Abstract
Although microRNAs (miRs) have been well recognized to play an important role in the pathogenesis of organ fibrosis, there is a lack of evidence as to whether miRs directly regulate the differentiation of myofibroblasts, the putative effector cells during pathological fibrogenesis. In this study, we found that levels of miR-27a-3p were up-regulated in transforming growth factor-β1–treated human lung fibroblasts in a Smad2/3-dependent manner and in fibroblasts isolated from lungs of mice with experimental pulmonary fibrosis. However, both basal and transforming growth factor-β1–induced expression of miR-27a-3p were reduced in lung fibroblasts from patients with idiopathic pulmonary fibrosis compared with that from normal control subjects. Overexpression of miR-27a-3p inhibited, whereas knockdown of miR-27a-3p enhanced, the differentiation of lung fibroblasts into myofibroblasts. We found that miR-27a-3p directly targeted the phenotypic marker of myofibroblasts, α-smooth muscle actin, and two key Smad transcription factors, Smad2 and Smad4. More importantly, we found that therapeutic expression of miR-27a-3p in mouse lungs through lentiviral delivery diminished bleomycin-induced lung fibrosis. In conclusion, our data suggest that miR-27a-3p functions via a negative-feedback mechanism in inhibiting lung fibrosis. This study also indicates that targeting miR-27a-3p is a novel therapeutic approach to treat fibrotic organ disorders, including lung fibrosis.
Keywords: microRNA-27a-3p, α-smooth muscle actin, Smad, myofibroblast, lung fibrosis
Clinical Relevance
This study discovered that microRNA (miR)-27a-3p was a negative regulator of lung myofibroblast differentiation and pulmonary fibrosis. These findings indicate that miR-27a-3p is a novel target for developing miR replacement therapy to treat lung fibrosis.
Pulmonary fibrosis is a group of respiratory disorders characterized by relentless accumulation of extracellular matrix (ECM), which can lead to scarring and stiffening of lung tissue, loss of normal alveolar architecture, and ultimate disruption of gas exchange and lethal respiratory failure (1–4).
Idiopathic pulmonary fibrosis (IPF) is the most common and devastating form of lung fibrosis that has unclear etiology and limited efficacious treatments (3, 5). The pathogenesis of IPF is complex, involving multiple cell types and a variety of cellular and molecular mechanisms (2, 6–9). Among these, lung fibroblast differentiation into myofibroblast has been widely recognized to be one of the most critical events during the pathological development of this disease (5, 10, 11).
The regulation of myofibroblast differentiation has been extensively studied at the genetic and molecular levels (12, 13). A number of profibrotic growth factors, represented by transforming growth factor (TGF)-β1, have been identified to be the key mediators in this process. Upon binding to its receptors, TGF-β1 induces numerous transcriptional and post-transcriptional events, in Smad-dependent and -independent manners, to promote the production of ECM and expression of α-smooth muscle actin (α-SMA) and other phenotypic markers of myofibroblasts (14–17).
MicroRNAs (miRs) are a class of 20- to 25-nucleotide small, noncoding RNAs that regulate gene expression in post-transcriptional manners (18–21). There has been plenty of evidence suggesting that dysregulation of miRs contributes to pulmonary fibrosis (22–26). However, the role of miRs that directly regulate lung myofibroblast differentiation is still undergoing further characterization.
In this study, we found that miR-27a-3p functioned via a negative-feedback mechanism to diminish lung myofibroblast differentiation. More importantly, we found that miR-27a-3p therapeutically mitigated bleomycin-induced pulmonary fibrosis in mice. Our data indicate that miR-27a-3p is a novel target that can be exploited to treat organ fibrotic disorders, including lung fibrosis. Some of the results of this study have been previously reported in the form of an abstract (27).
Materials and Methods
Additional information is available in the online data supplement.
Cell Lines
Human HEK-293T and pulmonary fibroblast line, MRC-5, were purchased from American Type Culture Collection (Manassas, VA).
Experimental Pulmonary Fibrosis Model
C57BL/6 mice (8–10 wk old) were from NCI Frederick (Frederick, MD). Pulmonary fibrosis was induced by intratracheal instillation of bleomycin (1.5 U/kg body weight in 50 μl saline) as previously described (25). The animal protocol was approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Isolation of Primary Lung Fibroblasts
Lung tissues were cut into small pieces and incubated with digestion buffer (0.1% collagenase, 0.05% trypsin, and 100 μg/ml DNase in 1× Hanks’ balanced salt solution) for 1 hour at 37°C. The digested tissue suspensions were filtered through a 40-μm cell strainer and centrifuged at 500 × g for 5 minutes. Pellets were resuspended in Eagle’s minimum essential medium (MEM), followed by lymphocytes/macrophages depletion in CD16/32- and CD45-coated Petri dishes for 30 minutes at 37°C. Selection for fibroblasts was performed by adherence of the suspension for an additional 45 minutes on cell culture dishes. The adherent lung fibroblasts were cultured in MEM that contains 10% FBS, and cells at passage 3–5 were used for experiments. The protocol was approved by the University of Alabama at Birmingham Institutional Review Board.
Immunohistochemistry and Masson’s Trichrome Staining
Immunohistochemistry was performed as described in our previous studies (25). The intensity of α-SMA staining was determined by Image-Pro Plus version 6.0 software (Media Cybernetics, Rockville, MD). Masson’s trichrome staining was performed using Trichrome Stain (Masson) kit (Sigma-Aldrich, St. Louis, MO).
Fibroblast Contraction Assay
Lung fibroblasts were resuspended in MEM that contains 1.5 mg/ml rat tail collagen and then plated into a 48-well plate (250 μl) at a concentration of 3 × 105 cells/ml. After 30-minute incubation at 37°C, the collagen gels were freed by a spatula from the walls and bottoms of the wells and overlaid with culture media with or without TGF-β1 (2 ng/ml). Pictures were taken and diameters of the gels were measured 2 days after gel release.
