulocyte/macrophage colony–stimulating factor (GM-CSF), and regulated upon activation, normal T cell expressed and secreted (RANTES) measured in cell culture supernatant collected 24 h after no stimulation or stimulation either by (A) TLR3 ligand poly I:C (4.5 μg/ml), (B) TLR4 ligand LPS (0.5 μg/ml), (C) TLR5 ligand flagellin (5 μg/ml), or (D) TLR9 ligand CpG (0.5 μg/ml). Results shown are representative of four independent experiments. The values are plotted as mean (±SD); n = 4–8 per group. ns, not significant. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Given the role of Lyn kinase in modulating cytokine and chemokine production in response to TLR stimulation in myeloid cells (32), we were interested in examining if Lyn might be playing a similar role in lung epithelial cells. For this, we TLR-stimulated equal numbers of ATII cells purified from lyn−/− mice alongside ATII cells purified from WT mice (Figure 7). Of note, the purified ATII cells from lyn−/− mice were similar to WT ATII cells in their levels of surfactant production, as measured by intracellular pro–SP-C staining (see Figure E2 in the online supplement). Upon stimulation with poly I:C, lyn−/− ATII cells produced significantly higher levels of RANTES compared with poly I:C–stimulated WT ATII cells. These results suggest that Lyn might be playing an inhibitory role in alveolar epithelial RANTES production downstream of TLR3 signaling, similar to its function in hematopoietic cells.
Discussion
Using an improved cell isolation method that yielded highly pure cell preparations, we re-evaluated the expression of a number of immune-related molecules in lung ATII cells and mTECs. We were driven to develop an improved ATII isolation method, as we were unable to convincingly reproduce previous reports (9, 10), suggesting that the Syk kinase, which we have studied extensively in immune cells (17, 33, 34), is present in ATII cells. Because high-quality anti-mouse Syk antibodies do not exist, we believed that it was important to look for Syk expression at the mRNA level. To convincingly address this question, we needed to begin with highly pure epithelial cells preparations. Because existing lung epithelial isolation strategies do not yield purities greater than 85–90%, we developed an improved ATII and mTEC isolation method that yielded highly pure preparations (>98%). In contrast to reports based only on immunostaining or whole lung tissue Western blotting, we found very little Syk mRNA in ATII cells and mTECs and no Syk protein in ATII cells by immunoblotting. By contrast, we did find expression of Lyn mRNA in both ATII cells and mTECs, as well as LynA protein isoform in ATII cells. This was surprising, as Lyn is thought to be expressed primarily in hematopoietic cells. Lyn has been reported to be present in other epithelial cell types (35–38). Lyn is unique in that it initiates both activating and inhibitory signaling in hematopoietic cells, with the inhibitory signaling role being dominant (25). Macrophages and dendritic cells from lyn−/− display exaggerated signaling in response to various TLR ligands. Similarly, ATII cells from lyn−/− mice overproduced the RANTES chemokine in response to poly I:C stimulation, suggesting that Lyn may regulate similar TLR pathways in ATII cells. The unanticipated finding that Lyn is present in ATII cells will certainly lead to further experiments to define what intracellular signaling pathways are regulated by this kinase.
Human airway epithelial cells have been reported to express TLR1–6 and -9 (39). By contrast, our highly purified murine ATII cells only expressed TLR2–5 and low levels of TLR9. Stimulation of purified ATII cells with TLR3–5 and -9 ligands resulted in a modest production of a limited number of cytokines, which seemed less dramatic than past studies using less-pure cell populations (40). More recent studies with low-passage human airway epithelial cells suggest that two stimuli (IL-4 plus poly I:C) are required for maximal cytokine production (41). Obviously, further studies with highly purified ATII cells, correlating with in vivo findings, will be required to fully dissect the role of these cells in pulmonary inflammation.