Luciferase Reporter Assay
The 3′ untranslated regions (UTRs) that contain the putative miR-27a-3p binding sequences of human α-SMA, Smad2, and Smad4 were cloned into a luciferase reporter vector, pMIR-REPORT, respectively (Ambion, Grand Island, NY). Luciferase activity was measured by Promega luciferase assay system (Promega, Madison, WI).
Collagen Content Determination
The collagen contents in right lungs were determined by Sircol collagen assay, as previously described (25).
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation assays were performed as previously described (28). Details are provided in the online supplement.
Lentivirus Production and Intratracheal Delivery
The lentiviral constructs that express mouse miR-27a-3p were generated using lentiviral vector pCDH-CMV-MCS-EF1-copGFP (CD511B-1; System Biosciences, Mountain View, CA). Details for construct generation, lentivirus production, and intratracheal delivery are provided in the online supplement.
Statistical Analysis
The two-tailed Student’s t test was used for two-group analyses. One-way ANOVA followed by post hoc Bonferroni test was performed for multiple group comparisons. Results are expressed as mean (±SD), and a P value less than 0.05 was considered statistically significant.
Results
miR-27a-3p Is Induced by TGF-β1 in Human Lung Fibroblasts
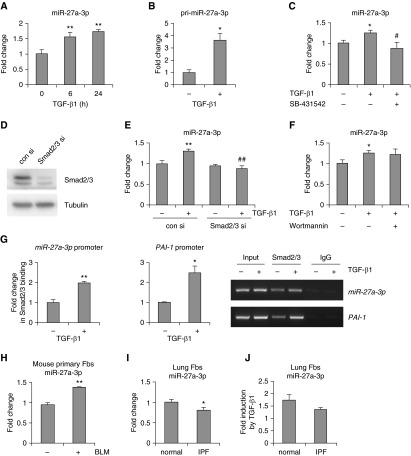
TGF-β1 has been well recognized to be one of the most important profibrotic mediators in IPF (12, 13, 16, 29). To identify TGF-β1–regulated miRs in lung fibroblasts, we previously performed miR arrays on RNAs isolated from untreated or TGF-β1–treated human lung fibroblasts (GSE43992) (30). We found that miR-27a-3p was significantly increased by TGF-β1. We performed real-time PCR assay and confirmed that miR-27a-3p was induced in TGF-β1–treated normal human lung fibroblasts (Figure 1A). In addition, the primary transcripts of miR-27a were also markedly increased by TGF-β1 (Figure 1B). We next demonstrated that TGF-β1–induced miR-27a-3p was mediated by type I TGF-β1 receptor, and the induction was dependent on Smad2/3, because either inhibitors against type I TGF-β1 receptor or specific Smad2/3 siRNAs attenuated the elevated expression of miR-27a-3p in TGF-β1–treated fibroblasts (Figures 1C–1E). In addition to activating the Smad transcriptional factors, TGF-β1 also affects Smad-independent pathways, such as the phosphoinositide 3-kinase/v-akt murine thymoma viral oncogene (PI3K/AKT) cascade (31). To determine if PI3K/AKT was also involved in miR-27a-3p induction by TGF-β1, we pretreated fibroblasts with the PI3K inhibitor, wortmannin, and found that wortamnnin had no effect on miR-27a-3p expression in TGF-β1–treated fibroblasts (Figure 1F). Next, we searched the promoter region of the human miR-27a gene and found multiple Smad-binding elements (AGAC) within 1,000 bp upstream of the transcription start site. To determine if Smads directly activated miR-27a-3p expression, we performed chromatin immunoprecipitation assays and confirmed that Smad2/3 bound to the miR-27a-3p promoter in TGF-β1–treated lung fibroblasts (Figure 1G). As a positive control, Smad2/3 also demonstrated greater binding to the PAI-1 promoter in TGF-β1–treated lung fibroblasts (Figure 1G). Altogether, these data suggest that the up-regulation of miR-27a-3p by TGF-β1 takes place at the transcriptional level in a Smad2/3-dependent manner.
Figure 1.
MicroRNA (miR)-27a-3p is induced by transforming growth factor (TGF)-β1 in human lung fibroblasts. (A and B) Human lung fibroblast line MRC-5 was treated with 2 ng/ml TGF-β1 for 0, 6, or 24 hours. RNA was isolated and levels of mature miR-27a-3p (A) and primary miR (pri-miR)-27a-3p (B) determined by real-time polymer chain reaction (PCR). (C) MRC-5 fibroblasts were pretreated with or without SB-431542 (10 μM) for 1 hour, followed by treatment with TGF-β1 (2 ng/ml) for 10 hours. RNA was isolated and levels of miR-27a-3p determined by real-time PCR. (D and E) MRC-5 fibroblasts were transfected with control siRNAs or siRNAs targeting Smad2 and Smad3 for 48 hours. Cells were then treated with TGF-β1 (2 ng/ml) for 10 hours. Protein levels of Smad2 and Smad3 were determined by Western blotting (D), and levels of miR-27a-3p were determined by real-time PCR (E). (F) MRC-5 cells were pretreated with or without wortmannin (1 μM) for 1 hour, followed by treatment with TGF-β1 (2 ng/ml) for 10 hours. RNA was isolated and levels of miR-27a-3p determined by real-time PCR. (A–F) n = 3; mean ± SD; *P < 0.05, **P < 0.01, compared with untreated control group; #P < 0.05, ##P < 0.01, compared with TGF-β1–treated control group. The experiments were performed two to three times with similar results. (G) MRC-5 fibroblasts were treated with or without TGF-β1 (2 ng/ml) for 6 hours. Cells were then fixed and lysed. Chromatin immunoprecipitation assays using either anti-Smad2/3 antibody or IgG were performed. The bindings of Smad2/3 to the Smad-binding element within the miR-27a promoter and the PAI-1 promoter were determined by real-time PCR, and representative gel images of PCR amplifications are shown. (H) Lung fibroblasts were isolated from control mice or mice that were exposed to intratracheal bleomycin for 14 days. Cells were cultured in Eagle’s minimum essential medium (MEM) with 10% FBS. At passage 2, levels of miR-27a-3p were determined by real-time PCR. miR-27a-3p levels were normalized to those in fibroblasts from control mice (n = 5, 5, respectively; mean ± SEM). **P < 0.01. (I) miR-27a-3p levels in normal lung fibroblasts and idiopathic pulmonary fibrosis (IPF) lung myofibroblasts were determined by real-time PCR (n = 6, 6, respectively; mean ± SEM). *P < 0.05 compared with the normal control group. (J) Normal lung fibroblasts and IPF lung myofibroblasts were treated with TGF-β1 (2 ng/ml) for 24 hours. Levels of miR-27a-3p were determined by real-time PCR (n = 3, 3, respectively; mean ± SEM). BLM, bleomycin; con si, control siRNA; Fbs, fibroblasts; PAI-1, plasminogen activator inhibitor-1; Smad2/3 si, Smad2/3 siRNA.