Our improved airway epithelial isolation method combines dispase-mediated release of ATII cells (12, 13) with positive selection using the specific epithelial cell marker, EpCAM. Most methods for isolation of murine ATII cells are based on the original description by Corti and colleagues (12), which uses dispase digestion of lung tissue, followed by magnetic bead depletion of CD45+ and CD32+ cells, then culture of ATII cells on fibronectin-coated surfaces. Improvements to the purity of murine ATII isolation have included culture on Matrigel, which favors ATII survival and maintenance of ATII phenotype (13), or inclusion of additional antibodies and flow cell sorting for negative staining cells (42). Although murine ATII cells have been known to express EpCAM (43), the use of this as a positive selection marker, in combination with magnetic bead–based negative selection against other cell types in the lung, is not commonly done. The use of EpCAM as a positive selection marker in flow cell sorting has been reported for isolation of human ATII cells; however, this protocol required careful removal of ATI cells and other epithelial cell types, as initial cell preparations were from tissue biopsy specimens (44). Inclusion of an anti–integrin β4 antibody in the protocol allowed us to deplete epithelial cells that were not ATII cells. Because the existing methods for isolation of murine ATII cells yield preparations of approximately 85–90% pure cells, this method is a significant improvement (12). Alternative methods for isolation of pure murine ATII cells require use of transgenic green fluorescent protein (GFP) reporter mice driven by the ATII-specific SP-C promoter, and even then tend to yield heterogeneous cell populations from adult animals (45). Our method is also the first described method to obtain high-purity mTEC preparations fresh from the mouse trachea. Existing methods require extensive culturing of crude digests of trachea on air–liquid interface, where the gene expression pattern of the cultured cells might be different from that of the starting material (46).
This improved lung epithelial cell isolation strategy can also be used as an important tool for examining gene deletion frequency in lung epithelial–specific conditional knockouts, especially in cases where specific, high-quality antibodies to proteins of interest are not available. Studies of highly purified ATII cells and mTECs isolated from normal versus diseased lung using this method should also significantly improve our understanding of the role of the epithelium in pulmonary diseases.
Acknowledgments
Acknowledgments
The authors thank Vinh Nguyen and Mike Lee at the Flow Cytometry and Cell Sorting Core Facility at University of California, San Francisco (UCSF), DeLaine Larsen and Kurt Thorn at the Nikon Imaging Center at UCSF for technical assistance, and Harold A. Chapman and Bao Duong for helpful discussions and advice.
Footnotes
This work was supported by National Institutes of Health grants AI65495 and AI68150 (C.A.L.) and, in part, by the University of California, San Francisco, Program for Breakthrough Biomedical Research Postdoctoral Fellowship (M.S.).
Author Contributions: M.S. designed the study, performed the experiments, analyzed data, prepared figures, and drafted the manuscript; M.S. and C.A.L. edited and revised the manuscript; M.S. and C.A.L. approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0171OC on November 17, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- 2.Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2:403–411. doi: 10.1513/pats.200508-090JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler KB, Tuvim MJ, Dickey BF. Regulated mucin secretion from airway epithelial cells. Front Endocrinol (Lausanne) 2013;4:129. doi: 10.3389/fendo.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganesan S, Comstock AT, Sajjan US. Barrier function of airway tract epithelium. Tissue Barriers. 2013;1:e24997. doi: 10.4161/tisb.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. The airway epithelium: more than just a structural barrier. Ther Adv Respir Dis. 2011;5:255–273. doi: 10.1177/1753465810396539. [DOI] [PubMed] [Google Scholar]