To further characterize the expression of miR-27a-3p in lung myofibroblasts, we compared the levels of miR-27a-3p in fibroblasts isolated from lungs of control normal mice and mice with experimental pulmonary fibrosis. As shown in Figure 1H, the expression of miR-27a-3p was significantly increased in lung fibroblasts from fibrotic lungs compared with that from normal mouse lungs. To investigate if miR-27a-3p was also up-regulated in pulmonary fibroblasts from other types of lung injuries, we examined its expression in fibroblasts from lungs of LPS-treated mice and found that miR-27a-3p levels were increased in these cells (see Figure E1A in the online supplement). The higher expression of miR-27a-3p in lung fibroblasts of LPS-treated mice was unlikely caused by LPS-activated signaling events in these cells, because LPS did not stimulate miR-27a-3p expression in lung fibroblasts (Figure E1B). Taken together, these data suggest that miR-27a-3p induction in fibroblasts from bleomycin- and LPS-exposed lungs most likely results from activation of common pathways in response to bleomycin and LPS treatments, such as up-regulation of TGF-β1 signaling events (32, 33).
Next, we assessed miR-27a-3p levels in lung fibroblasts from patients with IPF and normal control subjects. As shown in Figure 1I, miR-27a-3p displayed reduced expression in IPF myofibroblasts compared with normal control subjects. Furthermore, TGF-β1–induced miR-27a-3p in IPF myofibroblasts was less than that in normal lung fibroblasts (Figure 1J). These data suggest that the negative-feedback mechanism that suppresses overreactive fibrogenesis in normal lungs is defective in the lungs of patients with IPF.
miR-27a-3p Negatively Regulates TGF-β1–Induced Myofibroblast Differentiation
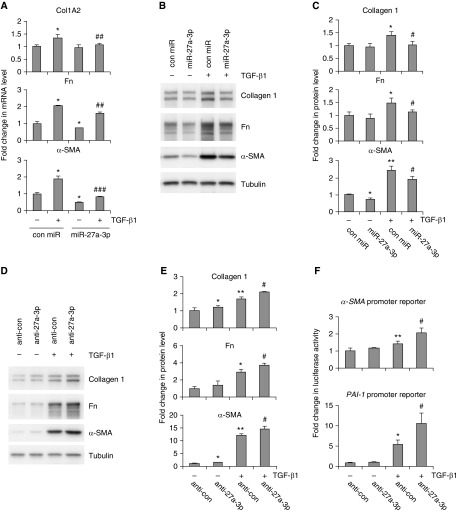
TGF-β1 promotes pulmonary fibrosis, in part, by inducing fibroblast differentiation into myofibroblast (12–14, 17). Given that TGF-β1–induced miR-27a-3p expression in lung fibroblasts, we next determined if miR-27a-3p regulated TGF-β1–induced myofibroblast differentiation. To experiment, we transfected human lung fibroblasts with control mimics or mimics for miR-27a-3p, followed by treatment with TGF-β1. As shown in Figures 2A–2C, TGF-β1 induced lung fibroblast differentiation into myofibroblasts, as demonstrated by enhanced expression of type 1 collagens, fibronectin, and α-SMA. More importantly, overexpression of miR-27a-3p significantly inhibited the expression of these phenotypic markers in TGF-β1–treated cells.
Figure 2.
miR-27a-3p inhibits TGF-β1–induced myofibroblast activation. (A) Human lung fibroblasts MRC-5 were transfected with 50 nM control mimics or mimics for miR-27a-3p. At 2 days after transfection, cells were starved in MEM containing 0.1% FBS overnight. The cells were then treated with 2 ng/ml TGF-β1 for 24 hours. RNA was isolated and levels of collagen type I α2 (Col1A2), fibronectin (Fn), and α-smooth muscle actin (α-SMA) determined by real-time PCR (n = 3; mean ± SD). *P < 0.05 compared with the untreated control miR group. ##P < 0.01, ###P < 0.001 compared with the TGF-β1–treated con miR group. (B and C) The experiments were performed as in A. Protein levels of collagen 1, Fn, and α-SMA were determined by Western blotting (B), and densitometric analyses were performed using ImageJ software (National Institutes of Health, Bethesda, MD) (C). (D and E) MRC-5 cells were transfected with 50 nM control inhibitors or inhibitors against miR-27a-3p. The cells were then treated as in B. Levels of collagen 1, Fn and α-SMA were determined by Western blotting and densitometric analyses performed using ImageJ. (F) MRC-5 cells were transfected with human α-SMA promoter or PAI-1 promoter reporter constructs, followed by transfection with 50 nM control inhibitors or miR-27a-3p inhibitors. After starvation, cells were treated with or without 2 ng/ml TGF-β1 for 24 hours, and luciferase activity in the cells was measured (n = 3; mean ± SD). *P < 0.05, **P < 0.01, compared with untreated control inhibitor group; #P < 0.05 compared with TGF-β1–treated control inhibitor group. The experiments were performed three times with similar results.
In the following experiments, we tested if miR-27a-3p blockage had an effect opposite to miR-27a-3p overexpression on TGF-β1–induced myofibroblast differentiation. We transfected lung fibroblasts with control inhibitors or specific inhibitors against miR-27a-3p, followed by treatment with TGF-β1. As shown in Figures 2D and 2E, knockdown of miR-27a-3p enhanced TGF-β1–induced expression of α-SMA, collagen 1, and fibronectin in lung fibroblasts. To further confirm the effect of miR-27a-3p silencing on the profibrotic activity of TGF-β1, we examined the activity of TGF-β1–responsive luciferase reporters that are under the control of the α-SMA or PAI-1 promoter in fibroblasts with or without miR-27a-3p knockdown. We found that TGF-β1–induced activation of both luciferase reporters was increased in fibroblasts with miR-27a-3p knockdown (Figure 2F). Taken together, these data suggest that miR-27a-3p functions in a negative-feedback loop to dampen TGF-β1–induced myofibroblast differentiation.
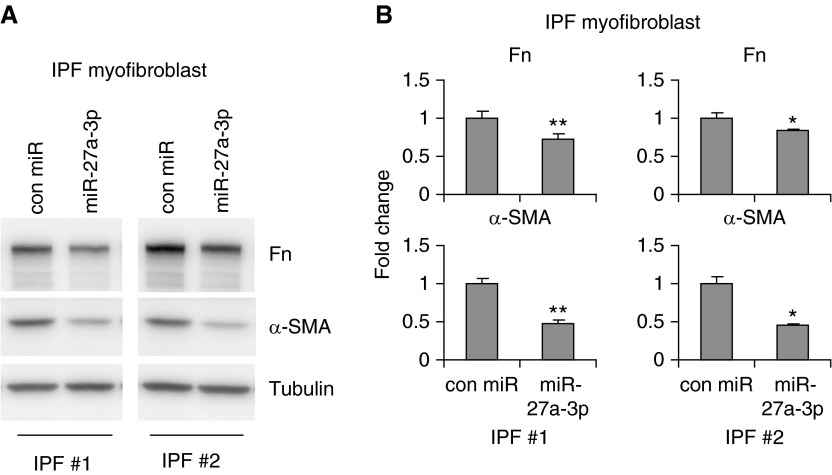
miR-27a-3p Inhibits the Profibrotic Activity of IPF Myofibroblasts
A pathologically defining feature of IPF lungs is the presence of a large number of fibrotic foci consisting of active myofibroblasts (34–36). IPF myofibroblasts are characterized by elevated fibrogenic activity, such as enhanced expression of smooth muscle actins, stress fiber formation, greater migration and contractility, and increased deposition of ECM proteins (34–38). To determine if miR-27a-3p regulated the fibrogenic activity of IPF myofibroblasts, we isolated fibroblasts from IPF lungs and transfected them with control mimics or miR-27a-3p mimics. As shown in Figures 3A and 3B, overexpression of miR-27a-3p in IPF myofibroblasts attenuated their profibrotic activities, as evidenced by diminished expression of α-SMA and fibronectin (Figure 3). These data suggest that increasing miR-27a-3p in myofibroblasts may demonstrate therapeutic benefits to curb pulmonary fibrosis.
Figure 3.
miR-27a-3p inhibits the profibrotic activity of IPF myofibroblasts. Human IPF fibroblasts were isolated as described in Materials and Methods. At passage 3–5, cells were transfected with 50 nM control mimics or mimics for miR-27a-3p for 48 hours. Levels of collagen 1, Fn, and α-SMA were determined by Western blotting (A), and densitometric analyses were performed using ImageJ (B). *P < 0.05, **P < 0.01 compared to control miR group. The experiments were performed two to three times with similar results.
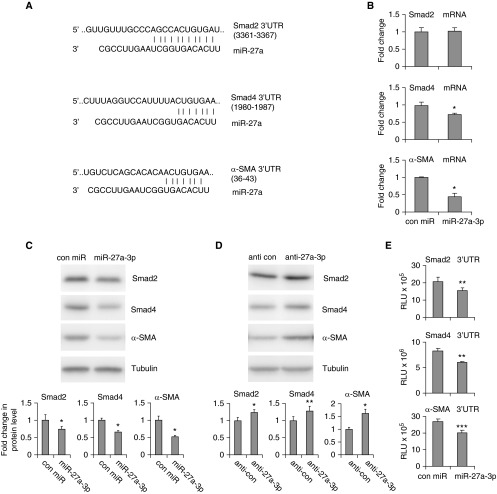
miR-27a-3p Directly Targets α-SMA, Smad2, and Smad4 in Human Lung Fibroblasts
miRs mainly function through regulating the expression of their target genes (20, 21). To delineate the mechanism by which miR-27a-3p regulates lung myofibroblast differentiation, we used the online program TargetScan (version 6.0; http://www.targetscan.org/vert_60/) to predict its targets. The potential miR-27a-3p targets that stood out were α-SMA and two key regulatory Smads, Smad2 and Smad4 (Figure 4A). Because these targets are known to participate in myofibroblast differentiation, they are plausible in mediating the antifibrotic role of miR-27a-3p. For this reason, we progressed to further characterize their regulations by miR-27a-3p.
Figure 4.
miR-27a-3p directly targets α-SMA, Smad2, and Smad4, in human lung fibroblasts. (A) Predicted targets of miR-27a-3p by TargetScan and illustrations of the putative miR-27a-3p binding sequences within the 3′ untranslated regions (UTRs) of the Smad2, Smad4, and α-SMA genes. (B and C) MRC-5 fibroblasts were transfected with 50 nM control mimics or mimics for miR-27a-3p. At 2 days after transfection, RNA was isolated and levels of Smad2, Smad4, and α-SMA determined by real-time PCR (B). Protein levels were determined by Western blotting and densitometric analyses performed using ImageJ software. (D) MRC-5 fibroblasts were transfected with 50 nM control inhibitors or inhibitors against miR-27a-3p. At 2 days after transfection, protein levels were determined by Western blotting and densitometric analyses performed using ImageJ software. (E) DNA fragments within the 3′ UTRs of the human α-SMA, Smad2, and Smad4 genes that contain the miR-27a-3p binding site were cloned into the luciferase reporter vector, pMIR-REPORT. Recombinant reporter constructs (5 ng) were cotransfected with 50 nM control mimics or mimics for miR-27a-3p. At 24 hours after transfection, luciferase activity in the cells was measured. (B–E) n = 3; mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control group. The experiments were performed three times with similar results. RLU, relative light units.