- 7.Guillot L, Nathan N, Tabary O, Thouvenin G, Le Rouzic P, Corvol H, Amselem S, Clement A. Alveolar epithelial cells: master regulators of lung homeostasis. Int J Biochem Cell Biol. 2013;45:2568–2573. doi: 10.1016/j.biocel.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Herzog EL, Brody AR, Colby TV, Mason R, Williams MC. Knowns and unknowns of the alveolus. Proc Am Thorac Soc. 2008;5:778–782. doi: 10.1513/pats.200803-028HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duta F, Ulanova M, Seidel D, Puttagunta L, Musat-Marcu S, Harrod KS, Schreiber AD, Steinhoff U, Befus AD. Differential expression of spleen tyrosine kinase Syk isoforms in tissues: effects of the microbial flora. Histochem Cell Biol. 2006;126:495–505. doi: 10.1007/s00418-006-0188-z. [DOI] [PubMed] [Google Scholar]
- 10.Ulanova M, Puttagunta L, Marcet-Palacios M, Duszyk M, Steinhoff U, Duta F, Kim MK, Indik ZK, Schreiber AD, Befus AD. Syk tyrosine kinase participates in β1-integrin signaling and inflammatory responses in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L497–L507. doi: 10.1152/ajplung.00246.2004. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Lau C, Wiehler S, Pow A, Mazzulli T, Gutierrez C, Proud D, Chow CW. Syk is downstream of intercellular adhesion molecule-1 and mediates human rhinovirus activation of p38 MAPK in airway epithelial cells. J Immunol. 2006;177:6859–6870. doi: 10.4049/jimmunol.177.10.6859. [DOI] [PubMed] [Google Scholar]
- 12.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 1996;14:309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- 13.Rice WR, Conkright JJ, Na CL, Ikegami M, Shannon JM, Weaver TE. Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol. 2002;283:L256–L264. doi: 10.1152/ajplung.00302.2001. [DOI] [PubMed] [Google Scholar]
- 14.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, et al. Epithelial cell α3β1 integrin links β-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, Lowell CA. Myeloid cells, BAFF, and IFN-γ establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010;207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham MT, Abram CL, Hu Y, Lowell CA. Expression of the TEL-Syk fusion protein in hematopoietic stem cells leads to rapidly fatal myelofibrosis in mice. PLoS One. 2013;8:e77542. doi: 10.1371/journal.pone.0077542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott ER, Van Ziffle JA, Scapini P, Sullivan BM, Locksley RM, Lowell CA. Deletion of Syk in neutrophils prevents immune complex arthritis. J Immunol. 2011;187:4319–4330. doi: 10.4049/jimmunol.1100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev. 2012;38:68–75. doi: 10.1016/j.ctrv.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Traverso V, Morris JF, Flower RJ, Buckingham J. Lipocortin 1 (annexin 1) in patches associated with the membrane of a lung adenocarcinoma cell line and in the cell cytoplasm. J Cell Sci. 1998;111:1405–1418. doi: 10.1242/jcs.111.10.1405. [DOI] [PubMed] [Google Scholar]
- 21.Shen H, Liu C, Shao P, Yi L, Wang Y, Mills Ko E, Tian Z, Zhao X, Wang J, Xing L, et al. Enhanced phenotypic alterations of alveolar type II cells in response to Aflatoxin G1–induced lung inflammation. J Cell Physiol. 2015;230:1199–1211. doi: 10.1002/jcp.24852. [DOI] [PubMed] [Google Scholar]
- 22.Edelson JD, Shannon JM, Mason RJ. Alkaline phosphatase: a marker of alveolar type II cell differentiation. Am Rev Respir Dis. 1988;138:1268–1275. doi: 10.1164/ajrccm/138.5.1268. [DOI] [PubMed] [Google Scholar]
- 23.Bradshaw JM. The Src, Syk, and Tec family kinases: distinct types of molecular switches. Cell Signal. 2010;22:1175–1184. doi: 10.1016/j.cellsig.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Abram CL, Lowell CA. The diverse functions of Src family kinases in macrophages. Front Biosci. 2008;13:4426–4450. doi: 10.2741/3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scapini P, Pereira S, Zhang H, Lowell CA. Multiple roles of Lyn kinase in myeloid cell signaling and function. Immunol Rev. 2009;228:23–40. doi: 10.1111/j.1600-065X.2008.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vautier S, MacCallum DM, Brown GD. C-type lectin receptors and cytokines in fungal immunity. Cytokine. 2012;58:89–99. doi: 10.1016/j.cyto.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 27.Drouin SM, Corry DB, Hollman TJ, Kildsgaard J, Wetsel RA. Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2002;169:5926–5933. doi: 10.4049/jimmunol.169.10.5926. [DOI] [PubMed] [Google Scholar]