To answer this question, we transfected lung fibroblasts with control mimics or miR-27a-3p mimics, and examined the expression of the three genes at both the mRNA and protein levels. As shown in Figure 4B, the mRNA levels of Smad4 and α-SMA were decreased in fibroblasts transfected with miR-27a-3p. miR-27a-3p also down-regulated the protein levels of these two genes in the cells (Figure 4C). However, different from Smad4 and α-SMA, Smad2 was decreased by miR-27a-3p only at the protein level, and not the mRNA level (Figures 4B and 4C). Next, we determined the regulation of mRNA stability of these targets by miR-27a-3p in MRC-5 cells. As shown in Figure E2, the rate of Smad2 mRNA decay was comparable in cells that were transfected with control and miR-27a-3p mimics. These data suggest that miR-27a-3p inhibits the translation of Smad2 mRNA. In contrast, miR-27a-3p promoted α-SMA mRNA decay, indicating the reduced α-SMA protein level in miR-27a-3p–transfected fibroblasts results from increased degradation of the mRNAs in these cells. Altogether, these data suggest that miR-27a-3p regulates the expression of its targets through different mechanisms, namely by promoting cleavage and degradation of the Smad4 and α-SMA mRNAs or repressing translation of the Smad2 mRNA. On the contrary, knockdown of miR-27a-3p by specific inhibitors enhanced the endogenous expression of these putative targets in lung fibroblasts (Figure 4D).
Next, we examined if miR-27a-3p directly targets Smad2, Smad4, and α-SMA. To approach this, the 3′ UTRs of these genes were cloned downstream of a luciferase reporter and cotransfected with either control or miR-27a-3p mimics. As shown in Figure 4E, miR-27a-3p significantly repressed the luciferase activities of the reporters that contain the 3′ UTR of the Smad2, Smad4, and α-SMA genes. Together, these data indicate that miR-27a-3p directly targets Smad2, Smad4, and α-SMA, and thus inhibits myofibroblast differentiation.
miR-27a-3p Inhibits the Contractility of Lung Fibroblasts
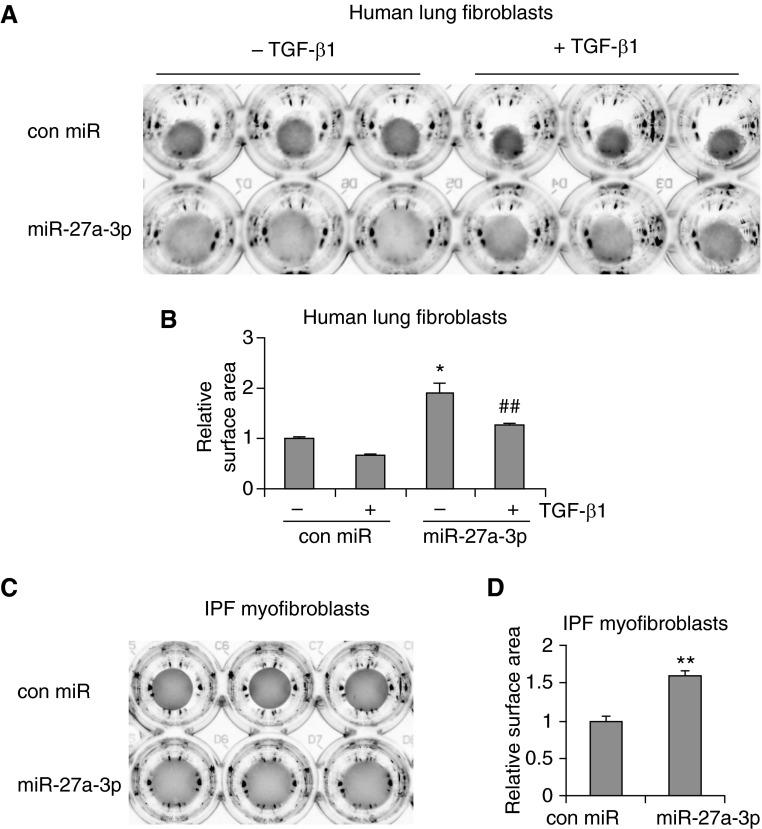
Enhanced contractility is another important profibrotic phenotype of lung myofibroblasts (34–36). α-SMA is one of the major components that are directly involved in the generation of contractile force (12, 39). We have shown that miR-27a-3p inhibits the basal and TGF-β1–induced expression of α-SMA in lung fibroblasts, indicating that miR-27a-3p regulates the contractility of lung fibroblasts. To test this hypothesis, we transfected human lung fibroblasts with control mimics or miR-27a-3p mimics and then evaluated their contractility by collagen gel contraction assays. As shown in Figures 5A and 5B, fibroblasts transfected with miR-27a-3p demonstrated decreased contraction of the collagen gels as compared with those transfected with control mimics. These data are concordant with the decreased α-SMA expression in the miR-27a-3p–transfected fibroblasts. miR-27a-3p also significantly diminished TGF-β1–induced contraction of collagen matrix by lung fibroblasts (Figures 5A and 5B). More importantly, miR-27a-3p overexpression attenuated the contractile activity of IPF myofibroblasts (Figures 5C and 5D).