- 28.Drouin SM, Kildsgaard J, Haviland J, Zabner J, Jia HP, McCray PB, Jr, Tack BF, Wetsel RA. Expression of the complement anaphylatoxin C3a and C5a receptors on bronchial epithelial and smooth muscle cells in models of sepsis and asthma. J Immunol. 2001;166:2025–2032. doi: 10.4049/jimmunol.166.3.2025. [DOI] [PubMed] [Google Scholar]
- 29.Mueller-Ortiz SL, Hollmann TJ, Haviland DL, Wetsel RA. Ablation of the complement C3a anaphylatoxin receptor causes enhanced killing of Pseudomonas aeruginosa in a mouse model of pneumonia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L157–L165. doi: 10.1152/ajplung.00358.2005. [DOI] [PubMed] [Google Scholar]
- 30.Schmudde I, Laumonnier Y, Köhl J. Anaphylatoxins coordinate innate and adaptive immune responses in allergic asthma. Semin Immunol. 2013;25:2–11. doi: 10.1016/j.smim.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 32.Lamagna C, Scapini P, van Ziffle JA, DeFranco AL, Lowell CA. Hyperactivated MyD88 signaling in dendritic cells, through specific deletion of Lyn kinase, causes severe autoimmunity and inflammation. Proc Natl Acad Sci USA. 2013;110:E3311–E3320. doi: 10.1073/pnas.1300617110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mócsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine–based activation motifs. Nat Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Ziffle JA, Lowell CA. Neutrophil-specific deletion of Syk kinase results in reduced host defense to bacterial infection. Blood. 2009;114:4871–4882. doi: 10.1182/blood-2009-05-220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi YL, Bocanegra M, Kwon MJ, Shin YK, Nam SJ, Yang JH, Kao J, Godwin AK, Pollack JR. LYN is a mediator of epithelial–mesenchymal transition and a target of dasatinib in breast cancer. Cancer Res. 2010;70:2296–2306. doi: 10.1158/0008-5472.CAN-09-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han J, Zhang G, Welch EJ, Liang Y, Fu J, Vogel SM, Lowell CA, Du X, Cheresh DA, Malik AB, et al. A critical role for Lyn kinase in strengthening endothelial integrity and barrier function. Blood. 2013;122:4140–4149. doi: 10.1182/blood-2013-03-491423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lepanto P, Bryant DM, Rossello J, Datta A, Mostov KE, Kierbel A. Pseudomonas aeruginosa interacts with epithelial cells rapidly forming aggregates that are internalized by a Lyn-dependent mechanism. Cell Microbiol. 2011;13:1212–1222. doi: 10.1111/j.1462-5822.2011.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W, Ye Y, Li J, Li X, Zhou X, Tan D, Jin Y, Wu E, Cui Q, Wu M. Lyn regulates cytotoxicity in respiratory epithelial cells challenged by cigarette smoke extracts. Curr Mol Med. 2014;14:663–672. doi: 10.2174/1566524014666140603095027. [DOI] [PubMed] [Google Scholar]
- 39.Iwamura C, Nakayama T. Toll-like receptors in the respiratory system: their roles in inflammation. Curr Allergy Asthma Rep. 2008;8:7–13. doi: 10.1007/s11882-008-0003-0. [DOI] [PubMed] [Google Scholar]
- 40.Matsukura S, Kokubu F, Kurokawa M, Kawaguchi M, Ieki K, Kuga H, Odaka M, Suzuki S, Watanabe S, Takeuchi H, et al. Synthetic double-stranded RNA induces multiple genes related to inflammation through Toll-like receptor 3 depending on NF-κB and/or IRF-3 in airway epithelial cells. Clin Exp Allergy. 2006;36:1049–1062. doi: 10.1111/j.1365-2222.2006.02530.x. [DOI] [PubMed] [Google Scholar]