Figure 5.
miR-27a-3p inhibits the contractility of lung fibroblasts. (A and B) Human lung fibroblasts MRC-5 were transfected with 50 nM control mimics or miR-27a-3p mimics. At 2 days after transfection, cells were trypsinized and resuspended in MEM that contained 1.5 mg/ml rat tail collagen. The mixtures were seeded into 48-well plates and incubated at 37°C for 30 minutes. The gels were then released from the wells and cells cultured in media that contained 0.1% FBS for 1 day. The cells were then treated with or without 4 ng/ml TGF-β1 for an additional 2 days. Images of the collagen gels were captured (A) and surface areas of the collagen gels determined and plotted (B). (C and D) IPF myofibroblasts were transfected with 50 nM control mimics or mimics for miR-27a-3p. At 2 days after transfection, cells were trypsinized and cultured in collagen-containing media. Images of the collagen gels were captured (C) and surface areas of the collagen gels determined and plotted (D). (A–D) n = 3; mean ± SD; *P < 0.05, **P < 0.01 compared with the untreated control miR group; ##P < 0.01 compared with the TGF-β1–treated control miR group. The experiments were performed three times with similar results.
Lentiviral Delivery of miR-27a-3p Attenuates Bleomycin-Induced Pulmonary Fibrosis in Mice
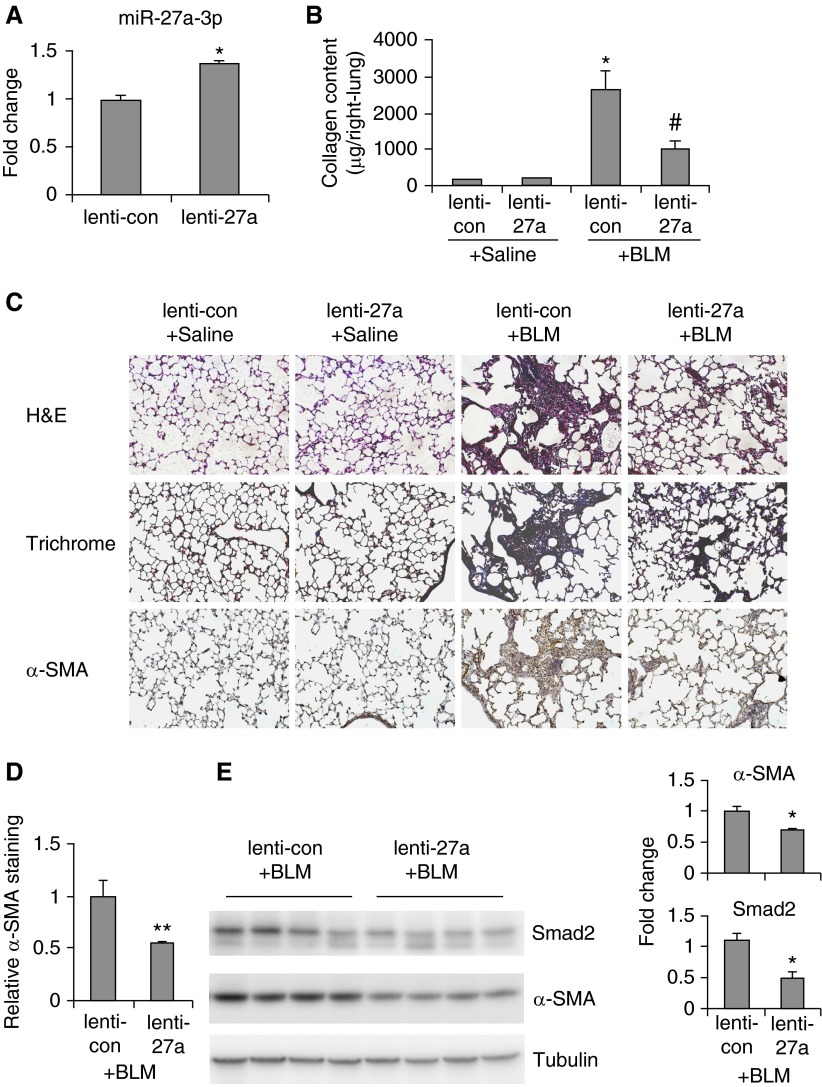
We have demonstrated previously here that miR-27a-3p is a negative regulator of myofibroblast differentiation. We next speculated if miR-27a-3p had a therapeutic efficacy to treat pulmonary fibrosis. To address this, mice were intratracheally instilled with bleomycin. At the 7th and 14th days after bleomycin administration, mice were given control lentiviruses or lentiviruses that express mouse miR-27a-3p (lenti-27a). At 3 weeks after bleomycin instillation, mice were killed and the severity of lung fibrosis was evaluated. First, we demonstrated that mice that received lenti-27a did have 1.5-fold increase of miR-27a-3p levels in the lungs compared with those given control virions (Figure 6A). Bleomycin instillation induced a marked increase of collagen deposition in the lungs. Most importantly, bleomycin-induced collagen deposition was significantly diminished in mice that received lenti-27a (Figure 6B).
Figure 6.
Lentiviral delivery of miR-27a-3p attenuates bleomycin induced pulmonary fibrosis in mice. C57BL/6 mice were intratracheally instilled with bleomycin (1.5 U/kg body weight in 50 μl saline). At Days 7 and 14 after bleomycin treatment, control lentiviruses (lenti-con) or lentiviruses that express miR-27a-3p precursors (lenti-27a) were intratracheally administered (3 × 106 TU/mouse in 50 μl PBS), respectively. At 3 weeks after bleomycin instillation, mice were killed and lungs collected. (A) Levels of miR-27a-3p in the lungs were determined by real-time PCR. (B) Collagen contents in right lungs were determined by Sircol collagen assays (n = 3, 3, 8, 8, respectively; mean ± SEM). *P < 0.05 compared with saline lenti-con group; #P < 0.05 compared with bleomycin lenti-con group. (C and D) Lung sections were prepared from experiments described previously here. Hematoxylin and eosin staining, Masson’s trichrome staining, and immunohistochemistry assay for α-SMA were performed (original magnification, ×20) (C). Quantification of α-SMA+ mesenchymal cells was performed by Image-Pro Plus software. **P < 0.01 compared with the bleomycin lenti-con group (D). (E) Levels of Smad2, α-SMA, and α-tubulin in the lungs were determined by Western blotting. Densitometric analyses were performed using ImageJ software. H&E, hematoxylin and eosin.
Therapeutic alleviation of bleomycin-induced lung fibrosis by miR-27a-3p was further confirmed by histological analysis of the lungs and Masson’s trichrome staining of lung collagen (Figure 6C). Corresponding with targeting of α-SMA by miR-27a-3p in fibroblasts in vitro, α-SMA expression in the lungs of bleomycin-treated mice that received lenti-27a was decreased (Figure 6C and 6D). We also examined which type of lung cell was infected by the injected lentivirus. Because the lentiviral construct used in the study is able to simultaneously express miR-27a-3p and the fluorescent marker, green fluorescent protein (GFP), in infected cells, we tracked lung cell populations that showed increased GFP fluorescence. As demonstrated in Figure E3, nearly all types of lung cells in bleomycin-treated mice had increased GFP fluorescence as compared with the background fluorescence in the lungs of bleomycin-treated mice that were not administered virions.
We have shown that miR-27a-3p directly targets Smad2, Smad4, and α-SMA in human lung fibroblasts (Figure 4). We then determined if miR-27a-3p acted in the same way in the lungs. miR-27a-3p was predicted by TargetScan to target mouse Smad2 and α-SMA, but not mouse Smad4. As shown in Figure 6D, levels of Smad2 and α-SMA were decreased in the lungs of mice that received miR-27a-3p expressing viruses compared with those that were given control viruses. Taken together, these data suggest that miR-27a-3p has similar antifibrotic activities in fibroblasts and lungs.
Discussion
miRs are small noncoding RNAs that play critical roles in various biological processes, such as cell proliferation, differentiation, apoptosis, and organ development (18–21, 40). There have been many successful efforts to harness miRs in treating human diseases, such as infectious and metabolic maladies (41, 42). The roles of miRs in respiratory diseases, including pulmonary fibrosis, have also attracted a great deal of interest in recent years (23, 25, 26, 30, 43–45). By targeting pro- or antifibrotic mediators, miRs can act as either negative or positive players in the pathogenesis of this disorder.
There is a growing list of miRs that has been shown to regulate the pathogenesis of pulmonary fibrosis (25, 26, 30, 43–45). For example, miR-26a and miR-21 regulates TGF-β1 activity through targeting Smad4 and Smad7, respectively, in lung fibroblasts (25, 44). miR-326 directly targets and represses the expression of TGF-β1 (43). miR-199a-5p promotes profibrotic activation of lung fibroblasts through both caveolin 1–dependent and –independent pathways (45). miR-145 promotes lung myofibroblast differentiation by targeting the negative regulator of α-SMA expression, Krüppel-like factor 4 (30). However, there is a lack of evidence that miRs are directly involved in myofibroblast differentiation. In the present study, we found that miR-27a-3p bound to the 3′ UTR of α-SMA and inhibited its expression in lung fibroblasts. We also discovered that miR-27a-3p targeted Smad2 and Smad4 in the TGF-β1 signaling cascade and decreased TGF-β1–induced myofibroblast differentiation. Therefore, the actions of miR-27a-3p appear to primarily center on regulating the myofibroblast phenotype. Given that myofibroblasts are well recognized therapeutic targets in designing treatments for IPF, it is definitely an intended focus to test the efficacy of miR-27a-3p introduction in this disease.
Although TGF-β1 is arguably the most important mediator of fibrotic organ disorders, therapies based on direct targeting of TGF-β1 have been unsatisfying (5). This is partly due to the fact that TGF-β1 is such a pleiotropic factor that participates in numerous essential cellular activities. Therefore, a complete blockade of TGF-β1 signaling could produce antagonistic effects, which will likely limit the efficacy of such an approach. However, as we have shown in the study that miR-27a-3p only partially down-regulates Smad2 and Smad4, strategies like this to fine tune TGF-β1 signaling by miRs may demonstrate advantages over direct targeting of TGF-β1 through antibodies or other approaches.
Of note, although we have shown that miR-27a-3p inhibits lung myofibroblast differentiation in vitro, the beneficial effect of miR-27a-3p introduction on bleomycin-induced pulmonary fibrosis may not be solely due to the diminished accumulation of myofibroblasts in the lungs. As we have shown previously here, lentiviruses that expressed miR-27a-3p also infected lung cell types other than interstitial fibroblasts, such as alveolar epithelial cells, endothelial cells, and macrophages, and most likely increased the levels of miR-27a-3p in, and affecting the phenotypes of, these cell populations. However, it remains to be determined how miR-27a-3p inhibits pulmonary fibrosis through affecting other pulmonary cells in addition to lung fibroblasts. Of note, miR-27a and miR-27b have been implicated in liver fibrosis as they demonstrate greater expression in, and decrease the proliferation of, activated hepatic stellate cells that play a critical role in the pathogenesis of this disease (46). This study, together with our findings, suggests that miR-27a has distinct roles in different types of cells and organs.
Despite the fact that we have demonstrated successful infection of lung cells by lenti-27a, as well as the resulting increase in pulmonary miR-27a-3p levels and attenuation in bleomycin-induced lung fibrosis, one should be aware that in vivo miR replacement/reconstitution in general faces more obstacles than miR blockage with regard to therapeutic translations (47). First, lack of cell-specific delivery of miR mimics risks potential off-target effects caused by uptake of the mimics by cells that normally do not express the miRs (47). It is also critical for miR replacement/reconstitution to restore their physiological levels, because miRs at supraphysiological levels could target gene sets that are quite different from those by miRs at endogenous levels, thus causing undesired side effects (48).
In all, we have identified another miR, miR-27a-3p, that has antifibrotic activity in treating pulmonary fibrosis. miR-27a-3p is induced by TGF-β1 in human lung fibroblasts and in fibroblasts isolated from fibrotic mouse lungs, whereas both basal and TGF-β1–induced expression of miR-27a-3p are reduced in lung fibroblasts from patients with IPF compared with that from normal control subjects. Mechanically, miR-27a-3p inhibits the profibrotic phenotypes of myofibroblasts through directly targeting α-SMA and the Smad transcriptional factors. Therefore, our study contributes significantly to the increasing pool of miR candidates for developing novel remedies to treat pulmonary fibrotic disorders.