- 41.Herbert C, Zeng QX, Shanmugasundaram R, Garthwaite L, Oliver BG, Kumar RK. Response of airway epithelial cells to double-stranded RNA in an allergic environment. Transl Respir Med. 2014;2:11. doi: 10.1186/s40247-014-0011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gereke M, Autengruber A, Gröbe L, Jeron A, Bruder D, Stegemann-Koniszewski S. Flow cytometric isolation of primary murine type II alveolar epithelial cells for functional and molecular studies. J Vis Exp. 2012;(70):pii:4322. doi: 10.3791/4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujino N, Kubo H, Ota C, Suzuki T, Suzuki S, Yamada M, Takahashi T, He M, Suzuki T, Kondo T, et al. A novel method for isolating individual cellular components from the adult human distal lung. Am J Respir Cell Mol Biol. 2012;46:422–430. doi: 10.1165/rcmb.2011-0172OC. [DOI] [PubMed] [Google Scholar]
- 45.Lee JH, Kim J, Gludish D, Roach RR, Saunders AH, Barrios J, Woo AJ, Chen H, Conner DA, Fujiwara Y, et al. Surfactant protein-C chromatin-bound green fluorescence protein reporter mice reveal heterogeneity of surfactant protein C-expressing lung cells. Am J Respir Cell Mol Biol. 2013;48:288–298. doi: 10.1165/rcmb.2011-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.You Y, Brody SL. Culture and differentiation of mouse tracheal epithelial cells. Methods Mol Biol. 2013;945:123–143. doi: 10.1007/978-1-62703-125-7_9. [DOI] [PubMed] [Google Scholar]
- 47.Kumagai N, Ohno K, Tameshige R, Hoshijima M, Yogo K, Ishida N, Takeya T. Induction of mouse c-src in RAW264 cells is dependent on AP-1 and NF-κB and important for progression to multinucleated cell formation. Biochem Biophys Res Commun. 2004;325:758–768. doi: 10.1016/j.bbrc.2004.10.094. [DOI] [PubMed] [Google Scholar]
- 48.Picard C, Gilles A, Pontarotti P, Olive D, Collette Y. Cutting edge: recruitment of the ancestral fyn gene during emergence of the adaptive immune system. J Immunol. 2002;168:2595–2598. doi: 10.4049/jimmunol.168.6.2595. [DOI] [PubMed] [Google Scholar]
- 49.Omri B, Crisanti P, Marty MC, Alliot F, Fagard R, Molina T, Pessac B. The Lck tyrosine kinase is expressed in brain neurons. J Neurochem. 1996;67:1360–1364. doi: 10.1046/j.1471-4159.1996.67041360.x. [DOI] [PubMed] [Google Scholar]
- 50.Goupil S, La Salle S, Trasler JM, Bordeleau LJ, Leclerc P. Developmental expression of SRC-related tyrosine kinases in the mouse testis. J Androl. 2011;32:95–110. doi: 10.2164/jandrol.110.010462. [DOI] [PubMed] [Google Scholar]
- 51.Irla M, Saade M, Kissenpfennig A, Poulin LF, Leserman L, Marche PN, Jouvin-Marche E, Berger F, Nguyen C. ZAP-70 restoration in mice by in vivo thymic electroporation. PLoS One. 2008;3:e2059. doi: 10.1371/journal.pone.0002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bao L, Osawe I, Haas M, Quigg RJ. Signaling through up-regulated C3a receptor is key to the development of experimental lupus nephritis. J Immunol. 2005;175:1947–1955. doi: 10.4049/jimmunol.175.3.1947. [DOI] [PubMed] [Google Scholar]
- 53.Sun L, Guo RF, Gao H, Sarma JV, Zetoune FS, Ward PA. Attenuation of IgG immune complex–induced acute lung injury by silencing C5aR in lung epithelial cells. FASEB J. 2009;23:3808–3818. doi: 10.1096/fj.09-133694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.del Pilar Jiménez-A M, Viriyakosol S, Walls L, Datta SK, Kirkland T, Heinsbroek SE, Brown G, Fierer J. Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of dectin-1 (Clec7a) Genes Immun. 2008;9:338–348. doi: 10.1038/gene.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ariizumi K, Shen GL, Shikano S, Ritter R, III, Zukas P, Edelbaum D, Morita A, Takashima A. Cloning of a second dendritic cell–associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J Biol Chem. 2000;275:11957–11963. doi: 10.1074/jbc.275.16.11957. [DOI] [PubMed] [Google Scholar]
- 56.Kurt-Jones EA, Sandor F, Ortiz Y, Bowen GN, Counter SL, Wang TC, Finberg RW. Use of murine embryonic fibroblasts to define Toll-like receptor activation and specificity. J Endotoxin Res. 2004;10:419–424. doi: 10.1179/096805104225006516. [DOI] [PubMed] [Google Scholar]
- 57.Xi Y, Tan K, Brumwell AN, Chen SC, Kim YH, Kim TJ, Wei Y, Chapman HA. Inhibition of epithelial-to-mesenchymal transition and pulmonary fibrosis by methacycline. Am J Respir Cell Mol Biol. 2014;50:51–60. doi: 10.1165/rcmb.2013-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He D, Zhang D, Wei G, Lin T, Li X. Cytoskeleton vimentin disruption of mouse sertoli cells injured by nitrogen mustard in vitro. J Androl. 2007;28:389–396. doi: 10.2164/jandrol.106.000455. [DOI] [PubMed] [Google Scholar]