Footnotes
This work was supported by National Institutes of Health grants HL105473 and HL076206 (G.L.), and HL114470 (V.J.T.).
Author Contributions: H.C. and G.L. designed the study; H.C., S.B., N.X., and J.G. performed the experiments; R.-M.L. contributed reagents; H.C., S.M., V.J.T., and G.L. wrote the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0205OC on November 24, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: ultimate and proximate causes. J Clin Invest. 2014;124:4673–4677. doi: 10.1172/JCI74368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkauskas CE, Noble PW. Cellular mechanisms of tissue fibrosis. 7. New insights into the cellular mechanisms of pulmonary fibrosis. Am J Physiol Cell Physiol. 2014;306:C987–C996. doi: 10.1152/ajpcell.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest. 2009;136:1364–1370. doi: 10.1378/chest.09-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol. 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chilosi M, Poletti V, Zamò A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, et al. Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan Z, Kui Z, Ping Z. Reviews and prospectives of signaling pathway analysis in idiopathic pulmonary fibrosis. Autoimmun Rev. 2014;13:1020–1025. doi: 10.1016/j.autrev.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Xu K, Moghal N, Egan SE. Notch signaling in lung development and disease. Adv Exp Med Biol. 2012;727:89–98. doi: 10.1007/978-1-4614-0899-4_7. [DOI] [PubMed] [Google Scholar]
- 9.Lekkerkerker AN, Aarbiou J, van Es T, Janssen RA. Cellular players in lung fibrosis. Curr Pharm Des. 2012;18:4093–4102. doi: 10.2174/138161212802430396. [DOI] [PubMed] [Google Scholar]
- 10.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122(6 suppl):286S–289S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 11.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 12.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-β 1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayaraman L, Massague J. Distinct oligomeric states of SMAD proteins in the transforming growth factor-β pathway. J Biol Chem. 2000;275:40710–40717. doi: 10.1074/jbc.M005799200. [DOI] [PubMed] [Google Scholar]
- 16.Leask A, Abraham DJ. TGF-β signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 17.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-β1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 19.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 20.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 21.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 22.Pandit KV, Milosevic J, Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res. 2011;157:191–199. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Pandit KV, Milosevic J. MicroRNA regulatory networks in idiopathic pulmonary fibrosis. Biochem Cell Biol. 2015;93:129–137. doi: 10.1139/bcb-2014-0101. [DOI] [PubMed] [Google Scholar]
- 24.Pagdin T, Lavender P. MicroRNAs in lung diseases. Thorax. 2012;67:183–184. doi: 10.1136/thoraxjnl-2011-200532. [DOI] [PubMed] [Google Scholar]
- 25.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lü J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui H, Tan Z, Banerjee S, Xie N, Antony V, Thannickal V, Liu G. Mir-27a is a negative regulator of lung fibrosis by targeting myofibroblast [abstract] Am J Respir Crit Care Med. 2015;A4925:191. [Google Scholar]
- 28.Liu G, Park YJ, Abraham E. Interleukin-1 receptor-associated kinase (IRAK)-1-mediated NF-κB activation requires cytosolic and nuclear activity. FASEB J. 2008;22:2285–2296. doi: 10.1096/fj.07-101816. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9:111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 30.Yang S, Cui H, Xie N, Icyuz M, Banerjee S, Antony VB, Abraham E, Thannickal VJ, Liu G. Mir-145 regulates myofibroblast differentiation and lung fibrosis. FASEB J. 2013;27:2382–2391. doi: 10.1096/fj.12-219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 32.Khalil N, Whitman C, Zuo L, Danielpour D, Greenberg A. Regulation of alveolar macrophage transforming growth factor-β secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J Clin Invest. 1993;92:1812–1818. doi: 10.1172/JCI116771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin TR, Matute-Bello G. Experimental models and emerging hypotheses for acute lung injury. Crit Care Clin. 2011;27:735–752. doi: 10.1016/j.ccc.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157:1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 35.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis: ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991;138:1257–1265. [PMC free article] [PubMed] [Google Scholar]
- 36.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 37.Ramos C, Montaño M, García-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24:591–598. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 38.Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, et al. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 41.Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93:98–104. doi: 10.1038/clpt.2012.192. [DOI] [PubMed] [Google Scholar]
- 42.Hydbring P, Badalian-Very G. Clinical applications of microRNAs. F1000 Res. 2013;2:136. doi: 10.12688/f1000research.2-136.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das S, Kumar M, Negi V, Pattnaik B, Prakash YS, Agrawal A, Ghosh B. MicroRNA-326 regulates profibrotic functions of transforming growth factor-β in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;50:882–892. doi: 10.1165/rcmb.2013-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang H, Xu C, Pan Z, Zhang Y, Xu Z, Chen Y, Li T, Li X, Liu Y, Huangfu L, et al. The antifibrotic effects and mechanisms of microrna-26a action in idiopathic pulmonary fibrosis. Mol Ther. 2014;22:1122–1133. doi: 10.1038/mt.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lino Cardenas CL, Henaoui IS, Courcot E, Roderburg C, Cauffiez C, Aubert S, Copin MC, Wallaert B, Glowacki F, Dewaeles E, et al. miR-199a-5p is upregulated during fibrogenic response to tissue injury and mediates TGFβ-induced lung fibroblast activation by targeting caveolin-1. PLoS Genet. 2013;9:e1003291. doi: 10.1371/journal.pgen.1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji J, Zhang J, Huang G, Qian J, Wang X, Mei S. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett. 2009;583:759–766. doi: 10.1016/j.febslet.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 47.van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med. 2014;6:851–864. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui H, Xie N, Thannickal VJ, Liu G. The code of non-coding RNAs in lung fibrosis. Cell Mol Life Sci. 2015;72:3507–3519. doi: 10.1007/s00018-015-1939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]